Abstract
Background:
Alzheimer’s disease (AD) is associated with age-associated central nervous sys-tem degeneration and dementia. This decline in the function correlates with deposition of Aβ peptide con-taining plaques and associated reactive gliosis. The inflammatory phenotype of microglia, in particular, is often considered detrimental to cognitive function in AD. In addition to the changes in the CNS, altered immune changes in the periphery have recently been observed in AD suggesting a critical immune-related communication between the periphery and the brain.
Objective:
We hypothesized that modulating the peripheral immune system may alter the proinflammatory gliosis associated with AD. Therapeutic antibodies against the α4β1 integrin receptor have been used clini-cally to attenuate the ability of various immune cells to adhere to endothelium and migrate into target tis-sues such as the intestines (Crohn’s disease) or brain (multiple sclerosis). We hypothesized that a similar peripheral antibody-based therapy would attenuate gliosis by altering immune cell infiltration or phenotype in peripheral organs and the brain using an APP/PS1 mouse model of Alzheimer’s disease.
Method:
Littermate control wild-type and APP/PS1 mice were tail vein injected with either saline, isotype control (IgG2b), or an antibody recognizing α4-integrin, anti-CD49d, once a week for 4 consecutive weeks. To understand CNS and peripheral immune changes, brains and spleen were used.
Results/Conclusion:
Our data suggests that the antibody therapy was able to reduce microgliosis, astro-gliosis, and synaptic changes in the APP/PS1 mice compared to isotype control injections without chang-ing amyloid-β plaque load. Interestingly, both isotype control and antibody therapy also reduced the number of proinflammatory cytokines in the spleen although changes in the brain were less robust. The anti-CD49d and isotype control treatments also reduced CD4 immunoreactivity in the brains, suggesting a possible mechanism for attenuation of inflammation in the brain. This data suggests that it is indeed feasi-ble to alter the immune component of AD brain changes using a clinically feasible strategy of delivering a particular subtype of IgG or epitope selective antibodies that target infiltration of the peripheral immune system.
Keywords: Alzheimer’s disease, APP/PS1, neuroinflammation, tysabri, peripheral immune system, Crohn’s disease
1. INTRODUCTION
Alzheimer’s disease is a progressive neurodegenerative disease characterized by the deposition of Aβ plaques [1-3]. These plaques are often associated with the presence of reactive microglia that have been implicated in an inflammatory phenotype in the Alzheimer’s disease brain [4-19]. Although brain innate immune cells, microglia, have been extensively examined in AD as well as other neurodegenerative diseases, a number of additional immune changes, both in the brain and the periphery have also been observed [20-24]. In the brain, in addition to the activation of microglia and astrocytes, the C1q complement pathway has been seen to upregulate in AD patients [25]. Activation of complement proteins has been associated with neuronal atrophy [25, 26]. Vascular abnormalities and peripheral inflammation have been found in many AD patients [27, 28]. In addition, numerous studies in a range of disease conditions including AD, PD and depression have demonstrated changes in peripheral immune cell behavior resulting in immune-related changes in the brain [29-31]. Different immune cell types peripherally have been implicated in the AD pathogenesis. These have been discussed in a recent review by Jevtic et al [32]. For example, mononuclear phagocytes [21, 33, 34], neutrophils [35], lymphocytes [36, 37], fibroblasts [38] and leukocytes [39] all undergo changes in their secretary phenotype in vivo in AD mice and AD patients. This systemic immune alteration has been demonstrated by an increase in proinflammatory markers such as IL-1β and TNF-α and coagulation factors in the serum [27, 28]. This suggests a general principle of immune cell communication between the peripheral immune system and the central nervous system and thus leads to the hypothesis that modulating peripheral immune cells may alter the proinflammatory phenotype in the AD brain.
One area of research that has emerged in the past decade as an intervention to AD has been immunization therapies. Development of Aβ vaccines or antibodies against Aβ peptides has been reported to show promise in clearing amyloid plaques or improving cognitive deficits in human or rodent models [40-43]. Another therapeutic intervention has been the administration of intravenous immunoglobulins (IVIG) in Alzheimer’s disease mouse models [40, 44]. IVIG have been reported to contain antibodies against Aβ as one possible mechanism for their efficacy. Based on these observations, we hypothesized that modulating the peripheral immune system may be useful in clearing Aβ-mediated gliosis and inflammation. Therapeutic antibodies against α4/β1 integrin receptor have been used clinically to attenuate the ability of various immune cells to adhere to endothelium and migrate into target tissues such as the intestines or brain [45-47]. One such antibody, marketed as Tysabri (Biogen Idec), has been clinically used to treat multiple sclerosis and Crohn’s disease [48-51].
We hypothesized that using an anti-CD49d antibody to target the α4 subunit of the α4/β1 integrin and modulate peripheral immune cell behavior may be able to alter the proinflammatory microglial changes in the brains of an Alzheimer’s disease mouse model. For this purpose, the anti-CD49d antibody was tail vein injected into wild-type (C57BL/6) littermate control and APP/PS1 mice once a week for 4 consecutive weeks. Saline vehicle and isotype control antibodies were injected into additional groups of mice. Interestingly, both the IgG2b isotype control and anti-CD49d antibody were able to reduce microgliosis in the brains of APP/PS1 mice. In addition, these antibodies were also able to reduce the levels of a number of proinflammatory cytokines in the spleens of the APP/PS1 mice. Both IgG2b isotype control and anti-CD49d antibodies were able to reduce CD4 positive cell immunoreactivity in the mice suggesting a mechanism of peripheral antibody therapy reducing T cell infiltration into the brain.
2. MATERIALS AND METHODS
2.1. Antibodies and Reagents
Anti-Aβ clone 4G8 was purchased from Biolegend (San Diego, CA). Anti-α-tubulin antibody and the horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All mouse cytokine ELISA kits were obtained from R&D Systems (Minneapolis, MN). Elite Vectastain ABC avidin and biotin kits, biotinylated anti-rabbit, anti-mouse, and anti-rat antibodies and the Vector VIP kits were from Vector Laboratories Inc. (Burlingame, CA). Anti-CD68 was obtained from Serotec (Raleigh, NC). Anti-PSD95 and anti-GFAP antibodies were purchased from Cell Signaling Technology (Danvers, MA). Synaptophysin antibody was purchased from Chemicon International, Inc. (Temecula, CA) while anti-Cox-2, anti-TMEM119 and biotinylated anti-mouse IgG2b antibodies were from Abcam (Cambridge, MA). The Anti-Iba-1 antibody was from Wako Chemicals USA, Inc. (Richmond, VA). The Anti-CD4 antibody was purchased from BD Pharmingen (BD Biosciences, San Jose, CA).
2.2. Animals
The APP/PS1 transgenic mouse line, strain 005864 B6. Cg-Tg (APPswe, PSEN1dE9)85Dbo/ Mmjax and wild-type mouse line, C57BL/6 (WT) were originally obtained from the Jackson Laboratory (Bar Harbor, Maine). APP/PS1 mice express the Swedish mutation in APP and deltaE9 mutation in the PS1 gene resulting in the expression of human APP and secretion of human Aβ. Female 9-10 months old littermate control WT and APP/PS1 mice were used for tail vein injections of vehicle (saline), IgG isotype control (purified NA/LE Rat IgG2b; BD Pharmingen) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (BD Pharmingen, San Jose, CA).
2.3. Animal Use
All animal use was approved by the University of North Dakota Institutional Animal Care and Use Committee (UND IACUC). Mice were provided food and water ad libitum and housed in a 12 h light/dark cycle. The investigation conforms to the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (8th edition).
2.4. Tail Vein Injections
Mice from both the strains (WT and APP/PS1) were injected intravenously (tail vein) with vehicle (saline), isotype control IgG (purified NA/LE Rat IgG2b; BD Pharmingen) antibody or 2 mg/kg purified NA/LE rat anti-mouse CD49d (BD Pharmingen, San Jose, CA) antibody once a week for 4 consecutive weeks. Mice were sacrificed 4 days after the last injection and brains and other tissues were collected and fixed.
2.5. Immunohistochemistry of Mouse Brains
The paraformaldehyde fixed right hemispheres for saline, IgG and anti-CD49d injected mice (WT and APP/PS1) were cut using a freezing microtome as previously described [52]. Briefly, paraformaldehyde fixed tissue was embedded in a 15% gelatin (in PBS) matrix and immersed in a 4% paraformaldehyde solution for 24h to harden the gelatin matrix. The blocks were then cryoprotected through cycles of 15% sucrose followed by 30% sucrose. The blocks were then flash frozen using dry-ice/iso methylpentane, and 40µm serial sections were cut using a freezing microtome. Serial sections (960µm apart) were immunostained using anti-Aβ (4G8), anti-TMEM119 and anti-GFAP antibodies at a dilution of 1:1000 or anti-CD68, anti-CD4 and anti-Iba-1antibodies at a dilution of 1:500 followed by incubation with biotinylated secondary antibodies (1:2000 dilution) (Vector Laboratories Inc., Burlingame, CA) and avidin/biotin solution (Vector ABC kit). For IgG2b, tissue was incubated with biotin-conjugated anti-mouse IgG2b antibody at a dilution of 1:500 for 2 hours, followed by avidin/biotin solution (Vector ABC kit). Immunoreactivity was visualized using Vector VIP as chromogen. The slides were dehydrated and coverslipped using VectaMount (Vector Laboratories) following a standard dehydrating procedure through a series of ethanol concentrations and Histo-Clear (National Diagnostics, Atlanta, GA). Images were taken using an upright Leica DM1000 microscope and Leica DF320 digital camera system. Quantitation of immunostaining was performed as previously described [53]. Briefly, optical densities from the temporal cortex region of the same serial sections were measured using Adobe Photoshop software (Adobe Systems, San Jose, CA). The values for each section were averaged (3 sections/brain, 3-6 brains per condition) and plotted ±SD.
2.6. Western blot Analyses of Mouse Brains
Flash frozen tissue from parietal cortices of mice was lysed, sonicated in RIPA buffer, and quantitated using the BCA assay (Life Technologies Corporation, Grand Island, NY). The lysates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes for western blotting using anti-GFAP, anti-PSD95, anti-synaptophysin and anti-Cox-2 antibodies with α-tubulin as the loading control. Antibody binding was detected using enhanced chemiluminescence for detection. Western blots were quantified using Adobe Photoshop software. Optical density (O.D.) of bands was normalized against its respective loading controls and averaged (±SD).
2.7. Amyloid-β (Aβ) Levels from Mouse Tissue
Parietal cortices from all groups of wild-type (WT) C57BL/6 and APP/PS1 mice were flash frozen following collection. Part of the parietal cortices was lysed in RIPA buffer and the supernatant was quantitated using the BCA assay. This was considered the soluble Aβ fraction. The pellet from the RIPA lysates was further lysed in guanidine hydrochloride. The lysates were quantitated using the BCA method and both RIPA and guanidine HCL fractions were used to determine levels of Aβ1-40 and Aβ1-42 as per the manufacturer’s protocol (High sensitivity human specific EZHS40 and EZHS42, MilliporeSigma, Burlington, Massachusetts). The concentrations were normalized against the total protein, averaged, and plotted ±SD.
2.8. Cytokine ELISA from Mouse Tissue
Parietal cortex and spleen samples from all groups of wild-type (WT) C57BL/6 and APP/PS1 mice were flash frozen following collection. Part of the parietal cortex and spleens were lysed in PBS and protein levels were quantitated using the BCA assay. Lysates were used for multiple cytokine measurements using ELISA reagents (R&D Systems, Minneapolis, MN) and the levels of various cytokines were determined as per the manufacturer protocol and concentrations were averaged and plotted ±SD.
2.9. Statistical Analyses
The data are presented as the mean ± standard deviation (SD). Values statistically different from controls were determined using one-way ANOVA (or two-way ANOVA where required). The Tukey-Kramer multiple comparisons post-test was used to determine p values.
3. RESULTS
3.1. Anti-CD49d Antibody Injections did not Alter Aβ Plaque Load or Aβ1-40 and Aβ1-42 Levels in APP/PS1 Mice
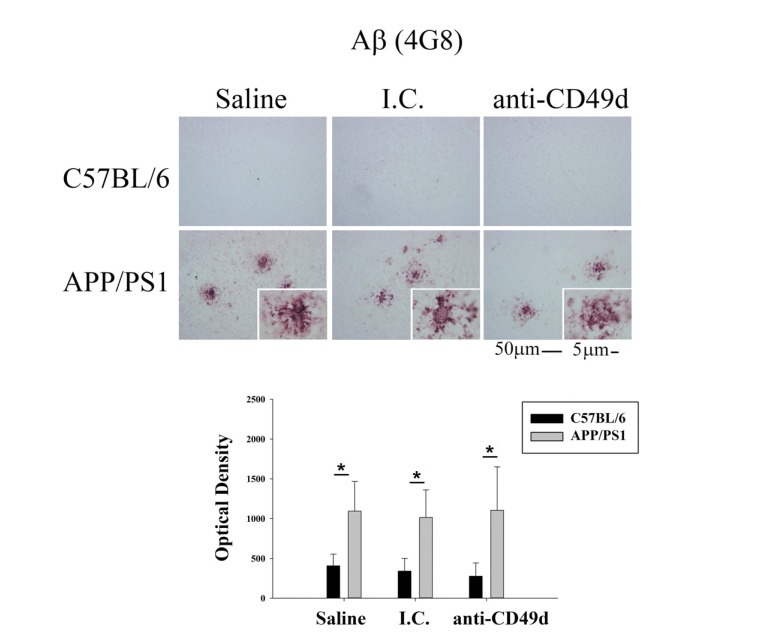
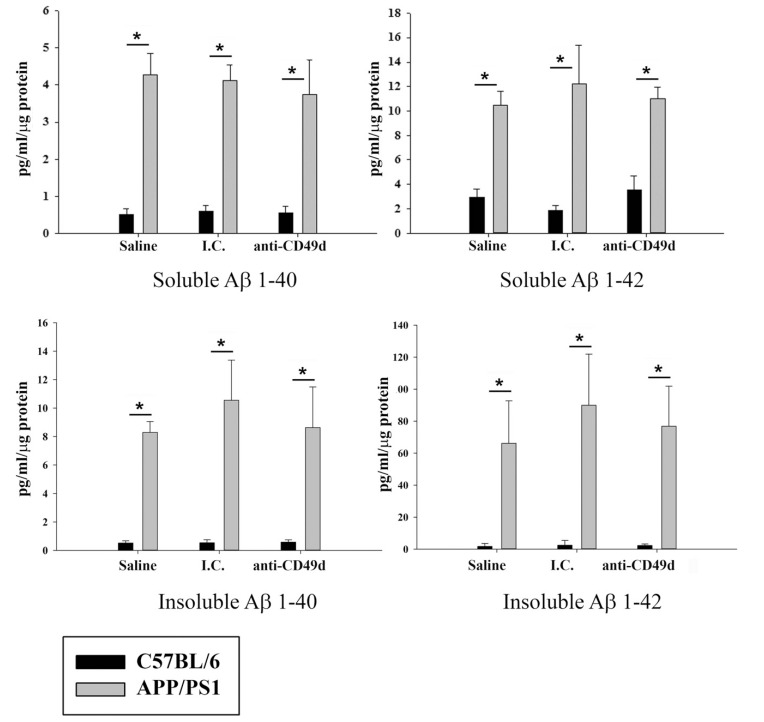
Since prior studies involving IVIG treatment of mice showed Aβ clearance (40, 44), we sought to determine whether IgG2b isotype control and anti-CD49d antibody injections could also affect Aβ levels in the mouse brains even though plaque removal was not part of our hypothesis. To assess the effects of anti-CD49d antibody on total Aβ plaque load, right brain hemispheres from WT and APP/PS1 mice injected with saline, isotype control (I.C.), and anti-CD49d antibody were immunostained using anti-Aβ (4G8) antibody. As expected, anti-CD49d antibody and isotype control injections did not have any effect on Aβ immunoreactivity in the brain (Fig. 1). To provide an additional means of quantifying Aβ, ELISAs were performed on the brain. The left brain hemispheres were flash frozen and parietal cortex dissected out. Frozen parietal cortex was then used to determine levels of Aβ1-40 and Aβ1-42, both soluble and insoluble fractions, using ELISA. APP/PS1 mice showed significantly higher levels of Aβ1-40 and Aβ1-42 in both soluble and insoluble fractions compared to WT mice (Fig. 2). However, similar to the immunostaining data, neither isotype control nor anti-CD49d antibody injections altered the concentrations of either form of the peptide in WT or APP/PS1 mice (Fig. 2).
Fig. (1).
Anti-CD49d antibody injections did not affect plaque load in APP/PS1 mice. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Right brain hemispheres from all the mice were fixed, serially sectioned, and immunostained using anti-Aβ (4G8) antibody. Representative images from 3-6 animals per group are shown at 20X magnification with 63X magnification insets. Quantitation of immunostaining was performed and O.D. values were averaged and plotted ± SD.
Fig. (2).
Anti-CD49d injections did not alter Aβ1-40 and Aβ1-42 levels in the brain. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Part of the parietal cortex from left brain hemispheres was dissected out, lysed and used for soluble and insoluble Aβ1-42 and Aβ1-40 measurements using ELISA. Aβ levels were determined from 3-6 animals per group ± SD (*p<0.05).
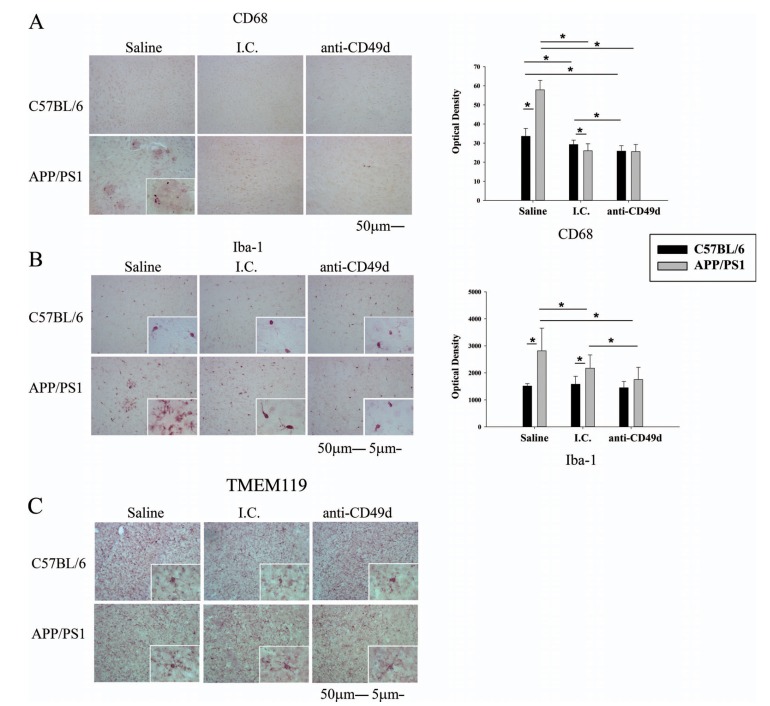
3.2. Both the Isotype Control and Anti-CD49d Antibody Injections Reduced Microgliosis in APP/PS1 Mice
In order to assess the immune response in the brain following antibody injections, right brain hemispheres from WT and APP/PS1 mice injected with saline, isotype control (I.C.), and anti-CD49d antibody were immunostained using antibodies against the microglial markers, CD68, Iba-1, and TMEM119. Interestingly, both I.C. and anti-CD49d antibody reduced microgliosis in APP/PS1 mice. CD68 immunoreactivity was significantly lower in I.C. and anti-CD49d antibody injected WT and APP/PS1 mice (Fig. 3a). Iba-1 immunoreactivity was also significantly reduced in I.C. injected compared to saline-injected APP/PS1 mice. However, anti-CD49d injections further reduced Iba-1 immunoreactivity compared to both saline and I.C. injections suggesting some additional benefit of anti-CD49d (Fig. 3b). Interestingly, the total number of resting microglia, as seen from TMEM119 positive immunostaining, did not seem to be altered with isotype control or anti-CD49d antibody injections suggesting that the antibody approaches were affecting microglial phenotype rather than numbers (Fig. 3c).
Fig. (3).
Both the isotype control (I.C.) and anti-CD49d antibody injections reduced microgliosis in APP/PS1 mice. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Right brain hemispheres from all the mice were fixed and serially sectioned. Microgliosis was assessed by immunostaining using anti-CD68 (3A) and anti-Iba-1 (3B) antibodies and total number of resting microglia were assessed using anti-TMEM119 antibody (3C). Representative images from 3-6 animals per group are shown at 20X magnification with 63X magnification insets. Quantitation of immunostaining was performed and O.D. values were averaged and plotted ± SD.
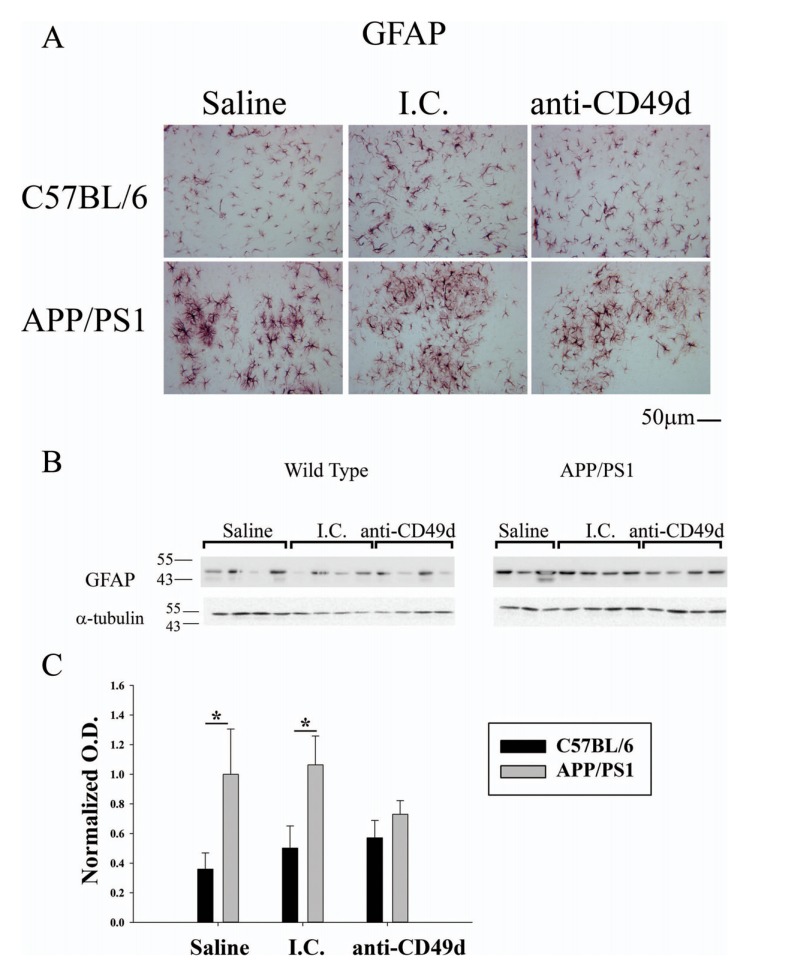
3.3. Anti-CD49d antibody injections reduced astrogliosis in APP/PS1 Mice
Since anti-CD49d injections reduced microgliosis in APP/PS1 mice, we next assessed the effect of antibody injections on astrocytes. Right brain hemispheres from WT and APP/PS1 mice were immunostained using anti-GFAP antibody. APP/PS1 mice in all groups had significantly higher
GFAP immunoreactivity compared to WT mice (Fig. 4). However, anti-CD49d antibody treated mice demonstrated a modest decrease in GFAP immunostaining (Fig. 4a). Western blotting for anti-GFAP antibody showed high GFAP levels in saline and isotype control injected APP/PS1 compared to WT mice, similar to the immunostaining data (Fig. 4b). Anti-CD49d antibody injected APP/PS1 mice had attenuated levels of GFAP down to WT values consistent with the microglial data that anti-CD49d treatment was able to affect gliosis in a manner slightly beyond that exerted by isotype control alone (Fig. 4c).
Fig. (4).
Anti-CD49d injections reduced astrogliosis in APP/PS1 mice. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Right brain hemispheres from all the mice were fixed and serially sectioned. Astrogliosis was assessed by immunostaining using anti-GFAP antibody (4A). Representative images from 3-6 animals per group are shown at 20X magnification. Parietal cortex from left brain hemispheres was dissected out, lysed and western blotted using anti-GFAP antibody (4B) with anti-α-tubulin as loading control. Optical densities for each antibody were normalized, averaged and graphed ± SD from 3-6 animal per group (*p<0.05).
3.4. Anti-CD49d Antibody Injections Reduced IL-12 and IFN-γ Levels in the Brain
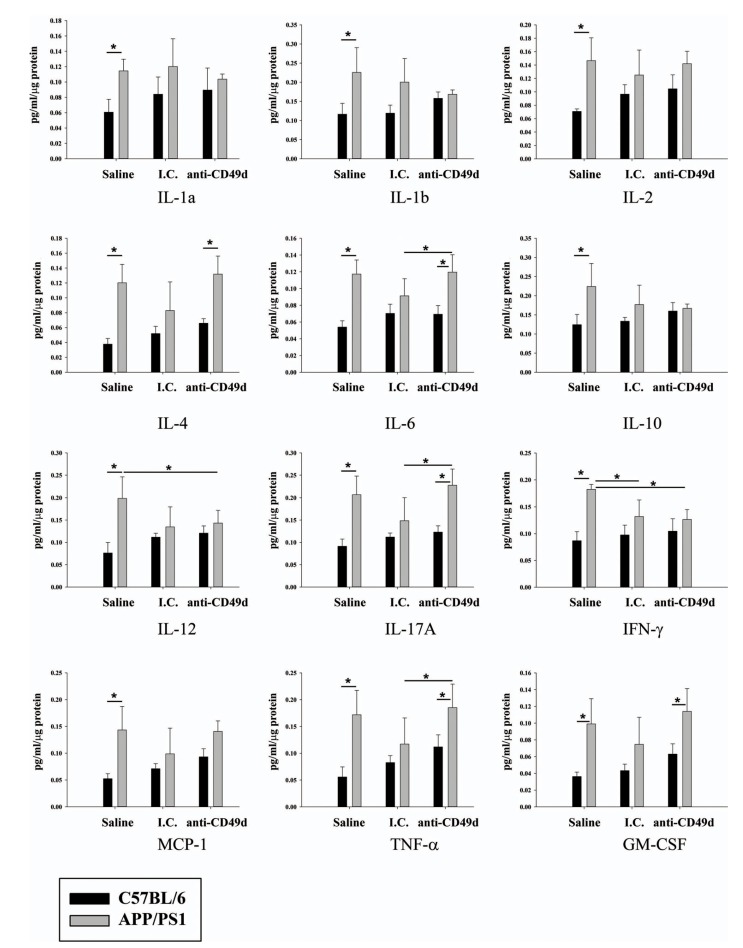
Activation of microglia has been implicated in the induction of cytokine secretion. In order to understand the effect of the anti-CD49d antibody on the inflammatory response in the brain, levels of different cytokines were determined from parietal cortex from left brain hemispheres of all groups of WT and APP/PS1 mice. Saline-injected APP/PS1 mice had increased levels of all cytokines assessed (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-17A, IFN-γ, MCP-1, TNF-α and GM-CSF) compared to WT mice (Fig. 5). Similar to the microgliosis immunostaining results, both I.C. and anti-CD49d antibodies attenuated levels of multiple cytokines in APP/PS1 compared to WT mice (Fig. 5). Interestingly, IL-12 and IFN-γ were significantly decreased in anti-CD49d treated compared to saline-injected APP/PS1 mice (Fig. 5).
Fig. (5).
Anti-CD49d injections reduced IL-12 and IFN-γ levels in the brain. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. A portion of parietal cortex from the left brain hemispheres was dissected out, lysed, and used for multiple cytokine measurements using ELISA. Cytokine levels were determined from 3-6 animals per group ± SD (*p<0.05).
3.5. Anti-CD49d Antibody Injections Reduced Levels of Multiple Proinflammatory Cytokines in the Spleen
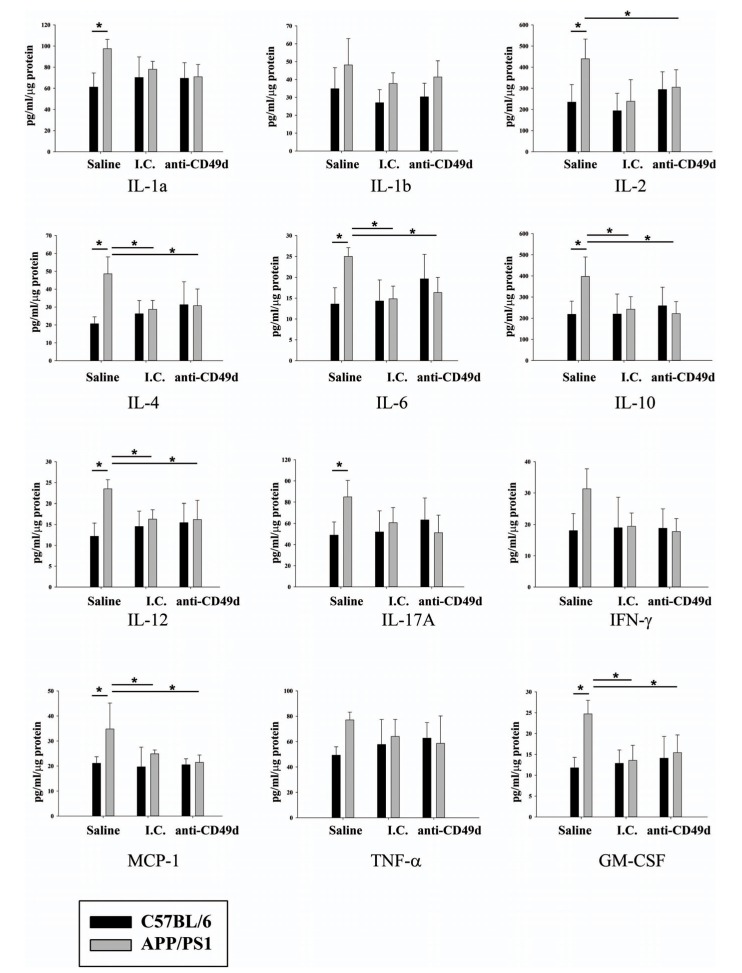
In order to correlate the inflammatory changes in the brain with any observed in the peripheral immune system, cytokines levels for all groups of mice were determined in the spleens. As expected, a number of cytokines (IL-1α, IL-2, IL-4, IL-6, IL-10, IL-12, IL-17A, MCP-1, and GM-CSF) were higher in APP/PS1 mice as compared to WT controls. Similar to the brain analysis, I.C. treated spleens of APP/PS1 mice demonstrated reduced cytokine levels compared to saline injected controls. However, unlike the brain, anti-CD49d antibodies attenuated the secretion of multiple cytokines including IL-2, IL-4, IL-6, IL-10, IL-12, MCP-1 and GM-CSF in the APP/PS1 mice compared to saline controls (Fig. 6). This data correlated with the observed attenuation of microgliosis using both IgG2b isotype control and anti-CD49d antibodies.
Fig. (6).
IgG and anti-CD49d injections reduced multiple cytokine levels in the spleen. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Spleens were dissected out, lysed, and used for multiple cytokine measurements using ELISAs. Cytokine levels were determined from 3-6 animals per group ± SD (*p<0.05).
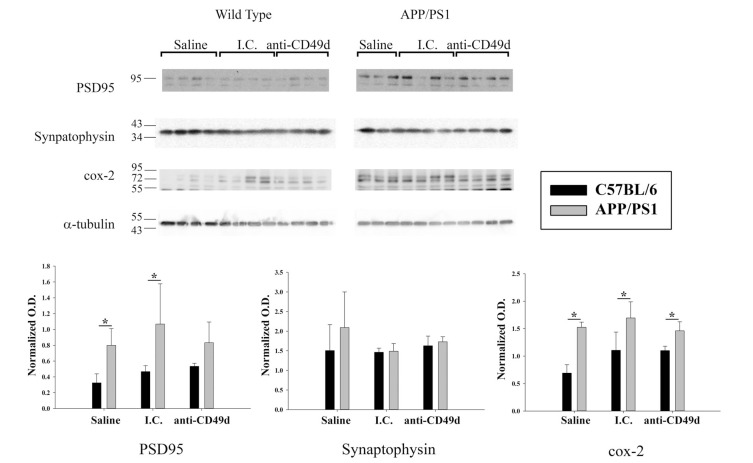
3.6. Anti-CD49d Antibody Injections Altered Post-Synaptic Proteins in APP/PS1 Mice
To examine the breadth of changes in other cell types that anti-CD49d antibody may cause, effects on synaptic markers were assessed. Parietal cortex from all groups of mice was lysed and western blotted for Cox-2, PSD95, and synaptophysin. APP/PS1 mice had no difference in presynaptic synaptophysin levels compared to WT mice (Fig. 7). However, APP/PS1 mice had significantly elevated post-synaptic PSD-95 and Cox-2 protein levels compared to wild-type mice (Fig. 7). Although antibody treatments did not alter Cox-2 protein levels, CD49d but not I.C. injections were sufficient to bring PSD-95 levels in APP/PS1 mice to WT levels (Fig. 7). This again suggested some additional benefit of the directed anti-CD49d therapy beyond the I.C.
Fig. (7).
Anti-CD49d antibody injections altered post-synaptic proteins APP/PS1 mice. C57BL/6 and APP/PS1 mice were injected intravenous (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Parietal cortex from left brain hemispheres was dissected out, lysed and western blotted using anti-PSD95, anti-synaptophysin and anti-Cox-2 antibodies with anti-α-tubulin as loading control. Optical densities for each antibody were normalized, averaged and graphed ± SD from 3-6 animal per group (*p<0.05).
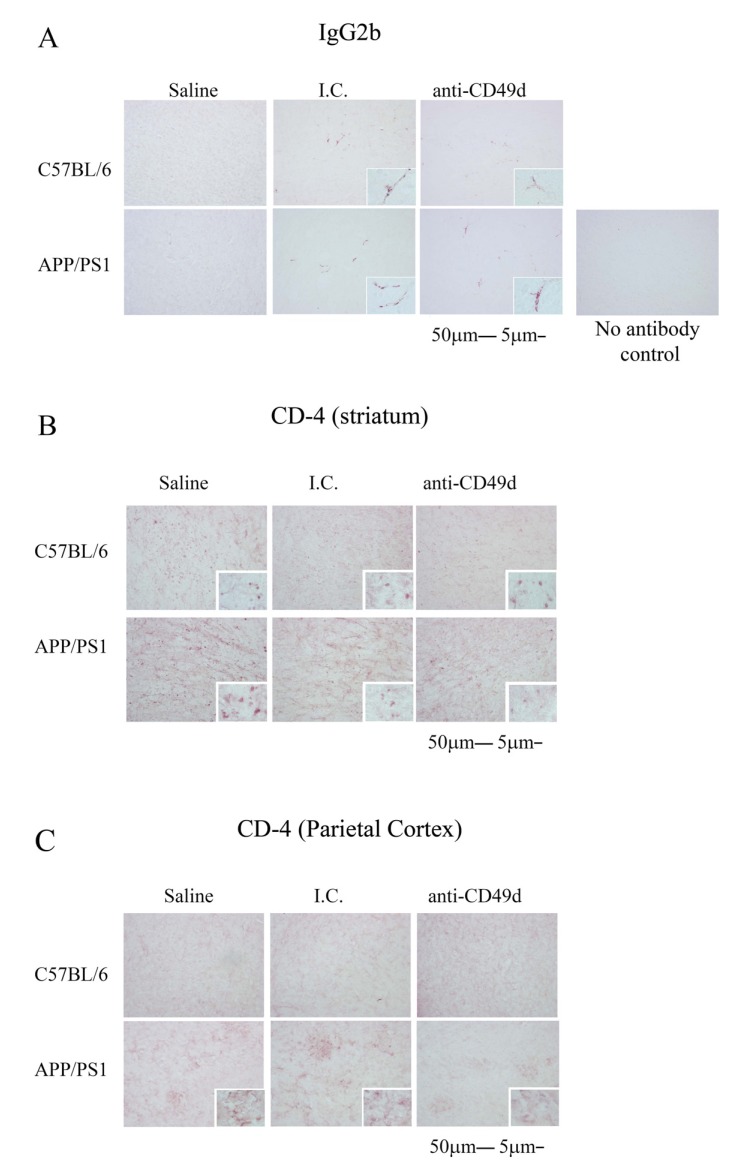
3.7. Both the Isotype Control and Anti-CD49d Antibody Injections Altered T cell Infiltration
In order to better understand the similarities and differences in the effects of the anti-CD49d and I.C. treatments, we determined whether antibody immunoreactivity was observed in the brain parenchyma. Since both antibodies are IgG2b, we visualized immunoreactivity for this subtype in the brain. Right brain hemispheres from WT and APP/PS1 mice injected with saline, I.C. and anti-CD49d antibody were immunostained using a biotinylated anti-mouse IgG2b antibody. Although brain parenchyma staining was not observed, both I.C. and anti-CD49d injected brains showed robust IgG2b immunoreactivity in the vasculature suggesting the possibility that the antibodies may enter the brain (Fig. 8a). As a negative control, a no antibody control was performed to demonstrate the specificity of the IgG2b detection (Fig. 8a).
Fig. (8).
Both the isotype control and anti-CD49d antibody injections affected brain CD4 immunoreactivity. C57BL/6 and APP/PS1 mice were injected intravenously (tail-vein) with Saline, IgG isotype control (purified NA/LE Rat IgG2b) (I.C.) or 2 mg/kg purified NA/LE rat anti-mouse CD49d (anti-CD49d) once a week for 4 weeks. Right brain hemispheres from all the mice were fixed and serially sectioned. The presence of IgG2b antibody in the brain was assessed by immunostaining using biotinylated anti-mouse IgG2b antibody (8A). The presence of T cells in the brain was visualized using anti-CD4 antibody and immunoreactivity observed in the striatum (8B) and parietal cortex (8C) was imaged. Representative images from 3-6 animals per group are shown at 20X magnification with 63X magnification insets.
In order to assess whether anti-CD49d antibody treatment preferentially affected T cell infiltration into the brain, we next immunostained sections from WT and APP/PS1 mice injected with saline, I.C., and anti-CD49d antibody using anti-mouse CD4 antibody. As expected, CD4 positive cell immunoreactivity was much higher in APP/PS1 compared to WT brains and was both plaque-associated and diffusely present in APP/PS1 mice (Fig. 8b, 8c). Although there were no differences observed in plaque-associated CD4 positive cells in the parietal cortices (Fig. 8c), there was a robust reduction in CD4 immunoreactivity observed in the striata of I.C. and anti-CD49d antibody injected WT and APP/PS1 mice compared to the saline-treated groups (Fig. 8b). This data suggested that the I.C. and anti-CD49d antibodies both attenuated T cell infiltration into the brain.
4. DISCUSSION
Our prior data suggests that the pathology of AD extends beyond the central nervous system [54-56]. This leads to the hypothesis that modulating the peripheral immune system may be useful in altering brain pathology during disease. Our study showed that intravenous delivery of an anti-CD49d and its isotype control antibody reduced microgliosis in an
Alzheimer’s disease mouse model, APP/PS1 mice, without affecting Aβ levels in the brains. Injections of the isotype control rat IgG2b and anti-mouse CD49d antibody were also both able to reduce a number of cytokines in the spleens and brains of these mice. Our prior work has suggested robust immune changes in the periphery of APP-/- mice correlating with the altered gastrointestinal function, adipocyte phenotype, and peripheral macrophage phenotype [57, 58]. This suggests that APP and/or its metabolites have a role in regulating the behavior of the peripheral immune system. More importantly, we recently observed a similar APP/APP metabolite regulatory role for the microglial phenotype in vitro and in vivo [59]. These data, coupled with the many studies demonstrating that AD is associated with altered immune cell behaviors outside of the brain support the premise that manipulating peripheral immune cell phenotypes may be effective against the proinflammatory changes during disease [21, 32-39, 57]. We hypothesize a possible role of immune cell activation in the periphery in parallel with those in the brain during AD.
A commercial anti-CD49d antibody, Natalizumab, has been shown to inhibit the influx of leukocytes into the CNS. Natalizumab is a humanized monoclonal antibody directed against the α-4 chain of the adhesion molecule VLA-4 (very late antigen-4) that is a member of integrin-type cell adhesion molecules responsible for leukocyte trafficking [60, 61]. It is an FDA approved drug used for the treatment of relapsing-remitting multiple sclerosis [49, 62, 63]. Natalizumab reduces CSF CD4+ T cells in MS patients [64]. Natalizumab is also reported to suppress CNS infiltration of natural killer cells and CD11b+CD4+ T cells in mouse models of EAE [65, 66]. These data correlate with our study where we demonstrate a reduction in gliosis in the brain without any change in Aβ plaque load in the APP/PS1 mice. Although these reports, including the current study, demonstrate immunomodulatory benefits from the use of anti-VLA-4 antibodies, we noted similar benefits from the IgG2b control injections. One possibility for this similarity of the control and therapeutic injections may be due to the subtype antibody used in our injections. It is well-known that IgG subtypes have differing abilities to modulate immune responses. For example, IgG1, the subtype of the commercial Natalizumab antibody used for EAE mouse models [66, 67], has the ability to bind to Fcγ Receptors I and II [68-70]. Therefore, injection of this anti-VLA4 IgG subtype might have similar immunomodulatory benefits as we observed along with an added benefit of increasing microglial Aβ phagocytic ability. Future work will define this potential.
The IgG2 subclass that was used in the current study can bind to FcγRIIb which is an inhibitory receptor on immune cells. Binding of both the I.C. and anti-CD49 antibodies on T cells, peripheral immune cells, as well as microglia could explain the overall inhibition of T cell immunoreactivity as well as reduced microgliosis, astrogliosis, and cytokine levels in the spleens and brain. Although our IgG2b immunostaining revealed only vascular reactivity it is possible that brain infiltration of the antibodies did occur. Indeed, infiltration into the brain may explain why the anti-CD49d treatment did have some additional effects on Iba-1 and GFAP immunoreactivity as well as PSD95 levels beyond the I.C. allowing the antibody to have selective effects on microglia in the brain in addition to the general effect of both I.C. and anti-CD49d on altering peripheral immune cell behavior and T cell infiltration. Further work is clearly needed to quantify antibody presence in the brain and effects on glial receptors.
It is known that IgG2b has a much lower affinity for FcγRIIb on monocytes and macrophages compared to IgG1 and hence shows lower complement activation [70]. This could explain the lack of effect of anti-CD49d and I.C. antibodies on Aβ plaque load or Aβ levels in our study as compared to studies using simply IVIG [68-70]. IVIG has been shown to increase Aβ clearance [71]. IVIGs are also known to contain anti-Aβ antibodies [71, 72]. Additionally, IVIGs contain a mixture of IgG subclasses with IgG1 being the most abundant [68, 69], suggesting that an increased binding to Fcγ receptor may be responsible for the increased phagocytic activity of microglia and in turn increased Aβ clearance in mice injected with IVIGs. This is further supported by a study by Marsh et al that shows mice lacking T, B, and natural killer cells show increased Aβ pathology and reduced phagocytic capacity in microglial cells [67]. A more comprehensive study of individual IgG subtype effects on both the peripheral and brain immune cells is needed in order to dissect apart subtleties of effect.
Although our initial hypothesis was to assess the role of anti-CD49d antibody in the immune response of the brain, we observed effects of isotype control similar to those of the anti-CD49d antibody in attenuation of T cell immunoreactivity, microgliosis, and cytokine production with the anti-CD49d approach providing a slightly increased benefit on only Iba-1, GFAP, and PSD95 changes compared to I.C. This suggests that the presence of an IgG2b antibody, irrespective of epitope specificity, was sufficient to alter both peripheral and brain immune cell phenotype superimposed upon any specific effects of inhibiting function of α4β1 integrins. Although we have not yet defined the precise mechanism of how the two antibody injection strategies were attenuating gliosis, we suggest that a dual mechanism of inhibition was exerted by the anti-CD49d approach combining the anti-α4β1 integrin effects with overall inhibitory effects of the IgG2b subclass to alter immune cell infiltration and phenotype changes in peripheral organs and within the brain. Further mechanistic studies are needed to understand the role of various IgG subclasses in the immune response in the periphery and brain of Alzheimer’s disease mice to better understand the utility of the straightforward approach to immunomodulation.
CONCLUSION
This study reports the use of an anti-CD49d antibody to attenuate microgliosis and astrogliosis in the brains of APP/PS1 mice. However, APP/PS1 mice injected with isotype control (rat IgG2b) and anti-CD49d antibody showed no changes in brain Aβ levels compared to mice injected with saline. Both antibody injections were also able to reduce T cell immunoreactivity and specific cytokine levels in the spleens and brains of APP/PS1 mice compared to WT mice. Collectively, our results support the notion that a directed antigen antibody, such as anti-CD49d, may have some benefits against immune cell activation in AD but the IgG2b subtype may be equally involved, if not more important, for consideration.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- Aβ
Amyloid-β
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- PS1
Presenilin 1
- CNS
Central Nervous System
- Aβ
Amyloid-β
- CD68
Cluster of Differentiation
- PSD95
Postsynaptic Density Protein 95
- GFAP
Glial Fibrillary Acidic Protein
- ELISA
Enzyme-linked Immunosorbent Assay
- WT
Wild-Type
- IgG
Immunoglobulin
- PBS
Phosphate-buffered Saline
- SD
Standard Deviation
- O.D.
Optical Density
- SDS-PAGE
Sodium Dodecyl Sulfate- polyacrylamide Gel Electrophoresis
- RIPA
Radioimmunoprecipitation Assay
- BCA
Bicinchoninic Acid
- GuHCL
Guanidine Hydrochloride
- ANOVA
Analysis of Variance
- I.C.
Isotype Control
- IL
Interleukin
- IFN
Interferon
- MCP-1
Monocyte Chemoattractant Protein-1
- TNF-α
Tumor Necrosis Factor Alpha
- GM-CSF
Granulocyte-macrophage Colony-stimulating Factor
- VLA
Very Late Antigen
Ethics Approval and Consent to Participate
All animals used were approved by the University of North Dakota Institutional Animal Care and Use Committee (UND IACUC).
Human and Animal Rights
No humans were used in this study. The investigation conforms to the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals (8th edition).
Consent for Publication
Not applicable.
conflict of interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Jarrett J.T., Berger E.P., Lansbury P.T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 3.Pike C.J., Burdick D., Walencewicz A.J., Glabe C.G., Cotman C.W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 1993;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama H., McGeer P.L. Brain microglia constitutively express beta-2 integrins. J. Neuroimmunol. 1990;30(1):81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- 5.Cras P., Kawai M., Siedlak S., Mulvihill P., Gambetti P., Lowery D., et al. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer’s disease. Am. J. Pathol. 1990;137(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- 6.Frautschy S.A., Yang F., Irrizarry M., Hyman B., Saido T.C., Hsiao K., et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998;152(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- 7.McGeer P.L., Itagaki S., Tago H., McGeer E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki A., Shoji M., Harigaya Y., Kawarabayashi T., Ikeda M., Naito M., et al. Amyloid cored plaques in Tg2576 transgenic mice are characterized by giant plaques, slightly activated microglia, and the lack of paired helical filament-typed, dystrophic neurites. Virchows Arch. 2002;441(4):358–367. doi: 10.1007/s00428-002-0643-8. [DOI] [PubMed] [Google Scholar]
- 9.Stalder M., Phinney A., Probst A., Sommer B., Staufenbiel M., Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 1999;154(6):1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Styren S.D., Civin W.H., Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer’s disease brain. Exp. Neurol. 1990;110(1):93–104. doi: 10.1016/0014-4886(90)90054-v. [DOI] [PubMed] [Google Scholar]
- 11.Wegiel J., Imaki H., Wang K.C., Wronska A., Osuchowski M., Rubenstein R. Origin and turnover of microglial cells in fibrillar plaques of APPsw transgenic mice. Acta Neuropathol. 2003;105(4):393–402. doi: 10.1007/s00401-002-0660-3. [DOI] [PubMed] [Google Scholar]
- 12.Wegiel J., Wang K.C., Imaki H., Rubenstein R., Wronska A., Osuchowski M., et al. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol. Aging. 2001;22(1):49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 13.Banati R.B., Gehrmann J., Schubert P., Kreutzberg G.W. Cytotoxicity of microglia. Glia. 1993;7(1):111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- 14.Combs C.K., Bates P., Karlo J.C., Landreth G.E. Regulation of beta-amyloid stimulated proinflammatory responses by peroxisome proliferator-activated receptor alpha. Neurochem. Int. 2001;39(5-6):449–457. doi: 10.1016/s0197-0186(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 15.Combs C.K., Johnson D.E., Karlo J.C., Cannady S.B., Landreth G.E. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J. Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. Beta-amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Bo R., Angeretti N., Lucca E., De Simoni M.G., Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and beta-amyloid production in cultures. Neurosci. Lett. 1995;188(1):70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 18.Giulian D., Haverkamp L.J., Li J., Karshin W.L., Yu J., Tom D., et al. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 1995;27(1):119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- 19.Klegeris A., Walker D.G., McGeer P.L. Interaction of Alzheimer beta-amyloid peptide with the human monocytic cell line THP-1 results in a protein kinase C-dependent secretion of tumor necrosis factor-alpha. Brain Res. 1997;747(1):114–121. doi: 10.1016/s0006-8993(96)01229-2. [DOI] [PubMed] [Google Scholar]
- 20.Eikelenboom P., Veerhuis R., van Exel E., Hoozemans J.J., Rozemuller A.J., van Gool W.A. The early involvement of the innate immunity in the pathogenesis of late-onset Alzheimer’s disease: neuropathological, epidemiological and genetic evidence. Curr. Alzheimer Res. 2011;8(2):142–150. doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y., Li L., Sun X.H. Monocytes and Alzheimer’s disease. Neurosci. Bull. 2011;27(2):115–122. doi: 10.1007/s12264-011-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimenez-Llort L., Mate I., Manassra R., Vida C., De la Fuente M. Peripheral immune system and neuroimmune communication impairment in a mouse model of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2012;1262:74–84. doi: 10.1111/j.1749-6632.2012.06639.x. [DOI] [PubMed] [Google Scholar]
- 23.Maccioni R.B., Rojo L.E., Fernandez J.A., Kuljis R.O. The role of neuroimmunomodulation in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009;1153:240–246. doi: 10.1111/j.1749-6632.2008.03972.x. [DOI] [PubMed] [Google Scholar]
- 24.Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol. Immunol. 2011;48(14):1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers J., Cooper N.R., Webster S., Schultz J., McGeer P.L., Styren S.D., et al. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1992;89(21):10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGeer P.L., Akiyama H., Itagaki S., McGeer E.G. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci. Lett. 1989;107(1-3):341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giunta B., Fernandez F., Nikolic W.V., Obregon D., Rrapo E., Town T., et al. Inflammaging as a prodrome to Alzheimer’s disease. J. Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diniz B.S., Teixeira A.L., Talib L., Gattaz W.F., Forlenza O.V. Interleukin-1beta serum levels is increased in antidepressant-free elderly depressed patients. Am. J. Geriatr. Psychiatry. 2010;18(2):172–176. doi: 10.1097/JGP.0b013e3181c2947f. [DOI] [PubMed] [Google Scholar]
- 30.Leonard B.E. Inflammation, depression and dementia: are they connected? Neurochem. Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 31.Scalzo P., Kummer A., Cardoso F., Teixeira A.L. Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s disease. J. Neuroimmunol. 2009;216(1-2):122–125. doi: 10.1016/j.jneuroim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Jevtic S., Sengar A.S., Salter M.W., McLaurin J. The role of the immune system in Alzheimer disease: etiology and treatment. Ageing Res. Rev. 2017;40:84–94. doi: 10.1016/j.arr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Sepuru K.M., Rajarathnam K. cxcl1/mgsa is a novel glycosaminoglycan (gag)-binding chemokine: structural evidence for two distinct non-overlapping binding domains. J. Biol. Chem. 2016;291(8):4247–4255. doi: 10.1074/jbc.M115.697888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K., Tian L., Liu L., Feng Y., Dong Y.B., Li B., et al. CXCL1 contributes to beta-amyloid-induced transendothelial migration of monocytes in Alzheimer’s disease. PLoS One. 2013;8(8):e72744. doi: 10.1371/journal.pone.0072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21(8):880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 36.Saresella M., Calabrese E., Marventano I., Piancone F., Gatti A., Calvo M.G., et al. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2010;21(3):927–938. doi: 10.3233/JAD-2010-091696. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira A.L., Reis H.J., Coelho F.M., Carneiro D.S., Teixeira M.M., Vieira L.B., et al. All-or-nothing type biphasic cytokine production of human lymphocytes after exposure to Alzheimer’s beta-amyloid peptide. Biol. Psychiatry. 2008;64(10):891–895. doi: 10.1016/j.biopsych.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Connolly G.P. Fibroblast models of neurological disorders: fluorescence measurement studies. Trends Pharmacol. Sci. 1998;19(5):171–177. doi: 10.1016/s0165-6147(98)01202-4. [DOI] [PubMed] [Google Scholar]
- 39.Dolman C.L. Diagnosis of neurometabolic disorders by examination of skin biopsies and lymphocytes. Semin. Diagn. Pathol. 1984;1(2):82–97. [PubMed] [Google Scholar]
- 40.Szabo P., Relkin N., Weksler M.E. Natural human antibodies to amyloid beta peptide. Autoimmun. Rev. 2008;7(6):415–420. doi: 10.1016/j.autrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 42.Morgan D., Diamond D.M., Gottschall P.E., Ugen K.E., Dickey C., Hardy J., et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 43.Brody D.L., Holtzman D.M. Active and passive immunotherapy for neurodegenerative disorders. Annu. Rev. Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodel R., Neff F., Noelker C., Pul R., Du Y., Bacher M., et al. Intravenous immunoglobulins as a treatment for Alzheimer’s disease: rationale and current evidence. Drugs. 2010;70(5):513–528. doi: 10.2165/11533070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Lobb R.R., Hemler M.E. The pathophysiologic role of alpha 4 integrins in vivo. J. Clin. Invest. 1994;94(5):1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayless K.J., Meininger G.A., Scholtz J.M., Davis G.E. Osteopontin is a ligand for the alpha4beta1 integrin. J. Cell Sci. 1998;111(Pt 9):1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 47.Theien B.E., Vanderlugt C.L., Nickerson-Nutter C., Cornebise M., Scott D.M., Perper S.J., et al. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood. 2003;102(13):4464–4471. doi: 10.1182/blood-2003-03-0974. [DOI] [PubMed] [Google Scholar]
- 48.Romme Christensen J., Ratzer R., Bornsen L., Lyksborg M., Garde E., Dyrby T.B., et al. Natalizumab in progressive MS: results of an open-label, phase 2A, proof-of-concept trial. Neurology. 2014;82(17):1499–1507. doi: 10.1212/WNL.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 49.Polman C.H., O’Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H., et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S., Goldin E., Gordon F.H., Malchow H.A., Rask-Madsen J., Rutgeerts P., et al. Natalizumab for active Crohn’s disease. N. Engl. J. Med. 2003;348(1):24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 51.Sandborn W.J., Colombel J.F., Enns R., Feagan B.G., Hanauer S.B., Lawrance I.C., et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005;353(18):1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 52.Nagamoto-Combs K., Manocha G.D., Puig K., Combs C.K. An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J. Neurosci. Methods. 2016;261:155–160. doi: 10.1016/j.jneumeth.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhawan G., Floden A.M., Combs C.K. Amyloid-beta oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiol. Aging. 2012;33(10):2247–2261. doi: 10.1016/j.neurobiolaging.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puig K.L., Lutz B.M., Urquhart S.A., Rebel A.A., Zhou X., Manocha G.D., et al. Overexpression of mutant amyloid-beta protein precursor and presenilin 1 modulates enteric nervous system. J. Alzheimers Dis. 2015;44(4):1263–1278. doi: 10.3233/JAD-142259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puig K.L., Manocha G.D., Combs C.K. Amyloid precursor protein mediated changes in intestinal epithelial phenotype in vitro. PLoS One. 2015;10(3):e0119534. doi: 10.1371/journal.pone.0119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulas J.A., Puig K.L., Combs C.K. Amyloid precursor protein in pancreatic islets. J. Endocrinol. 2017;235(1):49–67. doi: 10.1530/JOE-17-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puig KL, Brose SA, Zhou X, Sens MA, Combs GF, Jensen MD, et al. Amyloid precursor protein modulates macrophage phenotype and diet-dependent weight gain. 2017. [DOI] [PMC free article] [PubMed]
- 58.Puig K.L., Swigost A.J., Zhou X., Sens M.A., Combs C.K. Amyloid precursor protein expression modulates intestine immune phenotype. J. Neuroimmune Pharmacol. 2012;7(1):215–230. doi: 10.1007/s11481-011-9327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manocha G.D., Floden A.M., Rausch K., Kulas J.A., McGregor B.A., Rojanathammanee L., et al. APP regulates microglial phenotype in a mouse model of Alzheimer’s disease. J. Neurosci. 2016;36(32):8471–8486. doi: 10.1523/JNEUROSCI.4654-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yusuf-Makagiansar H., Anderson M.E., Yakovleva T.V., Murray J.S., Siahaan T.J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002;22(2):146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 61.Yang G.X., Hagmann W.K. VLA-4 antagonists: potent inhibitors of lymphocyte migration. Med. Res. Rev. 2003;23(3):369–392. doi: 10.1002/med.10044. [DOI] [PubMed] [Google Scholar]
- 62.Stuve O., Bennett J.L. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev. 2007;13(1):79–95. doi: 10.1111/j.1527-3458.2007.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havrdova E., Galetta S., Hutchinson M., Stefoski D., Bates D., Polman C.H., et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8(3):254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 64.Selter R.C., Biberacher V., Grummel V., Buck D., Eienbroker C., Oertel W.H., et al. Natalizumab treatment decreases serum IgM and IgG levels in multiple sclerosis patients. Mult. Scler. 2013;19(11):1454–1461. doi: 10.1177/1352458513477229. [DOI] [PubMed] [Google Scholar]
- 65.Mindur J.E., Ito N., Dhib-Jalbut S., Ito K. Early treatment with anti-VLA-4 mAb can prevent the infiltration and/or development of pathogenic CD11b+CD4+ T cells in the CNS during progressive EAE. PLoS One. 2014;9(6):e99068. doi: 10.1371/journal.pone.0099068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan Y., Liu R., Wu W., Bomprezzi R., Shi F.D. Antibody to alpha4 integrin suppresses natural killer cells infiltration in central nervous system in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2012;247(1-2):9–15. doi: 10.1016/j.jneuroim.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsh S.E., Abud E.M., Lakatos A., Karimzadeh A., Yeung S.T., Davtyan H., et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA. 2016;113(9):E1316–E1325. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.French M. Serum IgG subclasses in normal adults. Monogr. Allergy. 1986;19:100–107. [PubMed] [Google Scholar]
- 69.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruhns P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 71.St-Amour I., Pare I., Tremblay C., Coulombe K., Bazin R., Calon F. IVIg protects the 3xTg-AD mouse model of Alzheimer’s disease from memory deficit and Abeta pathology. J. Neuroinflammation. 2014;11:54. doi: 10.1186/1742-2094-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puli L., Tanila H., Relkin N. Intravenous immunoglobulins for Alzheimer’s disease. Curr. Alzheimer Res. 2014;11(7):626–636. doi: 10.2174/1567205011666140812113415. [DOI] [PubMed] [Google Scholar]