Abstract
Acute myeloid leukemia (AML) is a malignant blood disorder and the cure rate has been remarkably improved over the past decade. However, recurrent or refractory leu-kemia remains the major problem of the AML and no clearly effective therapy has been es-tablished so far. Traditional treatments such as chemotherapy and hematopoietic stem cell transplantation are both far dissatisfying the patients partly for their individual variety. Be-sides, conventional treatments usually have many side effects to result in poor prognosis. Therefore, an urgent need is necessary to update therapies of AML. To date, protein kinase inhibitors as new drugs offer hope for AML treatment and many of them are on clinical tri-als. Here, this review will provide a brief summary of protein kinase inhibitors investigated in AML thus far, mainly including tyrosine protein kinase inhibitors and serine/threonine kinase inhibitors. We also presented the sketch of signal pathways involving protein kinase inhibitors, as well as discussed the clinical applications and the challenges of inhibitors in AML treatment
Keywords: Acute myeloid leukemia, protein kinase, inhibitor, therapy, drug, FLT3, mTOR
1. Introduction
Acute myeloid leukemia (AML) is a kind of clonal disorders of malignant hematopoietic stem cells, remarkably characterized by the accumulation of primitive or immature leukemia cells in bone marrow and peripheral blood, resulting in inducing the disease and hampering the clinical treatment [1, 2]. When AML happened, the red blood cells, platelets and normal white blood cells became less, while the immature cells multiply accumulated, which further interfered with the normal bone marrow hematopoietic function. Based on clinical observation and related trials [3], it is commonly accepted that AML is a kind of refractory type of bone marrow cancer with poor prognosis and more commonly affects the elderly patients over 60 [4].
Although the recent advancement in understanding the treatment of AML has remarkably improved the cure rate over the past decade [5]; however, the 5-year survival rates of patients are only about 40% for young/middle-aged patients and less than 10% for elderly patients [6, 7]. The big difference between them might be caused by the inherently poor physical condition and more resistant leukemia cells for elderly patients, which lead to worse prognosis [2, 4, 8, 9]. In recent years, due to an aging population, the incidence of AML appears to be rising [4]. Encouragingly, the development of genomics and sequencing technology makes us have a better understanding of molecular genetic mutation and the pathogenesis of AML [10]. Furthermore, the targeted therapy has been becoming the most promising therapeutic approache [11, 12]. Especially, the key protein kinases as drug screening targets have shown effectives and become a focus [13]. Among the current clinical application of pharmacological targets in AML, kinase inhibitors account for the most, particularly tyrosine kinase inhibitors.
Developing new targeted drugs with high efficiency, low toxicity, strong specificity has become an attractive area of antitumor research. In spite of the mutation of the protein kinase and the drug resistant of the first generation of inhibitors, emerging effective therapies with recently designed compounds appear promising. In this review, we will provide a comprehensive overview of AML, kinase and kinase inhibitors, especially summarize the protein kinase inhibitors currently studied and developed in AML including tyrosine protein kinase inhibitors and serine/threonine kinase inhibitors.
2. THE DEVELOPMENT AND THERAPY OF AML
In 1900, leukemia has been divided into myeloid or lymphocytic leukemia according to the origin of the diseased red cells and non-lymphoid white blood cells (called the myeloid pathway) or a precursor of T and B cells (called the lymphoid pathway) [14]. Seventy-six years later, the French-American-British (FAB) classification system classified AML into eight subtypes, M0 to M7 [15]. The World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues, determining the types of AML based on the morphology and prognosis, was updated in 2008 and put up with the relative targeted therapy [16]. Clinically, doctors often adopt applicable treatment on the basis of the classification and patient’s own physical condition. According to the National Comprehensive Cancer Network (NCCN) Guidelines Version 1.2016 (Table 1) [17], the mutation of Fms-like tyrosine kinase3-intenal tandem duplication (FLT3-ITD), tumor protein 53 (TP53), c-KIT, NPM1 and CCAAT/enhancer-binding protein alpha (CEBPA) are all likely to add risk to AML, which may contribute to AML recurrence.
Table 1.
Risk status based on validated cytogenetics and molecular abnormalities.
| Risk status | Cytogenetics | Molecular Abnormalities |
|---|---|---|
| Favorable-risk | Core binding factor: inv(16) or t(16; 16) or t(8; 21) or t(15; 17) | Normal cytogenetics: NPM1 mutation in the absence of FLT3-ITD or isolated biallelic double CEBPA mutation |
| Intermediate-risk | Normal cytogenetics +8 alone t(9; 11) Other non-defined Complex (≥3 clonal chromosomal abnormalities) |
Core binding factor with KIT mutation |
| Poor-risk | Monosomal karyotype -5, 5q-, -7, 7q- 11q23 - non t(9; 11) inv(3), t(3; 3) t(6; 9) t(9; 22) |
Normal cytogenetics: with FLT3-ITD mutation TP53 mutation |
(NCCN - Evidence-Based Cancer Guidelines, Oncology Drug Compendium, Oncology Continuing Medical Education https://www.nccn.org//)
The conventional method in the treatment of AML aims to take advantage of the toxic compounds, namely chemotherapy drugs, to kill leukemia cells. However, the chemotherapy drugs surely ultimately attack the human’s normal immune cells, thus, it is hard to achieve the aim of complete remission (CR), which means no sign of poor symptom in patients and their returning to good health. During the formal therapy, it is expected to reduce more leukemia cells to an undetectable level to achieve a complete remission and the goal of consolidation therapy is to eliminate any residual undetectable disease and achieve a cure [18]. Young patients often can tolerate this kind of chemotherapy, but the tolerance to intensive chemotherapy will decrease with age. Adverse reaction to treatment, low induced relieving rate, high recurrence rate, and short survival period are tough problems that need to be solved in the treatment of AML.
Although chemotherapy will help to reduce tumor size, prolonging the use of chemotherapy would not result in more cancer destruction. In addition, it is hard to identify the diagnosis, stage and evaluate the treatment effect in AML. Thus, unnecessary chemotherapy, usually with high costs as well as side effects, makes it fail to cure AML. Moreover, the cancer cells may eventually develop resistance to multiple chemotherapeutics. For patients who are intolerant to standard chemotherapy, reducing the dose of induction chemotherapy regimens would greatly decrease the complete remission rate, specially for the recurrent AML. Despite a variety of clinical trials of new drugs, there is no breakthrough in classic “3 + 7” scheme of anthracycline-based drugs combined with cytarabine. Hematopoietic stem cell transplantation (HSCT) is usually considered if induction chemotherapy fails or AML relapses, however, the seriously scarce ligand resources and unaffordable treatment costs restrict the use.
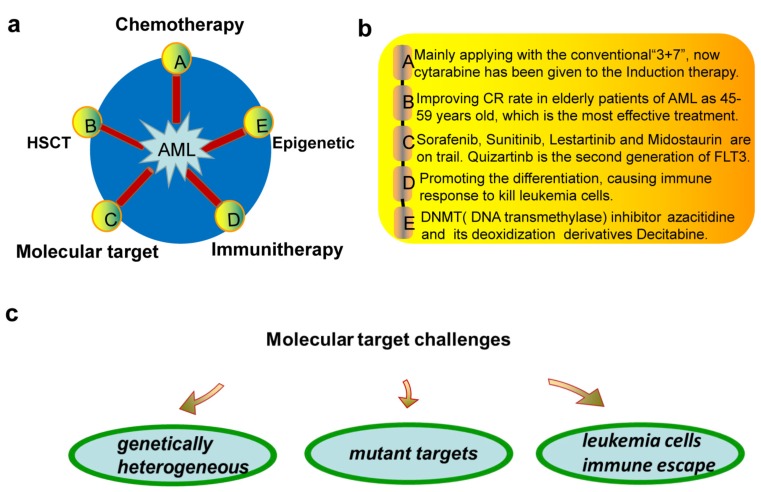
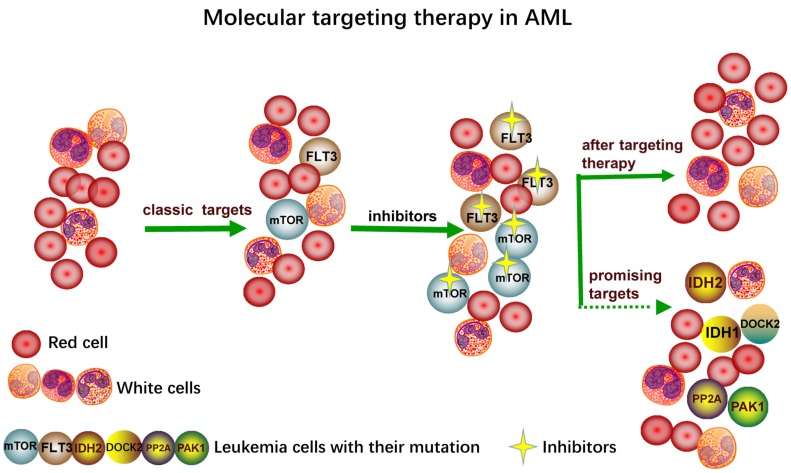
Now inhibitors are widely used in drug target therapy and can increase the effectiveness of the treatment, which has introduced a new era of tumor targeting therapy [19, 20]. Inhibitors generally attack specific sites and lessen the dependence on tissue-destroying chemotherapy. Targeted inhibitors have been developed as adjuncts or replacements for conventional chemotherapy for AML patients; however, the cure rate depends on a number of prognostic factors [21]. Recently, with the wide application of drugs, it has been found that the tumor has evolved the immune escape. The general treatment strategies for AML are summarized in Fig. (1a, b), mainly consisting of the traditional treatments of chemotherapy, HSCT and the novel treatments of molecular target, immunotherapy and epigenetic therapy. For molecular targeted therapy, the genetical heterogeneity, mutant targets and immune escape are the major challenges (Fig. 1c). The appearance of targeted therapy provides a more feasible and effective way for chemotherapy and promotes the emergence and development of new dosage forms [22]. Although the classic targeted inhibitors have had amazing effect on the specific targets like FLT3 and mTOR, new inhibitors specific to novel targets like IDH1/2, PP2A, DOCK2, PAK1 have been developed due to the emergence of many new mutation sites of new targets (Fig. 2). Besides the efforts to screen more related key enzyme targets, developing more stable and specific inhibitors is also urgently needed.
Fig. (1).
a. Five ways of AML therapy. A indicates the most traditional way called as chemotherapy “7 + 3” defined as 7 days of continuous infusion cytarabine (100–200 mg/m2/day) and 3 days of an anthracycline (most typically daunorubicin 45–90 mg/m2/day or idarubicin 12 mg/m2/day). B indicates hematopoietic stem cell transplantation (HSCT). C indicates the molecular targeted therapy including protein kinase inhibitors of targets like FLT3 and mTOR. D indicates immunotherapy. E indicates epigenetic therapy. DNA transmethylase inhibitors like azacitidine and its deoxidization derivatives decitabine have been applied to clinical. b. Illustrating of a. c. The challenges of molecular targeted therapy.
Fig. (2).
The mutation of the targets including classic FLT3, mTOR and new targets which are on trials like PP2A, PAK1, DOCK2, IDH1, IDH2.
3. PROTEIN KINASE INHIBITORS
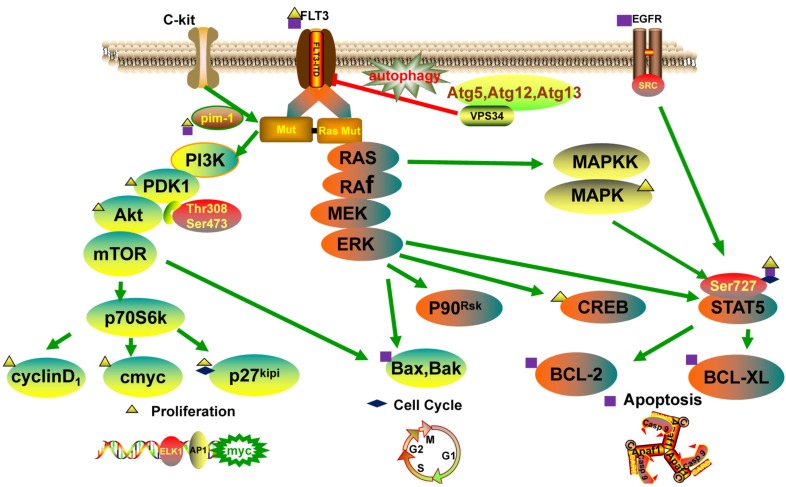
Protein kinase is a kind of protein phosphotransferase bringing the phosphate of ATP to the specific amino acid residue. It may conventionally be divided into five classes: tyrosine protein kinase, serine/threonine protein kinase, histidine protein kinase, tryptophan protein kinase and aspartyl/glutamoyl protein kinase. Protein kinases are associated with constitutive activation of many downstream pathways, such as phosphatidyl-inositol 3-kinase/v-akt murine thymoma viral oncogene homolog 1 (PIK3/AKT), mitogen-activated protein kinase/extracellular signal regulated kinase (MAPK/ERK) and signal transducer and activator of transcription 5 (STAT5) [23-25]. Protein kinases can regulate many kinds of signal transduction processes through phosphorylating the serine sites, threonine sites or tyrosine residues of substrate protein. It has been proved that abnormal activity of protein kinases is related to many diseases like cancer, immune system disease and inflammation. Thus, targeted protein kinase inhibitors have the potential to be new drugs, and more and more researchers are paying attention to develop new anti-tumor drugs from abnormal signal transduction [26]. Here, we discuss and summarize the main protein kinases involved in signal pathway in AML, including PIK3/AKT, MAPK/ERK, STAT5, autophagy (Fig. 3), and the protein kinase inhibitors (PKI) in AML (Table 2).
Fig. (3).
Signal pathways including PI3K/AKT, MAPK/ERK, STAT5 and autophagy, related to the proliferation, cycle and apoptosis of leukemia cells.
Table 2.
Protein kinase inhibitors in AML.
| Targets | Inhibitors | References |
|---|---|---|
|
FLT3 mTOR PI3K PDK1 AKT MEK Atg5/Atg12/Atg13 |
KW-2449/Tandutinib/Sorafenib/Sunitinib/Middostaurin/Estaurtinib/Su5416/Quizartinib/ Crenolanib/PLX-3397/Gliterinib/JI6 Rapamycin/CCI779/RAD001/AP23573/FAKBP12/OSI-027/PP242/AZD8055/Ciclopirox/ CCI779/Evorolimus/OSI-027/Torin1/ku-0063794/Way-600'/INK-128/MLN0128/ WYE687/NVPBGT226/NVP-BEZ235 BKM-120/IPI145/BYL719/Wortmannin/LY294002/IC87114 UCN01/L744832 NVPBGT226/NVP-BEZ235/AZD-5363/MK-2206 PD02325901/MEK162/Trametinib/E6201 Brotezomib |
[27] [32, 33] [32] [59, 60] [33] [28] [36, 38] |
FLT3 (Fms-like tyrosine kinase 3) is one of the class III family of receptor tyrosine kinases (RTKs), also called as fetal liver kinase-2. FLT3 comprises with the two most common types: one is internal tandem duplication (ITD) which is always associated with poor prognosis, and the other is a kind of point mutation called FLT3/tyrosine kinase domain (TKD) [27, 28]. FLT3-ITD approximately accounts for 30% of AML and indicates poorer prognosis [29]. Besides, accumulating evidences implicate that FLT3 is expected to be a promising therapeutic marker despite the historically poor prognosis of patients with AML. Now, most inhibitors are under clinical evaluation at phase I, II and III clinical trials
(Table 3), and a sustained effort has been taken to develop targeted inhibitors. The early first generation inhibitors of FLT3 principally comprise sunitinib (SU11248), midostaurin (PKC412), lestaurtinib (CEP-701) and tandutinib (MLN518) [30]. These inhibitors, rather than specifically target FLT3, are actually against a variety of other signal moleculars including c-KIT, VEGFR, PDGFRb and STAT5 or others [31]. Akt, also known as protein kinase B (PKB) or Rac, is a serine/threonine-specific protein kinase that plays a key role in cell proliferation, apoptosis and transcription. Akt family members consist of Akt1, Akt2 and Akt3, which have different functional roles [32]. The PKB kinase PDK1 phosphorylates its activation loop on the site of Thr308 and the COOH-terminus is activated by mammalian Target of Rapamycin (mTOR) complex 2 on Ser473. Akt1, also called Akt, is able to activate PIK3/AKT pathways and is a key signaling protein in the cellular pathways in the AML. Therefore, Akt1 is usually served as a target and there have been multiple inhibitors of Akt1 applied to the therapy of AML. Akt2 is an important signaling molecule in the insulin signaling pathway. As for Akt3, its function and mechanism are not yet clear now. GSK690693 is a novel ATP-competitive Akt kinase inhibitor and selective for the Akt isoforms, while it does inhibit additional members of the AGC kinase family [32]. MK-2206, is another kind of oral inhibitor of Akt1-3 that promotes apoptosis and cell cycle arrest in the AML; however, a trial study reported that only nearly 5% got good response during phase I, demonstrating its limited efficacy [33]. mTOR pathway is commonly activated in AML and other cancers which has been regarded as a critical target for AML therapy like FLT3. WYE-687, an mTOR kinase inhibitor, is a potential anti-acute myeloid leukemia agent.
Table 3.
Therapeutic inhibiors in AML.
| Inhibitors | Comments | Clinical Trials |
|---|---|---|
|
Sunitinib Sorafenib Midostaurin Lestaurtinib Tandutinib Quizartinib Crenolanib Bortezomib T315 Hsp90 Ibrutinib Alvocidib Volasertib Others |
It cooperates with cytarabine against AML cell lines with FL3-ITD, but not those with wild-type FLT3 It belongs to multi-kinase inhibitors, however it is often difficult to control the doses to inhibit FLT3 in clinical. It is an oral multitargeted kinase inhibitor to inihibit leukemia cells It is quite nonselective like inhibiting other tyrosine kinases like Janus kinase 2 (JAK2), and tropomycin receptor kinase (TRK) It inhibits FLT3-ITD AML cell lines The second-generation FLT3 inhibitor which has good effect in the treatment of AML The second-generation proteasome inhibitor Crenolanib usually combines with reinduction chemotherapy regimens in clinical It is associated with apoptotic and autophagic cell death of AML A new type of protein kinase inhibitor with the most promising results JAK-STAT and PI3K-AKT signaling networks are maintained by Hsp90 which promotes Hsp90-directed therapy Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive Alvocidib (IV bolus) in combination with cytarabine and mitoxantrone improves the outcome of the “7 + 3” It has been regarded as a promising agent for this patient population with AML Ciclopirox/AZD8055/WYE687/AG1109/SU5416/SU6668/BAG956 |
Phase III [82] Phase II-III [83] Phase III Phase I-II-III [84] Phase I Phase II [85] Earlier clinical trials Phase I-II [86] Earlier clinical trials [87] Phase I [88] Phase I-II-III Phase I-II [89] Phase III [90] Earlier clinical trials |
3.1. The First Generation Inhibitors
3.1.1. Sunitinib
Sunitinib is the first FLT3 inhibitor studied in the clinic and has the most clinically relevant data to be proved as a nonselective kinase inhibitor targeting FLT3, VEGF receptor, and Kit receptor tyrosine kinases [34]. It has been used together with cytarabine against AML cell lines with FL3-ITD mutation, rather than with wild-type FLT3 and achieved a CR rate of 59% in a phase I/II trial.
3.1.2. Midostaurin
Midostaurin can surpress a variety of kinases in many important cellular processes and interrupt leukemia cell growth and proliferation by inhibiting FTL3. In a phase III study, the addition of midostaurin to chemotherapy for AML patients had 23% improvement in overall survival (OS) and patients had significant prolongation in median survival [35].
3.1.3. Lestaurtinib
Lestaurtinib has multi-targets and can inhibit other tyrosine kinases, such as Janus kinase 2 (JAK2) and tropomycin receptor kinase (TRK) [36]. Lestaurtinib can promote apoptosis in FLT3-ITD leukemic blasts. In a phase II trial of oral lestaurtinib monotherapy, leukemia cells reduced at first place but not all the time, and now lestaurtinib has been combined with other drugs during the consolidation chemotherapy.
3.1.4. Tandutinib (MLN518)
Tandutinib, an inhibitor of FLT3, PDGFRb, and Kit, exhibited limited activity in phase I and II clinical trials in patients with AML and myelodysplastic syndrome. However, when combined with cytarabine and daunorubicin, tandutinib displayed promising anti-leukemic activity with 90% CR in a phase I/II trial in the newly diagnosed AML patients and inhibited FLT3-ITD leukemia cells proliferation and promoted apoptosis [37].
3.1.5. Bortezomib (PS-341)
Bortezomib, another proteasome inhibitor, can interfere with the transcription of FLT3-ITD and induce the early degradation of FLT3-ITD through autophagy. Bortezomib can trigger the inhibition of MAPK/ERK, PI3K/AKT and STAT5 pathways and subsequent activation of cell death in the AML [36, 38].
3.2. The Second Generation Inhibitors
3.2.1. Quizartinib (AC220)
Quizartinib is a second-generation FLT3 inhibitor that completely suppresses FLT3 phosphorylation in vivo at doses that are easily achievable and sustainable in the clinic [39]. There have been some trials proved that quizartinib can lead to favorable prognosis and it is promising that effective FLT3 inhibitors will become standard anti-leukemic approaches alongside traditional chemotherapies and HSCT.
3.2.2. Sorafenib (BAY43-9006)
With specific activity against the ITD mutation but not against other FLT3 mutations, sorafenib may suppress FLT3 phosphorylation and its downstream signaling ultimately promotes apoptosis in AML cells [40].
3.2.3. Gilteritinib (ASP2215)
Gilteritinib, a pyrazinecarboxamide derivative, is on trial at phase I-II-III and is a promising novel inhibitor of FLT3 inhibitors for its potential activity against all classes of FLT3-activating mutations [41].
3.2.4. Alvocidib (Formerly Flavopiridol)
Alvocidib is a multi-serine threonine cyclin-dependent kinase inhibitor and mainly downregulates CDK9 and CDK7 to inhibit cyclin D1, c-MYC, and MCL-1, which are all important for the proliferation of AML cells and cell cycle [42]. Alvocidib has currently been examined in a phase II study for the treatment of intermediate- and high-risk AML combined with standard of care agents.
3.2.5. AZD8055
AZD8055 has anti-tumor activity in AML cells by blocking mTOR signaling and can fully inhibit phosphorylation of eIF4E-binding protein 1, and subsequently arrests protein translation. Significantly, AZD8055 can decrease AML proliferation, hold back cell cycle progression and promote caspase-dependent apoptosis in leukemia cells but not in immature normal CD34+ cells. Interestingly, it can strongly induce autophagy, which may be protective autophagy in response to chemotherapy [43]. AZD8055 can markedly increase the survival of AML transplanted mice through a significant reduction of tumor growth without apparent drug toxicity [44]. Synergistic combinations of chemotherapy with low-dose AZD8055 could be more effective.
3.3. Novel Potential Inhibitors
3.3.1. Crenolanib (PLX-3397)
Crenolanib can be applied to the mutation of activation ring of FLT3 and the treatment strategy in which crenolanib is combined with induction chemotherapy of incipient FLT-ITD or FLT3-TKD mutations in AML is ongoing in clinical trials [45].
3.3.2. T315
T315 is an integrin-linked kinase (ILK) inhibitor which downregulates protein kinase B (Akt) and p-Akt and decreases cell activity of AML through promoting apoptotic and autophagic cell death [46, 47].
3.3.3. Gefitinib (ZD1839)
Gefitinib can selectively target EGFR, block the transduction pathway of tyrosine protein kinase and promote leukemia cell apoptosis. It was also found that BAG956, a kind of PI3K/PDK-1 inhibitor, can generate synergistic anti-proliferative effects when combined with midostaurin in the FLT3-mutated AML patients after HSCT [48, 49].
3.3.4. Volasertib
Volasertib, a Polo-like kinase inhibitor, is a potent drug with the combination therapy for those untreated patients who are ineligible for intensive induction therapy [50]. Volasertib is currently undergoing investigation in phase I and II trials and has yet to be licensed by the FDA.
3.3.5. Ciclopirox
Ciclopirox, an anti-fungal agent, is proved to be a novel specific mTOR kinase inhibitor and the combination of parthenolide and ciclopirox demonstrates greater toxicity against AML than treatment with either compound alone [51, 52].
3.3.6. HSP90
The heat-shock protein-90, also called HSP90, is a molecular chaperone connected with the co-chaperone Cdc37 which binds a variety of proteins, including tyrosine and serine/threonine protein kinases. Thus, finding valid inhibitor to disrupt such an interaction would help to block Akt function and further suppress leukemia cells [53].
3.3.7. Other Potential Inhibitors
Several trials demonstrated that PTEN and PIK3CA mutations may activate PDK1 downstream kinase [54-56], therefore, there are studies being conducted for the development of small molecule PDK1 inhibitors for the therapy of AML [57, 58]. Nevertheless, other PDK1 inhibitors have shown preclinical efficacy in AML [59, 60]. Recently, many inhibitors have emerged like HSP90, ibrutinib (PCI-32765) [61] which selectively target FLT3-ITD in mutant FLT3-positive AML [62, 63]. AG1109, which has been found as an effective inhibitor of protein tyrosine kinase, is a new potent inhibitor of protein kinase CK2. SU5416 and SU6668 are inhibitors of c-kit in AML, which is a receptor tyrosine kinase (RTK) with a pivotal role in disease [64]. There are many new candidate inhibitors which are still under development such as NVP-BEZ235 [65, 66], OSI-027 [67], PP242 [68], and CAL-101[69]. ACER3 has been found to be a potent target which promotes cell proliferation, inhibits apoptosis and activates AKT signal pathway of leukemia cells [70]. DOT1L is another new target and is potentially used in the chemotherapy of AML [71]. Therefore, developing their inhibitors is necessary.
conclusion and outlook
Although the treatment of AML has been improved over the past few decades, the pathogenesis of AML and the pathways involved are still not fully clear. Molecular targeted therapy and immunotherapy have both demonstrated impressive efficacy and promising impact on AML patients; however, further clinical trials incorporating patient diagnosis, prognosis, and in-depth investigation based on genetic and mechanistic biomarkers are urgently needed.
Many new targets have been studied on trials such as PAK1 [72], DOCK2 [73], IDH1/2 [74] and DOT1L [75]. PAK1 (p21-activated kinase) is a member of the PAK family of serine/threonine kinases, which promotes AML cells differentiation and apoptosis [72]. DOCK2 (Dedicator of cytokinesis 2) is a promising novel target for AML for the reason that knockdown of DOCK2 by shRNA enhances FLT3 activity and makes FLT3/ITD leukemic cells more sensitive to conventional antileukemic drugs [73]. IDH1/2 (isocitrate dehydrogenase 1/2) inhibitors change hypermethylation of DNA and histones in vitro therapy, interestingly, IDH2 mutations at the Arg140 usually bring a favorable outcome particularly in the intermediate-risk AML patients [74], providing novel candidate site to develop inhibitor for therapeutic targeting of specific AML subsets. DOT1L (Disruptor of telomeric silencing 1-like), required for the transforming activity of MLL fusion proteins, is a catalytic driver of leukemogenesis and its inhibitors EPZ-567 and EPZ004777 are on trial [75].
Currently, with the rapid development of biological information technology, multiple new types of protein kinase inhibitors such as FLT3 and mTOR inhibitors have been selected. PKIs have been used to treat AML patients either separately and in combination with traditional treatments. For example, combining sorafenib with standard induction chemotherapy yielded encouraging results in phase I/II [76]. Dasatinib was applied into the standard induction and consolidation chemotherapy in phase II patients with KIT-mutated/CBF AML and contributed to promising prognosis [77]. At the same time, plenty of clinical trials incorporate with the immune therapy, particularly in patients receiving transplantation or post-transplantation [78-81]. However, serious off-target effects and toxicity in patients have been observed, which might result from the genetic heterogeneity, the protein kinase mutation and evoluted immune escape.
Taken together, the incorporation of PKIs into the clinical standard chemotherapy still remains a considerable challenge and would result in the diverse curative rates due to many factors, such as specificity and stability of the PKIs, dose, inhibitors’ interaction, the clinical phase, patients’ individual variety, etc. Thus, further understanding of the pathogenesis of AML, screening new targets and seeking novel effective PKIs are necessary, especially with the increasing severity of drug-resistance. Moreover, highly specific kinase inhibitor drugs will hopefully provide truly effective and personalized treatments of cancers with less reliance on toxic chemotherapy.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (81300398), Natural Science Foundation of Guangdong Province, China (2015A030313528, 2015A030313518), and 2013 Sail Plan “Introduction of the Shortage of Top-Notch Talent” Project (YueRenCaiBan [2014] 1).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Haas M., Lonial S. Targeted therapy for haematological malignancies: clinical update from the American Society of Hematology, 2004. Expert Opin. Investig. Drugs. 2005;14(9):1161–1169. doi: 10.1517/13543784.14.9.1161. [DOI] [PubMed] [Google Scholar]
- 2.Leith C.P., Kopecky K.J., Godwin J., et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- 3.Leonard J.T., Raess P.W., Dunlap J., et al. Functional and genetic screening of acute myeloid leukemia associated with mediastinal germ cell tumor identifies MEK inhibitor as an active clinical agent. J. Hematol. Oncol. 2016;9:31. doi: 10.1186/s13045-016-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallman M.S., Gilliland D.G., Rowe J.M. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 5.Schulenburg A., Blatt K., Cerny-Reiterer S., et al. Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J. Hematol. Oncol. 2015;8:16. doi: 10.1186/s13045-015-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohner H., Weisdorf D.J., Bloomfield C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 7.Colvin G.A., Elfenbein G.J. The latest treatment advances for acute myelogenous leukemia. Med. Health R. I. 2003;86(8):243–246. [PubMed] [Google Scholar]
- 8.Gregory T.K., Wald D., Chen Y., et al. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J. Hematol. Oncol. 2009;2:23. doi: 10.1186/1756-8722-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appelbaum F.R., Gundacker H., Head D.R., et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [J]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Necochea-Campion R., Shouse G.P., Zhou Q., et al. Aberrant splicing and drug resistance in AML. J. Hematol. Oncol. 2016;9(1):85. doi: 10.1186/s13045-016-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naymagon L., Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J. Hematol. Oncol. 2016;9(1):52. doi: 10.1186/s13045-016-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Liu C., Tsui S.T., et al. Second-generation inhibitors of Bruton tyrosine kinase. J. Hematol. Oncol. 2016;9(1):80. doi: 10.1186/s13045-016-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masetti R., Bertuccio S.N., Astolfi A., et al. Hh/Gli antagonist in acute myeloid leukemia with CBFA2T3-GLIS2 fusion gene. J. Hematol. Oncol. 2017;10(1):26. doi: 10.1186/s13045-017-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z.Y. Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis. Hematology (Am. Soc. Hematol. Educ. Program) 2003;•••:1–13. doi: 10.1182/asheducation-2003.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Bennett J.M., Catovsky D., Daniel M.T., et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br. J. Haematol. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 16.Sabattini E., Bacci F., Sagramoso C., et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87. [PubMed] [Google Scholar]
- 17.Grimwade D., Walker H., Oliver F., et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1, 612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 18.Wu H., Hu C., Wang A., et al. Discovery of a BTK/MNK dual inhibitor for lymphoma and leukemia. Leukemia. 2016;30(1):173–181. doi: 10.1038/leu.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cang S., Iragavarapu C., Savooji J., et al. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J. Hematol. Oncol. 2015;8:129. doi: 10.1186/s13045-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y., Gao H., Zhang Y., et al. Alantolactone selectively ablates acute myeloid leukemia stem and progenitor cells. J. Hematol. Oncol. 2016;9(1):93. doi: 10.1186/s13045-016-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estey E.H. Prognostic factors in acute myelogenous leukemia. Leukemia. 2001;15(4):670–672. doi: 10.1038/sj.leu.2402057. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y., He D., Peng Z., et al. Circular RNAs in cancer: an emerging key player. J. Hematol. Oncol. 2017;10(1):2. doi: 10.1186/s13045-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuki M., Fenski R., Halfter H., et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96(12):3907–3914. [PubMed] [Google Scholar]
- 24.Brandts C.H., Sargin B., Rode M., et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65(21):9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J. Hematol. Oncol. 2011;4:13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharabi M.B., Chevet E., Panse J., et al. Drugging the unfolded protein response in acute leukemias. J. Hematol. Oncol. 2015;8:87. doi: 10.1186/s13045-015-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacher U., Haferlach C., Kern W., et al. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood. 2008;111(5):2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 28.Thiede C., Steudel C., Mohr B., et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 29.Gilliland D.G., Griffin J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 30.Weisberg E., Boulton C., Kelly L.M., et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 31.Kayser S., Levis M.J. FLT3 tyrosine kinase inhibitors in acute myeloid leukemia: clinical implications and limitations. Leuk. Lymphoma. 2014;55(2):243–255. doi: 10.3109/10428194.2013.800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martelli A.M., Evangelisti C., Chiarini F., et al. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1(2):89–103. doi: 10.18632/oncotarget.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konopleva M.Y., Walter R.B., Faderl S.H., et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin. Cancer Res. 2014;20(8):2226–2235. doi: 10.1158/1078-0432.CCR-13-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiernik P.H. FLT3 inhibitors for the treatment of acute myeloid leukemia. Clin. Adv. Hematol. Oncol. 2010;8(6):429–436, 444. [PubMed] [Google Scholar]
- 35.Hassanein M., Almahayni M.H., Ahmed S.O., et al. FLT3 inhibitors for treating acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2016;16(10):543–549. doi: 10.1016/j.clml.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Degryse S., Cools J. JAK kinase inhibitors for the treatment of acute lymphoblastic leukemia. J. Hematol. Oncol. 2015;8:91. doi: 10.1186/s13045-015-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y., Paz K. Tandutinib, an oral, small-molecule inhibitor of FLT3 for the treatment of AML and other cancer indications. IDrugs. 2008;11(1):46–56. [PubMed] [Google Scholar]
- 38.Larrue C., Saland E., Boutzen H., et al. Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood. 2016;127(7):882–892. doi: 10.1182/blood-2015-05-646497. [DOI] [PubMed] [Google Scholar]
- 39.James J., Pratz K., Stine A., et al. Clinical pharmacokinetics and FLT3 phosphorylation of AC220, a highly potent and selective inhibitor of FLT3. Blood. 2008;112(11):912. [Google Scholar]
- 40.Pratz K.W., Cho E., Levis M.J., et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng F., Wang L., Shen Y., et al. Preclinical evaluation of WYE-687, a mTOR kinase inhibitor, as a potential anti-acute myeloid leukemia agent. Biochem. Biophys. Res. Commun. 2016;470(2):324–330. doi: 10.1016/j.bbrc.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X., Wang X., Qin L., et al. The dual mTORC1 and mTORC2 inhibitor PP242 shows strong antitumor activity in a pheochromocytoma PC12 cell tumor model. Urology. 2015;85(1):271–273. doi: 10.1016/j.urology.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Bhutia S.K., Kegelman T.P., Das S.K., et al. Astrocyte elevated gene-1 induces protective autophagy. Proc. Natl. Acad. Sci. USA. 2010;107(51):22243–22248. doi: 10.1073/pnas.1009479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt N., O’Neill H.M., Kleinert M., et al. Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2015;309(2):E142–E153. doi: 10.1152/ajpendo.00313.2014. [DOI] [PubMed] [Google Scholar]
- 45.Smith C.C., Lasater E.A., Lin K.C., et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc. Natl. Acad. Sci. USA. 2014;111(14):5319–5324. doi: 10.1073/pnas.1320661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu C.F., Weng J.R., Jadhav A., et al. T315 decreases acute myeloid leukemia cell viability through a combination of apoptosis induction and autophagic cell death. Int. J. Mol. Sci. 2016;17(8) doi: 10.3390/ijms17081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okabe S., Tauchi T., Tanaka Y., et al. Anti-leukemic activity of axitinib against cells harboring the BCR-ABL T315I point mutation. J. Hematol. Oncol. 2015;8:97. doi: 10.1186/s13045-015-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weisberg E., Wright R.D., McMillin D.W., et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol. Cancer Ther. 2008;7(5):1121–1129. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao M.W., Lai M.J., Liou J.P., et al. The synergic effect of vincristine and vorinostat in leukemia in vitro and in vivo. J. Hematol. Oncol. 2015;8:82. doi: 10.1186/s13045-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gjertsen B.T., Schoffski P. Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29(1):11–19. doi: 10.1038/leu.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen S., Hassane D.C., Corbett C., et al. Novel mTOR inhibitory activity of ciclopirox enhances parthenolide antileukemia activity. Exp. Hematol. 2013;41(9):799–807. doi: 10.1016/j.exphem.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi P.J., Xu L.H., Lin K.Y., et al. Synergism between the mTOR inhibitor rapamycin and FAK down-regulation in the treatment of acute lymphoblastic leukemia. J. Hematol. Oncol. 2016;9:12. doi: 10.1186/s13045-016-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearl L.H. Hsp90 and Cdc37 - a chaperone cancer conspiracy. Curr. Opin. Genet. Dev. 2005;15(1):55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Konopleva M.Y., Walter R.B., Faderl S.H., et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin. Cancer Res. 2014;20(8):2226–2235. doi: 10.1158/1078-0432.CCR-13-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce L.R., Komander D., Alessi D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 56.Vasudevan K.M., Barbie D.A., Davies M.A., et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman R.I., Wu J.M., Polokoff M.A., et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J. Biol. Chem. 2005;280(20):19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 58.Peifer C., Alessi D.R. Small-molecule inhibitors of PDK1. ChemMedChem. 2008;3(12):1810–1838. doi: 10.1002/cmdc.200800195. [DOI] [PubMed] [Google Scholar]
- 59.Najafov A., Sommer E.M., Axten J.M., et al. Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. Biochem. J. 2011;433(2):357–369. doi: 10.1042/BJ20101732. [DOI] [PubMed] [Google Scholar]
- 60.Medina J.R., Becker C.J., Blackledge C.W., et al. Structure-based design of potent and selective 3-phosphoinositide-dependent kinase-1 (PDK1) inhibitors. J. Med. Chem. 2011;54(6):1871–1895. doi: 10.1021/jm101527u. [DOI] [PubMed] [Google Scholar]
- 61.Maffei R., Fiorcari S., Martinelli S., et al. Targeting neoplastic B cells and harnessing microenvironment: the “double face” of ibrutinib and idelalisib. J. Hematol. Oncol. 2015;8:60. doi: 10.1186/s13045-015-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H., Hu C., Wang A., et al. Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive AML. Leukemia. 2016;30(3):754–757. doi: 10.1038/leu.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rushworth S.A., Murray M.Y., Zaitseva L., et al. Identification of Bruton’s tyrosine kinase as a therapeutic target in acute myeloid leukemia. Blood. 2014;123(8):1229–1238. doi: 10.1182/blood-2013-06-511154. [DOI] [PubMed] [Google Scholar]
- 64.Malaise M., Steinbach D., Corbacioglu S. Clinical implications of c-Kit mutations in acute myelogenous leukemia. Curr. Hematol. Malig. Rep. 2009;4(2):77–82. doi: 10.1007/s11899-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 65.Airiau K., Mahon F.X., Josselin M., et al. PI3K/mTOR pathway inhibitors sensitize chronic myeloid leukemia stem cells to nilotinib and restore the response of progenitors to nilotinib in the presence of stem cell factor. Cell Death Dis. 2013;4:e827. doi: 10.1038/cddis.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertacchini J., Guida M., Accordi B., et al. Feedbacks and adaptive capabilities of the PI3K/Akt/mTOR axis in acute myeloid leukemia revealed by pathway selective inhibition and phosphoproteome analysis. Leukemia. 2014;28(11):2197–2205. doi: 10.1038/leu.2014.123. [DOI] [PubMed] [Google Scholar]
- 67.Gruppuso P.A., Boylan J.M., Sanders J.A. The physiology and pathophysiology of rapamycin resistance: implications for cancer. Cell Cycle. 2011;10(7):1050–1058. doi: 10.4161/cc.10.7.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu P.P., Kang S.A., Rameseder J., et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Wang A., Liang X., et al. Simultaneous inhibition of Vps34 kinase would enhance PI3Kdelta inhibitor cytotoxicity in the B-cell malignancies. Oncotarget. 2016;7(33):53515–53525. doi: 10.18632/oncotarget.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C., Yin Y., Li C., et al. ACER3 supports development of acute myeloid leukemia. Biochem. Biophys. Res. Commun. 2016;478(1):33–38. doi: 10.1016/j.bbrc.2016.07.099. [DOI] [PubMed] [Google Scholar]
- 71.Liu W., Deng L., Song Y., et al. DOT1L inhibition sensitizes MLL-rearranged AML to chemotherapy. PLoS One. 2014;9(5):e98270. doi: 10.1371/journal.pone.0098270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pandolfi A., Stanley R.F., Yu Y., et al. PAK1 is a therapeutic target in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2015;126(9):1118–1127. doi: 10.1182/blood-2014-12-618801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu M., Hamaker M., Li L., et al. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia. 2017;31(3):688–696. doi: 10.1038/leu.2016.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdel-Wahab O., Levine R.L. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121(18):3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daigle S.R., Olhava E.J., Therkelsen C.A., et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20(1):53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ravandi F., Cortes J.E., Jones D., et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J. Clin. Oncol. 2010;28(11):1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marcucci G., Geyer S., Zhao W., et al. Adding KIT inhibitor dasatinib (DAS) to chemotherapy overcomes the negative impact of KIT mutation/over-expression in core binding factor (CBF) acute myeloid leukemia (AML): Results from CALGB 10801 (Alliance). Blood. 2014;124(21) [J]. [Google Scholar]
- 78.Metzelder S.K., Schroeder T., Finck A., et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 79.Sammons S.L., Pratz K.W., Smith B.D., et al. Sorafenib is tolerable and improves clinical outcomes in patients with FLT3-ITD acute myeloid leukemia prior to stem cell transplant and after relapse post-transplant. Am. J. Hematol. 2014;89(9):936–938. doi: 10.1002/ajh.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herrero-Sanchez M.C., Rodriguez-Serrano C., Almeida J., et al. Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft-versus-host disease development. J. Hematol. Oncol. 2016;9(1):113. doi: 10.1186/s13045-016-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang E., Xu H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J. Hematol. Oncol. 2017;10(1):1. doi: 10.1186/s13045-016-0379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miano M., Micalizzi C., Calvillo M., et al. New targets in pediatric Acute Myeloid Leukemia. Immunol. Lett. 2013;155(1-2):47–50. doi: 10.1016/j.imlet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Crump M., Hedley D., Kamel-Reid S., et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (National Cancer Institute of Canada) Clinical Trials Group Study. Leuk. Lymphoma. 2010;51(2):252–260. doi: 10.3109/10428190903585286. [DOI] [PubMed] [Google Scholar]
- 84.Strock C.J., Park J.I., Rosen M., et al. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003;63(17):5559–5563. [PubMed] [Google Scholar]
- 85.Ostronoff F., Estey E. The role of quizartinib in the treatment of acute myeloid leukemia. Expert Opin. Investig. Drugs. 2013;22(12):1659–1669. doi: 10.1517/13543784.2013.842973. [DOI] [PubMed] [Google Scholar]
- 86.Csizmar C.M., Kim D.H., Sachs Z. The role of the proteasome in AML. Blood Cancer. 2016;6(12):e503. doi: 10.1038/bcj.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiu C.F., Weng J.R., Jadhav A., et al. T315 decreases acute myeloid leukemia cell viability through a combination of apoptosis induction and autophagic cell death. Int. J. Mol. Sci. 2016;17(8) doi: 10.3390/ijms17081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zong H., Gozman A., Caldas-Lopes E., et al. A hyperactive signalosome in acute myeloid leukemia drives addiction to a tumor-specific Hsp90 species. Cell Reports. 2015;13(10):2159–2173. doi: 10.1016/j.celrep.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeidner J.F., Karp J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015;39(12):1312–1318. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Hao Z., Kota V. Volasertib for AML: clinical use and patient consideration. OncoTargets Ther. 2015;8:1761–1771. doi: 10.2147/OTT.S60762. [DOI] [PMC free article] [PubMed] [Google Scholar]