Abstract
Patients are exposed to numerous prescribed and over-the-counter medications. Unfortunately, drugs remain a relatively common cause of acute and chronic kidney injury. A combination of factors including the innate nephrotoxicity of drugs, underlying patient characteristics that increase their risk for kidney injury, and the metabolism and pathway of excretion by the kidneys of the various agents administered enhance risk for drug-induced nephrotoxicity. This paper will review these clinically relevant aspects of drug-induced nephrotoxicity for the clinical nephrologist.
Keywords: Acute Kidney Injury, acute renal failure, drug nephrotoxicity, Drug Transporters, Humans, kidney, metabolism, Nephrologists, Nonprescription Drugs, Pharmacology, Proximal Tubulopathy, Renal Elimination, Risk
Introduction
Medications are a relatively common cause of kidney injury (1–12). The epidemiology of drug-induced nephrotoxicity is currently based on literature focusing on AKI. Drug-induced nephrotoxicity in adults is approximately 14%–26% in prospective cohort studies of AKI, whereas 16% of hospitalized AKI is due to drugs in the pediatric population (1–4). Drug-induced nephrotoxicity is more common in hospitalized patients, in particular intensive care unit patients (2,5).
Importantly, the general population is exposed to a large number of prescribed and over-the-counter drugs as well as a variety of substances available at health food stores (natural products, supplements, herbal remedies) (6–20). Various imaging agents used for diagnostic purposes are also associated with nephrotoxicity (21–23). However, not all patients exposed to the various potential nephrotoxins develop kidney disease. Thus, the nephrotoxicity of medications, drugs, and other ingested substances is a complicated process that involves a combination of factors. These include the inherent nephrotoxic potential of the drug, underlying patient characteristics that enhance their risk for kidney injury, and the metabolism and excretion of the potential offending agent by the kidney (6–9).
As part of the Clinical Journal of the American Society of Nephrology series “Nephropharmacology for the Clinician,” this review will cover some of the common nephrotoxic drugs that the kidney is exposed to in clinical practice, the factors that increase vulnerability of the kidney to these potential toxins, provide insight into the mechanisms by which kidney injury occurs, and cover some of the associated clinical kidney syndromes that develop in response to these agents (1–33).
Factors Associated with Drug-Induced Nephrotoxicity
The development of drug-induced nephrotoxicity can be best understood by examining the factors that contribute to nephrotoxicity (1–9). Exposure to a potentially nephrotoxic medication is an obvious requirement. Drugs may be modestly nephrotoxic or maintain high risk to cause kidney injury on the basis of their structure, dose, metabolic handling, excretory pathway through the kidney, and other characteristics (5–9). Underlying patient characteristics, such as comorbid conditions, genetic determinants of drug metabolism and transport, and immune response genes, are also important in drug nephrotoxicity (5–9). As the kidney metabolizes and excretes (through filtration and tubular secretion) many ingested drugs, the interaction of these substances with various parts of the nephron may be associated with nephrotoxicity (5–9). For kidney injury to occur, some combination of these three risk factors is generally present. More often than not, more than one is present. It is the differences in structure of the ingested drug, underlying patient characteristics, and alterations in kidney handling of the ingested substance that likely explain the variability and heterogeneity observed with drug-induced nephrotoxicity.
The Drug
The initial step in the development of kidney injury involves exposure to a potentially toxic offending agent. The general population is exposed to a variety of potential nephrotoxic substances including prescribed therapeutic agents, over-the-counter products, diagnostic agents, and environmental substances (Table 1). Examples of potentially nephrotoxic drugs that are utilized to treat various disease processes include antimicrobial agents, anticancer drugs, analgesics, and immunosuppressive agents (1–34). Furthermore, a large number of new medications with unknown nephrotoxic potential make it through clinical trials and are subsequently released into clinical practice where they cause kidney injury. This is likely related to exposure of these new drugs in patients who have comorbidities or other characteristics that increase nephrotoxic risk that were not included in clinical trials. Although clinicians prescribe the vast majority of potentially nephrotoxic medications, many are also available as over-the-counter preparations. Radiocontrast agents, in particular those delivered intra-arterially at high dose, are another potential cause of AKI (22,23).
Table 1.
Nephrotoxic drugs and intoxicants
| Therapeutic medications |
| Antimicrobial |
| Aminoglycosides |
| Antiviral agents |
| Amphotericin B |
| Colistin |
| Polymixin B |
| Sulfadiazine |
| Quinolones |
| Vancomycin |
| Chemotherapy |
| Platins |
| Ifosfamide |
| Mitomycin |
| Gemcitabine |
| Methotrexate |
| Pentostatin |
| Interleukin-2 (high dose) |
| Antiangiogenesis agents |
| Immunotherapies (immune checkpoint inhibitors, chimeric antigen receptor T cells) |
| Analgesics |
| NSAIDs |
| Selective COX-2 inhibitors |
| Phenacetin |
| Analgesic combinations |
| Immunosuppressives |
| Calcineurin inhibitors |
| Sirolimus, everolimus |
| Other |
| ACE inhibitors/ARBs/renin inhibitors |
| SGLT-2 inhibitors (canagloflozin, dapagliflozin) |
| Methoxyflurane |
| Sucrose (IVIg excipient), hydroxyethyl starch, mannitol, dextran |
| Pamidronate, Zolendronate |
| Topiramate, Zonisamide |
| Orlistat |
| Statins |
| Mesalamine |
| Alternative/health products |
| Herbal remedies |
| Aristolochic acid |
| Ephedra sp. |
| Glycyrrhiza sp. |
| Datura sp. |
| Taxus celebica |
| Uno degatta |
| Cape aloes |
| Adulterants |
| Mefenamic acid |
| Dichromate |
| Cadmium |
| Phenylbutazone |
| Melamine |
| Diagnostic agents |
| Radiocontrast |
| High osmolar |
| Low osmolar |
| Iso-osmolar |
| Other agents |
| Gadolinium (in high dose) |
| Oral NaP solution (colonoscopy prep) |
| Environmental intoxicants |
| Heavy metals |
| Lead |
| Mercury |
| Cadmium |
| Uranium |
| Copper |
| Bismuth |
| Solvents |
| Hydrocarbons |
| Other toxins |
| Silicon |
| Germanium |
NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclo-oxygenase; ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers; SGLT-2, sodium glucose transporter-2; NaP, sodium phosphate; IVIg, intravenous immunoglobulin; sp., species.
In addition to Food and Drug Administration (FDA)–approved medications, unregulated sources of potentially nephrotoxic substances are the alternative/complementary products, which are widely available at most health food stores (17–20). Included are items described as herbal remedies, natural products, and nutritional supplements (16). Another concern is that these products often contain a number of harmful chemicals and/or contaminants that are not listed on the label (16–20). Not uncommonly, the substances listed on the package label are present in varying amounts ranging from large, to small, to even nonexistent. In addition to direct nephrotoxicity, herbal products may interact with conventional drugs producing another potential avenue of nephrotoxicity. Examples of such unlisted contents include Ephedra species and aristolochic acid as well herbal products adulterated with phenylbutazone and other nonsteroidal anti-inflammatory drugs (NSAIDs), cadmium, and dichromate (16–20).
Drug Dose and Duration of Therapy
One of the most important parts of drug-induced nephrotoxicity is the innate kidney toxicity of the offending agent. A number of drug characteristics and their varied mechanisms of action play a role in causing kidney injury (Figure 1). High doses and prolonged courses of certain nephrotoxins will enhance risk for kidney injury via excessive exposure of the kidney, even in patients with minimal or no underlying risk. Several drugs such as the aminoglycosides, platinums, amphotericin B, and colistin fall into this category (24–28).
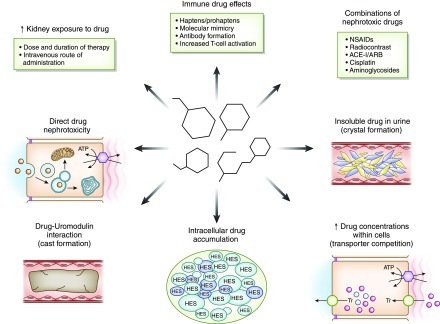
Figure 1.
Drug factors associated with increased risk for nephrotoxicity. Medications cause kidney injury through various mechanisms. Increased exposure of the kidney on the basis of route, dose, and duration of drug exposure; drug-related immune effects (such as B-lactams, PPIs, NSAIDs, and immune checkpoint inhibitors); combined nephrotoxic drug exposure; and drug and metabolite insolubility in the urine (such as methotrexate, acyclovir, and sulfadiazine) lead to kidney injury. In addition, increased drug concentrations within tubular cells are due to transport effects (such as tenofovir and cisplatin), intracellular accumulation of certain drugs due to lack of metabolizing enzymes (such as sucrose and hydroxyethyl starch), innate direct cell toxicity (such as aminoglycosides, colistin, and amphotericin B), and intratubular cast formation from drugs interacting with uromodulin (vancomycin). ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; HES, hydroxyethyl starch; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor; Tr, transporter.
Drug Characteristics (Solubility, Structure, and Charge)
Drugs and metabolites that are insoluble in the urine may cause acute crystalline nephropathy by precipitating in distal tubular lumens (11,29–31). This process is enhanced further by reduced urinary flow rates, urine pH (depending on drug pKa), excessive drug dosing, and rapid infusion rates. In addition to obstructing urinary flow, precipitated crystals induce inflammation in the surrounding interstitium. Medications associated with development of crystalline nephropathy include methotrexate, acyclovir, indinavir/atazanavir, sulfadiazine, vitamin C, foscarnet, oral sodium-phosphate, and triamterene.
A number of medications used for intravascular volume repletion (dextran, hydroxyethyl starch) or as carrier molecules (sucrose with intravenous immunoglobuling) are associated with osmotic nephropathy (32,33). These drugs accumulate within phagolysosomes of proximal tubular cells. Because of their structure, these molecules cannot be metabolized and ultimately cause lysosomal dysfunction and cell swelling.
An interesting drug characteristic that enhances nephrotoxicity is the positive charge of polycationic aminoglycosides, which are attracted to the negatively charged proximal tubular membrane phospholipids (24,34). This facilitates drug binding to the megalin/cubilin receptor complex. For example, aminoglycoside nephrotoxicity is in part related to their cationic charge—neomycin has higher cationic charge and is more nephrotoxic than amikacin, which has a lower cationic charge.
Drug Combinations
Combinations of potential nephrotoxic drugs can increase risk for kidney injury with examples including vancomycin+piperacillin/tazobactam, aminoglycosides+cephalothin, NSAIDs+radiocontrast, and cisplatin+aminoglycosides (35–39). As will be reviewed, the pathway of excretion by the kidney represents another risk for drug nephrotoxicity. Medications compete with endogenously produced substances (and other drugs) for transport proteins and influx/efflux transporters, which can increase intracellular drug concentration and risk for kidney injury (5–7). These drug-drug interactions increase kidney injury and overall drug toxicity.
Innate Drug Nephrotoxicity.
A number of medications maintain higher potential for causing kidney injury on the basis of their more significant innate nephrotoxicity. These drugs, which include the aminoglycosides, amphotericin B, the polymyxins, and cisplatin, may cause kidney injury with therapeutic doses and brief durations of exposure (5–7,40–42). Accumulation of high concentrations of the polycationic aminoglycosides within intracellular lysosomes causes lysosomal injury, which is associated with phospholipid membrane injury, oxidative stress, and mitochondrial dysfunction. This promotes proximal tubular cell apoptosis and necrosis with clinical manifestations such as an isolated proximal tubulopathy or AKI (5–7,40–42).
Amphotericin B, and the lipid/liposomal formulations to a lesser degree, cause kidney injury by disrupting tubular cell membranes and increasing permeability to cations, which result in tubular dysfunction due to cell swelling/dysfunction (40). In general, the lipid/liposomal formulations are less nephrotoxic. The polymixin antimicrobial agents, colistin and polymyxin B, are highly nephrotoxic with a very narrow therapeutic window. Nephrotoxicity is related to their D-amino content and fatty acid component, which increases cellular membrane permeability and allows cation influx (41). This effect leads to tubular cell swelling and lysis with AKI development.
The acyclic nucleotide phosphonates (adefovir, cidofovir, tenofovir) enter the cell via basolateral human organic anion transporter–1(hOAT-1) and promote cellular injury primarily through disturbing mitochondrial function. Mitochondrial injury is manifested by mitochondrial enlargement, clumped cristae, and convoluted contours that impair cellular energetics (8,10,26,43). Tenofovir, which is employed widely to treat hepatitis B virus and HIV infection, is associated with proximal tubulopathy and AKI (8,10,26,43).
Antiangiogenesis therapy with monoclonal antibodies against vascular endothelial growth factor (VEGF), circulating soluble VEGF receptors, and small molecule tyrosine kinase inhibitors that impair intracellular VEGF signaling pathways are associated with various forms of kidney injury (11,44–47). In the kidney, VEGF is produced by podocytes and binds glomerular and peritubular capillary endothelial cell VEGF receptors. Glomerular endothelial VEGF receptor binding maintains normal fenestrated endothelial health and is important for normal functioning of the glomerular basement membrane (11,44–47). Reduction in VEGF levels or signaling pathways by antiangiogenic drugs promotes loss of the healthy fenestrated endothelial phenotype and promotes microvascular injury and thrombotic microangiopathy, causing proteinuria and AKI. Reduced nephrin expression in the slit diaphragms may also contribute to the development of proteinuria. Although other kidney lesions occur with these drugs, endothelial injury and thrombotic microangiopathy are most common (11,44–47). By interfering with local alternative complement pathway regulators, these drugs may also activate complement and increase risk for TMA (48).
Drug-Induced Inflammation
Another pathway of drug-induced nephrotoxicity is through induction of an inflammatory response by the host, which can target the kidney (49–53). Through multiple mechanisms (hapten/prohapten, molecular mimicry, immune-complex formation), medications can promote the development of acute interstitial nephritis (AIN) leading to AKI and/or various urinary abnormalities such as tubular proteinuria, pyuria, and hematuria (49–52). Classic drugs associated with AIN include antimicrobial agents (in particular B-lactams and sulfonamides), NSAIDs, proton pump inhibitors, and aminosalicylates (49–53). Newer agents such as the immune checkpoint inhibitors (ipilimumab, nivolumab, pembrolizumab) cause AIN via activation of T cells and perhaps reducing tolerance to exogenous drugs (54–56). As will be discussed, the patient’s genetic makeup may enhance immunogenicity to exogenous agents.
Drug-Induced Cast Nephropathy
Another intriguing drug-related kidney injury is vancomycin-related obstructive tubular cast formation. Using immunohistologic staining techniques to detect vancomycin in kidney tissue, casts composed of noncrystal nanospheric vancomycin aggregates entangled with uromodulin have been observed in patients with AKI (57). In these patients, high vancomycin trough plasma levels were observed. These same vancomycin casts were reproduced experimentally in mice using in vivo imaging techniques. Thus, the interaction of uromodulin with nanospheric vancomycin aggregates represents a new mode of tubular injury with development of vancomycin-associated cast nephropathy (57).
The Patient
There are a number of patient-specific factors that increase risk for medication-induced nephrotoxicity (Figure 2, Table 2). Underlying risk factors for nephrotoxicity may be nonmodifiable, such as older age and female sex, which are associated with decreased lean body mass and reduced total body water that can lead to excess drug dosing (6–9). A “normal serum creatinine” in these patients may actually be a lower GFR. Women and the elderly have lower serum albumin concentrations—hypoalbuminemia results in reduced drug binding and increased free drug concentrations that can be nephrotoxic (6–9,35–38). In addition to these factors, the elderly have an increased propensity to vasoconstriction from excessive circulating angiotensin II and endothelin levels and have higher levels of oxidatively modified biomarkers (58). These factors combine to increase patient exposure to excess drug concentrations and nephrotoxicity risk.
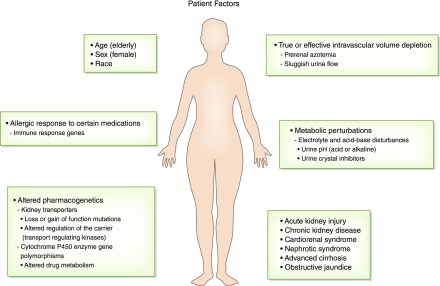
Figure 2.
Patient factors that increase risk for drug-induced nephrotoxicity. Patients have risk factors from nonmodifiable characteristics such as age, sex, race, and the genetic makeup of immune response genes and drug metabolizing enzymes and transport pathways that enhance the nephrotoxicity of drugs. Comorbid conditions such as liver disease, heart disease, and CKD and acutely developed diseases such as intravascular volume depletion, metabolic perturbations, and AKI are also important risk factors for drug-induced nephrotoxicity.
Table 2.
Risk factors for drug nephrotoxicity
| Drug factors |
| Prolonged dosing periods and nephrotoxic drug exposure |
| Potent direct nephrotoxic drug effects |
| Combinations of toxins/drugs promoting enhanced nephrotoxicity |
| Competition between endogenous and exogenous toxins for transporters, increasing drug accumulation within the tubular cell |
| Insoluble drug and/or metabolite with intratubular crystal precipitation |
| Drug that accumulates in lysosome due to lack of enzymes to metabolize the drug |
| Patient factors |
| Female sex |
| Old age (>65 yr of age) |
| Nephrotic syndrome |
| Cirrhosis/obstructive jaundice (nephrotoxic bile acids) |
| AKI |
| CKD |
| True or effective volume depletion (kidney hypoperfusion) |
| Decreased GFR |
| Enhanced proximal tubular toxin reabsorption |
| Sluggish distal tubular urine flow rates |
| Metabolic perturbations |
| Hypokalemia, hypomagnesemia, hypercalcemia |
| Alkaline or acid urine pH |
| Immune response genes increasing allergic drug response |
| Pharmacogenetics favoring drug toxicity |
| Gene mutations in hepatic and kidney P450 system |
| Gene mutations in kidney transporters and transport proteins |
| Kidney factors |
| High rate of blood delivery to the kidneys (approximately 25% of cardiac output) |
| Increased drug concentrations within the kidney medulla and interstitium |
| Biotransformation of drugs to nephrotoxic metabolites and reactive oxygen species |
| High metabolic rate of tubular cells (i.e., loop of Henle) within a hypoxic environment |
| Proximal tubular uptake of drugs |
| Apical drug uptake via endocytosis or pinocytosis with drug accumulation |
| Basolateral drug transport via hOAT or hOCT with drug accumulation |
| Reduced drug efflux via apical transporters with drug accumulation |
hOAT, human organic anion transporters; hOCT, human organic cation transporters.
Genetic Makeup
Along the lines of nonmodifiable risk factors is the patient’s underlying genetic makeup. In fact, the role of pharmacogenetics as an explanation for the heterogeneous patient response to drugs (underdosing, therapeutic dosing, and overdosing) reflects genetic makeup and supports the need for “personalized” or “precision” medicine. As such, underlying host genetic makeup can enhance vulnerability of the kidney to potential nephrotoxins (59–63). There are data that suggest that metabolic pathways, transport proteins, and drug transporters vary between patient populations due to the effect of genetic composition. Several enzymes that comprise the hepatic cytochrome P450 (CYP450) enzyme system have gene polymorphisms that are associated with reduced drug metabolism and subsequent end organ toxicity. Because the kidney also possesses CYP450 enzymes that participate in drug metabolism (59–63), it is not surprising that gene polymorphisms favoring reduced drug metabolism could increase nephrotoxic risk.
Polymorphisms of genes encoding proteins involved in the metabolism and subsequent elimination of drugs by the kidney as well as the repair pathways after drug injury are correlated with various levels of drug sensitivity. Polymorphisms in genes encoding ERCC1, a key enzyme in the DNA repair pathway by which cells repair platinum-induced DNA damage, may be associated with increased nephrotoxicity (64). Polymorphisms in cytosolic glutathione-S-transferase enzymes, which normally function to detoxify reactive molecules such as cisplatin, increase risk for nephrotoxicity with exposure to this drug (65).
Loss-of-function mutations in apical secretory transporters that reduce drug efflux from the cell into the urine, and mutations in kinases that regulate drug carrier proteins, can impair drug elimination and promote nephrotoxicity by elevating intracellular drug concentrations (59–63). It is probable that patients differ in the function and regulation of receptors, channels, carriers, and transporters that regulate the metabolism and elimination of drugs by the kidneys. Tenofovir-induced Fanconi syndrome represents one such example (66). Patients with HIV receiving tenofovir who developed Fanconi syndrome were noted to have a single nucleotide polymorphism: 1249 G→A single nucleotide polymorphism in the gene coding the multidrug-resistant protein-2 efflux transporter, which transports tenofovir out of the cell into the urine. In contrast, treated patients with HIV who did not develop Fanconi syndrome did not have the gene polymorphism (66).
Genetic alterations in a patient’s immune system may also enhance risk for drug nephrotoxicity via inflammatory injury. The administered drug or its metabolite may form adducts that modify their physical structure, which enhances their immunogenicity (49,53). Heterogeneity in patient response to drugs and exogenous agents exists, with one example being the heightened allergic response of some individuals as compared with others. As such, differences in innate host immune response genes can predispose some patients to developing an allergic reaction to a medication (49,53). In fact, the variability of immune responses has been demonstrated in patients who develop drug-induced AIN, which appears to be a T cell–driven process (49). Thus, enhanced vulnerability to an allergic response in the kidney and the associated development of AIN reflect yet another form of drug nephrotoxicity.
Comorbid Diseases
Underlying AKI and CKD are also important risk factors for increasing vulnerability to nephrotoxic injury (6–9,35–37). The decline in GFR and increase in tubular secretion of endogenous substances (and medications) increase risk for adverse drug-related kidney effects. GFR reduction can also result in excessive drug dosing for medications excreted by the kidneys, increased drug exposure in a reduced number of functioning nephrons and ischemia preconditioned tubular cells, and more robust oxidative injury response to various medications by the kidney. In addition, increased tubular secretion of drugs that are cleared by both glomerular filtration and tubular secretion may enhance kidney tubular toxicity (6–9).
Other types of systemic and kidney disease may also increase the nephrotoxic effects of drugs. Nephrotic syndrome and cirrhosis enhance nephrotoxic risk through multiple mechanisms that include altered kidney perfusion from reduced effective circulating blood volume, hypoalbuminemia with increased free circulating drug levels, and unrecognized kidney impairment (6–9,35–38). Obstructive jaundice also enhances toxicity to certain drugs, such as the aminoglycosides, through altered hemodynamics such as decreased renal blood flow and direct toxic effects of bile salts on tubular epithelia (67). True volume depletion from vomiting, diarrhea, and diuretics as well as effective volume depletion associated with congestive heart failure, ascites, and sepsis increase risk for drug nephrotoxicity. Induction of kidney hypoperfusion and prerenal physiology by these comorbidities increases the nephrotoxicity of many drugs (6–9,35–38). Ultimately, reduced kidney perfusion enhances nephrotoxicity in drugs excreted through the kidneys by fostering drug overdosing, increasing drug concentrations within tubular cells in drugs reabsorbed by the proximal tubule, and enhancing drug/metabolite crystal precipitation within distal tubular lumens in the setting of sluggish urinary flow rates of insoluble drugs (6–9,35–38).
Metabolic Disturbances
A number of metabolic abnormalities can also increase risk for adverse kidney effects with certain drugs. For example, electrolyte disorders such as hypokalemia, hypomagnesemia, and hypocalcemia increase the nephrotoxicity associated with the aminoglycosides (6–9,35–38,68). Severe hypercalcemia leads to afferent arteriolar vasoconstriction and tubular sodium and water wasting, which induces prerenal physiology, which enhances nephrotoxic drug injury. Metabolic disorders that alter urinary pH also increase risk for intratubular crystal deposition with certain drugs (6–9,29–31,68). Systemic metabolic acidosis or alkalosis may decrease or increase urine pH, whereas proximal and distal renal tubular acidoses are associated with alkaline urine due to impaired ability of the kidney to excrete H+ ion. Acidic urinary pH (<5.5) increases intratubular crystal deposition with drugs such as sulfadiazine, methotrexate, and triamterene that have limited solubility in a low-pH environment (11,25–27). Alkaline urine (pH>6.0) increases crystal precipitation within tubular lumens from drugs such as indinavir, atazanavir, oral sodium phosphate solution, and ciprofloxacin (10,11,21,29–31). In addition, drugs such as topiramate, zonisamide, and acetazolamide induce the formation of an alkaline urine by inhibiting carbonic anhydrase thereby promoting precipitation of calcium-phosphate within tubules and enhancing risk for nephrolithiasis (30,31).
The Kidney
The mechanism by which the kidney metabolizes and excretes various drugs and toxins importantly contributes to drug nephrotoxicity (Figure 3). The high rate of drug and toxin delivery to the kidney, a result of high renal blood flow, which approximates 25% of cardiac output, exposes the kidney to significant drug concentrations (6–9). In addition, many tubular cells, particularly those in the loop of Henle, reside in a relatively hypoxic environment due to the high metabolic requirements associated with active solute transport by Na+-K+-ATPase–driven transport (6–9,68,69). Excessive cellular workload of these cells in this relatively hypoxic environment enhances risk for a nephrotoxic-related injury. High concentrations of certain medications and their metabolites develop in the kidney medulla and interstitium from the enormous concentrating ability of the kidney, which can induce kidney injury through direct toxicity as well as ischemic damage from reduced prostaglandin and increased thromboxane production (6–9,68,69).
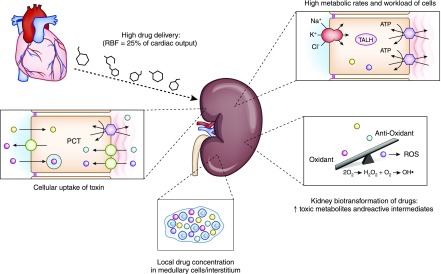
Figure 3.
Kidney factors that enhance risk for drug-induced nephrotoxicity. High RBF increases drug delivery and exposure to the kidney. High metabolic rates of TALH tubular cells increase risk for drug nephrotoxicity. Kidney metabolism of drugs to toxic metabolites and ROS overwhelms local antioxidants and promotes tubular injury. Increased concentrations of potentially nephrotoxic drugs in the medulla and interstitium increase kidney injury. Apical uptake of certain drugs (aminoglycosides, hydroxyethyl starch) and basolateral transport of drugs through the organic anion transporter (tenofovir) and organic cation transporter (cisplatin) increase kidney toxicity. PCT, proximal convoluted tubule; RBF, renal blood flow; ROS, reactive oxygen species; TALH, thick ascending loop of Henle.
Drug Metabolism
In addition to hepatic metabolism, a number of drugs undergo biotransformation by kidney enzyme systems, including the CYP450 and flavin-containing monooxygenases (6–9,68–71). This leads to the potential formation of nephrotoxic metabolites and reactive oxygen species as seen with the aminoglycosides, platinums, and several other medications (6–9,34,68–74). These byproducts of biotransformation may swing the balance in favor of oxidative stress, which outstrips natural antioxidants and increases kidney injury via DNA strand breaks, nucleic acid alkylation or oxidation, lipid peroxidation, and protein damage (6–9,34,68–74).
Drug Excretory Pathway
Drugs are excreted from the body by both glomerular filtration and tubular secretion. An important avenue of kidney injury occurs with excretion of drugs via the active transporters in proximal tubular cells (6–9,75–79). Extensive tubular cell uptake of potential nephrotoxic drugs via both apical and basolateral transport systems underlies development of kidney injury. From the urinary space, apical uptake of drugs occurs via endocytosis/pinocytosis and other active/passive transport pathways (6–9,32–34). Medications taken up via this pathway include polycationic aminoglycosides (Figure 4A), heavy metals, and various complex sugars and starches. In the case of aminoglycosides, after endocytic receptor (megalin/cubilin) binding and uptake of these cationic ligands, these drugs are translocated into the lysosomal compartment where they accumulate and subsequently form myeloid bodies (6,34,68,69). Myeloid bodies are membrane fragments and damaged organelles formed as a consequence of aminoglycoside inhibition of lysosomal enzymes. This apical pathway of uptake leads to accumulation of a critical concentration of aminoglycoside within cells, which triggers an injury cascade leading to cell injury and death, which present clinically as a proximal tubulopathy and/or AKI. Filtered dextran, sucrose, and hydroxyethyl starch may cause tubular injury when they undergo pinocytosis by proximal tubular cells (6,9,34,35). Similar to the aminoglycosides, after pinocytosis these substances are taken up by and collect in lysosomes (Figure 4B). The absence of cellular enzymes capable of metabolizing these substances allows them to build up within the cytoplasm and cause tubular cell injury and AKI (6,9,34,35).
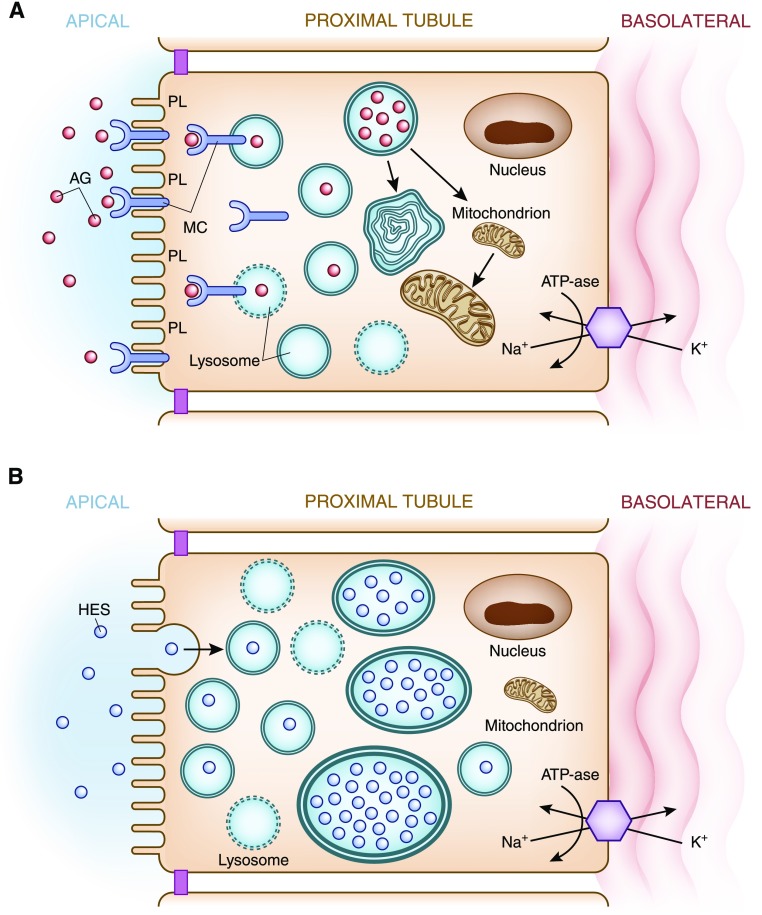
Figure 4.
Apical transport of drugs in the proximal tubule. (A) Aminoglycosides Apical membrane handling of substances, in this example aminoglycosides, by proximal tubular cells increases cellular uptake of this nephrotoxic drug. Polycationic aminoglycosides are attracted to the anionic phospholipid membranes where they interact with megalin-cubilin receptor on the apical surface. The aminoglycosides are endocytosed and enter the cell where they are translocated into lysosomes. Lysosomal injury and rupture along with mitochondrial injury result in tubular cell injury. (B) Hydroxyethyl starch. Apical membrane handling of hydroxyethyl starch by proximal tubular cells increases cellular uptake of this potentially nephrotoxic drug. Hydroxyethyl starch as well as sucrose (carrier for IVIg), dextran, and mannitol undergo pinocytosis and enter the cell where they are translocated into lysosomes. The lack of enzymes necessary to metabolize these substances allows accumulation within lysosomes, which causes cell swelling (occluding tubular lumens) and eventual lysosomal rupture resulting in tubular cell injury. AG, aminoglycosides; HES, hydroxyethyl starch; IVIg, intravenous immunoglobulin; K+, potassium; MC, megalin-cubilin; Na+, sodium; PL, anionic phospholipids.
In addition to apical uptake of drugs, another pathway of proximal tubular cell drug exposure occurs via basolateral delivery via the peritubular capillaries (6,26,43,72–76). After delivery of potentially nephrotoxic drugs by the peritubular capillaries, uptake into proximal tubular cells occurs via a family of active transporters (6,26,43,72–76). These include the hOAT for negatively charged drugs and the human organic cation transporters (hOCT) for positively charged drugs (6,26,43,72–76). Endogenously produced anionic and cationic substances, as well as exogenously administered drugs, compete for transport via these pathways. Classic examples of potentially nephrotoxic drugs utilizing these transport pathways are the acyclic nucleotide phosphonates such as tenofovir (Figure 5A), which are transported via hOAT-1 (6,26,43), and cisplatin, which is transported via hOCT-2 (Figure 5B) (72–74,76). Upon transport of drugs into proximal tubular cell cytoplasm, they move through the intracellular space by various regulated carrier proteins, and subsequently exit from cells via apical transport proteins (5,6,26,43,72–74,76). Transport of drugs through proximal tubular cells, as well as the buildup of drug concentrations when transport out of cells is blunted (or transport into the cell is increased), enhances risk for nephrotoxicity (6,9,26,43,72–74,76). Examples of the former are loss-of-function mutations in and competition for apical secretory transporters (6,9,26,43,66,72–74,76). This reduces nephrotoxin efflux from cell into urine, which may promote accumulation of toxic substances within proximal tubular cells and cause cellular injury via apoptosis or necrosis (Figure 5). An example of the latter is reduced glomerular filtration of drug, which increases proximal tubular drug secretion and increases tubular cell drug exposure (6–9). Ultimately, this extensive trafficking of drugs increases tubular exposure and risk for elevated concentration of potentially nephrotoxic drugs when other risk factors supervene.
Figure 5.
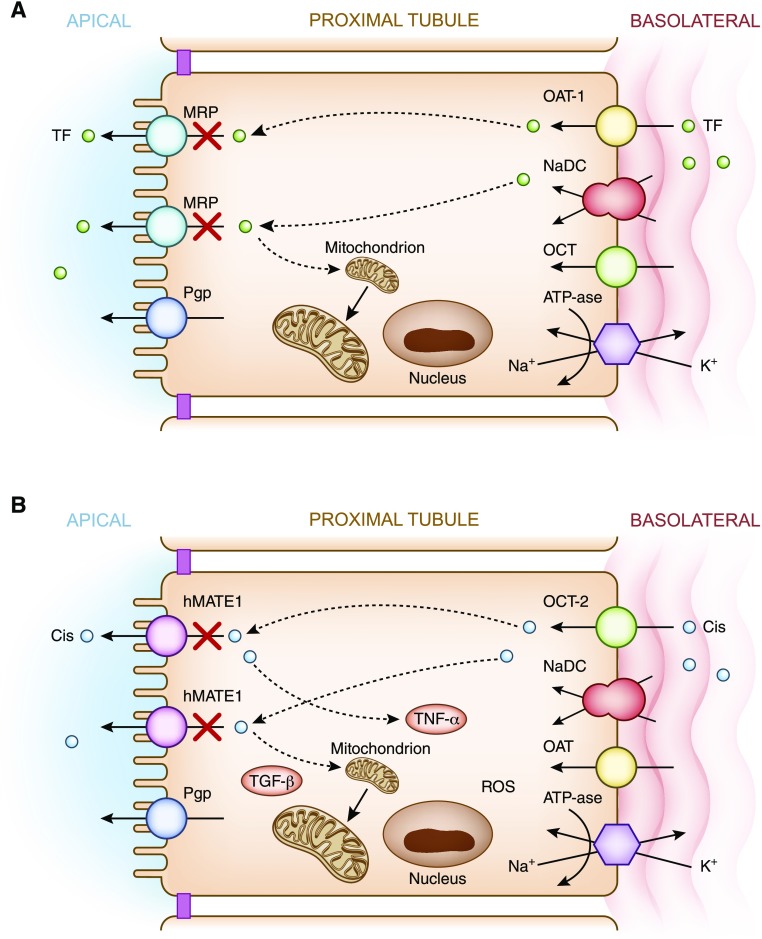
Basolateral transport of drugs. (A) Tenofovir. Basolateral handling of certain drugs, in this example tenofovir, by proximal tubular cells may lead to cellular injury. Tenofovir is delivered to the basolateral membrane, transported into the cell via the human organic anion transporter-1, and excreted by various apical transporters into the urinary space. In this example, transport by the multidrug-resistance protein transporters is inhibited or dysfunctional, causing intracellular accumulation of drug and nephrotoxicity via mitochondrial toxicity. (B) Cisplatin. Basolateral handling of certain drugs such as cisplatin by proximal tubular cells may lead to cellular injury. Cisplatin is delivered to the basolateral membrane, transported into the cell via the human organic cation transporter-2, and excreted by various apical transporters into the urinary space. Intracellular accumulation of cisplatin due to increased basolateral uptake or deficient efflux by the hMATE1 transporters into the urine leads nephrotoxicity via production of a number of substances (TNF-α, TGF-β, and ROS), which promote mitochondrial toxicity. Cis, cisplatin; hMATE1, human multidrug and toxin extrusion protein transporter; K+, potassium; MRP, multidrug resistance protein transporter; Na+, sodium; NaDC, sodium dicarboxylate transporter; OAT-1, organic anion transporter-1; OCT-1, organic cation transporter-1; Pgp, P-glycoprotein transporter; ROS, reactive oxygen species; TF, tenofovir; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
Preclinical and Clinical Tests for Drug-Induced Nephrotoxicity
Kidney-on-a-chip technology is being employed in the drug discovery field using in vitro models that mimic kidney physiologic structures and continuous flow conditions (74,80,81). Most systems consist of kidney tubular epithelial cells embedded on the surface of an extracellular matrix, which is attached to perfusable microchannels that allow for nutrient enrichment, waste clearance, and flow (81). These in vitro models, in particular the 3D models, are thought to more reliably replicate the in vivo environment and predict nephrotoxicity that occurs with certain drugs in the clinical setting (81). Proximal tubular cells cultured under these physiologic conditions demonstrate various markers of drug cytotoxicity. Kidney-on-a-chip models have been successfully employed with known nephrotoxins such as cisplatin (74).
Novel biomarkers of injury are also useful to examine for the possibility of structural kidney injury due to various drugs. To this point, the FDA and European Medicines Agency (EMEA) approved seven novel kidney biomarkers, along with traditional clinical chemistry and histopathology, for preclinical animal studies to detect nephrotoxicity in the development of new drugs (82). Biomarkers were added to preclinical studies on the basis of their superior sensitivity and specificity in detecting drug-induced nephrotoxicity as compared with traditional tests. Because these biomarkers detect injury in various parts of the nephron, they would be well suited not only to signal the occurrence of parenchymal kidney injury, but also point to the site of injury. Thus, animal experiments measuring these biomarkers after administration of a medication under development would provide insight into potential nephrotoxicity. In addition to drug development, the FDA and EMEA recommend that biomarkers should eventually be evaluated for their utility in clinical studies to promote patient safety and guide therapeutic clinical decisions (83). Novel biomarkers could also be measured in stored urine samples from patients participating in clinical trials studying the efficacy and safety of various drugs. The results of animal and human studies would provide a potential avenue to identify drug-induced structural kidney injury and allow recognition of drug-induced nephrotoxicity at earlier time points to allow drug discontinuation before further kidney injury occurs. Kidney-on-a-chip technology in combination with the urine microscopy (84) and novel biomarkers may allow clinicians to better understand if a drug is nephrotoxic and, if so, the site of injury and mechanism underlying development of kidney injury.
Summary
Medications are widely prescribed and ingested by patients and remain a relatively common cause of kidney injury. Drug nephrotoxicity is a complicated process that involves a combination of factors including the innate nephrotoxicity of drugs, underlying patient characteristics that enhance their risk for kidney injury, and the metabolism and excretion of the potential offending agent by the kidney.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM; Program to Improve Care in Acute Renal Disease: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani V, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Moffett BS, Goldstein SL: Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6: 856–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perazella MA: Drug use and nephrotoxicity in the intensive care unit. Kidney Int 81: 1172–1178, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Perazella MA: Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 4: 1275–1283, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Markowitz GS, Perazella MA: Drug-induced renal failure: A focus on tubulointerstitial disease. Clin Chim Acta 351: 31–47, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Perazella MA: Drug-induced nephropathy: An update. Expert Opin Drug Saf 4: 689–706, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Perazella MA: Drug-induced renal failure: Update on new medications and unique mechanisms of nephrotoxicity. Am J Med Sci 325: 349–362, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Izzedine H, Harris M, Perazella MA: The nephrotoxic effects of HAART. Nat Rev Nephrol 5: 563–573, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Perazella MA: Crystal-induced acute renal failure. Am J Med 106: 459–465, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Eras J, Perazella MA: NSAIDs and the kidney revisited: Are selective cyclooxygenase-2 inhibitors safe? Am J Med Sci 321: 181–190, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Gambaro G, Perazella MA: Adverse renal effects of anti-inflammatory agents: Evaluation of selective and nonselective cyclooxygenase inhibitors. J Intern Med 253: 643–652, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA, Markowitz GS: Bisphosphonate nephrotoxicity. Kidney Int 74: 1385–1393, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gurevich F, Perazella MA: Renal effects of anti-angiogenesis therapy: Update for the internist. Am J Med 122: 322–328, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Luciano RL, Perazella MA: Aristolochic acid nephropathy: Epidemiology, clinical presentation, and treatment. Drug Saf 38: 55–64, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Luciano RL, Perazella MA: Nephrotoxic effects of designer drugs: Synthetic is not better! Nat Rev Nephrol 10: 314–324, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Isnard Bagnis C, Deray G, Baumelou A, Le Quintrec M, Vanherweghem JL: Herbs and the kidney. Am J Kidney Dis 44: 1–11, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Blowey DL: Nephrotoxicity of over-the-counter analgesics, natural medicines, and illicit drugs. Adolesc Med Clin 16: 31–43, x, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Wang IJ, Chen PC, Hwang KC. Melamine and nephrolithiasis in children in Taiwan. N Engl J Med 360: 1157–1158, 2009. [DOI] [PubMed]
- 21.Markowitz GS, Perazella MA: Acute phosphate nephropathy. Kidney Int 76: 1027–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Perazella MA: Radiocontrast-induced nephropathy: An update. Minerva Urol Nefrol 61: 215–233, 2009 [PubMed] [Google Scholar]
- 23.Perazella MA, Reilly RF: Imaging patients with kidney disease: How do we approach contrast-related toxicity? Am J Med Sci 341: 215–221, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Rougier F, Ducher M, Maurin M, Corvaisier S, Claude D, Jelliffe R, Maire P: Aminoglycoside dosages and nephrotoxicity: Quantitative relationships. Clin Pharmacokinet 42: 493–500, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Perazella MA, Moeckel GW: Nephrotoxicity from chemotherapeutic agents: Clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol 30: 570–581, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Perazella MA: Tenofovir-induced kidney disease: An acquired renal tubular mitochondriopathy. Kidney Int 78: 1060–1063, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Perazella MA: Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 7: 1713–1721, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Perazella MA, Izzedine H: New drug toxicities in the onco-nephrology world. Kidney Int 87: 909–917, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Stratta P, Lazzarich E, Canavese C, Bozzola C, Monga G: Ciprofloxacin crystal nephropathy. Am J Kidney Dis 50: 330–335, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Vega D, Maalouf NM, Sakhaee K: Increased propensity for calcium phosphate kidney stones with topiramate use. Expert Opin Drug Saf 6: 547–557, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wroe S: Zonisamide and renal calculi in patients with epilepsy: How big an issue? Curr Med Res Opin 23: 1765–1773, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Orbach H, Tishler M, Shoenfeld Y: Intravenous immunoglobulin and the kidney--a two-edged sword. Semin Arthritis Rheum 34: 593–601, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Dickenmann M, Oettl T, Mihatsch MJ: Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 51: 491–503, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Nagai J, Takano M: Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet 19: 159–170, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Evenepoel P: Acute toxic renal failure. Best Pract Res Clin Anaesthesiol 18: 37–52, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Singh NP, Ganguli A, Prakash A: Drug-induced kidney diseases. J Assoc Physicians India 51: 970–979, 2003 [PubMed] [Google Scholar]
- 37.Guo X, Nzerue C: How to prevent, recognize, and treat drug-induced nephrotoxicity. Cleve Clin J Med 69: 289–290, 293–294, 296–297 passim, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wyatt CM, Arons RR, Klotman PE, Klotman ME: Acute renal failure in hospitalized patients with HIV: Risk factors and impact on in-hospital mortality. AIDS 20: 561–565, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL: Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: A systematic review and meta-analysis. Crit Care Med 46: 12–20, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Alexander BD, Wingard JR: Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis 40[Suppl 6]: S414–S421, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Falagas ME, Kasiakou SF. Nephrotoxicity of intravenous colistin: A prospective evaluation. Crit Care 10: R27 1–13, 2006.
- 42.Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, Park J, Nasr SH, Hoh S, Siegel DS, D'Agati VD: Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 64: 281–289, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Izzedine H, Launay-Vacher V, Deray G: Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 45: 804–817, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R: Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Izzedine H, Escudier B, Lhomme C, Pautier P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R, Hollebecque A, Sahali D, Soria JC: Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): An 8-year observational study at a single center. Medicine (Baltimore) 93: 333–339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC, Kuzmuk V, Coward RJ, Goult J, Bull KR, Sharma R, Bharti K, Westenskow PD, Michael IP, Saleem MA, Friedlander M: VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest 127: 199–214, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, Gugger M, Mohaupt M, Pichler WJ: Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 17: 2919–2927, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Perazella MA, Markowitz GS: Drug-induced acute interstitial nephritis. Nat Rev Nephrol 6: 461–470, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Moledina DG, Perazella MA: Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 12: 2046–2049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moledina DG, Perazella MA: Proton pump inhibitors and CKD. J Am Soc Nephrol 27: 2926–2928, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishnan N, Perazella MA: Drug-induced acute interstitial nephritis: Pathology, pathogenesis, and treatment. Iran J Kidney Dis 9: 3–13, 2015 [PubMed] [Google Scholar]
- 54.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with Programmed Cell Death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perazella MA: Checkmate: Kidney injury associated with targeted cancer immunotherapy. Kidney Int 90: 474–476, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Luque Y, Louis K, Jouanneau C, Placier S, Esteve E, Bazin D, Rondeau E, Letavernier E, Wolfromm A, Gosset C, Boueilh A, Burbach M, Frère P, Verpont MC, Vandermeersch S, Langui D, Daudon M, Frochot V, Mesnard L: Vancomycin-associated cast nephropathy. J Am Soc Nephrol 28: 1723–1728, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jerkić M, Vojvodić S, López-Novoa JM: The mechanism of increased renal susceptibility to toxic substances in the elderly. Part I. The role of increased vasoconstriction. Int Urol Nephrol 32: 539–547, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Harty L, Johnson K, Power A: Race and ethnicity in the era of emerging pharmacogenomics. J Clin Pharmacol 46: 405–407, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Ciarimboli G, Koepsell H, Iordanova M, Gorboulev V, Dürner B, Lang D, Edemir B, Schröter R, Van Le T, Schlatter E: Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. J Am Soc Nephrol 16: 1562–1570, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Ulrich CM, Bigler J, Potter JD: Non-steroidal anti-inflammatory drugs for cancer prevention: Promise, perils and pharmacogenetics. Nat Rev Cancer 6: 130–140, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Awdishu L, Nievergelt CM, Davenport A, Murray PT, Macedo E, Cerda J, Chakaravarthi R, Ramachandra Rao SP, Holden A, Goldstein SL, Mehta RL: Rationale and Design of the Genetic Contribution to Drug Induced Renal Injury (DIRECT) Study. Kidney Int Rep 1: 288–298, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Y, Paules RS: Use of transcriptomics in understanding mechanisms of drug-induced toxicity. Pharmacogenomics 11: 573–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suk R, Gurubhagavatula S, Park S, Zhou W, Su L, Lynch TJ, Wain JC, Neuberg D, Liu G, Christiani DC: Polymorphisms in ERCC1 and grade 3 or 4 toxicity in non-small cell lung cancer patients. Clin Cancer Res 11: 1534–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, Peters WP, Jones RB, Hall J, Marks JR: Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol 23: 6117–6125, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray AG: Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis 194: 1481–1491, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Lucena MI, Andrade RJ, Cabello MR, Hidalgo R, Gonzalez-Correa JA, Sanchez de la Cuesta F: Aminoglycoside-associated nephrotoxicity in extrahepatic obstructive jaundice. J Hepatol 22: 189–196, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Cummings BS, Schnellmann RG: Pathophysiology of nephrotoxic cell injury. In: Diseases of the Kidney and Urogenital Tract, edited by Schrier RW, Philadelphia, Lippincott Williams & Wilkinson, 2001, pp 1071–1136 [Google Scholar]
- 69.Kaloyanides GJ, Bosmans J-L, DeBroe ME: Antibiotic and Immunosuppression-related renal failure. In: Diseases of the Kidney and Urogenital Tract, edited by Schrier RW, Philadelphia, PA, Lippincott Williams & Wilkinson, 2001, pp 1137–1174 [Google Scholar]
- 70.Aleksa K, Matsell D, Krausz K, Gelboin H, Ito S, Koren G: Cytochrome P450 3A and 2B6 in the developing kidney: Implications for ifosfamide nephrotoxicity. Pediatr Nephrol 20: 872–885, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Fanos V, Cataldi L: Renal transport of antibiotics and nephrotoxicity: A review. J Chemother 13: 461–472, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Hucke A, Ciarimboli G: The role of transporters in the toxicity of chemotherapeutic drugs: Focus on transporters for organic cations. J Clin Pharmacol 56[Suppl 7]: S157–S172, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, Ciarimboli G, Sparreboom A: Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res 20: 4026–4035, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE: Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5: 1119–1129, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Enomoto A, Endou H: Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin Exp Nephrol 9: 195–205, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E: Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167: 1477–1484, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang F: Regulating renal drug elimination? J Am Soc Nephrol 16: 1535–1536, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Alexander BD, Wingard JR. Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis 40[Suppl 6]: S414–S421, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Falagas ME, Kasiakou SF: Nephrotoxicity of intravenous colistin: A prospective evaluation. Crit Care 10: R27 1–13, 2006. 16507149 [Google Scholar]
- 80.Paoli R, Samitier J: Mimicking the kidney: A key role in organ-on-chip development. Micromachines (Basel) 7: 126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R: Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 34: 156–170, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Blank M, De Felice A, Goodsaid F, Harlow P, Hausner E, Jacobson-Kram D, et al. Review of qualification data for biomarkers of nephrotoxicity submitted by the predictive safety testing consortium, edited by Administration CfDEaRUSFaD, 2009 [Google Scholar]
- 83.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F: Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perazella MA: The urine sediment as a biomarker of kidney disease. Am J Kidney Dis 66: 748–755, 2015 [DOI] [PubMed] [Google Scholar]