Humans encounter mycobacterial species due to their ubiquity in different environmental niches. In many individuals, pathogenic mycobacterial species may breach our first-line barrier defenses of the innate immune system and modulate the activation of phagocytes to cause disease of the respiratory tract or the skin and soft tissues, sometimes resulting in disseminated infection.
KEYWORDS: Buruli ulcer, Mycobacterium, Mycobacterium kansasii, Mycobacterium marinum, Mycobacterium ulcerans, cutaneous, leprosy, mycobacteria, nontuberculous mycobacteria, tuberculosis
SUMMARY
Humans encounter mycobacterial species due to their ubiquity in different environmental niches. In many individuals, pathogenic mycobacterial species may breach our first-line barrier defenses of the innate immune system and modulate the activation of phagocytes to cause disease of the respiratory tract or the skin and soft tissues, sometimes resulting in disseminated infection. Cutaneous mycobacterial infections may cause a wide range of clinical manifestations, which are divided into four main disease categories: (i) cutaneous manifestations of Mycobacterium tuberculosis infection, (ii) Buruli ulcer caused by Mycobacterium ulcerans and other related slowly growing mycobacteria, (iii) leprosy caused by Mycobacterium leprae and Mycobacterium lepromatosis, and (iv) cutaneous infections caused by rapidly growing mycobacteria. Clinically, cutaneous mycobacterial infections present with widely different clinical presentations, including cellulitis, nonhealing ulcers, subacute or chronic nodular lesions, abscesses, superficial lymphadenitis, verrucous lesions, and other types of findings. Mycobacterial infections of the skin and subcutaneous tissue are associated with important stigma, deformity, and disability. Geography-based environmental exposures influence the epidemiology of cutaneous mycobacterial infections. Cutaneous tuberculosis exhibits different clinical phenotypes acquired through different routes, including via extrinsic inoculation of the tuberculous bacilli and dissemination to the skin from other sites, or represents hypersensitivity reactions to M. tuberculosis infection. In many settings, leprosy remains an important cause of neurological impairment, deformity, limb loss, and stigma. Mycobacterium lepromatosis, a mycobacterial species related to M. leprae, is linked to diffuse lepromatous leprosy of Lucio and Latapí. Mycobacterium ulcerans produces a mycolactone toxin that leads to subcutaneous tissue destruction and immunosuppression, resulting in deep ulcerations that often produce substantial disfigurement and disability. Mycobacterium marinum, a close relative of M. ulcerans, is an important cause of cutaneous sporotrichoid nodular lymphangitic lesions. Among patients with advanced immunosuppression, Mycobacterium kansasii, the Mycobacterium avium-intracellulare complex, and Mycobacterium haemophilum may cause cutaneous or disseminated disease. Rapidly growing mycobacteria, including the Mycobacterium abscessus group, Mycobacterium chelonei, and Mycobacterium fortuitum, are increasingly recognized pathogens in cutaneous infections associated particularly with plastic surgery and cosmetic procedures. Skin biopsies of cutaneous lesions to identify acid-fast staining bacilli and cultures represent the cornerstone of diagnosis. Additionally, histopathological evaluation of skin biopsy specimens may be useful in identifying leprosy, Buruli ulcer, and cutaneous tuberculosis. Molecular assays are useful in some cases. The treatment for cutaneous mycobacterial infections depends on the specific pathogen and therefore requires a careful consideration of antimicrobial choices based on official treatment guidelines.
INTRODUCTION
Human Societies and the Origin and Spread of Major Mycobacterial Pathogens
Mycobacteria are aerobic rod-shaped bacteria that do not form spores and that are lipid rich with long-chain mycolic acids in their cell walls, which are largely responsible for their acid fastness (1). Modern genomic, phylogenetic, and ecological studies have shed light on the origins of most important mycobacterial infections affecting humans (1–20). For example, leprosy and tuberculosis (TB) have had a profound effect on human suffering for thousands of years (16). Modern molecular genomic analysis of ancient human remains and records of ancient texts demonstrate that the spread of some mycobacterial diseases, including TB and leprosy, track historical milestones of human societies (7–10). These events include waves of human expeditionary, military, or commercial migrations (8). Phylogeographic studies uncovered the origin of leprosy in eastern Africa and its spread through the Silk Road or the transatlantic slave migration trade routes (7, 8, 10, 12). The domestication of animals and the development of water distribution systems also influenced the transmission dynamics of mycobacterial infections (16). Similarly, phylogeographic studies of the tuberculous bacilli have shown that the dominant clone of smooth tubercle bacilli (Mycobacterium canettii) emerged in eastern Africa and later diversified into the Mycobacterium tuberculosis complex during the worldwide spread of TB by waves of human migration (5, 11).
Mycobacterial Species as Human Pathogens
The genus Mycobacterium is part of the order Actinomycetales and the phylum Actinobacteria and belongs to a variety of environmental habitats, including natural waters, soils, and drinking water distribution systems (1, 20–23). Mycobacterial species reside in a wide-variety of environments due to multiple adaptations. Some of the features include the presence of a lipid-rich hydrophobic outer membrane, which is a major determinant of surface adherence, biofilm formation, aerosolization, and antibiotic/disinfectant resistance. Additionally, mycobacteria have the ability to replicate at a low rate, providing them with a decreased susceptibility to most antimicrobial agents, and they also possess the ability to grow at low carbon levels, thus making them effective competitors in low-nutrient environments (oligotrophs) (21, 22).
From a large mycobacterial pool, some species have evolved into potential major human pathogens (20, 23–25) (Fig. 1). Genomic events such as genome reduction, critical gene acquisition, gene transfer, mutations, and recombination permitted environmental mycobacteria to evolve into host-associated pathogens (2, 5, 9, 11, 14–16, 19). Phylogenetic reconstructions of genomic sequences suggest that Mycobacterium marinum, Mycobacterium leprae, Mycobacterium ulcerans, and M. tuberculosis evolved from a common environmental ancestor (2, 15, 16, 19). Their gene loss or acquisition reflects fluctuating environmental challenges and host-specific pathoadaptations (2, 3, 5) (Table 1). The molecular mechanisms by which M. tuberculosis and M. leprae have evolved to cause disease involved complex interactions between the pathogen and the host. In contrast, the pathogenicity of M. ulcerans derives from the acquisition of a plasmid encoding the polyketide toxin mycolactone (2, 5).
FIG 1.
Classification of major pathogenic mycobacteria.
TABLE 1.
Comparison of epidemiological and clinical features of the four categories of cutaneous mycobacterial infections: cutaneous tuberculosis, leprosy, Buruli ulcer, and disease caused by nontuberculous mycobacteria
| Parameter | Cutaneous tuberculosis | Leprosy | Buruli ulcer | Disease caused by NTM |
|
|---|---|---|---|---|---|
| Rapidly growing | Slowly growinga | ||||
| Mycobacterial species | M. tuberculosis, M. bovis, bacillus Calmette-Guérin | M. leprae, M. lepromatosis | M. ulcerans | M. abscessus, M. fortuitum, M. chelonae | M. marinum, M. ulcerans, M. haemophilum |
| Microbiological diagnosis | Culture; clinical diagnosis supported by histopathology and molecular detection methods (PCR) | M. leprae and M. lepromatosis are noncultivable; clinical diagnosis supported by histopathology and molecular detection methods (PCR) | M. ulcerans grows at 29–33oC, colonies are yellowish, rough and distinct from one another; clinical diagnosis supported by histopathology and molecular detection methods (PCR) | M. abscessus complex grows within 7 days on Middlebrook 7H10 or 7H11 agar and Bactec 12B; some strains of M. chelonae grow at 30°C | M. haemophilum requires iron or hemin for growth at 30°C |
| Genome | Genome of 4.4 Mb with 4,000 genes and only 6 pseudogenes (91% coding capacity); no major reductive evolution | M. leprae suffered reductive evolution and large pseudogene (1, 116) formation resulting in the 3.31-Mb genome; only 50% coding capacity by 1,605 genes; M. lepromatosis has approximately 13% genomic-scale difference compared to M. leprae | M. marinum and M. ulcerans share 97% of genes; selected genes facilitate occupying an aerobic niche environment and osmotically stable, dark, and extracellular environments | M. abscessus genome is 5 Mb with some homologous genes that determine virulence, similar to M. tuberculosis, with some genes acquired via horizontal transfer from Pseudomonas and Burkholderia cepacia | M. ulcerans downsized from 6.6 MB (M. marinum) to 5.8-Mb genome associated with lateral gene transfer and reductive evolution; M. marinum, M. ulcerans, and M. haemophilum are phylogenetically closely related and produce similar cutaneous forms of the disease |
| Target cells of infection | Intracellular: macrophages (alveolar) and in the reticuloendothelial system; nonclassical immune cells (epithelial cells, endothelial cells, fibroblasts, adipocytes, glia, and neurons) | Intracellular: Schwann cells, histiocytes, keratinocytes | Extracellular: extracellular matrix in subcutaneous tissues where M. ulcerans directs the production of the polyketide toxin mycolactone, leading to tissue necrosis and local tissue and systemic immunosuppression | Intracellular: histiocytes, macrophages | Intracellular: histiocytes, macrophages |
| Pathogenic mechanisms | Intracellular persistence in macrophages and histiocytes causes necrotizing granulomas that cause tissue destruction of skin and soft tissues | Infection of Schwann cells leads to peripheral nerve dysfunction secondary to demyelination; reprogramming of Schwann cells is linked to disseminated disease | Destructive ulcerative ulcers (Buruli or Bairnsdale ulcer) with subcutaneous fat necrosis | Granulomatous inflammation with tissue destruction | Granulomatous inflammation with tissue destruction |
| Environmental determinants | Members of the Mycobacterium tuberculosis complex can be shed by infected hosts to the environment in sputum, feces, and urine by humans, in milk from dairy animals (cattle), and in infected body tissues from other domestic and wild animals; free-living amoebas may act as macrophage-like niches in the environment | Armadillo is a natural host in the Western Hemisphere and causes a zoonosis in the southern US; red squirrel identified as a reservoir in the UK; in some settings some primate species may become reservoirs of M. leprae; there is evidence of viable M. leprae found in soil, water, and free-living amoebas (Acanthamoeba spp.) | Mycobacterium ulcerans transmission cycle involves aquatic insect vectors, aquatic plants, and aquatic animals; aquatic plants (e.g., Rhizoclonium spp., Hippeastrum reticulatum) favor growth of and biofilm production by M. ulcerans; there are two different ways that M. ulcerans persists in the environment and infects aquatic animals (in the savanna landscape residing in stagnant or slowly moving waters and in tropical rainforests dwelling in alkaline waters, and its growth is dependent on climate changes) | Biofilms in urban water networks; free-living amoebas act as reservoirs | Fish and aquatic environments (biofilms in rocks and coral); free-living amoebas act as reservoirs |
| Vector transmission | The ancestor of the smooth bacillus M. canettii and the ancestor of M. tuberculosis complex likely had nonmammalian reservoirs (e.g., plant or insect) prior to the Neolithic revolution | Some mosquitoes (Culex fatigans and Cimex hemipterus caught in the field in an area of endemicity in India harbor viable M. leprae); laboratory-bred Culex fatigans and Cimex hemipterus are able to take up leprosy bacilli from blood of patients with untreated lepromatous leprosy; the feasibility of biting arthropods amplifying the transmission of leprosy through mechanical studies demonstrated that large numbers of bacilli are readily available to the biting apparatus of arthropods among individuals with untreated multibacillary leprosy; bacteremia in cases of leprosy may make viable bacilli available to biting arthropods | Naucoridae (aquatic insects) in West African countries (Ghana, Benin, Togo) harbor M. ulcerans | Unknown | Unknown |
Slowly growing mycobacteria include a large number of species other than the ones included in this table. Other than the three species included in this table, M. kansasii often produces skin and soft tissue involvement.
Mycobacterial species are present in the environment in water and soil niches that are shared with humans (19, 21, 22). In the human host, mycobacterial infections may affect many anatomical sites, but since they enter through the skin and mucosal barriers, they lead mostly to pulmonary or cutaneous infections (25–28). The pathogenesis of cutaneous mycobacterial infections is the result of hematogenous dissemination, local or regional spread from a deep-seated infection, or direct inoculation into the skin and soft tissues (24). The modes of transmission of the different mycobacterial species involved in cutaneous disease include zoonotic transmission (e.g., foodborne transmission of Mycobacterium bovis by ingestion of unpasteurized dairy products) (16, 23), person-to-person transmission (e.g., of leprosy) (29, 30), vector-borne transmission (possibly for Mycobacterium ulcerans) (3, 31–33), and acquisition of infection from environmental exposures (e.g., freshwater or salt water injuries leading to Mycobacterium marinum, M. ulcerans, or Mycobacterium haemophilum infection) (24, 33–38) (Table 1). Nontuberculous mycobacteria (NTM), i.e., those mycobacterial species that do not cause tuberculosis or leprosy, are frequently present in municipal water systems, residing mostly in the pipeline biofilms (21, 22, 38, 39). Free-living amoebas, including Acanthamoeba or Vermamoeba, may act as reservoirs of M. leprae and NTM (38–41).
The most common cutaneous forms of acquisition of NTM involve direct inoculation via trauma (33), postsurgical infections (42), or iatrogenic acquisition with indwelling medical devices, plastic surgery, cosmetic procedures, or prosthetic implants (24, 42). Mycobacteria may seed the skin and soft tissues during systemic dissemination in immunosuppressed individuals (24, 25, 37, 42–44). There is some evidence of potential human-to-human transmission of Mycobacterium abscessus subsp. massiliense among patients with cystic fibrosis (43, 44).
The precise mode of transmission of leprosy remains uncertain but probably involves human-to-human contact through respiratory droplets (29, 30, 45–47) or through blood transfusion (48). Ecological data suggest that environmental factors, such as trauma or skin breaks during soil and water exposures, insect vectors, free-living amoebas, and animal reservoirs (e.g., armadillos, squirrels, felines, or other animals), influence leprosy transmission (39, 47, 49–63). Zoonotic transmission from armadillos acting as reservoirs of infection has been confirmed for autochthonous southeast United States cases (51, 52). Armadillos may also play a role in the transmission of leprosy in some areas in Colombia (55) and in Brazil (56). In some of the British Isles, red squirrels may develop leprosy-like lesions due to either M. leprae or Mycobacterium lepromatosis (53).
In this review, we group cutaneous mycobacterial infections into four major categories: (i) infection due to Mycobacterium tuberculosis complex, (ii) infection caused by Mycobacterium leprae and M. lepromatosis, (iii) infection caused by Mycobacterium ulcerans and other slowly growing mycobacteria (SGM), and (iv) infection due to rapidly growing mycobacteria (RGM).
CUTANEOUS TUBERCULOSIS
Cutaneous forms of tuberculosis are a rare clinical manifestation of M. tuberculosis or M. bovis infection, comprising approximately only 1 to 2% of all TB cases (47, 64–67). In settings where immunization programs administer the bacillus Calmette-Guérin (BCG) vaccine, an attenuated form of M. bovis, cutaneous complications, including local reactions, abscess formation, ulcerations, scrofuloderma, and, rarely, disseminated infections, may occur (64, 65). Visceral tuberculosis (pulmonary or extrapulmonary) is rarely associated with concomitant cutaneous involvement (68). However, when it occurs, it is usually in the form of scrofuloderma or lupus vulgaris (47, 67–70). The ability to culture M. tuberculosis facilitates the diagnosis of the cutaneous disease, in contrast to M. leprae (47). Therefore, the gold standard for diagnosing cutaneous tuberculosis is mycobacterial culture of skin biopsy specimens or via molecular detection (47, 71). Depending on the BCG vaccination status, tuberculin skin testing using purified protein derivative has a specificity of 63% and a sensitivity between 33 and 96% for cutaneous tuberculosis (47, 72). Interferon gamma (IFN-γ) release assays (IGRAs) demonstrate a sensitivity of 92% and a specificity of 76% in individuals with cutaneous TB (73).
Cutaneous forms of TB are currently classified according to clinical morphological patterns, the route of acquisition (exogenous inoculation, hematogenous spread, or regional extension), and the host immune status (47, 64–66, 71). In both tuberculosis and leprosy, well-organized epithelioid granulomas are associated with a high degree of cell-mediated immunity (CMI) and a reduced bacterial load (47). Histologically, perineural granulomas assist in distinguishing tuberculoid leprosy from cutaneous TB. Cutaneous TB cases associated with increased CMI and few noted bacilli are classified as high-immune forms (i.e., tuberculosis verrucose cutis, lupus vulgaris, and tuberculids) (47, 69) (Table 2).
TABLE 2.
Modes of acquisition, history of previous sensitization to Mycobacterium tuberculosis, and clinical features of the cutaneous presentations of tuberculosis
| Clinical presentation | Mode of acquisition | Bacillary loada | Previous sensitization to M. tuberculosisb | Clinical features |
|---|---|---|---|---|
| Tuberculosis verrucosa cutis | Exogenous inoculation | Paucibacillary | ++ | Verrucous lesions in dorsum of hands or digits, ankles, or buttocks |
| Primary inoculation tuberculosis | Exogenous inoculation | Multibacillary | − | Nodular lesion in the face or in the upper or lower extremities with associated lymphadenopathy |
| Scrofuloderma | Contiguous extension | Multibacillary | + | More common in children, adolescents, and the elderly; most common site is the neck; may coexist with pulmonary TB |
| Lupus vulgaris | Contiguous extension or hematogenous spread | Paucibacillary | ++ | Affects women predominantly and manifests as smoldering nodules, annular plaques, or hypertrophic or vegetative lesions; initial lesions start as a collection of reddish papules that coalesce to form plaques with serpiginous or verrucous borders with central clearing and atrophy |
| Tuberculosis cutis orificialis | Contiguous extension | Multibacillary | − | Periorificial nonhealing ulcers of the nasal, oral, or anogenital skin or mucosa among individuals with advanced forms of pulmonary, gastrointestinal, or genitourinary TB |
| Acute miliary tuberculosis | Hematogenous spread | Multibacillary | − | Immunocompromised individuals, may present with single or multiple subcutaneous nodules that may potentially evolve into ulcers or draining sinuses without regional adenopathy |
| Metastatic tuberculosis abscess | Hematogenous spread | Multibacillary | − | May appear as a cellulitis or as purpuric papules that may umbilicate and become crusted among individuals with severe immunosuppression (i.e., AIDS or severe malnutrition) |
| Tuberculidsc | Hypersensitivity reactions to M. tuberculosis infection | Negative cultures and stains of affected lesionsd | +++ | Erythema induratum of Bazin (more common in females) and presents as granulomatous lobular painful panniculitis, usually in the lower extremities; papulonecrotic tuberculid (more common in children and young adults), may present with lupus vulgaris and scrofuloderma, presents with dark red or violaceous papules that evolve to pustules or become necrotic, fever and constitutional symptoms present; Lichen scrofulosorum (more common in children and young adults) with lymphatic or pulmonary TB (this condition has also been associated with BCG vaccination and with M. avium-intracellulare infection |
The bacillary load correlates with the degree of cell-mediated immune response (CMI). High immunity determines the occurrence of tuberculosis verrucose cutis, lupus vulgaris, and all forms of the tuberculids. Low immunity against M. tuberculosis is a risk factor for tuberculosis cutis orificialis, acute military tuberculosis, and metastatic tuberculous abscess.
A plus sign indicates a previous history of sensitization to M. tuberculosis. A stronger history of sensitization is indicated with two or three plus signs. A minus sign indicates the absence of a previous history of sensitization to M. tuberculosis.
The diagnosis of tuberculids is supported by a strongly positive tuberculin skin test or a positive IFN-γ release assay, indicating history of exposure to the tuberculous bacilli, with absence of M. tuberculosis in stains or cultures of skin biopsy specimens but evidence of granulomatous inflammation. The response to antituberculosis therapy is also important in confirming these conditions.
M. tuberculosis DNA may be detected in some cases of erythema induratum and papulonecrotic tuberculid.
Tuberculosis verrucosa cutis represents primary M. tuberculosis infection. It occurs predominantly in the extremities and manifests as violaceous or brownish warty plaque-like lesions that present in a previously sensitized host because of direct inoculation of the TB bacillus (47). Lesions consist of usually single painless indurated warty plaques that may potentially ulcerate. This clinical form presents predominantly in children, but when it is present in adults, it tends to occur among those with occupational exposures, such as butchers or farmers. The most frequent sites of involvement include the fingers and dorsum of the hands, followed by ankles or buttocks (Fig. 2) (47, 69). The differential diagnosis of cutaneous TB includes other granulomatous conditions, including sarcoidosis, leprosy, leishmaniasis, fungal conditions (blastomycosis and chromoblastomycosis), Majocchi’s granuloma, halogenoderma, squamous cell carcinomas, orf disease (parapox virus), and syphilis (24, 47).
FIG 2.
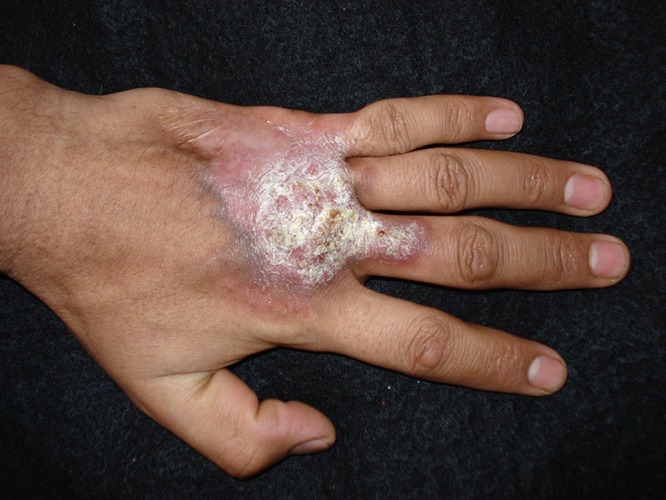
Tuberculosis verrucosa cutis of the hand, manifesting as verrucous plaques caused by direct inoculation of the tuberculous bacilli into the skin of an individual previously sensitized to this pathogen.
Primary-inoculation TB occurs after exogenous inoculation in individuals not previously sensitized to M. tuberculosis, and it represents a phenomenon analogous to the Ghon complex in the lung (47, 69). An initial nodular or papular lesion frequently identified in the extremities or in the face evolves into a shallow ulcer with associated regional lymphadenopathy (67, 69, 71). Some identified risk factors for developing this clinical variant include minor trauma, tattoos, piercing, and surgical procedures with unsterilized equipment (47, 71). Untreated primary-inoculation TB may resolve in a period of 12 months or longer or may progress to disseminated forms of the disease by hematogenous spread of the bacillus to other organs. Some patients may have associated erythema nodosum. Other diseases that need to be considered in the differential diagnosis include bartonellosis, tularemia, leishmaniasis, syphilis, eumycetoma, and yaws (47, 69).
Scrofuloderma is a form of TB that is caused by M. tuberculosis or M. bovis and commonly affects children, adolescents, and older adults (47, 69). This variant of TB is the result of contiguous spread to the overlying skin from adjacent structures such as a lymph node, joint, bone, or the epididymis. When M. bovis infection manifests as scrofuloderma, it is often the result of consumption of unpasteurized milk (47, 69, 71). The most common sites of involvement are the neck, axillae, or groin (Fig. 3). Scrofuloderma initially presents as a firm subcutaneous nodule or nodules that gradually enlarge, become confluent, ulcerate, and form draining sinus tracts of purulent or caseous material. These lesions eventually lead to significant scarring (47, 69). Scrofuloderma may be associated with concomitant pulmonary tuberculosis, particularly when it is associated with right supraclavicular and cervical lymphadenitis (47, 66, 67, 69). Scrofuloderma needs to be distinguished from infections caused by nontuberculous mycobacteria (i.e., Mycobacterium avium-intracellulare complex [MAC], M. haemophilum, or Mycobacterium scrofulaceum), hidradenitis suppurativa, actinomycosis, and eumycetoma (47, 66, 69).
FIG 3.
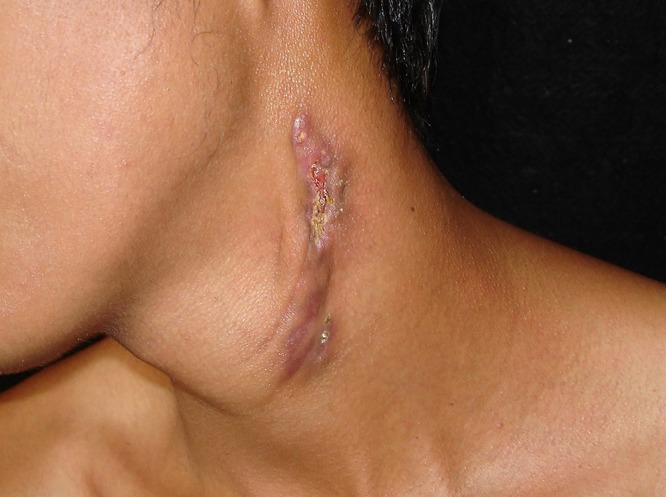
Scrofuloderma presenting in the neck, resulting from direct extension of an infected left cervical lymph node into the overlying cutaneous structures. This form is also known as tuberculosis colliquative cutis. This form of cutaneous tuberculosis is also associated with infection caused by Mycobacterium bovis or bacillus Calmette-Guérin.
Tuberculosis cutis orificialis occurs among severely immunocompromised middle-aged and older adults with advanced pulmonary, gastrointestinal, or genitourinary tuberculosis (47). This rare type of cutaneous TB occurs on the nasal, oral, or anogenital skin or mucosa and is clinically important to consider in individuals with periorificial nonhealing ulcers. The lesions originate from autoinoculation of the mucosal orifices by other cutaneous draining sites from internal organ infections. The differential diagnosis of this condition includes paracoccidioidomycosis, syphilis, lymphogranuloma venereum, pyoderma gangrenosum, and skin malignancies (47, 69).
Lupus vulgaris is a chronic form of cutaneous TB that may occur due to regional lymphatic or hematogenous spread in individuals with reinfection or reactivation of latent TB infection or BCG vaccination (47, 69, 71). Lupus vulgaris may occur concomitantly with scrofuloderma, or it rarely may be associated with primary-inoculation TB. Lupus vulgaris occurs predominantly in Asia and southern Africa. This progressive clinical form originates through lymphatic spread or by contiguous spread from a lymph node or bone (47). This clinical form affects women predominantly and manifests as smoldering nodules and annular plaques, or it may present with hypertrophic or vegetative lesions. Sometimes it may start as a collection of reddish papules that coalesce to form plaques with serpiginous or verrucous borders with central clearing and atrophy. The sites most frequently affected include the lower extremities or buttocks in tropical and subtropical settings, whereas in temperate areas lesions occur most frequently in the head and neck. Lesions of lupus vulgaris may have the appearance of “apple jelly” on diascopy (47, 69, 71). The differential diagnosis of lupus vulgaris is with conditions such as discoid lupus, sarcoidosis, Spitz nevus, chromoblastomycosis, tuberculoid leprosy, and leishmaniasis. Untreated cases of lupus vulgaris may evolve into verrucous squamous cell carcinoma (67, 69).
Hematogenous metastatic tuberculous abscesses occur among immunocompromised individuals and may present with single or multiple subcutaneous nodules that may potentially evolve into ulcers or draining sinuses without regional adenopathy (47, 69). Some lesions may mimic scrofuloderma. Similarly, acute military tuberculosis represents primary infection in individuals with advanced immunosuppression, including those with HIV infection/AIDS (71). This clinical form may appear as a cellulitis or as purpuric papules that may become umbilicated and crusted (49).
Tuberculids are cutaneous disorders that represent hypersensitivity reactions to mycobacterial antigens. The most frequent tuberculid is erythema induratum of Bazin, but some individuals may manifest lichen scrofulosorum, or papulonecrotic tuberculid (47, 67, 69) (Table 2).
Treatment of cutaneous TB follows the same recommendations as for other forms of TB, with multidrug therapy (MDT) and ideally adjusted by culture and susceptibility data (47, 67, 69). The management of extensive scrofuloderma sometimes requires surgical intervention. Reconstructive surgery may be indicated for severe forms of cutaneous TB such as lupus vulgaris (69, 71).
LEPROSY
Leprosy is a mycobacterial infection caused by Mycobacterium leprae that tends to be chronic and to compromise human societies by producing peripheral nerve damage, limb loss, blindness, and disfiguring skin lesions (4, 72, 74). Leprosy occupies a prominent position among infectious diseases due to its high frequency of disability and associated stigma (75–78).
M. leprae is a noncultivable obligate intracellular pathogen with a slow division time that targets peripheral nerves by predominantly infecting Schwann cells and histiocytes and keratinocytes in the skin (72, 74, 78–90). The entry of the M. leprae bacillus into the Schwann cell activates the cell to enter into a dedifferentiation process. The infection may then be carried to other sites by immature cells (83, 84). Innate immune responses by macrophages in human tissues are responsible for initiating nerve damage in leprosy by interaction with phenolic glycolipid 1 (PGL-1) with myelinating glia (88, 89).
The clinical manifestations of leprosy are related to the immune response to the leprosy bacillus (Table 3) (4, 47, 72, 74, 78). The Ridley-Jopling staging system divides leprosy into tuberculoid, borderline (borderline tuberculoid, borderline borderline, and borderline lepromatous), and lepromatous forms (Fig. 4) (72, 74, 78). Leprosy reactions, due to their potential inflammatory compromise of the nerve fibers, lead to sensory and motor loss (85, 90–92). Histologically, intraneural or perineural granulomas may assist the pathologist in distinguishing leprosy from cutaneous tuberculosis (47).
TABLE 3.
Clinical spectrum of leprosy and leprosy reactions (reversal reactions and erythema nodosum leprosum)
| Parameter | Ridley-Jopling classification: |
||||
|---|---|---|---|---|---|
| Tuberculoid | Borderline tuberculoida | Borderline borderline | Borderline lepromatous | Lepromatous | |
| Reversal reactionsb | − | ++ | ++ | ++ | − |
| Erythema nodosum leprosumc | − | − | − | ++ | ++ |
| WHO classification | Paucibacillary | Paucibacillary | Multibacillary | Multibacillary | Multibacillary |
| Clinical features | Single or very few hypopigmented macules or plaques with a raised edge, dry, scaly, hairless with hypoesthesia or anesthesia; few peripheral nerves are commonly enlarged | Skin lesions (hypopigmented anesthetic patches that become confluent); moderate nerve involvement; may have late neural thickening with asymmetrical anesthesia and paresis | Skin lesions (hypopigmented anesthetic patches with punched-out centers and raised erythematous borders); multiple nerve involvement with symmetrical thickened nerves; asymmetrical anesthesia and paresis may be present | Skin lesions (widely distributed nodules with diffuse skin infiltration); multiple nerve involvement with symmetrical involvement (thickened nerves); symmetrical glove and stocking anesthesia; deformity, amputation, and disability | Widely distributed skin lesions (macules, nodules, erythematous papules); diffuse skin infiltration with thickened peripheral nerves; symmetrical glove-and-stocking anesthesia; deformity, amputation, and disability |
| Multidrug treatment recommendations | |||||
| U.S. National Hansen’s Disease Program | Dapsone at 100 mg/day for 12 mo; rifampin at 600 mg/day for 12 mo |
Dapsone at 100 mg/day for 24 mo; rifampin at 600 mg/day for 24 mo; clofazimine at 50 mg/day for 24 mo (may substitute daily minocycline) |
|||
| WHO | Dapsone at 100 mg/day for 6 mo; rifampin at 600 mg once monthly under supervision for 6 mo |
Dapsone at 100 mg/day for 12 mo; rifampin at 600 mg once monthly under supervision for 12 mo; clofazimine at 50 mg/day for 12 mo plus 300 mg every month under supervision for 12 mo |
|||
Borderline forms represent a mixture of signs and symptoms of polar forms.
Management of reversal reaction (type 1 reaction) requires prednisone or prednisolone (40 to 80 mg daily tapered over a 12- to 20-week period.
Treatment of erythema nodosum leprosum (type 2 reaction) involves the use of prednisone or prednisolone (40 to 80 mg daily tapered over a 12- to 24-week period) but sometimes requires a longer taper. Thalidomide at a dose of 200 to 400 mg in divided doses is sometimes used in combination with corticosteroids to control severe erythema nodosum leprosum. Clofazimine may be used in those intolerant to corticosteroids or in combination.
FIG 4.
Clinical manifestations of leprosy: borderline tuberculoid (BT) (A), borderline borderline (BB) (B), and lepromatous (LL) (C).
Since the early 1980s, multidrug therapy (MDT) has been universally instituted through active case finding in highly affected communities. This strategy has helped to reduce the prevalence of this infection (93–97). Nevertheless, since 2005, the number of reported new cases has remained consistently stable despite continued use of multidrug therapy (4, 93, 95). The number of new cases will reach the 4 million mark by 2020 (since 2000) (76). Many of these new patients already have grade 2 neurological disability by the time of their diagnosis (96, 97). Since cases of leprosy in children indicate ongoing transmission of M. leprae in settings of endemicity, targeted screening involving school-based surveillance followed by continuous household surveillance increases early detection of new leprosy cases (97). Molecular detection methods and phenolic glycolipid 1 (PGL-1) serological data in combination with spatial epidemiology increase detection of leprosy cases (98). Early identification of new cases likely prevents further transmission, but, importantly, it may also reduce the risk of neurological dysfunction and disability associated with leprosy (96–98). There are multiple remaining hazards in the epidemiology of leprosy that may make it impossible to eliminate leprosy transmission by the year 2020 (96, 97).
The management of leprosy requires the use of MDT in combination with steroids or other anti-inflammatory drugs among those with leprosy reactions (72, 74, 78). Since M. leprae is not cultivable, the bacteriostatic and bactericidal effects of antimycobacterial drugs against M. leprae have been assessed in laboratory studies (47). The WHO recommended the institution of multidrug therapy with dapsone, rifampin, and clofazimine in 1982 (47, 97, 99). Relapse or reinfection is considered a rare clinical phenomenon (47, 78, 86, 87). New cutaneous lesions presenting during or after completing MDT are most likely caused by leprosy reactions (47, 85, 91, 92). Among patients with relapse, some researchers have detected drug resistance with the use of rapid DNA-based molecular assays (99).
Diffuse Lepromatous Leprosy of Lucio and Latapí
Mycobacterium lepromatosis was the cause of leprosy in two patients of Mexican origin who died of diffuse lepromatous leprosy (DLL) (100). This form of leprosy is associated with a large bacillary burden that often affects many organs and endothelial cells in subcutaneous tissues, producing dermal vascular occlusion with associated skin necrosis and ulceration (100–105). Infection caused by M. lepromatosis is responsible for this unique clinicopathological presentation, which is known as Lucio’s phenomenon (101, 102, 104, 106–108). Originally described in Mexico, this clinical form of leprosy also occurs in other countries (102–104). Comparative genomic analyses have demonstrated that M. lepromatosis and M. leprae are related mycobacterial species that are distinguishable at the genomic level but cause similar clinical manifestations (104–107). There is also some evidence suggesting that M. lepromatosis may be associated with severe leprosy reactions, but this association requires further confirmation (103, 107). Clinical studies need to determine whether differences between infection with M. leprae and M. lepromatosis are clinically distinguishable (102, 107, 108) or whether coinfection with M. leprae and M. lepromatosis may potentially predispose individuals to experience more severe leprosy reactions (108). In England, Ireland, and Scotland, red squirrels may be infected and develop leprosy-like lesions due to M. lepromatosis (53). This strain of M. lepromatosis appears to have diverged from the two human strains from Mexico (53).
BURULI ULCER
In 1947, Mycobacterium ulcerans was identified as the cause of Buruli ulcer (BU) (2, 3, 31, 32, 109–111). The first cases of BU (formerly known as Bairnsdale ulcer) were identified in Australia in the 1930s (110). BU is considered a neglected tropical disease (NTD) because most cases occur among impoverished populations, often causing an important disability burden (2, 15, 31, 32, 109). M. ulcerans and all mycolactone-producing mycobacterial species evolved from M. marinum and have become specialized variants living in restricted environments (2, 3). Currently, most cases of Buruli ulcer occur in parts of western and central Africa, but cases occur in at least 33 countries, mostly in South America and Western Pacific regions (110). WHO identifies approximately 3,000 to 5,000 cases annually, affecting predominantly children less than 15 years of age (20, 31, 32, 109). From an ecological standpoint, BU is a mycobacterial disease identified in rural areas with wetlands, such as ponds, swamps, marshes, impoundments, backwaters, slow-moving rivers, and flooding areas (31, 32). The precise mode of transmission remains to be elucidated, but M. ulcerans living in contaminated water can enter the host through insect bites, puncturing injuries, or skin trauma (31, 33). A similar mechanism of transmission maybe responsible for some cases of leprosy (33).
Clinically, BU affects predominantly the lower extremities (>55%) and less often the upper extremities or other body parts (31, 109, 111, 112) The toxin (polyketide), mycolactone secreted by M. ulcerans causes tissue destruction (111, 113), local immunosuppression through the inhibition of protein translocation into the endoplasmic reticulum of cytokines of the innate immune system, membrane receptors, adhesion molecules, and T-cell-dependent cytokines (114). Additionally, this toxin induces hypoesthesia by altering the signaling pathways of the type 2 angiotensin II receptors, leading to hyperpolarization of neurons (115). Untreated cases or those with extensive and deep ulcerations develop scarring contractures, deformity, osteonecrosis, and limb loss (31, 109). Other environmental mycobacteria can produce the lipid toxin mycolactone (111, 113). Collectively, these bacteria constitute the group of mycolactone producers, including Mycobacterium shinshuense (identified in human cases in Japan), Mycobacterium pseudoshottsii (found in striped bass in the United States), M. marinum DL240490 (found in European sea bass in the Red Sea), and others (111, 113). All of these species have been isolated from humans, frogs, and fish. They share phenotypic and genotypic features, including the large virulence plasmid (pMUM) required for mycolactone production. Based on these similarities, researchers have proposed recognizing all these bacteria as M. ulcerans (31, 32, 111, 113). In fact, there is less than 1% nucleotide variation among all mycolactone-producing mycobacteria (113).
M. ulcerans is a slowly growing environmental mycobacterium causing infection that is considered to have an incubation period of 5 to 8 weeks, but this may be as long as six months in areas of endemicity (31, 109). BU often presents as a painless nodule, as a large indurated plaque, or as diffuse painless swelling of the lower extremities, upper extremities, or face (109). In a period of approximately 4 weeks, the nodule, plaque, or edematous area evolves into an ulcer with undermined borders. The diagnosis of Buruli ulcer is mostly a clinical one and is based on the age of presentation, geographic area, and location (31, 109, 112). The most important conditions that should be considered in the differential diagnosis of BU include tropical phagedenic ulcers, cutaneous tuberculosis, vascular (venous or arterial) ulcerations, diabetic foot ulcerations, pyoderma gangrenosum, infections due to Haemophilus ducreyi, cutaneous leishmaniasis, ulcerative yaws, fungal infections (e.g., chromoblastomycosis), and pyogenic ulcerations (e.g., caused by Staphylococcus aureus) (24, 109, 112). The diagnosis of BU maybe confirmed by direct microscopy of suspicious lesions, histopathology of skin biopsy specimens, culture, and IS2404 PCR (PCR) (24, 31, 109). Rapid diagnostic tests to detect mycolactone are currently under evaluation for use as point-of-care tests in areas of high endemicity (109). The histopathology of BU demonstrates large numbers of extracellular bacilli during the acute phase of infection (24, 47, 109).
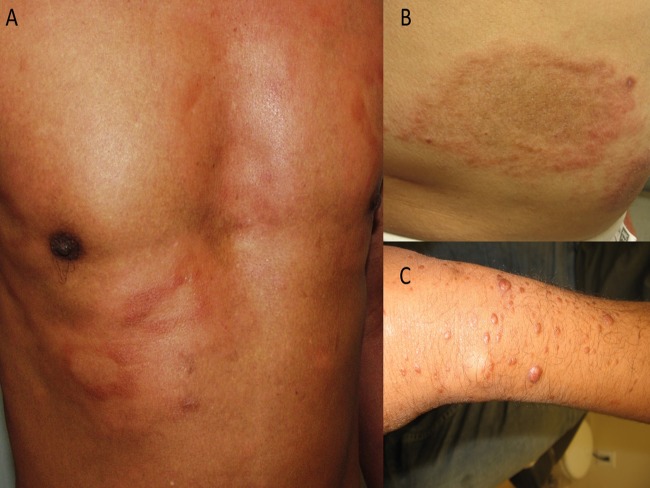
For clinical staging purposes, BU is divided into three categories by the degree of cutaneous involvement. Category I is a single small lesion. Category II is defined by the presence of nonulcerative or ulcerative plaques and edematous forms (Fig. 5). Category III is when there is evidence of severe disease with dissemination, osteitis, osteomyelitis, or joint involvement (31, 109). Early diagnosis and treatment are crucial to minimize morbidity and prevent long-term disability (2, 3, 109). However, in most countries, at least 70% of all cases are diagnosed in the stage with deep ulceration. HIV coinfection/AIDS appears to foster the rapid progression of lesions into severe ulcerative stages (31, 109).
FIG 5.
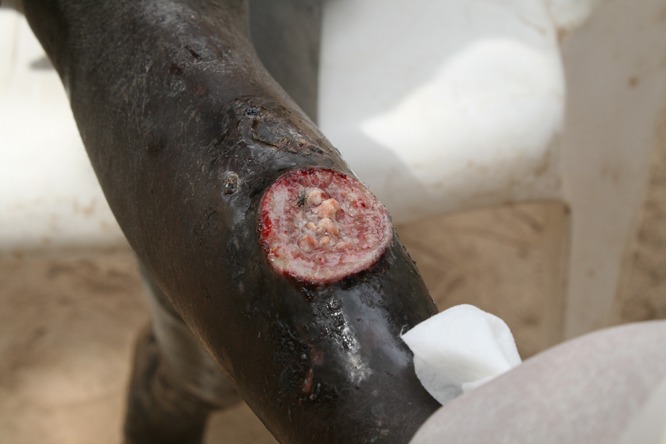
An 11-year-old male demonstrating a destructive panniculitis causing ulceration with undermined borders, characteristic of Buruli ulcer.
Treatment of BU consists of a combination of antimycobacterial drugs and wound management interventions (109, 112). Surgical debridement and skin grafting are used to speed wound healing in those with large lesions. Antimicrobial regimens of 8 weeks or longer are recommended, irrespective of the clinical staging, and include a combination of rifampin and streptomycin (31, 109). Alternatively, a combination of rifampin and clarithromycin or rifampin and moxifloxacin could be used (109, 112).
NONTUBERCULOUS MYCOBACTERIA
The NTM group constitutes mycobacterial species other than those belonging to the M. tuberculosis complex and that do not cause leprosy (1, 42). Historically, NTM were classified according to the Runyon classification based on their growth rates and their ability to produce pigment in response to light (1, 115). Currently, the NTM group is also divided into two major subgroups defined by their ability to grow on solid culture media: (i) rapidly growing mycobacteria (RGM) and (ii) slowly growing mycobacteria (SGM) (Fig. 1) (1). Infections due to NTM can produce pulmonary or extrapulmonary disease in immunocompromised hosts (1, 24, 42).
Recent studies have demonstrated that the prevalence of nontuberculous mycobacterial infections is increasing in many settings (24–28). The clinical spectrum of disease associated with mycobacterial pathogens depends on the route of exposure and host susceptibility factors (25–27). Pulmonary nontuberculous mycobacterial infections are multisystem and multigenic diseases (26). Affected individuals tend to have more frequent protein variants in immune, cystic fibrosis transmembrane conductor regulator (CFTR), and connective tissue genes (26, 27). However, those suffering from cutaneous involvement of NTM usually possess other risk factors (Table 4) (24). Disseminated NTM infections affect severely immunocompromised human hosts, including those with primary immunodeficiencies, such as genetic or acquired defects of the IFN-γ–interleukin-12 (IL-12) pathway (e.g., GATA2 deficiency or anti-IFN-γ autoantibodies), or acquired immunodeficiencies, such as HIV infection/AIDS, transplant-associated immunosuppression, and treatment with biological agents such as anti-tumor necrosis factor alpha (anti-TNF-α) receptor blockers (26, 27, 116).
TABLE 4.
Risk factors for acquiring major nontuberculous mycobacterial infections of the skin and soft tissues and medical and surgical recommendation
| Mycobacterial species | Risk factors for cutaneous disease | Therapy |
|---|---|---|
| M. fortuitum | Dermal piercing, tattoos, mesotherapy, acupuncture, intravascular devices (e.g., central venous catheters), peritoneal dialysis catheters |
Combination of macrolide, fluoroquinolones, doxycycline, trimethoprim-sulfamethoxazole; surgical excision may be indicated |
| M. abscessus complex | Cosmetic surgery, postsurgical infections, acupuncture, mesotherapy, pedicure sessions |
Combination of macrolide (azithromycin or clarithromycin), cefoxitin, imipenem, amikacin; surgical excision and/or debridement need to be considered for severe deep tissue involvement |
| M. chelonae | Tattoos, mesotherapy, acupuncture | Combination of macrolide (azithromycin or clarithromycin), cefoxitin, imipenem, fluoroquinolones, amikacin; surgical excision may be indicated |
| M. haemophilum | Cosmetic procedures, permanent makeup, HIV infection/AIDS, anti-TNF-α blockers, freshwater or salt water injuries |
Combination of macrolide (clarithromycin), fluoroquinolones, rifamycin; surgical excision may be indicated, similarly to infections caused by M. abscessus complex |
| M. marinum | Freshwater or salt water injuries associated with fishing injuries, coral trauma, and other related injuries |
Combination of clarithromycin and ethambutol or trimethoprim-sulfamethoxazole and rifampin; alternative drugs include doxycycline or minocycline |
| M. kansasii | HIV infection/AIDS, renal transplant, chronic pulmonary diseases | Combination of isoniazid, rifampin, ethambutol; clarithromycin may be used instead of isoniazid; linezolid is an alternative drug |
Rapidly Growing Mycobacteria
Cutaneous NTM infections are transmitted via direct inoculation through skin barrier breaks, which may occur during trauma, surgical procedures, plastic surgery (including liposuction), injections, tattoos, acupuncture, and body piercings (Table 4) (1, 24, 42, 117). Cosmetic procedures such as mesotherapy (multiple injections of pharmaceutical products, plant extracts, homeopathic substances, vitamins, or other compounds into subcutaneous fat) have been involved in the transmission of rapidly growing mycobacteria (1, 24, 42, 117). The clinical manifestations of cutaneous involvement include cellulitis, papular lesions, nodules with purple discoloration, abscesses, draining sinuses, subcutaneous nodules (pseudoerythema nodosum), and ulcerations (117). Some individuals may manifest with a single lesion, but others manifest with multiple lesions, depending on the mode of acquisition and level of host immunity (42, 117). NTM infections of the skin may spread to cause tenosynovitis, myositis, osteomyelitis, and septic arthritis (24, 42, 117).
Mycobacterium abscessus was first identified in a patient with a knee infection and subcutaneous abscesses in 1950 (117). Among the rapidly growing mycobacteria, it is the most common cause of lung disease (117). Along with Mycobacterium fortuitum and Mycobacterium chelonae, members of the M. abscessus complex (M. abscessus, Mycobacterium massiliense, and Mycobacterium bolletii) are the major NTM associated with cutaneous involvement (Fig. 6) (1, 24, 42, 117). Localized cutaneous infections are due to posttraumatic wound infection, catheter-associated infections (e.g., from peritoneal dialysis or central venous catheters), postsurgical infections, and trauma-associated infections (Fig. 7) (24, 42, 117). M. chelonae and M. abscessus usually present with multiple skin lesions, while M. fortuitum tends to present as a single lesion (24, 42, 43, 117). Susceptibilities to antimicrobials depend on the species. Members of the M. abscessus complex tend to be susceptible to macrolides, amikacin, cefoxitin, and imipenem (115). However, it is important to confirm the detection of inducible macrolide (clarithromycin) resistance by the presence of the erm41 gene (1, 117, 118). Detection of in vivo resistance to macrolides requires incubation of NTM isolates with clarithromycin prior to determining an MIC (117, 118). Azithromycin is the preferred agent in M. abscessus infections, whereas clarithromycin or azithromycin is effective in cases of M. massiliense (117, 118). M. fortuitum, M. abscessus, and M. chelonae are resistant to all of the antituberculosis agents (1, 24, 42, 115). M. fortuitum is susceptible to macrolides, amikacin, doxycycline, fluoroquinolones, and trimethoprim-sulfamethoxazole. Finally, M. chelonae is often susceptible to macrolides, cefoxitin, fluoroquinolones, and tobramycin (1, 42).
FIG 6.

An adult with Mycobacterium abscessus infection presenting as scrofuloderma with extensive tissue destruction in the right cervical and supraclavicular areas.
FIG 7.
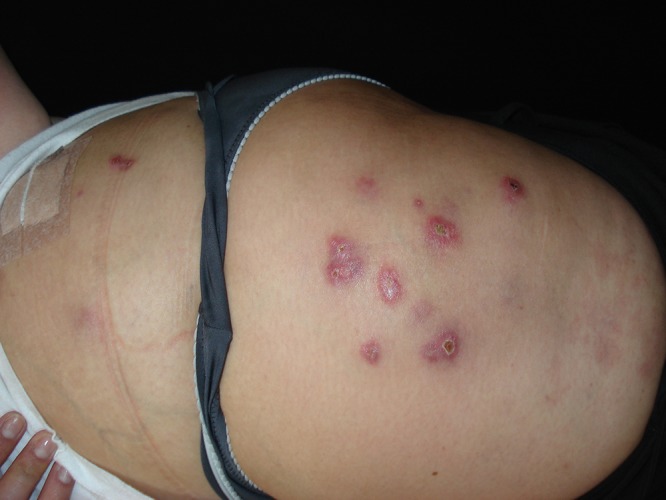
Infection caused by Mycobacterium fortuitum associated with mesotherapy.
Mycobacterium kansasii
M. kansasii was identified in 1953 as causing an infectious disease that produces lung cavitary lesions resembling those in pulmonary TB (119). M. kansasii has been identified only in municipal water systems (21, 22, 42). M. kansasii infection manifests predominantly as pulmonary disease. Cutaneous involvement of M. kansasii is usually present in immunocompromised hosts and sometimes with concomitant pulmonary disease or disseminated disease (24, 37). Cutaneous infection may present as nodules, pustules, verrucous lesions, erythematous plaques, ulcers, and abscesses (Fig. 8). It may also be associated with osteomyelitis and septic arthritis. This infection may occur among immunocompetent and immunocompromised hosts, including those with HIV infection/AIDS or with renal transplantation. Similarly to M. tuberculosis, M. kansasii expresses ESAT-6 and CFP-10 analogs, and therefore IFN-γ release assays (IGRAs) are not useful in distinguishing this infection from M. tuberculosis infection (37). M. kansasii is susceptible to isoniazid, rifampin, ethambutol, clarithromycin, fluoroquinolones, and aminoglycosides but is intrinsically resistant to pyrazinamide (37, 119). Susceptibility testing is recommended only for rifampin given the fact that the best clinical outcomes are associated with rifampin susceptibility (37, 119).
FIG 8.
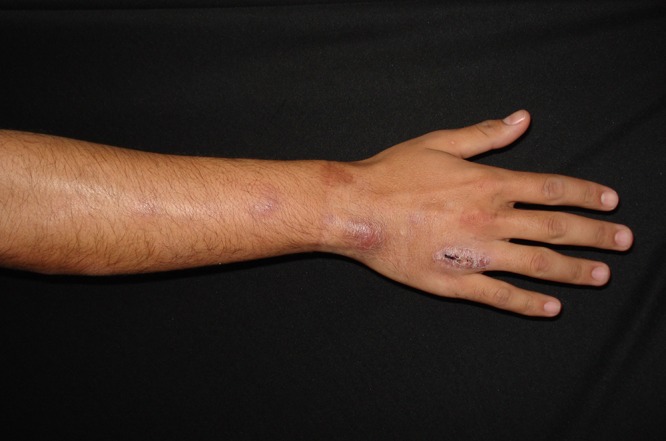
Characteristic sporotrichoid nodular lymphangitic spread of Mycobacterium marinum.
Mycobacterium haemophilum
M. haemophilum requires iron or hemin supplementation for growth (35, 36, 42, 119). M. haemophilum was identified in 1978 in individuals with skin infections. M. leprae and M. haemophilum have important similarities, including the presence of large quantities of docosanoic acid, and M. haemophilum possesses a phenolic glycolipid antigen analogous to the one expressed by M. leprae. Furthermore, M. leprae and M. haemophilum are phylogenetically related and also share ancestry with other mycobacterial species, such as M. marinum and M. ulcerans (35, 36, 119). Patients with M. haemophilum may also experience immune reconstitution events analogous to leprosy reactions or to paradoxical immune reactions seen after initiating antimycobacterial therapy in patients with M. tuberculosis infection (35, 36, 119). This infection may present as a localized or disseminated disease in immunocompromised hosts, including those with HIV infection/AIDS, transplant recipients, and those receiving biological agents such as anti-TNF-α agents (119). This organism preferentially grows at 30°C, explaining its predilection for causing lesions in the upper and lower extremities (34–36). However, M. haemophilum has been associated with subcutaneous infections, lymphadenitis, septic arthritis, osteomyelitis, pneumonitis, and disseminated disease. Like disease caused by M. marinum or M. ulcerans, the cutaneous disease caused by M. haemophilum may present after salt water injuries (24, 36).
The clinical spectrum of cutaneous manifestations of M. haemophilum includes multiple skin lesions presenting as erythematous or violaceous papules, plaques, or nodules. Some of these lesions evolve into necrotic abscesses or deep-seated ulcerations (36). Most of these presentations occur in the extremities, particularly over joints. Earlier lesions presenting as papules or nodules are usually painless but when these lesions evolve into ulcerations or abscesses, patients may experience significant pain. In children, this infection usually presents as cervical lymphadenitis (35, 36). Treatment involves a combination of clarithromycin, ciprofloxacin, and rifampin or rifabutin for 12 to 24 months (34–36, 42).
Mycobacterium marinum
M. marinum is a slowly growing pigmented organism responsible for “fish tank granuloma” due to its ability to cause localized skin and soft tissue infections in individuals with exposure to contaminated freshwater or salt water (24, 42, 120). Disease caused by M. marinum is associated with minor to moderate skin infections presenting as granulomatous lesions similar to those caused by M. tuberculosis or M. haemophilum. Most cases of cutaneous infections take place among individuals who suffered puncture injuries or other types of trauma in freshwater or salt water. The clinical spectrum of cutaneous disease caused by M. marinum includes a solitary papule or nodule that may ulcerate and then spreads in a sporotrichoid pattern (lymphangitic spread) (Fig. 9) (120). Because its optimal temperature for growth is around 30°C, cutaneous lesions most frequently occur in the upper or lower extremities and sometimes in the tip of the nose. M. marinum may produce deep tissue involvement (Fig. 10) or disseminated disease among severely immunocompromised hosts (120). Treatment of this mycobacterial infection requires a combination of at least two drugs, including a macrolide, ethambutol, trimethoprim-sulfamethoxazole, or rifamycin, with a duration of therapy ranging from two to six months depending on the degree of cutaneous involvement (24, 42, 120).
FIG 9.
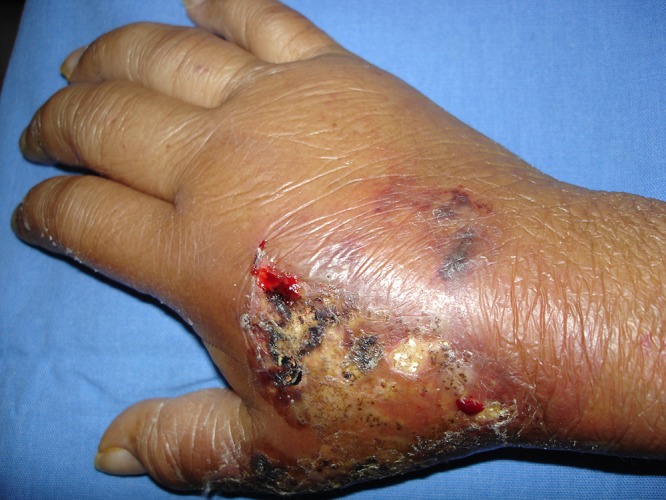
Severe hand swelling and nodular lymphangitic lesions caused by Mycobacterium marinum infection.
FIG 10.
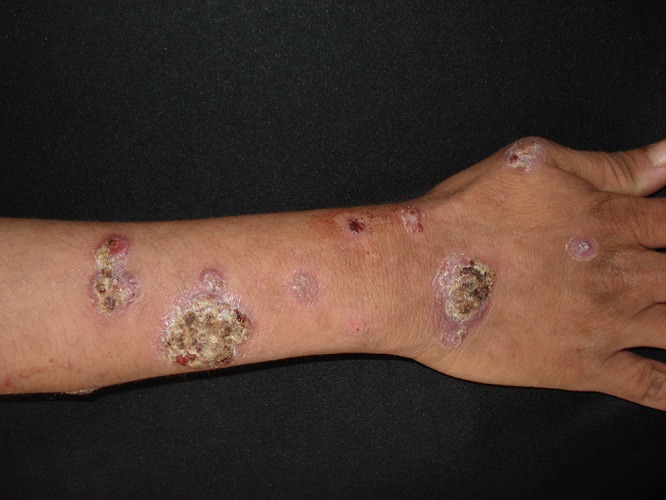
Mycobacterium kansasii leading to a sporotrichoid nodular lymphangitis of the right arm.
Mycobacterium avium-intracellulare
Cutaneous involvement of M. avium-intracellulare complex (MAC) infections has been rarely reported (121–125). MAC is composed of several different slowly growing mycobacterial species, including M. avium, M. intracellulare, Mycobacterium indicus pranii, Mycobacterium chimera, Mycobacterium arosiense, and many others. The spectrum of clinical manifestations includes papular, nodular lesions with a sporotrichoid pattern, verrucous ulcers, inflammatory pseudotumors, draining sinuses, and cold abscesses (Fig. 11) (121–125). Modes of acquisition of MAC infection include trauma, cosmetic procedures (such as pedicures, footbaths, and leg waxing), and postsurgical infections (126). Recent outbreaks of severe, life-threatening infections caused by M. chimera were associated with extracorporeal circulation following cardiothoracic surgery procedures. Many of these patients presented with surgical wound infections (41, 116, 125).
FIG 11.
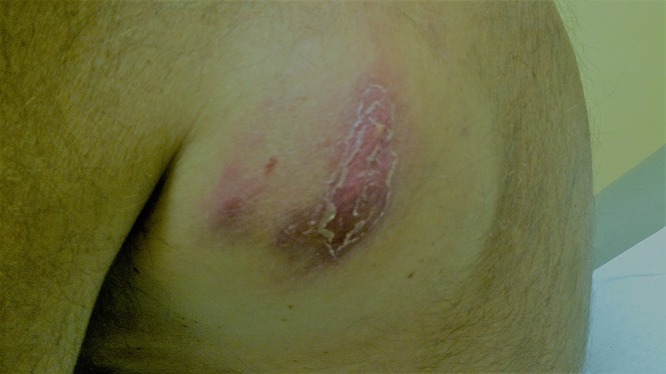
Cold abscess caused by Mycobacterium avium-intracellulare complex infection in a 60-year-old male.
DIAGNOSIS OF CUTANEOUS MYCOBACTERIAL INFECTIONS
The diagnosis of mycobacterial infections of the skin and soft tissues requires a low threshold of clinical suspicion given the broad spectrum of potential clinical presentations. Confirming a diagnosis of cutaneous mycobacterial infections requires tissue biopsies of cutaneous lesions to assess for the presence of acid-fast bacilli and cultures of tissue specimens or material obtained from draining lesions (1, 42). Some mycobacterial species have specific growth requirements in solid or liquid culture media. Molecular techniques such as 16S rRNA gene sequencing, PCR analysis, and high-performance liquid chromatography are methods that improve the ability to identify mycobacterial pathogens in tissue specimens.
The clinical diagnosis of leprosy relies on the identification of characteristic plaques, macules, or nodules concomitantly with sensory loss in the form of hypoesthesia or anesthesia and the presence of thickened nerves (72, 74). Tissue biopsies of lesions may demonstrate, using Fite-Faraco staining, the presence of acid-fast bacilli residing inside nerves and perineural or intraneural granulomas (47). In addition, histopathological evaluation of tissue samples contributes to defining the immunopathological spectrum of polar and borderline forms of leprosy (47). The identification of Buruli ulcer often relies on the presence of characteristic nodules or ulcers, ecological risk factors, and at-risk age groups residing in settings of endemicity. Culturing M. ulcerans is cumbersome since it requires a low oxygen concentration and a temperature between 29°C and 33°C. Molecular identification of M. ulcerans by employing quantitative PCR (qPCR) assays is an alternative methodology for confirming a diagnosis of Buruli ulcer. The use of point-of-care diagnosis of Buruli ulcer is under evaluation in field studies in settings of endemicity (1, 42). Identifying M. tuberculosis in tissue specimens through culture or molecular detection is of paramount significance when suspecting most clinical forms of cutaneous tuberculosis.
Treatment guidelines recommend performing susceptibility testing of mycobacterial isolates with the goal of optimizing the choice of specific antimycobacterial drug combinations, since the MIC to specific antimicrobials correlates clinically with in vivo responses to antimicrobial therapy for many mycobacterial species (1, 42). It is recommended that rapidly growing mycobacteria be tested against selected antibacterial drugs of different classes (1, 42). Susceptibility testing for M. leprae involves assessments of specific genetic markers of antimycobacterial resistance (99).
CONCLUSIONS
The most common clinical presentations of mycobacterial infections include pulmonary, cutaneous and disseminated forms in immunocompromised hosts. Cutaneous mycobacterial infections may manifest with localized or diffuse lesions. Disruption of skin and soft tissues frequently constitutes the portal of entry of NTM from environmental niches (soil, natural water systems, engineered water networks, etc.) (21, 22). In addition, some mycobacterial infections affecting cutaneous structures occur after exposures to infected animals or their products (127, 128). The most frequently identified mycobacterial pathogens involving the skin and soft tissues include Mycobacterium leprae Mycobacterium ulcerans and M. tuberculosis. However, NTM are becoming important emerging pathogens in different geographical areas. Of these, rapidly growing mycobacteria, M. haemophilum, and M. marinum are important agents involving cutaneous structures. Further clinical and epidemiological research that advances our understanding of mycobacterial pathogens that infect the skin and soft tissues may improve our ability to prevent these infections and optimize their medical management.
ACKNOWLEDGMENT
We have no conflicts of interest to disclose.
Biographies

Carlos Franco-Paredes, M.D., M.P.H., is an infectious diseases clinician with expertise in tropical medicine and neglected tropical diseases. Dr. Franco-Paredes obtained his medical degree from L Salle University in Mexico City. He completed his residency in internal medicine and fellowship in infectious diseases at Emory University in Atlanta, GA. He also obtained a Masters in Public Health in Global Health from the Rollins School of Public Health of Emory University. Prior to joining the Division of Infectious Diseases at the University of Colorado as an Associate Professor of Medicine in 2017, Dr. Franco-Paredes was an Associate Professor of Medicine and Global Health at Emory University (2004 to 2011) and a Staff Physician in Infectious Diseases at Phoebe Memorial Hospital in Albany, GA (2011 to 2017). Dr. Franco-Paredes has participated in multiple projects in global health, focusing mainly on leprosy and Chagas disease. He is deputy editor for PLoS Neglected Tropical Diseases and Annals of Clinical Microbiology and Antimicrobials. He has 172 publications in peer-reviewed journals. In 2016, he edited a textbook, Neglected Tropical Diseases in Latin America and the Caribbean (Springer-Verlag), and he has written a textbook on infectious diseases (Core Concepts in Clinical Infectious Diseases [Elsevier]). Dr. Franco-Paredes has an adjunct faculty appointment as a research professor at the Hospital Infantil de Mexico Federico Gomez in Mexico City. His current research activities include the epidemiology and clinical spectrum of leprosy and other cutaneous mycobacterial diseases.

Luis A. Marcos, M.D., M.P.H., received his M.D. from Universidad Peruana Cayetano Heredia in Lima, Peru, in 2003. He completed an internship and residency in internal medicine at the University of Texas Health Science Center, followed by a fellowship in infectious diseases at Washington University. He received his M.P.H. from the Bloomberg School of Public Health at Johns Hopkins University. He is currently an Associate Professor of Clinical Medicine in the Division of Infectious Diseases at Stony Brook University and serves as the Fellowship Program Director. He is also the Associate Director for Research for the Global Health Institute at Stony Brook University. He serves as a member of the Global Health Committee at IDSA. His main research interests are vector-borne diseases and emerging infectious diseases in the United States and overseas. He is the Director of the Tick-Borne Disease Center at Stony Brook.

Andrés F. Henao-Martínez, M.D., graduated from Universidad del Valle, School of Medicine, in Colombia in 2003. He completed an internship and residency at the University of Texas Health Science Center in San Antonio in 2010, followed by a fellowship in Infectious Diseases with an advanced research year at the University of Colorado, Denver, in 2014. He has served on the faculty of the Division of Infectious Diseases, Department of Medicine, since then. He has been the director of the travel clinic at University of Colorado Hospital since 2016. In addition, Dr. Henao-Martínez directs the Outpatient Infectious Diseases Rotation for the internal medicine and preventive medicine residency programs. His research interest is the study of factors in host susceptibility to different fungal and tropical infections, including Chagas disease and Cryptococcus infection.

Alfonso J. Rodríguez-Morales, M.D., M.Sc., D.T.M.&H., F.R.S.T.M.H.(Lon), F.F.T.M R.C.P.S.(Glasg), F.A.C.E, Ph.D.(c), Hon.D.Sc, is an expert in tropical diseases, particularly in zoonotic and vector-borne diseases, but also including tuberculosis and mycobacterial diseases. He is President of the Travel Medicine Committee of the Pan American Infectious Diseases Association (API), as well Secretary of the Colombian Infectious Diseases Association (ACIN). He is a member of the Committee on Tropical Medicine, Zoonoses and Travel Medicine of ACIN. He is part of the Executive Board of the Latin American Society for Travel Medicine (SLAMVI). Since 2014, has been recognized as Senior Researcher by the National Agency of Science in Colombia, Colciencias. He is Professor of Medicine and Veterinary Medicine and Director of Scientific Research of the Faculty of Health Sciences of the Universidad Tecnológica de Pereira (UTP) in Pereira, Risaralda, Colombia. He is Co-Director of the Public Health and Infection Research Group, UTP, classified A1 by Colciencias. His H index is currently 24.

Wilmer E. Villamil-Gómez, M.D., Ph.D.(c), is a medical doctor (graduated from the University of Cartagena), family medicine specialist (University of Cartagena), specialist in epidemiology (School of Medicine, Juan N Corpas University), candidate for Ph.D. in tropical medicine and infectious diseases (University of Cartagena and University of the Atlantic), and fellow in infectology (University of Buenos Aires). He graduated from the Field Epidemiology Training Program (FETP) of the CDC and Tephinet in Colombia. He is Professor of Infectology and Tropical Medicine (University of Sucre, Colombia), Professor of the Graduate Promotion and Prevention of the University of Sucre (Colombia), member of the Research Committee of the University Hospital of Sinclejo (Colombia), Colciencias peer reviewer, coordinator of the Tropical Medicine and Zoonoses Committee of the Colombian Association of Infectology (2017 to 2019), and member of the editing and arbitration committees of multiple international journals. He has published more than 50 articles in journals indexed in ScienceCitationIndex, Medline, and Scopus (including Lancet Infectious Diseases). He has written four chapters of books. He has a Scopus H index of 17. He is an Associate Researcher, Colciencias and winner of the Wiliam Jarvis 2014 Award, Best International Research, awarded by SHEA. He is a member of the Committee of Travel Medicine Committee of API (Pan-American Association of Infectology), former president of the Colombian Association of the Infectious Region of the Caribbean Region, and a member of the International Infection Control Consortium (INICC).

Eduardo Gotuzzo, M.D., is a world-recognized leader in the epidemiology and clinical manifestations of infectious diseases. He is the former director of the Tropical Medicine Institute “Alexander von Humboldt” and Professor of Medicine and Infectious Diseases at Universidad Peruana Cayetano Heredia in Lima, Peru. He has authored more than 350 peer-reviewed publications on infectious diseases and tropical medicine.

Alexandro Bonifaz is Head of the Department of Mycology, Dermatology Service, Hospital General de Mexico “Dr. Eduardo Liceaga.” He is also a Researcher of the Health Systems, Health Secretary, Mexican Government, and Senior Researcher of the National System of Researchers (CONACYT). He is a member of multiple national and international associations in dermatology, medical mycology, and tropical medicine (especially in mycetoma, chromoblastomycosis, sporotrichosis, mucormycosis, superficial cutaneous mycoses, mycobacterial infections, leprosy, and cutaneous parasitoses).
REFERENCES
- 1.Forbes BY, Hall GS, Miller MB, Novak S, Rowlinson MC, Salfinger M, Somoskövi A, Warshauer DM, Wilson ML. 2018. Practice guidelines for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 31:e00038-17. doi: 10.1128/CMR.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Röltgen K, Stinear TP, Pluschke G. 2012. The genome, evolution and diversity of Mycobacterium ulcerans. Infect Genet Evol 12:522–529. doi: 10.1016/j.meegid.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Doig KD, Holt KE, Fyfe JAM, Lavender CJ, Eddyani M, Portaels F, Yeboah-Manu D, Pluschke G, Seeman T, Stinear TP. 2012. On the origin of Mycobacterium ulcerans, the causative agent of Buruli Ulcer. BMC Genomics 13:258. doi: 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco-Paredes C, Rodriguez-Morales AJ. 2016. Unsolved matters in leprosy: a descriptive review and call for further research. Ann Clin Microbiol Antimicrob 15:33. doi: 10.1186/s12941-016-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottai D, Stinear TP, Supply P, Brosch R. 2014. Mycobacterial pathogenomics and evolution. Microbiol Spectr 2:MGM2-0025-2013. doi: 10.1128/microbiolspec.MGM2-0025-2013. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Valero L, Rocha EPC, Latorre A, Silva F. 2007. Reconstructing the ancestor of Mycobacterium leprae: the dynamics of gene loss and genome reduction. Genome Res 17:1178–1185. doi: 10.1101/gr.6360207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trueba G, Dunthorn M. 2012. Many neglected tropical diseases may have originated in the Paleolithic or before: new insights from genetics. PLoS Negl Trop Dis 6:e1393. doi: 10.1371/journal.pntd.0001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monot M, Honoré N, Garnier T, Araoz R, Coppée JY, Lacroix C, Sow S, Spencer JS, Truman RW, Williams DL, Gelber R, Virmond M, Flageul B, Cho SN, Ji B, Paniz-Mondolfi A, Convit J, Young S, Fine PE, Rasolofo V, Brennan PJ, Cole ST. 2005. On the origin of leprosy. Science 308:1040–1042. doi: 10.1126/science/1109759. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Cole ST. 2011. Mycobacterium leprae: genes, pseudogenes, and genetic diversity. Future Microbiol 6:57–71. doi: 10.2217/fmb.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuenemann VJ, Singh P, Mendum TA, Krause-Kyora B, Jäger G, Bos KI, Herbig A, Economou C, Benjak A, Busso P, Nebel A, Boldsen JL, Kjellström A, Wu H, Stewart GR, Taylor GM, Bauer P, Lee OY, Wu HH, Minnikin DE, Besra GS, Tucker K, Roffey S, Sow SO, Cole ST, Nieselt K, Krause J. 2013. Genome-wide comparison of medieval and modern Mycobacterium leprae. Science 341:179–183. doi: 10.1126/science.1238286. [DOI] [PubMed] [Google Scholar]
- 11.Prasanna AN, Mehra S. 2013. Comparative phylogenomics of pathogenic and non-pathogenic Mycobacterium. PLoS One 8:e71248. doi: 10.1371/journal.pone.0071248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardona-Castro N, Cortes N, Cortés E, Beltrán C, Romero M, Badel-Mogollón JE, Bedoya G. 2015. Human genetic ancestral composition correlates with the origin of Mycobacterium leprae strains in a leprosy endemic population. PLoS Negl Trop Dis 9:e0004045. doi: 10.1371/journal.pntd.0004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman SA, Singh Y, Kohli S, Ahmad J, Ehtesham NZ, Tyagi AK, Hasnain SE. 2014. Comparative analyses of nonpathogenic, opportunistic, and totally pathogenic mycobacteria reveal genomic and biochemical variabilities and highlight the survival attributes of Mycobacterium tuberculosis. mBio 5:e02020-14. doi: 10.1128/mBio.02020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PDR, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PLC, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boritsch EC, Supply P, Honoré N, Seemann T, Seeman T, Stinear TP, Brosch R. 2014. A glimpse into the past and predictions for the future: the molecular evolution of the tuberculosis agent. Mol Microbiol 93:835–852. doi: 10.1111/mmi.12720. [DOI] [PubMed] [Google Scholar]
- 16.Mostowy S, Behr MA. 2005. The origin and evolution of Mycobacterium tuberculosis. Clin Chest Med 26:207–216. doi: 10.1016/j.ccm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Stone AC, Wilbur AK, Buikstra JE, Roberts CA. 2009. Tuberculosis and leprosy in perspective. Am J Phys Anthropol 140:66–94. doi: 10.1002/ajpa.21185. [DOI] [PubMed] [Google Scholar]
- 18.Marsollier L, Stinear T, Aubry J, Saint Andre JP, Robert R, Legras P, Manceau AL, Audrain C, Bourdon S, Kouakou H, Carbonnelle B. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol 70:1097–1103. doi: 10.1128/AEM.70.2.1097-1103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, Tichit M, Porter JL, Ryan J, Johnson PDR, Davies JK, Jenkin GA, Small PLC, Jones LM, Tekaia F, Laval F, Daffe M, Parkhill J, Cole ST. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res 17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown-Elliott BA, Wallace RJ Jr.. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkinham JO., III. 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 107:356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- 22.Falkinham JO., III. 2015. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Behr MA. 2008. Mycobacterium du jour: what’s on tomorrow’s menu? Microbes Infect 10:968–972. doi: 10.1016/j.micinf.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang SH, Pancholi P. 2014. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep 16:438. doi: 10.1007/s11908-014-0438-5. [DOI] [PubMed] [Google Scholar]
- 25.Wu U, Holland SM. 2015. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis 15:968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 26.Szymanski EP, Leung JM, Fowler CJ, Haney C, Hsu AP, Chen F, Duggal P, Oler AJ, McCormack R, Podack E, Drummond RA, Lionakis MS, Browne SK, Prevots DR, Knowles M, Cutting G, Liu X, Devine SE, Fraser CM, Tettelin H, Olivier KN, Holland SM. 2015. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am J Respir Crit Care Med 192:618–628. doi: 10.1164/rccm.201502-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu YJ, Koh WJ, Daley CL. 2016. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians’ perspectives. Tuberc Respir Dis (Seoul) 79:74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davey TF, Rees RJ. 1974. The nasal discharge in leprosy: clinical and bacteriological aspects. Lepr Rev 45:121–134. [DOI] [PubMed] [Google Scholar]
- 30.Job CK, Jayakumar J, Kearney M, Gillis TP. 2008. Transmission of leprosy: a study of skin and nasal secretions of household contacts of leprosy patients using PCR. Am J Trop Med Hyg 78:518–521. doi: 10.4269/ajtmh.2008.78.518. [DOI] [PubMed] [Google Scholar]
- 31.Merritt RW, Walker ED, Small PL, Wallace JR, Johnson PD, Benbow ME, Boakye DA. 2010. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis 4:e911. doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garchitorena A, Guégan JF, Léger L, Eyangoh S, Marsollier L, Roche B. 2015. Mycobacterium ulcerans dynamics in aquatic ecosystems are driven by a complex interplay of abiotic and biotic factors. Elife 4:e07616. doi: 10.7554/eLife.07616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace JR, Mangas KM, Porter JL, Marcsisin R, Pidot SJ, Howden B, Omansen TF, Zeng W, Axford JK, Johnson PDR, Stinear TP. 2017. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis 11:e0005553. doi: 10.1371/journal.pntd.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah MK, Sebti A, Kiehn TE, Massarella SA, Sepkowitz KA. 2001. Mycobacterium haemophilum in immunocompromised patients. Clin Infect Dis 33:330–337. doi: 10.1086/321894. [DOI] [PubMed] [Google Scholar]
- 35.Smith S, Taylor GD, Fanning EA. 2003. Chronic cutaneous Mycobacterium haemophilum infection acquired from coral injury. Clin Infect Dis 37:e100–e101. doi: 10.1086/377267. [DOI] [PubMed] [Google Scholar]
- 36.Lindeboom JA, Lesla E, Bruijnesteijn van C, Soolingen D, Prins JM, Kuijper EJ. 2011. Clinical manifestations, diagnosis, and treatment of Mycobacterium haemophilum infections. Clin Microbiol Rev 24:701–717. doi: 10.1128/CMR.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Feng M, He JQ. 2017. Disseminated Mycobacterium kansasii infection with cutaneous lesions in an immunocompetent patient. Int J Infect Dis 62:59–63. doi: 10.1016/j.ijid.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Delafont V, Mougari F, Cambau E, Joyeux M, Bouchon D, Héchard Y, Moulin L. 2014. First evidence of amoebae-mycobacteria association in drinking water network. Environ Sci Technol 48:11872–11882. doi: 10.1021/es5036255. [DOI] [PubMed] [Google Scholar]
- 39.Wheat WH, Casali AL, Thomas V, Spencer JS, Lahiri R, Williams DL, McDonnell GE, Gonzalez-Juarrero M, Brennan PJ, Jackson M. 2014. Long-term survival and virulence of Mycobacterium leprae in amoebal cysts. PLoS Negl Trop Dis 8:e3405. doi: 10.1371/journal.pntd.0003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahiri R, Krahenbuhl JL. 2008. The role of free-living pathogenic amoeba in the transmission of leprosy: a proof of principle. Lepr Rev 79:401–409. [PubMed] [Google Scholar]
- 41.Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, Rössle M, Falk V, Kuster SP, Böttger EC, Weber R. 2015. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis 61:67–75. doi: 10.1093/cid/civ198. [DOI] [PubMed] [Google Scholar]
- 42.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Disease Subcommittee, American Thoracic Society, Infectious Diseases Society of America . 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 43.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aitken ML, Limaye A, Pottinger P, Whimbey E, Goss CH, Tonelli MR, Cangelosi GA, Dirac MA, Olivier KN, Brown-Elliott BA, McNulty S, Wallace RJ. Jr, 2012. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. Am J Respir Crit Care Med 185:231–232. doi: 10.1164/ajrccm.185.2.231. [DOI] [PubMed] [Google Scholar]
- 45.Turankar RP, Lavania M, Singh M, Siva Sai KSR, Jadhav RS. 2012. Dynamics of Mycobacterium leprae transmission in environmental context: deciphering the role of environment as potential reservoir. Infect Gen Evol 12:121–126. doi: 10.1016/j.meegid.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Araujo S, Freitas LO, Goulart LR, Goulart IMB. 2016. Molecular evidence for the aerial route of infection of Mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Clin Infect Dis 63:1412–1420. doi: 10.1093/cid/ciw570. [DOI] [PubMed] [Google Scholar]
- 47.Scollard DM, Dacso MM, Abad-Venida ML. 2015. Tuberculosis and leprosy. Classical granulomatous diseases in the twenty-first century. Dermatol Clin 33:541–562. doi: 10.1016/j.det.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Goulart IM, Araujo S, Filho AB, de Paiva PH, Goulart LR. 2015. Asymptomatic leprosy infection among blood donors may predict disease development and suggest a potential mode of transmission. J Clin Microbiol 53:3345–3348. doi: 10.1128/JCM.01305-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sreevatsa 1993. Leprosy and arthropods. Indian J Lepr 65:189–200. [PubMed] [Google Scholar]
- 50.Matsuoka M, Izumi S, Budiawan T, Nakata N, Saeki K. 1999. Mycobacterium leprae DNA in daily using water as a possible source of leprosy infection. Indian J Lepr 71:61–67. [PubMed] [Google Scholar]
- 51.Truman RW, Singh P, Sharma R, Busso P, Rougemont J, Paniz-Mondolfi A, Kapopoulou A, Brisse S, Scollard DM, Gillis TP, Cole ST. 2011. Probable zoonotic leprosy in the southern United States. N Engl J Med 364:1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balamayooran G, Pena M, Sharma R, Truman RW. 2015. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin Dermatol 33:108–115. doi: 10.1016/j.clindermatol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Avanzi C, del-Pozo J, Benjak A, Stevenson K, Simpson VR, Busso P, McLuckie J, Loiseau C, Lawton C, Schoening J, Shaw DJ, Piton J, Vera-Cabrera L, Velarde-Felix JS, McDermott F, Gordon SV, Cole ST, Meredith AL. 2016. Red squirrels in the British Isles are infected with leprosy bacilli. Science 354:744–747. doi: 10.1126/science.aah3783. [DOI] [PubMed] [Google Scholar]
- 54.Malik R, Hughes MS, James G, Martin P, Wigney DI, Canfield PJ, Chen SC, Mitchell DH, Love DN. 2002. Feline leprosy: two different clinical syndromes. J Feline Med Surg 4:43–59. doi: 10.1053/jfms.2001.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardona-Castro N, Beltran JC, Ortiz-Bernal A, Vissa V. 2009. Detection of Mycobacterium leprae DNA in nine-banded armadillos (Dasypus novemcinctus) from the Andean region of Colombia. Lepr Rev 80:424–431. [PubMed] [Google Scholar]
- 56.da Silva MB, Portela JM, Li W, Jackson M, Gonzalez-Juarrero M, Hidalgo AS, Belisle JT, Bouth RC, Gobbo A, Barreto JG, Minervino AHH, Cole ST, Avanzi C, Busso P, Frade MAC, Geluk A, Salgado CG, Spencer JS. 2018. Evidence of zoonotic leprosy in Para, Brazilian Amazon, and risks associated with human contact or consumption of armadillos. PLoS Negl Trop Dis 12:e0006532. doi: 10.1371/journal.pntd.0006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Job CK, Harris EB, Allen JL, Hastings RC. 1986. Thorns in armadillo ears and noses and their role in the transmission of leprosy. Arch Pathol Lab Med 110:1025–1028. [PubMed] [Google Scholar]
- 58.Dungal N. 1961. Is leprosy transmitted by arthropods? Lepr Rev 32:28–35. [DOI] [PubMed] [Google Scholar]
- 59.Narayanan E, Sreevatsa KWF, Bedi BMS. 1977. Transfer of leprosy bacilli from patients to mouse foot pads by Aedes aegypti. Lepr India 49:181–186. [PubMed] [Google Scholar]
- 60.Narayanan E, Sreevatsa RAD, Kirchheimer WF, Bedi BMS. 1978. Persistence and distribution of M. leprae in Aedes aegyptii and Culex fatigans experimentally fed on leprosy patients. Lepr India 50:26–36. [PubMed] [Google Scholar]
- 61.Narayanan E, Manja KS, Bedi BM, Kirchheimer WF, Balasubrahmanyan M. 1972. Arthropod feeding experiment in lepromatous leprosy. Lepr Rev 43:188–193. [PubMed] [Google Scholar]
- 62.Kirchheimer WF. 1976. The role of arthropods in the transmision of leprosy. Int J Lepr Other Mycobact Dis 44:104–107. [PubMed] [Google Scholar]
- 63.Joshua V, Mehendale S, Gupte MD. 2016. Bayesian model, ecologial factors & transmission of leprosy in an endemic area of south India. Indian J Med Res 143:104–106. doi: 10.4103/0971-5916.178618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravo FG, Gotuzzo E. 2007. Cutaneous tuberculosis. Clin Dermatol 25:173–180. doi: 10.1016/j.clindermatol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Handog EB, Gabriel TG, Pineda RT. 2008. Management of cutaneous tuberculosis. Dermatol Ther 21:154–161. doi: 10.1111/j.1529-8019.2008.00186.x. [DOI] [PubMed] [Google Scholar]
- 66.MacGregor RR. 1995. Cutaneous tuberculosis. Clin Dermatol 13:245–255. doi: 10.1016/0738-081X(95)00019-C. [DOI] [PubMed] [Google Scholar]
- 67.Semaan R, Traboulsi R, Kanj S. 2008. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis 12:472–477. doi: 10.1016/j.ijid.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Kivanç-Altunay l, Baysal Z, Ekmekçi TR, Köslü A. 2003. Incidence of cutaneous tuberculosis in patients with organ tuberculosis. Int J Dermatol 42:197–200. doi: 10.1046/j.1365-4362.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 69.Van Zyl L, Du Plessi J, Viljoen J. 2015. Cutaneous tuberculosis overview and current treatment strategies. Tuberculosis 95:629–638. doi: 10.1016/j.tube.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Yew WW, Lee J. 1995. Pathogenesis of cervical tuberculous lymphadenitis: pathways to anatomic localization. Tuber Lung Dis 76:275–276. doi: 10.1016/S0962-8479(05)80019-X. [DOI] [PubMed] [Google Scholar]
- 71.Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. 2002. Cutaneous tuberculosis: diagnosis and treatment. Am J Clin Dermatol 3:319–328. doi: 10.2165/00128071-200203050-00004. [DOI] [PubMed] [Google Scholar]
- 72.Britton WJ, Lockwood DNJ. 2004. Leprosy. Lancet 363:1209–1219. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 73.Lai CC, Tan CK, Lin SH, Liu WL, Liao CH, Huang YT, Hsueh PR. 2011. Diagnostic value of an enzyme-linked immunospot assay for interferon-γ in cutaneous tuberculosis. Diagn Microbiol Infect Dis 70:60–64. doi: 10.1016/j.diagmicrobio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 74.Walker SL, Lockwood DNJ. 2007. Leprosy. Clin Dermatol 25:165–172. doi: 10.1016/j.clindermatol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 75.Lockwood DNJ, Shetty V, Penna GO. 2014. Hazards of setting targets to eliminate disease: lessons from the leprosy elimination campaign. Br Med J 348:g1136. doi: 10.1136/bmj.g1136. [DOI] [PubMed] [Google Scholar]
- 76.Smith WC, van Brakel W, Gillis T, Saunderson P, Richardus JH. 2015. The missing millions: a threat to the elimination of leprosy. PLoS Negl Trop Dis 9:e0003658. doi: 10.1371/journal.pntd.0003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White C. 2011. Leprosy and stigma in the context of international migration. Lepr Rev 82:147–154. [PubMed] [Google Scholar]
- 78.White C, Franco-Paredes C. 2015. Leprosy in the 21st century. Clin Microbiol Rev 28:80–94. doi: 10.1128/CMR.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blake LA, West BC, Lary CH, Todd JRIV. 1987. Environmental nonhuman sources of leprosy. Rev Infect Dis 9:561–577. [DOI] [PubMed] [Google Scholar]
- 80.Kerr-Pontes LRS, Barreto ML, Evangelist CMN, Rodrigues LC, Heukelbach J, Feldmeier H. 2006. Socioeconomic, environmental, and behavioural risk factors for leprosy in Northeast Brazil: results of a case-control study. Int J Epidemiol 35:994–1000. doi: 10.1093/ije/dyl072. [DOI] [PubMed] [Google Scholar]
- 81.Kerr-Pontes LRS, Montenegro ACD, Barreto ML, Werneck GL, Feldmeier H. 2004. Inequality and leprosy in Northeast Brazil: an ecological study. Int J Epidemiol 33:262–269. doi: 10.1093/ije/dyh002. [DOI] [PubMed] [Google Scholar]
- 82.Lockwood DNJ. 2004. Commentary: leprosy and poverty. Int J Epidemiol 33:269–270. doi: 10.1093/ije/dyh115. [DOI] [PubMed] [Google Scholar]
- 83.Polycarpou A, Walker SL, Lockwood DN. 2013. New findings in the pathogenesis of leprosy and implications for the management of leprosy. Curr Opin Infect Dis 26:413–419. doi: 10.1097/QCO.0b013e3283638b04. [DOI] [PubMed] [Google Scholar]
- 84.Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. 2013. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell 152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leon KE, Jacob JT, Franco-Paredes C, Kozarsky PE, Wu HM, Fairley JK. 2016. Delayed diagnosis, leprosy reactions, and nerve injury among individuals with Hansen’s disease seen at a United States clinic. Open Forum Infect Dis 3:ofw063. doi: 10.1093/ofid/ofw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scollard DM, Truman RW, Ebenezer GJ. 2015. Mechanisms of nerve injury in leprosy. Clin Dermatol 33:46–54. doi: 10.1016/j.clindermatol.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Scollard DM. 2008. The biology of nerve injury in leprosy. Lepr Rev 79:242–253. [PubMed] [Google Scholar]
- 88.Madigan CA, Cambier CJ, Kelly-Scumpia KM, Scumpia PO, Cheng T-Y, Zailaa J, Bloom BR, Moody DB, Smale ST, Sagasti A, Modlin RL, Ramakrishnan L. 2017. A macrophage response to Mycobacterium leprae phenolic glycolipid initiates nerve damage in leprosy. Cell 170:973–985. doi: 10.1016/j.cell.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teles RMB, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, Sarno EN, Rea TH, Hewison M, Adams JS, Popper SJ, Relman DA, Stenger S, Bloom BR, Cheng G, Modlin RL. 2013. Type I interferon suppresses type II interferon-triggered human antimycobacterial responses. Science 339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Brakel WH, Nicholls PG, Wilder-Smith EP, Das L, Barkataki P, Lockwood DN, INFIR Study Group . 2008. Early diagnosis of neuropathy in leprosy—comparing diagnostic tests in a large prospective study (the INFIR cohort study). PLoS Negl Trop Dis 2:e212. doi: 10.1371/journal.pntd.0000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacob J, Kozarsky P, Dismukes R, Bynoe V, Margoles L, Leonard M, Tellez I, Franco-Paredes C. 2008. Five-year experience with type 1 and type 2 reactions in Hansen’s disease at a U.S. travel clinic. Am J Trop Med Hyg 79:452–454. doi: 10.4269/ajtmh.2008.79.452. [DOI] [PubMed] [Google Scholar]
- 92.Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, Lockwood DNJ. 2006. Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg 74:868–879. doi: 10.4269/ajtmh.2006.74.868. [DOI] [PubMed] [Google Scholar]
- 93.Bratschi MW, Steinmann P, Wickenden A, Gillis TP. 2015. Current knowledge on Mycobacterium leprae transmission: a systematic literature review. Lepr Rev 86:142–155. [PubMed] [Google Scholar]
- 94.John TJ, Dandona L, Sharma VP, Kakkar M. 2011. Continuing challenge of infectious diseases in India. Lancet 377:252–269. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 95.Mensah-Awere D, Bratschi MW, Steinmann P, Fairley JK, Gillis TP. 2015. Developing strategies to block the transmission of leprosy. Lepr Rev 86:156–164. [PubMed] [Google Scholar]
- 96.Lockwood DNJ, Suneetha S. 2005. Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ 83:230–235. doi: 10.1590/S0042-96862005000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith CS, Noordeen SK, Richardus JH, Sansarricq H, Cole ST, Soares RC, Savioli L, Aerts A, Aertsh A, Baruaf S. 2014. A strategy to halt leprosy transmission. Lancet Infect Dis 14:96–97. doi: 10.1016/S1473-3099(13)70365-7. [DOI] [PubMed] [Google Scholar]
- 98.Barreto JG, Bisanzio D, Frade MA, Moraes TM, Gobbo AR, de Souza Guimarães L, da Silva MB, Vazquez-Prokopec GM, Spencer JS, Kitron U, Salgado CG. 2015. Spatial epidemiology and serologic cohorts increase the early detection of leprosy. BMC Infect Dis 15:527. doi: 10.1186/s12879-015-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cambau E, Saunderson P, Matsuoka M, Cole ST, Kai M, Suffys P, Rosa PS, Williams D, Gupta UD, Lavania M, Cardona-Castro N, Miyamoto Y, Hagge D, Srikantam A, Hongseng W, Indropo A, Vissa V, Johnson RC, Cauchoix B, Pannikar VK, Cooreman EAWD, Pemmaraju VRR, Gillini L, WHO Surveillance Network of Antimicrobial Resistance in Leprosy . 2018. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009-15. Clin Microb Infect. doi: 10.1016/j.cmi.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, Li W, Nair RG. 2008. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol 130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 101.Vargas-Ocampo F. 2007. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev 78:248–260. [PubMed] [Google Scholar]
- 102.Sotiriou MC, Stryjewska BM, Hill C. 2016. Case report: two cases of leprosy in siblings caused by Mycobacterium lepromatosis and review of the literature. Am J Trop Med Hyg 95:522–527. doi: 10.4269/ajtmh.16-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han XY, Aung FM, Choon S-E, Werner B. 2014. Analysis of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis in four countries. Am J Clin Pathol 142:524–532. doi: 10.1309/AJCP1GLCBE5CDZRM. [DOI] [PubMed] [Google Scholar]
- 104.Han XY, Mistry NA, Thompson EJ, Tang HL, Khanna K, Zhang L. 2015. Draft genome sequence of new leprosy agent Mycobacterium lepromatosis. Genome Announc 3:e00513–e00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, Nieselt K, Krause J, Vera-Cabrera L, Cole ST. 2015. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci U S A 112:4459–4464. doi: 10.1073/pnas.1421504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han XY, Sizer KC, Thompson EJ, Kabanja J, Li J, Hu P, Gómez-Valero L, Silva FJ. 2009. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J Bacteriol 191:6067–6074. doi: 10.1128/JB.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han XY, Jessurun J. 2013. Severe leprosy reactions due to Mycobacterium lepromatosis. Am J Med Sci 345:65–69. doi: 10.1097/MAJ.0b013e31826af5fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han XY, Sizer KC, Velarde-Felix JS, Frias Castro LO, Vargas-Ocampo F. 2012. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol 51:952–959. doi: 10.1111/j.1365-4632.2011.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yotsu RR, Murase C, Sugawara M, Suzuki K, Nakanaga K, Ishii N, Asiedu K. 2015. Revisiting Buruli ulcer. J Dermatol 42:1033–1041. doi: 10.1111/1346-8138.13049. [DOI] [PubMed] [Google Scholar]
- 110.Alsop DG. 1972. The bairnsdale ulcer. Aust N Z J Surg 41:317–319. doi: 10.1111/j.1445-2197.1969.tb06535.x. [DOI] [PubMed] [Google Scholar]
- 111.MacCallum P, Tolhurst JC, Buckle G, Sissons HA. 1948. A new mycobacterial infection in man. J Pathol Bacteriol 60:93–122. doi: 10.1002/path.1700600111. [DOI] [PubMed] [Google Scholar]
- 112.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass MK, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimycobacterial treatment for early, limited Mycobacterium ulcerans infection: a randomized controlled trial. Lancet 375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 113.Pidot SJ, Asiedu K, K Ser M, Fyfe JAM, Stinear TP. 2010. Mycobacterium ulcerans and other mycolactone-producing mycobacteria should be considered a single species. PLoS Negl Trop Dis 4:e663. doi: 10.1371/journal.pntd.0000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hall BS, Hill K, McKenna M, Ogbechi J, High S, Willis AE, Simmonds RE. 2014. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog 10:e1004061. doi: 10.1371/journal.ppat.1004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marion E, Song O-R, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guérineau NC, Saint André J-P, Gersbach P, Altmann K-H, Stinear TP, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. 2014. Mycobacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell 157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 116.Henkle E, Winthrop KL. 2015. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med 36:91–96. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. 2016. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti-Infect Ther 14:1139–1154. doi: 10.1080/14787210.2016.1238304. [DOI] [PubMed] [Google Scholar]
- 118.Runyon EH. 1959. Anonymous mycobacteria in pulmonary disease. Med Clin North Am 43:273–290. doi: 10.1016/S0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 119.Tan HH, Tan A, Theng CC, Ng SK. 2004. Cutaneous Mycobacterium haemophilum infections in immunocompromised patients in a dermatology clinic in Singapore. Ann Acad Med Singapore 33:532–536. [PubMed] [Google Scholar]
- 120.Aubry A, Mougari F, Reibel F, Cambau E. 2017. Mycobacterium marinum. Microbiol Spectr 5 doi: 10.1128/microbiolspec.TNMI7-0038-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou L, Wang HS, Feng SY, Wang QL. 2013. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venerol 93:711–714. doi: 10.2340/00015555-1595. [DOI] [PubMed] [Google Scholar]
- 122.Vedvyas C, Shvartsbeyn M, Brinster N, Femia A. 2015. Mycobacterium avium complex infection. Dermatol Online J 21:16. [PubMed] [Google Scholar]
- 123.Rahmani M, Alroy J, Zoukhri D, Wein RO, Tischler AS. 2013. Mycobacterial pseudotumor of the skin. Virchows Arch 463:843–846. doi: 10.1007/s00428-013-1491-4. [DOI] [PubMed] [Google Scholar]
- 124.Cox SK, Strausbaugh LJ. 1981. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol 117:794–796. doi: 10.1001/archderm.1981.01650120040019. [DOI] [PubMed] [Google Scholar]
- 125.Allen KB, Yuh DD, Schwartz SB, Schwartz SB, Lange RA, Hopkins R, Bauer K, Marders JA, Delgado Donayre J, Milligan N, Wentz C. 2017. Nontuberculous Mycobacterium infections associated with heater-cooler devices. Ann Thorac Surg 104:1237–1242. doi: 10.1016/j.athoracsur.2017.04.067. [DOI] [PubMed] [Google Scholar]
- 126.Kaplan R, Gonzalez E, Rebolledo P, Franco-Paredes C. 2007. An unexpected visitor. Am J Med 120:415–416. doi: 10.1016/j.amjmed.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 127.Henao-Martinez A, Olague N, Franco-Paredes C. 2018. Spontaneous Mycobacterium bolletti skin abscesses—an underrecognized zoonsois from raw bovine milk. Travel Med Infect Dis Epub 2018 Jan 31. doi: 10.1016/j.tmaid.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 128.Vargas J, Gamboa C, Negrin D, Correa M, Sandoval C, Aguiar A, Prieto M, Rodriguez-Morales AJ, De Waard J, Yakrus M. 2005. Disseminated Mycobacterium mucogenicum infection in a patient with idiopathic CD4+ T lymphocytopenia manifesting as fever of unknown origin. Clin Infect Dis 41:759–760. doi: 10.1086/432622. [DOI] [PubMed] [Google Scholar]