Abstract
Background and objectives
The burden of CKD is greater in ethnic and racial minorities and persons living in rural communities, where access to care is limited.
Design, setting, participants, & measurements
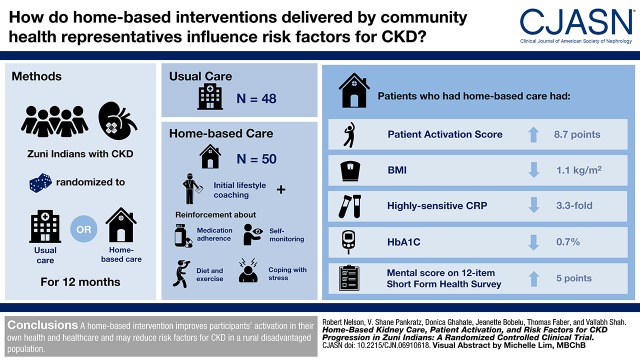
A 12-month clinical trial was performed in 98 rural adult Zuni Indians with CKD to examine the efficacy of a home-based kidney care program. Participants were randomized by household to receive usual care or home-based care. After initial lifestyle coaching, the intervention group received frequent additional reinforcement by community health representatives about adherence to medicines, diet and exercise, self-monitoring, and coping strategies for living with stress. The primary outcome was change in patient activation score, which assesses a participant’s knowledge, skill, and confidence in managing his/her own health and health care.
Results
Of 125 randomized individuals (63 intervention and 62 usual care), 98 (78%; 50 intervention and 48 usual care) completed the 12-month study. The average patient activation score after 12 months was 8.7 (95% confidence interval, 1.9 to 15.5) points higher in the intervention group than in the usual care group after adjusting for baseline score using linear models with generalized estimating equations. Participants randomized to the intervention had 4.8 (95% confidence interval, 1.4 to 16.7) times the odds of having a final activation level of at least three (“taking action”) than those in the usual care group. Body mass index declined by 1.1 kg/m2 (P=0.01), hemoglobin A1c declined by 0.7% (P=0.01), high-sensitivity C-reactive protein declined by 3.3-fold (P<0.001), and the Short-Form 12 Health Survey mental score increased by five points (P=0.002) in the intervention group relative to usual care.
Conclusions
A home-based intervention improves participants’ activation in their own health and health care, and it may reduce risk factors for CKD in a rural disadvantaged population.
Keywords: clinical trial; Epidemiology and outcomes; chronic kidney disease; outcomes; risk factors; C-Reactive Protein; Glycated Hemoglobin A; Patient Participation; Public Health; Vulnerable Populations; Linear Models; Body Mass Index; Mentoring; Renal Insufficiency, Chronic; Life Style; Chronic Disease, kidney; Diet
Visual Abstract
Introduction
CKD, defined by an eGFR of 15–59 ml/min per 1.73 m2 or a urine albumin-to-creatinine ratio ≥30 mg/g, affects nearly 30 million adults in the United States (1) and is associated with increased morbidity and mortality (2–4). The burden of CKD is greater in ethnic and racial minorities and persons living in rural communities, where access to care is limited (5). More than 17% of American Indians in the southwestern United States have CKD (6), and many of them also live in remote locations. Appropriated Indian Health Service funds cover only 55% of the cost of care for American Indians, and therefore, effective and low-cost approaches are needed to manage CKD in these disadvantaged populations.
The prevalence of CKD in the Zuni Indians is >2.5-fold higher than in the United States general population (7). A combination of geography, economic factors, cultural beliefs, and an underlying suspicion of Indian Health Service has hindered the delivery of health care in the Zuni Pueblo. In this study, we report the results of a pilot home-based kidney care treatment program for management of CKD in the Zuni Indians that used community health representatives under physician supervision to deliver state-of-the-art health care in the patient’s home environment using point-of-care technology. The goal of the study was to determine whether this approach might encourage these high-risk patients to take a more active role in their care and if a more active role might improve various clinical measures, including metrics of adherence to treatment and quality of life.
Materials and Methods
We performed a two-arm randomized clinical trial to assess the effect of home-based intervention versus usual care on patient activation and other clinical measures in Zuni Indians with CKD (ClinicalTrials.gov number NCT02915029). All participants in the intervention group received the same curriculum-based intervention. Baseline patient activation level was not used to tailor the intervention. This home-based intervention was developed using information from focus groups performed as part of the Zuni Health Initiative (8) and experience gained while recruiting participants for both the Zuni Kidney Project (7) and the Zuni Health Initiative, which indicated that the Zuni people strongly prefer their health care to be delivered in their home environment. We, therefore, used home-based care as the cornerstone of this comparative effectiveness study.
To develop the intervention, we worked with the Zuni tribal leadership and other health programs in the Zuni Pueblo to create a Tribal Advisory Panel. The panel met quarterly throughout the study; it provided community input into the design of the clinical trial and actively monitored the progress of the study, advising the investigators on recruitment strategies and how to improve health literacy about diabetes and kidney disease in the community. The intervention, therefore, incorporated the culture and traditions of the Zuni Indians into the program of care, which was intended to bolster levels of kidney-specific knowledge, self-efficacy, and CKD self-management, enabling the participants to more effectively carry out the recommendations of their health care providers.
The intervention consisted of biweekly home visits by two community health representatives employed by the University of New Mexico who provided education on healthy lifestyles that focused on diet; exercise; alcohol abuse and smoking; and management of diabetes, hypertension, and hyperlipidemia. They also performed data collection, data entry, and community engagement activities. Descriptions of the training received by the community health representatives, the intervention that they provided, and the standardization of the intervention are found in Supplemental Material. Further details are also found at https://www.pcori.org/research-results/2013/reducing-health-disparity-chronic-kidney-disease-zuni-indians.
Participants in the intervention arm were trained by the community health representatives to use home BP and glucose monitors as a means of encouraging them to take a more active role in their health care. Community health representatives also provided educational pamphlets from the Indian Health Service, the National Kidney Foundation, the American Association of Kidney Patients, the National Kidney Disease Education Program, and the American Diabetes Association. Additional educational materials were prepared by the investigators and given to those in the intervention group. Point-of-care testing for glycated hemoglobin A1c (HbA1c) and albuminuria was conducted in the participants’ homes at the 3‐ and 9‐month follow‐up visits, and the results of these tests were reported to the participant along with an explanation of their implications. About 200 lifestyle and diet-related motivational messages were transmitted by the community representatives to each participant in the intervention group via smartphone during the first 4–6 months of the intervention. Examples of those messages are shown in Supplemental Table 1. Participants in the intervention arm were also invited to attend quarterly group sessions at the Zuni Health Initiative Clinic located adjacent to the community that were facilitated by the community health representatives.
Participants randomized to the usual care group received standard clinical care and lifestyle advice from their Indian Health Service providers. They also received publicly available information about diabetes prevention, weight loss, diet, and exercise consistent with current recommendations for a healthy lifestyle. They did not have direct contact with study staff aside from the data collection visits at baseline and 6 and 12 months.
Participants
Potential participants were identified using clinical data obtained from a previously established cohort (9). In that study, 1416 individuals were screened for relevant clinical factors, and a subset of them was enrolled as families. In this study, 315 individuals who were members of families enrolled in that cohort were screened for eligibility; 127 met criteria for inclusion in the study, which included being between 21 and 80 years of age and having a urine albumin-to-creatinine ratio ≥30 mg/g and at least one other risk factor that included body mass index (BMI) >30 kg/m2, HbA1c>7.0%, or a family history of diabetes and kidney disease. Two individuals declined to participate, and the remaining 125 were enrolled in the study.
At baseline, participants were randomized by the community health representatives to either the usual care group or the intervention group according to the randomization sequence described below. Because more than one person in a household could participate, we randomized households in a 1:1 allocation to the intervention or usual care group to ensure that members of the same household were not allocated to different treatment groups. The randomization sequence was generated using the PROC PLAN procedure in SAS to permute the two levels of treatment randomly and without replacement within blocks containing two, four, or six households. Ninety-six households were enrolled in this study; 39 participants enrolled in the usual care group and 37 participants enrolled in the intervention group were from single-participant households. Neither the investigators nor the participants were blinded at randomization, because it was clear which participants were receiving the behavioral intervention, but outcome measures were assessed by individuals who had no knowledge of the treatment assignment. Community representatives were assigned to ensure that the same representative did not work with members of extended families in different households. All participants received health evaluations by study personnel at baseline and 6 and 12 months. These evaluations included measurements of height without shoes and weight in light indoor clothing. BP was measured three times about 5 minutes apart with the participants resting in a seated position, and the results were averaged. Education level was obtained by self-report.
This study was approved by the University of New Mexico Health Sciences Center Human Research Review Committee and the Indian Health Service Institutional Review Board, and it adhered to the ethical principles in the Declaration of Helsinki. All participants provided written consent.
Laboratory Testing
Serum and urine creatinine values were measured by an enzymatic method, and eGFR was computed using the Chronic Kidney Disease Epidemiology Collaboration equation (10). Urine albumin was measured by nephelometric immunoassay, and all concentrations were above the detection limit of the assay (5.0 mg/L). HbA1c was measured by high-performance liquid chromatography. Serum total cholesterol, HDL cholesterol, and triglycerides were measured using enzymatic methods, and LDL was calculated using the Friedewald equation.
Study Outcomes
The patient activation score was the primary outcome of the clinical trial. The patient activation measure is a validated tool that assesses a patient’s ability to effectively participate in their care (11–13). The 13 questions in the short form questionnaire use a categorical agreement scale with four response options of (1) strongly disagree, (2) disagree, (3) agree, and (4) strongly agree as well as not applicable. The raw score is calculated by adding responses to the 13 questions. If all questions are answered, the range of raw scores is from 13 to 52. If there is at least one item with a response of not applicable, the total score is divided by the number of items completed and multiplied by 13 to yield a normalized raw score. A nomogram provided under a licensing agreement (Insignia Health, Portland, OR) converts raw scores to an “activation score” ranging from zero to 100 with a classification level between one and four: level 1, believing that the patient’s role is important but not taking action; level 2, having the confidence and knowledge necessary to take action; level 3, taking action to maintain and improve one’s health; and level 4, staying the course even under stress.
Several secondary clinical outcomes were also assessed. All screening and follow‐up laboratory results were shared with the participant’s primary care physician who was also notified of any change in the participant’s clinical status during the study. Data on hospitalizations and other health care utilization were not collected in this 12-month pilot study.
Health-related quality of life was assessed by the Kidney Disease Quality of Life Survey (KDQOL) (14,15). The Morisky scale was used to assess adherence with prescribed medicines (16). Higher scores for each of these scales reflected improved quality of life and greater adherence to prescribed medicines, respectively.
Statistical Analyses
We designed the study to detect between-group differences in postintervention patient activation scores corresponding to an expected between-group difference in meaningful clinical outcomes of at least 20%. Because a one-point difference in patient activation score can be equated with a 2% improvement in clinical outcomes (http://www.insigniahealth.com/products/pam-survey), we, therefore, powered the study to detect a between-group difference of nine points. Assuming an SD of 14 points (17), we estimated that 30 households per arm, each with two participants and a within-household correlation of 0.50, would provide at least 80% power to detect this difference in the total patient activation score between the treatment groups. If all participants were recruited from individual households, the detectable difference in total score would be seven points between the treatment groups, corresponding to an expected improvement of 16% in meaningful clinical outcomes.
Clinical features at baseline were expressed as mean (SD), median (interquartile range), or n (percentage). The primary analysis of efficacy of the study treatment compared changes in activation score from baseline to 12 months between treatment groups using linear models that accounted for within-family clustering via generalized estimating equations. The same approach was used to test differences in secondary outcomes between groups after applying transformations when necessary to meet the modeling assumptions of normality and homoscedasticity of residuals. Changes over time in each variable of interest were made while adjusting for the baseline values of those variables to account for correlations between baseline measurements and the observed changes from baseline. A logistic regression model, with generalized estimating equations to account for household clustering, was used to estimate the odds of participants in the intervention group having activation levels of greater than or equal to three at 12 months relative to usual care while adjusting for each participant’s baseline activation level. We did not apply corrections for multiple comparisons for the secondary outcomes. Analyses were performed in the R statistical package (version 3.4.4) (18), and P values below 0.05 were considered statistically significant.
Results
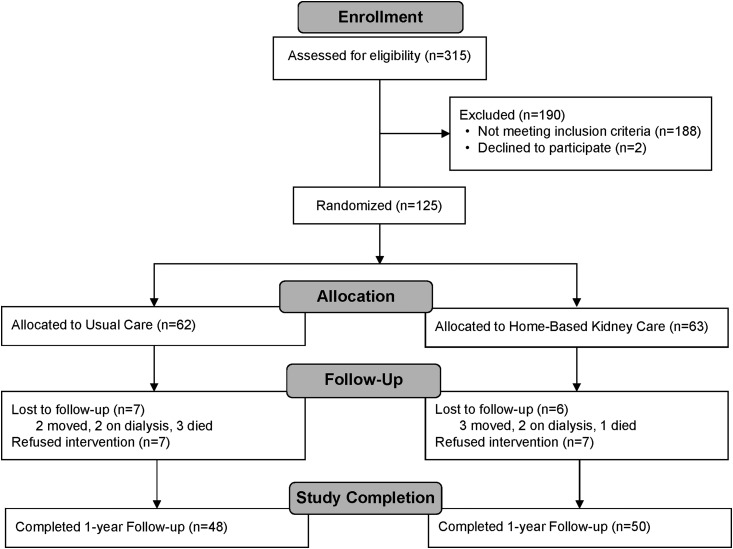
Of the 125 participants enrolled in the study, 63 were randomized to the intervention group, and 62 were randomized to usual care (the Consolidated Standards of Reporting Trials diagram is shown in Figure 1). Table 1 shows baseline characteristics for the two randomized groups. Thirty-three (52%) participants in the intervention group and 39 (63%) participants in the usual care group had type 2 diabetes, and all participants had a urine albumin-to-creatinine ratio ≥30 mg/g. Baseline characteristics were similar in both groups. Of those enrolled into the study, 98 (78%) completed the 12-month follow-up period. Fourteen of those not included in the analysis were allocated to usual care, and 13 were allocated to the intervention. Seven participants in each group refused to complete the study, with the remainder withdrawing for other reasons. Results for these 98 individuals (48 in the usual care group and 50 in the intervention group) were used to evaluate the effectiveness of the intervention at 12 months. Baseline characteristics of the 98 participants with complete study data were virtually identical to those of the 27 participants who did not complete the study (Supplemental Table 2).
Figure 1.
The flow chart of the study population describes the enrollment, randomization, and follow-up of the study participants according to treatment assignment. All participants who completed the baseline and 12-month follow-up visit were included in the analysis.
Table 1.
Baseline characteristics of the participants by treatment group
| Characteristic | Usual Care, n=62 | Home-Based Kidney Care, n=63 | ||
|---|---|---|---|---|
| Mean or N | SD or % | Mean or N | SD or % | |
| Age, yr | 48 | 12 | 46 | 11 |
| Women, % | 26 | 42 | 31 | 49 |
| Body mass index, kg/m2 | 32 | 7 | 32 | 8 |
| Diabetes, % | 39 | 63 | 33 | 52 |
| BP, mm Hg | ||||
| Systolic | 132 | 19 | 129 | 17 |
| Diastolic | 85 | 13 | 83 | 13 |
| Hypertension | 42 | 68 | 36 | 63 |
| High school graduate, % | 37 | 60 | 41 | 66 |
| HbA1c, % | 7.5 | 2.3 | 7.5 | 2.6 |
| Serum total protein, g/dl | 7.6 | 0.6 | 7.7 | 0.6 |
| Serum cholesterol, mg/dl | 181 | 38 | 201 | 58 |
| Serum triglycerides, mg/dla | 133 | 90–200 | 139 | 100–182 |
| Serum HDL cholesterol, mg/dla | 50 | 39–63 | 46 | 39–62 |
| Serum LDL cholesterol, mg/dl | 109 | 32 | 117 | 42 |
| eGFR, ml/min per 1.73 m2 | 101 | 29 | 105 | 31 |
| Urine ACR, mg/ga | 190 | 57–714 | 139 | 57–377 |
| hsCRP, mg/La | 2.6 | 1.3–4.1 | 3.8 | 0.9–10.0 |
| Morisky scoreb | 4.6 | 2.3 | 5.4 | 2.3 |
| KDQOL measures | ||||
| Symptom/problem list | 86.6 | 14.4 | 86.4 | 11.3 |
| Effects of kidney disease | 93.2 | 12.0 | 92.7 | 7.3 |
| Burden of kidney disease | 74.3 | 23.8 | 70.3 | 20.5 |
| SF-12 physical score | 45.4 | 8.5 | 45.5 | 9.5 |
| SF-12 mental score | 51.2 | 10.0 | 47.4 | 9.6 |
| Patient activation total score | 65.1 | 14.6 | 60.7 | 20.5 |
| Patient activation level ≥3 | 52 | 84 | 43 | 68 |
HbA1c, hemoglobin A1c; ACR, albumin-to-creatinine ratio; hsCRP, high-sensitivity C-reactive protein; KDQOL, Kidney Disease Quality of Life Survey; SF-12, Short-Form 12 Health Survey.
Median and interquartile range.
Use of the Morisky Medication Adherence Scale is protected by United States copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, Department of Community Health Sciences, University of California, Los Angeles School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095–1772.
Adherence was satisfactory in the intervention group. Forty-five of the 50 (90%) participants who completed the study in the intervention group received at least 20 of the 24 biweekly home visits, and 40 (80%) were present at all four group sessions. No adherence data were available for those receiving usual care.
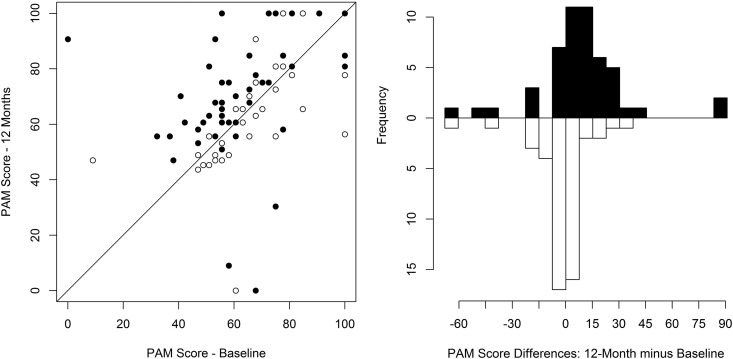
Primary Study Outcome
The relationships between the baseline and 12-month patient activation total scores for the usual care and intervention groups are shown in Figure 2. The mean (SD) activation scores at baseline among those completing the study were 64.3 (15.3) and 61.1 (21.2) in the usual care and intervention groups, respectively. After 12 months, the mean scores were 62.9 (17.1) and 70.3 (21.6), respectively. After adjusting for baseline scores, the average activation score was 8.7 points higher (95% confidence interval [95% CI], 1.9 to 15.5; P=0.01) in the intervention group than in the usual care group (Table 2), showing a statistically significant beneficial effect of the intervention on the primary outcome. Moreover, the odds that participants randomized to the intervention group had a poststudy activation of level 3 or higher was 4.8 times as high as in those in the usual care group (95% CI, 1.4 to 16.7; P=0.01) after controlling for their baseline activation level. Differences in outcome measures between the baseline and 12-month visits in the intervention and usual care groups are shown in Supplemental Table 3.
Figure 2.
The patient activation score improved in those receiving home-based kidney care during the 12-month intervention but did not improve in those receiving standard care. The left panel shows absolute values at baseline and after 12 months of treatment. Participants will fall along the diagonal line if there is no change in patient activation score over time. The white circles represent the usual care group, and the black circles represent the intervention group. The right panel shows the change from baseline by treatment group. The white bars represent the usual care group, and the black bars represent the intervention group. PAM, patient activation measure.
Table 2.
Comparison of direct (intervention-usual care) or relative (intervention/usual care) changes in clinical measures between treatment groups in the participants who completed the study
| Characteristic | Treatment Effect of Intervention Compared with Usual Carea | ||
|---|---|---|---|
| Difference | 95% Confidence Interval | P Value | |
| Primary outcome measures | |||
| Patient activation total score | 8.7 | 1.9 to 15.5 | 0.01 |
| Patient activation level ≥3b | 4.8 | 1.4 to 16.7 | 0.01 |
| Secondary outcome measures | |||
| Body mass index, kg/m2 | −1.1 | −1.9 to −0.3 | 0.01 |
| BP, mm Hg | |||
| Systolic | −2.9 | −8.6 to 2.8 | 0.32 |
| Diastolic | −4.6 | −12.3 to 3.1 | 0.24 |
| HbA1c, % | −0.7 | −1.3 to −0.2 | 0.01 |
| Serum total protein, g/dl | −0.1 | −0.2 to 0.1 | 0.26 |
| Serum cholesterol, mg/dl | 5.3 | −42.1 to 52.7 | 0.83 |
| Serum triglycerides, mg/dlc | 1.0 | 0.8 to 1.2 | 0.74 |
| Serum HDL cholesterol, mg/dlc | 1.0 | 0.9 to 1.2 | 0.45 |
| Serum LDL cholesterol, mg/dl | 18.5 | −19.3 to 56.3 | 0.34 |
| eGFR, ml/min per 1.73 m2 | 4.7 | −0.5 to 9.9 | 0.08 |
| Urine ACR, mg/gc | 0.6 | 0.3 to 1.2 | 0.15 |
| hsCRP, mg/Lc | 0.3 | 0.2 to 0.5 | <0.001 |
| Morisky scored | −0.4 | −1.1 to 0.4 | 0.34 |
| KDQOL measures | |||
| Symptom/problem list | −2.5 | −7.0 to 2.0 | 0.28 |
| Effects of kidney disease | 0.2 | −2.4 to 2.7 | 0.91 |
| Burden of kidney disease | 8.1 | −0.3 to 16.4 | 0.06 |
| SF-12 physical score | 1.8 | −1.3 to 4.9 | 0.26 |
| SF-12 mental score | 5.0 | 1.8 to 8.2 | 0.002 |
HbA1c, hemoglobin A1c; ACR, albumin-to-creatinine ratio; hsCRP, high-sensitivity C-reactive protein; KDQOL, Kidney Disease Quality of Life Survey; SF-12, Short-Form 12 Health Survey.
Adjusted for the baseline value of each clinical characteristic.
Odds ratio reported for this categorical variable.
Relative differences reported as fold changes. Relative differences greater than one reflect larger positive changes in home-based kidney care than usual care, whereas relative differences less than one reflect larger negative changes in home-based kidney care than usual care.
Use of the Morisky Medication Adherence Scale is protected by United States copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, Department of Community Health Sciences, University of California, Los Angeles School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095–1772.
Prespecified Secondary Study Outcomes
One year of intervention improved several clinical characteristics relative to usual care (Table 2). Mean (SD) BMI declined by 0.2 (1.9) kg/m2 in the usual care group and 1.3 (2.1) kg/m2 in the intervention group for an average decline that was 1.1 kg/m2 (95% CI, 0.3 to 1.9; P=0.01) greater in the intervention group than in the usual care group after adjusting for baseline BMI. Mean (SD) HbA1c levels increased, on average, by 0.1% (1.4%) and median (interquartile range) high-sensitivity C-reactive protein (hsCRP) concentrations increased by 1.7 (0.5–6.5) mg/L in the usual care group; corresponding declines of 0.5% (1.4%) and 1.5 (0–5.8) mg/L, respectively, were observed in the intervention group. After adjustment for baseline levels, participants in the intervention had their HbA1c levels decline by 0.7% more than those in the usual care group (P=0.01), and hsCRP levels in the intervention group averaged 31% of the levels observed in the usual care group (P<0.001). For quality of life indicators, the average Short-Form 12 Health Survey (SF-12) mental score increased by 5.0 more points (95% CI, 1.8 to 8.2; P=0.002) in the intervention group than in the usual care group. None of the remaining differences in clinical measures were statistically significant (Table 2).
Adverse Events
No adverse events related to the study or the intervention were reported during the clinical trial. Four participants initiated dialysis during the study: two in the intervention group and two in the usual care group. There were four deaths: one in the intervention group and three in the usual care group.
Discussion
Zuni Indian adults in the home-based kidney care intervention group experienced significantly greater improvement in the primary outcome of patient activation than participants in the usual care group by the end of the 12-month intervention. Our pilot study was not designed to determine which components of the intervention contributed to the beneficial changes in patient activation. However, the findings suggest that the intervention empowered patients to become more active participants in their care than those assigned to usual care. One factor that may have beneficially affected the activation scores was the use of health representatives who were members of the participant community and therefore, knowledgeable about the culture, language, resources, and barriers encountered in this remote community. By administering the intervention using community health representatives, we hoped to provide education in a culturally sensitive manner and at a level appropriate for the target population. Numerous studies show beneficial outcomes from having community health representatives as part of the health care team (19–22). The intervention was also associated with general improvement in a range of clinical measures and quality of life indicators. Statistically significant declines in BMI, HbA1c, and hsCRP, a marker of chronic inflammation in which a higher concentration reflects greater inflammation, and an increase in the SF-12 KDQOL indicator were observed in the intervention group relative to those receiving usual care, suggesting that patient activation could have health benefits.
The patient activation instrument has been validated in several populations (12,13), including our previous work in Zuni Indians with diabetes (23). Patient activation scores reflect a patient’s general approach to their health. Patients with lower activation scores do not take control of their own health and often lack basic knowledge about their condition, whereas patients with higher activation scores tend to possess the knowledge, skills, and confidence to self‐manage their disease, even under adverse circumstances. In addition, patients with higher activation scores are more likely to exercise on a regular basis, eat a healthy diet, and abstain from smoking, resulting in better self‐reported health and health outcomes and lower rates of health care utilization, such as emergency department use and hospitalization (24–32). Past studies report that activation levels can be increased with education and appropriate intervention (33,34), but they have not shown this increase to translate into improvements in clinical measures of diabetes and CKD (33,34). A cross-sectional study of adults with controlled (HbA1c≤7%) and uncontrolled (HbA1c>7%) type 2 diabetes showed that high levels of patient activation correlated with self‐management behaviors but did not correlate with glycemic control, and it concluded that, for patient activation measures to affect glycemic control, the highest level of activation may need to be achieved (25).
This study adds to our understanding of patient activation among Zuni Indians with CKD. The mean patient activation level of respondents was >60 on a scale of 0–100, and it was in accordance with activation levels among people with diabetes and other chronic diseases (31,35–40).
Strengths of this pilot study included the use of a well tolerated intervention designed to be sensitive to the culture and beliefs of the individuals being studied. Engaging the community in the study design and employing community members to deliver the intervention may have contributed to its success. Although an intervention that focuses on the culture and beliefs of a specific group of people may not be relevant to another community, the approach to developing a community-specific intervention may be generalizable. The small sample size, short follow-up period, and lack of interventions tailored to the baseline patient activation score (34) are clear limitations of this study, but the knowledge gained from this study will be used to implement a larger study of home-based kidney care in another high-risk population. Another limitation is that 22% of those enrolled and randomized did not complete the 1-year intervention, despite efforts to make the study appealing to community members. Attrition was not related to enrollment in the intervention group, because the level of attrition was comparable in the intervention and usual care groups. Although the usual care group was intended to receive treatment routinely provided to members of the community, any additional attention that they received as part of the clinical trial would likely reduce the differences between the treatment groups. Information regarding hospitalizations and other health care utilization, which could affect patient activation scores, was not collected as part of this study, but any effect of this health care utilization should be balanced between the randomized treatment groups, limiting its effect on the differences observed between groups.
In conclusion, we found that patient activation was greater in Zuni Indians who received home-based kidney care from trained Zuni health representatives than in those who received usual care. Trained community health representatives successfully promoted the patients’ active involvement in their daily diabetes and/or CKD care. These results suggest that interventions involving trained community members are efficacious in enhancing the educational process and getting patients more involved in their own care. Higher activation was also associated with modest improvement in several clinical and KDQOL measures, including decreases in BMI, HbA1c, and hsCRP and an increase in mental health quality of life assessed by the SF-12 questionnaire. These findings suggest that delivery of effective care for complex diseases is possible using local community resources. Demonstration of these findings in other high-risk populations will help us determine whether this approach to patient care should be adopted in clinical practice.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Kathleen Colleran and Dr. Bruce Struminger for their role in the design and conduct of the study. We also thank the tribal stakeholders, including the Zuni tribal Governor and his council members, as well as the tribal advisory panel members who contributed to the logistics of study-related activities and the development of this manuscript. Finally, we sincerely thank the Zuni people for welcoming us into their lives. Individual deidentified participant data will be shared according to the policies of the Patient-Centered Outcomes Research Institute (PCORI) as described at https://www.pcori.org/research-results/2013/reducing-health-disparity-chronic-kidney-disease-zuni-indians. The data will become available in September 2018. Related documents, including the study protocol, will be available at the same website.
This research was supported by PCORI award AD-12-11-5532 (to V.O.S.) and the Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases.
Parts of this study were presented in abstract form at the Annual Meeting of the American Society of Nephrology November 2–5, 2017 in New Orleans, Louisiana and the 78th Scientific Sessions of the American Diabetes Association June 22–26, 2018 in Orlando, Florida.
D.M.G. and J.B. are Zuni community members and stakeholders.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06910618/-/DCSupplemental.
References
- 1.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, Barregård L, Barrett-Connor E, Basu S, Bello AK, Bensenor I, Bergstrom J, Bikbov B, Blosser C, Brenner H, Carrero JJ, Chadban S, Cirillo M, Cortinovis M, Courville K, Dandona L, Dandona R, Estep K, Fernandes J, Fischer F, Fox C, Gansevoort RT, Gona PN, Gutierrez OM, Hamidi S, Hanson SW, Himmelfarb J, Jassal SK, Jee SH, Jha V, Jimenez-Corona A, Jonas JB, Kengne AP, Khader Y, Khang YH, Kim YJ, Klein B, Klein R, Kokubo Y, Kolte D, Lee K, Levey AS, Li Y, Lotufo P, El Razek HMA, Mendoza W, Metoki H, Mok Y, Muraki I, Muntner PM, Noda H, Ohkubo T, Ortiz A, Perico N, Polkinghorne K, Al-Radaddi R, Remuzzi G, Roth G, Rothenbacher D, Satoh M, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Ukwaja K, Umesawa M, Vollset SE, Warnock DG, Werdecker A, Yamagishi K, Yano Y, Yonemoto N, Zaki MES, Naghavi M, Forouzanfar MH, Murray CJL, Coresh J, Vos T; Global Burden of Disease 2013 GFR Collaborators; CKD Prognosis Consortium; Global Burden of Disease Genitourinary Expert Group : Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28: 2167–2179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schöttker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Ärnlöv J; CKD Prognosis Consortium : Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris K, Nissenson AR: Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19: 1261–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sánchez-Lozada LG, Johnson RJ: Diabetes and kidney disease in American Indians: Potential role of sugar-sweetened beverages. Mayo Clin Proc 90: 813–823, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Scavini M, Stidley CA, Paine SS, Shah VO, Tentori F, Bobelu A, Welty TK, MacCluer JW, Zager PG: The burden of chronic kidney disease among the Zuni Indians: The Zuni Kidney Project. Clin J Am Soc Nephrol 2: 509–516, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Shah VO, Ghahate DM, Bobelu J, Sandy P, Newman S, Helitzer DL, Faber T, Zager P: Identifying barriers to healthcare to reduce health disparity in Zuni Indians using focus group conducted by community health workers. Clin Transl Sci 7: 6–11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacCluer JW, Scavini M, Shah VO, Cole SA, Laston SL, Voruganti VS, Paine SS, Eaton AJ, Comuzzie AG, Tentori F, Pathak DR, Bobelu A, Bobelu J, Ghahate D, Waikaniwa M, Zager PG: Heritability of measures of kidney disease among Zuni Indians: The Zuni Kidney Project. Am J Kidney Dis 56: 289–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibbard JH, Mahoney ER, Stockard J, Tusler M: Development and testing of a short form of the patient activation measure. Health Serv Res 40: 1918–1930, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D: Promoting glycemic control through diabetes self-management: Evaluating a patient activation intervention. Patient Educ Couns 56: 28–34, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Stepleman L, Rutter MC, Hibbard J, Johns L, Wright D, Hughes M: Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil 32: 1558–1567, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Ricardo AC, Hacker E, Lora CM, Ackerson L, DeSalvo KB, Go A, Kusek JW, Nessel L, Ojo A, Townsend RR, Xie D, Ferrans CE, Lash JP; CRIC Investigators : Validation of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36) US Spanish and English versions in a cohort of Hispanics with chronic kidney disease. Ethn Dis 23: 202–209, 2013 [PMC free article] [PubMed] [Google Scholar]
- 16.Morisky DE, Ang A, Krousel-Wood M, Ward HJ: Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 10: 348–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Moljord IE, Lara-Cabrera ML, Perestelo-Pérez L, Rivero-Santana A, Eriksen L, Linaker OM: Psychometric properties of the Patient Activation Measure-13 among out-patients waiting for mental health treatment: A validation study in Norway. Patient Educ Couns 98: 1410–1417, 2015 [DOI] [PubMed] [Google Scholar]
- 18.R Core Team : R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2017 [Google Scholar]
- 19.Brownstein JN, Bone LR, Dennison CR, Hill MN, Kim MT, Levine DM: Community health workers as interventionists in the prevention and control of heart disease and stroke. Am J Prev Med 29[Suppl 1]: 128–133, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Thompson JR, Horton C, Flores C: Advancing diabetes self-management in the Mexican American population: A community health worker model in a primary care setting. Diabetes Educ 33[Suppl 6]: 159S–165S, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, Jack L Jr., Satterfield DW: Effectiveness of community health workers in the care of persons with diabetes. Diabet Med 23: 544–556, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Spencer MS, Kieffer EC, Sinco B, Piatt G, Palmisano G, Hawkins J, Lebron A, Espitia N, Tang T, Funnell M, Heisler M: Outcomes at 18 months from a community health worker and peer leader diabetes self-management program for Latino adults. Diabetes Care 41: 1414–1422, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah VO, Carroll C, Mals R, Ghahate D, Bobelu J, Sandy P, Colleran K, Schrader R, Faber T, Burge MR: A home-based educational intervention improves patient activation measures and diabetes health indicators among Zuni Indians. PLoS One 10: e0125820, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbard JH, Greene J, Shi Y, Mittler J, Scanlon D: Taking the long view: How well do patient activation scores predict outcomes four years later? Med Care Res Rev 72: 324–337, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Mayberry R, Willock RJ, Boone L, Lopez P, Qin H, Nicewander D: A high level of patient activation is observed but unrelated to glycemic control among adults with type 2 diabetes. Diabetes Spectr 23: 171–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell SE, Gardiner PM, Sadikova E, Martin JM, Jack BW, Hibbard JH, Paasche-Orlow MK: Patient activation and 30-day post-discharge hospital utilization. J Gen Intern Med 29: 349–355, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibbard JH, Mahoney ER, Stock R, Tusler M: Do increases in patient activation result in improved self-management behaviors? Health Serv Res 42: 1443–1463, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parchman ML, Zeber JE, Palmer RF: Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: A STARNet study. Ann Fam Med 8: 410–417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rost KM, Flavin KS, Cole K, McGill JB: Change in metabolic control and functional status after hospitalization. Impact of patient activation intervention in diabetic patients. Diabetes Care 14: 881–889, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Rask KJ, Ziemer DC, Kohler SA, Hawley JN, Arinde FJ, Barnes CS: Patient activation is associated with healthy behaviors and ease in managing diabetes in an indigent population. Diabetes Educ 35: 622–630, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Nijman J, Hendriks M, Brabers A, de Jong J, Rademakers J: Patient activation and health literacy as predictors of health information use in a general sample of Dutch health care consumers. J Health Commun 19: 955–969, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Greene J, Hibbard JH: Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 27: 520–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terry PE, Fowles JB, Xi M, Harvey L: The ACTIVATE study: Results from a group-randomized controlled trial comparing a traditional worksite health promotion program with an activated consumer program. Am J Health Promot 26: e64–e73, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Hibbard JH, Greene J, Tusler M: Improving the outcomes of disease management by tailoring care to the patient’s level of activation. Am J Manag Care 15: 353–360, 2009 [PubMed] [Google Scholar]
- 35.Remmers C, Hibbard J, Mosen DM, Wagenfield M, Hoye RE, Jones C: Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? J Ambul Care Manage 32: 320–327, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Rademakers J, Nijman J, van der Hoek L, Heijmans M, Rijken M: Measuring patient activation in The Netherlands: Translation and validation of the American short form Patient Activation Measure (PAM13). BMC Public Health 12: 577, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maindal HT, Sokolowski I, Vedsted P: Translation, adaptation and validation of the American short form Patient Activation Measure (PAM13) in a Danish version. BMC Public Health 9: 209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker ER, Roblin DW: Translating primary care practice climate into patient activation: The role of patient trust in physician. Med Care 46: 795–805, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Begum N, Donald M, Ozolins IZ, Dower J: Hospital admissions, emergency department utilisation and patient activation for self-management among people with diabetes. Diabetes Res Clin Pract 93: 260–267, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Hendriks M, Rademakers J: Relationships between patient activation, disease-specific knowledge and health outcomes among people with diabetes; a survey study. BMC Health Serv Res 14: 393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.