Abstract
Background:
Our previous study demonstrated that Myosin Phosphatase Targeting subunit 1 (MYPT1) may function as a direct target of microRNA-30d, which promotes tumor angiogenesis and tumor growth of prostate cancer (PCa). Here, we aimed to investigate the clinical significance of MYPT1 expression and its functions in PCa.
Methods:
Roles of MYPT1 deregulation in tumor angiogenesis of PCa was determined in vitro and in vivo experiments. Expression patterns of MYPT1 and CD31 proteins were examined by immunohistochemistry and immunofluorescence, respectively. Associations of MYPT1/CD31 combination with various clinicopathological features and patients' prognosis of PCa were also statistically evaluated.
Results:
Through gain- and loss-of-function experiments, MYPT1 inhibited capillary tube formation of endothelial cells and in vivo tumor angiogenesis in a mouse model with the downregulation of VEGF and CD31 expression. In addition, MYPT1 expression was significantly decreased, while CD31 expression was dramatically increased in PCa tissues compared to benign prostate tissues. Notably, MYPT1 expression levels in PCa tissues were negatively correlated with that of CD31. Statistically, MYPT1-low/CD31-high expression was distinctly associated with high Gleason score, positive biochemical recurrence, and reduced overall survival of PCa patients. Moreover, PCa patients with MYPT1-low/CD31-high expression more frequently had shorter overall, biochemical recurrence-free and metastasis-free survivals. MYPT1/CD31 combination was identified as an independent factor to predict biochemical recurrence-free and metastasis-free survivals of PCa patients.
Conclusions:
Our findings indicate that MYPT1 may inhibit angiogenesis and contribute favorable prognosis in PCa patients, implying that MYPT1 might be a potential drug candidate in anticancer therapy.
Keywords: Prostate cancer, myosin phosphatase targeting subunit 1, prognosis, tumor angiogenesis, metastasis, anticancer therapy
1. Background
Prostate cancer (PCa), as the most frequently non-cutaneous malignancy affecting western men, represents the second leading cause of cancer-related mortality accounting for more than 164,690 new cases and 29,430 deaths in the United States in 2018 [1]. Growing evidence from clinics and experiments suggests that the aggressive progression of human cancers may be critically dependent upon attaining an 'angiogenic phenotype', which is involved by vascular endothelia growth factor (VEGF) signaling pathway [2]. More interestingly, recent studies statistically found that the angiogenic level of malignant tissues may be positively correlated with Gleason score, tumor stage, invasion, metastasis and survival of PCa patients [3]. It also has been indicated that therapy targeting angiogenesis may be efficient to inhibit the aggressive cancer progression, to prevent the onset of metastasis and to improve the quality of patients' life [4]. However, the underlying mechanisms of angiogenesis during PCa progression have not been fully elucidated.
Myosin Phosphatase Targeting subunit 1 (MYPT1), together with MYPT2, MYPT3, MBS85 and TIMAP, belongs to the mammalian MYPT family which functions as regulatory subunits controlling the subcellular localization and specificity of the corresponding substrates [5]. Members in the MYPT family have several common conserved domains, including an RVxF motif for PP1c binding and several ankyrin repeats, and functionally are involved into diverse pathological events, including hypertension, nervous system diseases and cancer [6]. Among them, the human MYPT1 gene, located on chromosome 12q15-q21.2, is expressed in many tissues with abundant in smooth muscles [7]. Similar to other MYPT family members which can be regulated via phosphorylation at many sites by various protein kinases, Rho kinase (ROK) phosphorylates MYPT1, leading to inhibition of phosphatase activity and Ca2+ sensitization of smooth muscle contraction [8]. Accumulating studies have reported that MYPT1 plays an important role in diverse cellular processes, including cell development, cell cycle, cell adhesion and cell migration [9, 10]. Interestingly, Somlyo et al. [10] indicated that MYPT1 was phosphorylated in PCa PC3 cells and endothelial cells (HUVECs), and phosphorylation of MYPT1 was reduced by the ROK inhibitor Wf-536, leading to the inhibition of vasculation by endothelial cells and lumen formation, as well as the earliest detectable stages of angiogenesis. Consistently, our previous study also found that MYPT1 may function as a direct target of microRNA-30d, which promotes tumor angiogenesis and tumor growth, as well as predicts aggressive outcome in PCa [11]. However, the clinical significance of MYPT1 and its functions in PCa remain unknown.
To address this problem, we determined the roles of MYPT1 deregulation in tumor angiogenesis of PCa by in vitro and in vivo experiments. Then, expression patterns of MYPT1 and CD31 proteins were examined by immunohistochemistry and immunofluorescence, respectively. Associations of MYPT1/CD31 axis with various clinicopathological features and patients' prognosis of PCa were also statistically evaluated.
2. Methods
2.1. Ethic Statement
This study was approved by the human study ethics committees at MGH, Boston, MA and the Ministry of Public Health of P.R. China. All specimens were handled and made anonymous according to the ethical and legal standards.
All animal experiments in this study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research at Guangzhou Medical University, Guangzhou, P.R. China.
2.2. Patients and Tissue Samples
The same cohorts of patients and tissue samples were used in the current study with our previous study [12, 13]. Briefly, for human PCa tissue microarrays (TMA), 232 consecutive PCa patients who underwent radical prostatectomy included in our study. Relative clinicopathological data were included.
2.3. Cell Culture
Human PCa cell lines, LNCaP and DU145 were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibico, USA), 2 mM L-glutamine, and antibiotics. All cell lines were maintained at 37°C in a humidified chamber supplemented with 5% CO2.
2.4. Generation of the In Vivo Xenograft Model
The in vivo xenograft model was constructed according to the protocol described in our previous study [14]. DU145 or LNCaP cells transfected with MYPT-1/NC or sh-MYPT1/ sh-NC vectors were trypsinized and suspended in phosphate-buffered saline (PBS). Then, the cells were subcutaneously injected into the flanks of each nude mouse. Cells were subcutaneously injected as a mixture of 2 × 106 cells with an equal volume of Matrigel (Cat No.: 356234, BD Biosciences), reaching a total concentration of 10 mg/mL. The xenograft tumors were measured every 4-day, and the mice were sacrificed on day 44 for LNCaP and day 36 for DU145 groups.
2.5. Cell Transfection
To enforce and inhibit the expression of MYPT1 in PCa cells, the myosin phosphatase targeting protein 1 (MYPT1) coding sequence cloned into pLL3.7-CMV-IRES-puro-Vector (provided by Huijun company of China)/ blank vector (NC), and shRNA-targeting human MYPT1 (sh-MYPT1, Cat. No.: GV248, Genechem, China) and the shRNA non-targeting (sh-NC, Cat. No.: CON077, Genechem, China) were transfected into PCa cells using Lipofectamine 2000 Reagent (Cat. No.: 11668019, Invitrogen, USA) according to the manufacturer’s protocol. Forty-eight hours after the transfection, PCa cells were collected and used for the functional analyses.
2.6. Tube Formation
HUVECs (2x104) were plated onto matrigel-coated (10 mg/ml, BD Pharmingen, San Jose, CA) 12-well plates with condition media of MYPT1 / NC and sh-MYPT-1/NC PCa cells. After 12 hours of the incubation at 37°C, HUVECs were fixed with 4% paraformaldehyde and the formation of capillary-like structures was captured under a light microscope (OLYMPUS, CKX41, U-CTR30-2, Japan). The number of branch points of the tube structures, as the degree of angiogenesis, was counted in three fields at 100× magnification.
2.7. In Vivo Tumor Angiogenesis Assay
Xenograft tumors were removed five weeks later for MYPT1 and NC PCa cells while seven weeks later for sh-MYPT1 and sh-NC, then fixed in formalin and embedded in paraffin. Expression patterns of vascular endothelial growth factor A (VEGFA) proteins in different groups were respectively detected by Western blot analysis. The microvessel density (MVD) in tumor tissues was evaluated based on the immunostaining for vascular marker CD31.
2.8. Western Blot Analysis
Proteins were extracted 48 hour post-transfection for Western blot analyses. Proteins (40 lg) were fractioned on SDS-PAGE and transferred onto Hybond nitrocellulose membranes (GE Healthcare). The membranes were blocked with 5% skim milk in PBS-Tween 20 and probed with anti-MYPT1 (ab59235, Abcam Co. Ltd., USA) or anti–β-actin antibody (M30017, Abmart, China). β-actin was used as an internal loading control.
2.9. Immunohistochemistry
Cellular distribution and expression level of MYPT1 protein in clinical PCa tissues, and those of CD31 protein in subcutaneous tumor xenografts of nude mice were respectively examined by immunohistochemistry and immunofluorescence according to protocol. The slides were incubated with the primary antibody (anti-MYPT1, ab59235, Abcam Co. Ltd., USA or anti-CD31, ZSGB-BIO, China).
2.10. Statistical Analyses
SPSS software for Windows (version 17.0, SPSS Inc., IL, USA) was used to perform all statistical analyses in the current study. Data of continuous variables were expressed as mean±S.D. For functional analyses in vitro and in vivo, the differences between groups were analyzed using a Student’s t test when comparing only two groups or one-way analysis of variance when comparing more than two groups. Data obtained from Western blot were conducted using Wilcoxon signed-rank test. Associations of MYPT1/CD31 expression with various clinicopathological parameters were evaluated by Fisher’s exact test for any 2×2 tables and Pearson χ2 test for non-2×2 tables. Survival analysis was performed by Kaplan-Meier method and Cox regression model. Correlation between MYPT1 and CD31 expression in clinical PCa tissue samples was assessed by the Spearman correlation analysis. Differences were considered statistically significant when the P value was less than 0.05.
3. Results
3.1. MYPT1 Suppresses the Abilities of PCa Cells to Recruit Endothelial Cells with the Downregulation of VEGF Expression
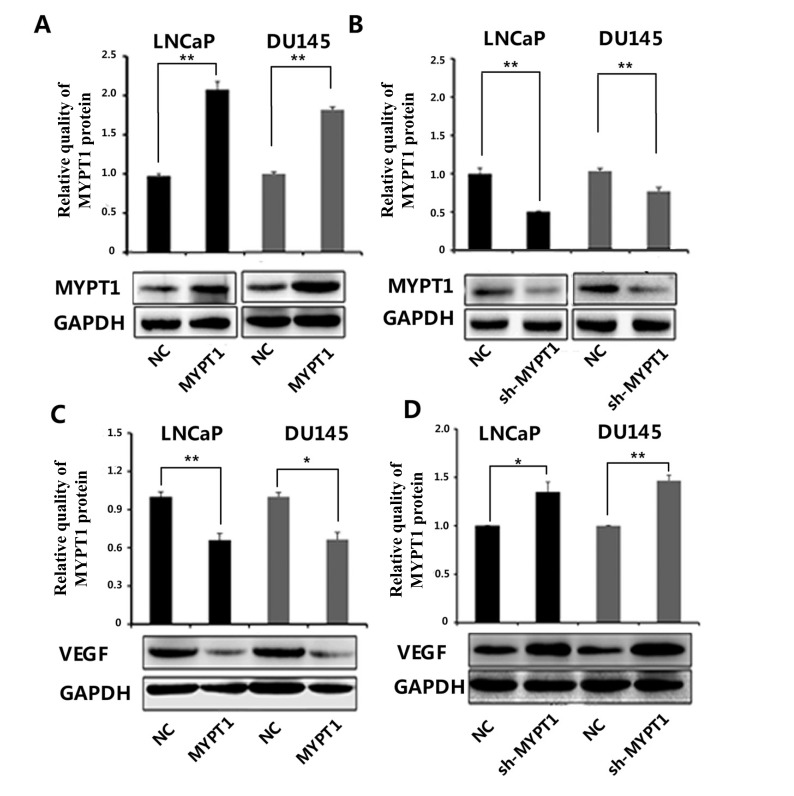
Western blot analysis showed that the expression levels of MYPT1 in PCa cells (LNCaP and DU145) transfected with MYPT1 and sh-MYPT1 expression vector were respectively lower and higher than those with control and sh-NC expression vectors (all P < 0.01, Fig. 1A and B). In contrast, VEGF protein expression was significantly decreased in both LNCaP and DU145 cells with enforced expression of MYPT1, but was dramatically increased in those cells with knockdown of MYPT1 (all P < 0.05, Fig. 1C and D).
Fig. (1).
Expression levels of MYPT1 (A and B) and VEGF (C and D) proteins in LNCaP and DU145 transfected with MYPT1 and sh-MYPT1 expression vector. '*' P < 0.05, compared with negative controls; '**' P < 0.01, compared with negative control.
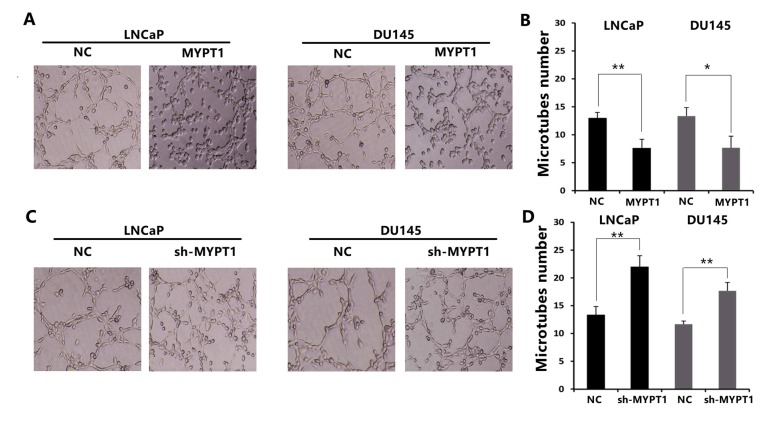
To investigate the influence of MYPT1 in the abilities of PCa cells to recruit endothelial cells, the condition medium of PCa cells (LNCaP and DU145) was collected and the tube formation assay was performed. As a result, the culture medium of MYPT1-transfected LNCaP and DU145 cells obviously reduced the abilities of capillary tube formation (Fig. 2A and B), while the medium of sh-MYPT1 transfected LNCaP and DU145 cells significantly promoted the capillary tube formation (Fig. 2C and D).
Fig. (2).
MYPT1 suppresses the capillary tube formation of endothelial cells. (A and B) Capillary tube formation assays of HUVECs with the culture medium of MYPT1 expression vector-transfected LNCaP and DU145 cells. (C and D) Capillary tube formation assays of HUVECs with the culture medium of sh-MYPT1 transfected LNCaP and DU145 cells. Data were presented as Mean ± SD. * P < 0.05; ** P < 0.01.
3.2. MYPT1 Suppresses Angiogenesis In Vivo with the Downregulation of CD31 and VEGF Proteins
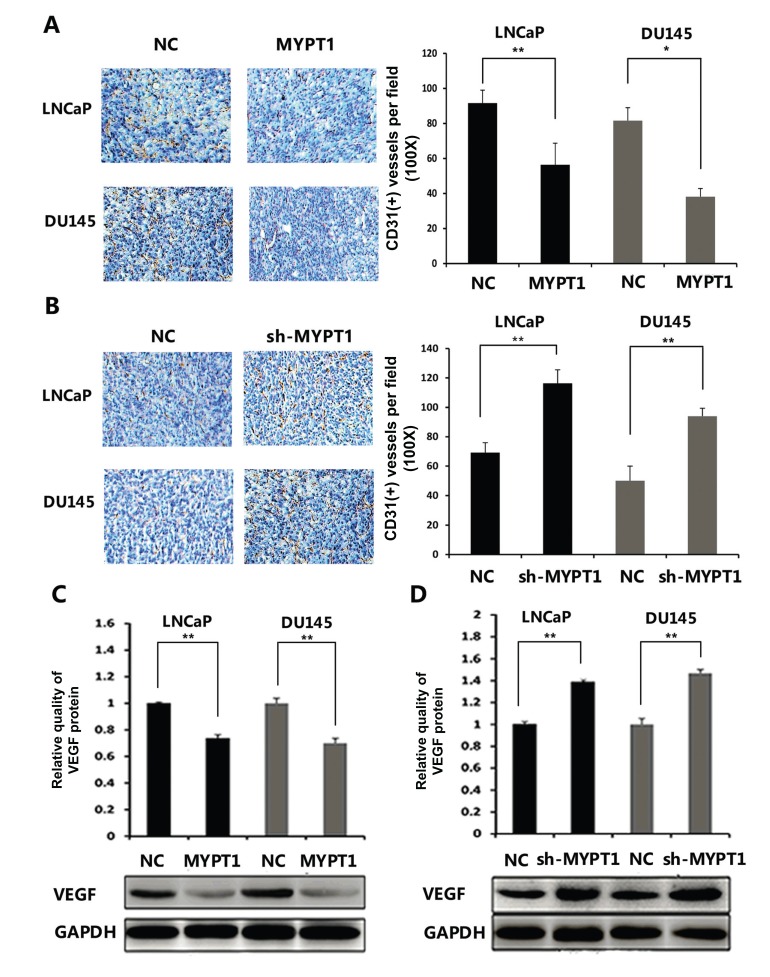
To assess the effects of MYPT1 in the angiogenesis of the tumor xenografts, immunohistochemical analysis using pan-endothelial marker CD31 antibody were performed. As shown in Fig. 3A and B, the immunostaining of CD31 protein in the tumor xenografts established by sh-MYPT1-transfected LNCaP or DU145 cells were markedly stronger than that in the control groups (both P < 0.05). Consistently, the expression levels of VEGF protein in tumor tissues of the subcutaneous models bearing MYPT1 and sh-MYPT1 expression vector transfected PCa cells were significantly decreased and increased in the comparison with the respective controls (both P < 0.01, Fig. 3C and D).
Fig. (3).
MYPT1 suppresses angiogenesis in vivo with the downregulation of CD31 and VEGF proteins. (A and B) Immunohistochemical analysis using pan-endothelial marker CD31 antibody. (C and D) VEGFA protein expression in different groups detected by Western blot analysis. Data were presented as Mean ± SD. * P < 0.05; ** P <0.01.
3.3. Associations Between MYPT1/CD31 Combined Expression and Various Clinicopathological Parameters of Human PCa
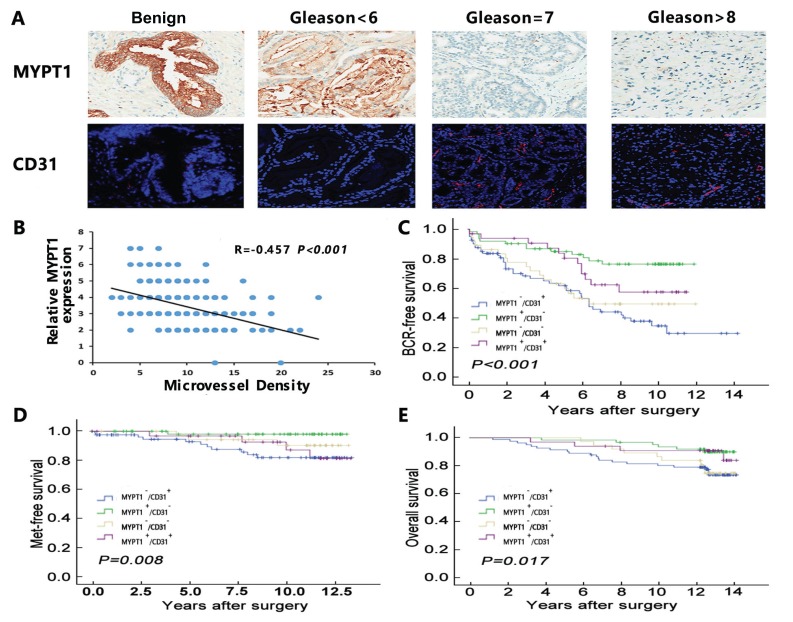
Immunohistochemistry and immunofluorescence analysis were respectively performed to examine the expression patterns of MYPT1 and CD31 proteins in human PCa tissues and benign prostate tissues. As shown in Fig. 4A, the positive immunostainings of MYPT1 protein in benign prostate tissues were distinctly stronger than that in PCa tissues. In addition, its immunostainings were decreased in PCa tissues with the increasing Gleason score. In contrast, the positive expression of CD31 protein was significantly enhanced in PCa tissues in a Gleason score-increasing manner, when compared with benign prostate tissues.
Fig. (4).
Expression patterns and prognostic values of MYPT1 and CD31 expression in human PCa. (A) Immunohistochemistry and immunofluorescence results of MYPT1 and CD31 proteins in benign prostate tissues and PCa tissues. (B) Spearman analysis on the correlation between MYPT1 and CD31 protein expression in PCa tissues. (C-E) Kaplan-Meier analysis on the associations between MYPT1/CD31 combined expression and BCR-free survival, metastasis-free survival and overall survival.
The spearman correlation analysis demonstrated that MYPT1 expression levels in PCa tissues were negatively correlated with that of CD31 (r=-0.457, P < 0.001, Fig. 4B).
To evaluate the associations of MYPT1/CD31 combined expression with various clinicopathological parameters of PCa, all 234 PCa patients were divided into MYPT1-low/CD31-high (n=61), MYPT1-high/CD31-low (n=71), MYPT1-low/CD31-low (n=50) and MYPT1-high/CD31-high (n=50) groups by setting cut-off value at the median MYPT1 or CD31 expression level. As shown in Table 1, PCa patients with MYPT1-low/CD31-high expression more frequently had higher Gleason score (P=0.004), higher pathological stage (P=0.047), positive biochemical recurrence (P=0.022), and shorter overall survival (P=0.031) than those with other combination of MYPT1/CD31 expression.
Table 1.
Associations between MYPT1/CD31 combined expression and various clinicopathological parameters of patients with prostate cancer.
|
Clinicopathological
Parameters |
Case
no. |
MYPT1(low)/
CD31(high) (n, %) |
MYPT1(high)/
CD31(low) (n, %) |
MYPT1(low)/
CD31(low) (n, %) |
MYPT1(high)/
CD31(high) (n, %) |
P |
|---|---|---|---|---|---|---|
| Age (year) | ||||||
| < 66 | 155 | 40 (25.80) | 52 (33.50) | 35 (22.60) | 28 (18.10) | 0.238 |
| ≥66 | 77 | 21 (27.30) | 19 (24.70) | 15 (19.50) | 22 (28.60) | |
| Serum PSA (ng/ml) | ||||||
| <4 | 30 | 7(23.30) | 9(30.00) | 6(20.00) | 8(26.70) | 0.856 |
| ≥4 | 159 | 47(29.60) | 49(30.8) | 30(18.90) | 33(20.80) | |
| Gleason score | ||||||
| < 7 | 93 | 13(14.00) | 36(38.70) | 23(24.70) | 21(22.60) | 0.004 |
| ≥7 | 139 | 48(34.50) | 35(25.20) | 27(19.40) | 29(20.90) | |
| Pathological stage | ||||||
| T2A-T2C | 173 | 38(22.00) | 53(30.6) | 42(24.30) | 40(23.10) | 0.047 |
| T3A-T4 | 59 | 23(39.00) | 18(30.50) | 8(13.6) | 10(16.9) | |
| Metastasis | ||||||
| Negative | 212 | 55(25.90) | 68(32.10) | 47(22.20) | 42(19.80) | 0.125 |
| Positive | 20 | 6(30.00) | 3(15.00) | 3(15.00) | 8(40.900) | |
| Overall survival | ||||||
| Live | 187 | 43(23.00) | 61(32.60) | 43(23.00) | 40(21.40) | 0.102 |
| Death | 45 | 18(40.00) | 10(22.20) | 7(15.36) | 10(22.20) | |
| Biochemical recurrence | ||||||
| Negative | 146 | 31(21.20) | 54(37.00) | 32(21.90) | 29(19.90) | 0.022 |
| Positive | 86 | 30(34.90) | 17(19.80) | 18(20.90) | 21(24.4) |
3.4. MYPT1/CD31 Combination is an Efficient Prognostic Factor for Human PCa
In survival analysis, pairwise comparisons showed significant differences in the BCR-free survival (P < 0.001, Fig. 4C), metastasis-free survival (P = 0.008, Fig. 4D) and overall survival (P = 0.017, Fig. 4E) between patients with MYPT1-low/CD31-high, MYPT1-high/CD31-low, MYPT1-low/CD31-low and MYPT1-high/CD31-high expression. Further univariate and multivariate analyses using a COX regression model revealed that MYPT1/CD31 combined expression was significantly associated with BCR-free, metastasis-free and overall survival of PCa patients, and it was demonstrated to be an independent prognostic factor for this cancer (Table 2).
Table 2.
Prognostic value of MYPT1/CD31 combined expression for the biochemical recurrence-free, metastasis-free and overall survival in univariate and multivariate analysis using Cox Regression models.
| Features | BCR-free survival | Met-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
|
Univariate analysis |
|||||||||
| MYPT1 /CD31 | 0.281 | 0.150-0.525 | <0.001 | 0.105 | 0.014-0.814 | 0.031 | 2.343 | 1.081-5.078 | 0.031 |
| CD31 | 1.834 | 1.173-2.867 | 0.008 | 3.663 | 1.215-11.039 | 0.021 | 0.975 | 0.465-2.045 | 0.946 |
| AJCC patho- logical stage |
1.552 | 0.861-2.795 | 0.144 | 1.796 | 0.482-6.689 | 0.383 | 1.351 | 0.624-2.927 | 0.446 |
| Gleason score | 6.031 | 2.548-14.272 | <0.001 | 4.783 | 0.598-28.251 | 0.140 | 3.023 | 1.149-7.953 | 0.025 |
| PSA level | 3.764 | 0.910-15.570 | 0.067 | 1.176 | 0.147-9.426 | 0.878 | 1.118 | 0.333-3.748 | 0.857 |
| Age | 1.426 | 0.777-2.620 | 0.252 | 0.747 | 0.155-3.599 | 0.716 | 1.990 | 0.941-4.212 | 0.072 |
|
Multivariate analysis |
|||||||||
| MYPT1 /CD31 | 0.366 | 0.183-0.734 | 0.005 | 0.139 | 0.017-1.113 | 0.043 | 1.551 | 0.506-2.910 | 0.353 |
| CD31 | 1.271 | 0.755-2.139 | 0.367 | 3.413 | 0.937-12.430 | 0.063 | 1.214 | 0.615-3.914 | 0.664 |
| AJCC patho- logical stage |
1.042 | 0.558-1.945 | 0.897 | 1.158 | 0.342-3.923 | 0.814 | 0.887 | 0.358-2.197 | 0.796 |
| Gleason score | 3.360 | 1.740-6.491 | <0.001 | 2.727 | 0.759-9.797 | 0.124 | 1.646 | 0.535-5.065 | 0.385 |
| PSA level | 1.678 | 0.650-4.329 | 0.284 | 0.402 | 0.112-1.440 | 0.162 | 0.631 | 0.168-2.372 | 0.496 |
| Age | 1.244 | 0.678-2.284 | 0.481 | 0.981 | 0.245-3.924 | 0.979 | 2.157 | 0.918-5.068 | 0.078 |
4. Discussion
Angiogenesis, one of the critical steps during tumor development and progression, contributes to the formation of new capillaries from preexisting blood vessel, as well as promotes tumor growth and metastasis by providing essential nutrients and oxygen to tumor [15]. Thus, anti-angiogenesis has been indicated to be one of the most important anticancer therapies. However, the molecular mechanisms underlying tumor angiogenesis have not been fully elucidated due to its complexity. In our previous study, microRNA-30d was identified as an onco-microRNA which promoted angiogenesis of human PCa through regulating its target gene MYPT1 [11]. Currently, we would like to investigate the influence of MYPT1 in tumor angiogenesis and its clinical significance in PCa. There are three main findings according to our data as following: firstly, MYPT1 inhibited capillary tube formation of endothelial cells in vitro and tumor angiogenesis in vivo with the downregulation of VEGF and CD31 expression; secondly, MYPT1 expression was markedly decreased in clinical PCa tissues with a negative correlation of CD31 expression; thirdly, the combined expression of MYPT1-downregulation/CD31-upregulation was significantly associated with advanced progression and poor prognosis in patients with PCa. These findings support the evidence of the contribution of MYPT1 to tumor angiogenesis of human PCa.
MYPT family members have been reported to be involved into various pathological events, including human cancers. To our interests, Umelo et al. [16] determined the suppressive role of RhoA/ROCK/MYPT1 pathway in lung cancer-related invasion by pharmacological inhibition and RNA interference techniques; Yang et al. [17] indicated that the change in MYPT1 phosphorylation (P-MYPT1) was one of the molecular mechanisms by which Rho-kinase inhibitor suppresses proliferation and metastasis of small lung cancer; Our previous study also revealed that microRNA-30d promoted angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway [11]. Here, we further investigated the involvement of MYPT1 in tumor angiogenesis, clinical progression and patients' prognosis of PCa. VEGF, as one of the known angiogenic molecules, functions as the key mediator that promotes angiogenesis. CD31 is an angiogenesis-related biomarker. Our data obtained from gain- and loss-of-function experiments in vitro and in vivo demonstrated that MYPT1 inhibited capillary tube formation of endothelial cells and tumor angiogenesis in a mouse model with the downregulation of VEGF and CD31 expression, suggesting its suppressive role in PCa angiogenesis.
The clinical significance of MYPT1 in human PCa had been investigated in our previous study [11]. It showed that low MYPT1 protein was significantly associated with high Gleason score, positive metastasis and BCR and shorter overall survival of PCa patients. In the current study, we confirmed that downregulation of MYPT1 protein in PCa tissue was negatively correlated with CD31 expression. Interestingly, we demonstrated the significant associations of MYPT1-low/CD31-high combined expression with aggressive clinicopathological characteristics such as higher Gleason score, advanced pathological stage and biochemical recurrence, shorter BCR-free and overall survivals of PCa patients. Another important finding of this study was that MYPT1/CD31 combination might be novel independent prognostic markers for BCR-free, metastasis free and overall survival of PCa patients. Especially, the Cox proportional hazards multivariate model based on the PCa tissues revealed that the prediction efficiency of MYPT1/CD31 combination to BCR or metastasis of PCa patients was stronger than CD31 alone. However, it needs more information and data to support the role of MYPT1/CD31 combination in predicting the prognosis of PCa patients.
Conclusions
Our findings indicate that MYPT1 may inhibit angiogenesis and its combination with CD31 may be an efficient prognostic factor of human PCa. Since anti-angiogenesis therapy has been a crucial strategy for the treatment of human cancers, MYPT1 may be a potential drug candidate in anticancer therapy.
ACKNOWLEDGEMENTS
Declared none.
Declarations
Part of this article has previously been published in MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer, Molecular Cancer 2017; 16: 48.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the human study ethics committees at MGH, Boston, MA and the Ministry of Public Health of P.R. China. All specimens were handled and made anonymous according to the ethical and legal standards. All animal experiments in this study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research at Guangzhou Medical University, Guangzhou, P.R. China.
HUMAN AND ANIMAL RIGHTS
All the procedures involving human subjects were compliant with the ethical guideline of the 1975 Declaration of Helsinki and its subsequent revisions. For animal models, this study was performed in compliance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
CONSENT FOR PUBLICATION
Informed consent was signed by all patients or their families.
AUTHORS’ CONTRIBUTIONS
Yuxiang Liang and Zhuoyuan Lin: participated in study design and coordination, analysis and interpretation of data, material support for obtained funding, and supervised study. Yangjia Zhuo, Jianming Lu, Weimin Dong and Xuejun Zhu: performed most of the experiments and statistical analysis and drafted the manuscript. Other author: carry out the experiments and sample collection. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
FUNDING
This work was supported by grants from Guangzhou Planned Project of Science and Technology (201710010081), National Natural Science Foundation of China (81571427, 81600620), Guangzhou General Science and Technology Project of Health and Family Planning (20171A011303).
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Ward J.F., Moul J.W. Rising prostate-specific antigen after primary prostate cancer therapy. Nat. Clin. Pract. Urol. 2005;2(4):174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 3.Georgiou H.D., Namdarian B., Corcoran N.M., Costello A.J., Hovens C.M. Circulating endothelial cells as biomarkers of prostate cancer. Nat. Clin. Pract. Urol. 2008;5:445–454. doi: 10.1038/ncpuro1188. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grassie M.E., Moffat L.D., Walsh M.P., MacDonald J.A. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1δ. Arch. Biochem. Biophys. 2011;510(2):147–159. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Ito M., Nakano T., Erdodi F., Hartshorne D.J. Myosin phosphatase: structure, regulation and function. Mol. Cell. Biochem. 2004;259(1-2):197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 7.Lartey J., Taggart J., Robson S., Taggart M. Altered Expression of Human Smooth Muscle Myosin Phosphatase Targeting (MYPT) Isovariants with Pregnancy and Labor. PLoS One. 2016;11(10):e0164352. doi: 10.1371/journal.pone.0164352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi N., Ito M., Tanaka J., et al. Localization of the gene coding for myosin phosphatase, target subunit 1 (MYPT1) to human chromosome 12q15-q21. Genomics. 1997;44:150–152. doi: 10.1006/geno.1997.4859. [DOI] [PubMed] [Google Scholar]
- 9.Machida H., Ito M., Okamoto R., et al. Molecular cloning and analysis of the 5′-flanking region of the human MYPT1 gene. Biochim. Biophys. Acta. 2001;1517:424–429. [Google Scholar]
- 10.Somlyo A.V., Phelps C., Dipierro C., et al. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xeno-transplants. FASEB J. 2003;17(2):223–234. doi: 10.1096/fj.02-0655com. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z.Y., Chen G., Zhang Y.Q., et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol. Cancer. 2017;16:48. doi: 10.1186/s12943-017-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Cai C., Chen Q.B., Han Z.D., et al. miR-195 Inhibits Tumor Progression by Targeting RPS6KB1 in Human Prostate Cancer. Clin. Cancer Res. 2015;21:4922–4934. doi: 10.1158/1078-0432.CCR-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z.Y., Huang Y.Q., Zhang Y.Q., et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int. J. Cancer. 2014;135:541–550. doi: 10.1002/ijc.28707. [DOI] [PubMed] [Google Scholar]
- 14.Chen G., Liang Y.X., Zhu J.G., et al. CC Chemokine Ligand 18 Correlates with Malignant Progression of Prostate Cancer. BioMed Res. Int. 2014;2014:230183. doi: 10.1155/2014/230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Cozzi P.J. Angiogenesis as a strategic target for prostate cancer therapy. Med. Res. Rev. 2010;30:23–66. doi: 10.1002/med.20161. [DOI] [PubMed] [Google Scholar]
- 16.Umelo I.A., Wever O.D., Kronenberger P., et al. Combined inhibition of rho-associated protein kinase and EGFR suppresses the invasive phenotype in EGFR-dependent lung cancer cells. Lung Cancer. 2015;90(2):167–174. doi: 10.1016/j.lungcan.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Di J., Zhang Y., et al. The Rho-kinase inhibitor inhibits proliferation and metastasis of small cell lung cancer. Biomed. Pharmacother. 2012;66(3):221–227. doi: 10.1016/j.biopha.2011.11.011. [DOI] [PubMed] [Google Scholar]