Highlights
-
•
Transcriptional feedback is insufficient to account for circadian rhythm generation.
-
•
Post-translational regulators of daily cellular clocks are conserved among eukaryotes.
-
•
Eukaryotic circadian timekeeping may result from divergent evolution.
Abstract
‘Biological clocks’ orchestrate mammalian biology to a daily rhythm. Whilst ‘clock gene’ transcriptional circuits impart rhythmic regulation to myriad cellular systems, our picture of the biochemical mechanisms that determine their circadian (∼24 hour) period is incomplete. Here we consider the evidence supporting different models for circadian rhythm generation in mammalian cells in light of evolutionary factors. We find it plausible that the circadian timekeeping mechanism in mammalian cells is primarily protein-based, signalling biological timing information to the nucleus by the post-translational regulation of transcription factor activity, with transcriptional feedback imparting robustness to the oscillation via hysteresis. We conclude by suggesting experiments that might distinguish this model from competing paradigms.
Current Opinion in Physiology 2018, 5:117–132
This review comes from a themed issue on Circadian rhythms
Edited by Martin Young, Karen Gamble and Hugh Piggins
For a complete overview see the Issue and the Editorial
Available online 6th November 2018
https://doi.org/10.1016/j.cophys.2018.10.003
2468-8673/© 2018 MRC Laboratory of Molecular Biology. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction: the utility of biological timekeeping at multiple levels of organisation
Life consists of the spatiotemporal organisation of chemical processes in order to maintain homeostasis. As the Earth rotates, organisms encounter daily variations in environmental factors such as light, temperature and food availability. Most organisms have developed internal daily timing systems that allow them to reliably anticipate and adapt to these external cycles, conferring a competitive advantage over organisms that do not [1, 2, 3, 4, 5, 6, 7]. The persistence of circadian (about daily) rhythms when organisms are removed from external stimuli demonstrates their endogenous nature.
The history of circadian research begins with Jean-Jacques de Mairan’s fascinating observation in 1729 [8], that the leaves of Mimosa pudica open and close on a daily basis, and that this biological rhythm persists in constant darkness; this shows that the behaviour is under the control of an endogenous circadian timekeeping mechanism — a daily ‘biological clock’. Subsequently the circadian organisation of very many biological activities has been observed, from the eclosion activity of Drosophila pseudoobscura to the wheel running activity of Mus musculus [8]. Colin Pittendrigh, a founding figure in chronobiology [9], laid out a set of ‘Empirical Generalisations’ at the 1960 Cold Spring Harbour Symposium on Quantitative Biology [10] that define the fundamental characteristics of a circadian rhythm: 1) An endogenous oscillation with an approximately 24 hour period under constant conditions; 2) Period length is temperature-compensated (temperature coefficient, Q10, of 0.9–1.2); 3) The phase of oscillation is entrained by (i.e. synchronised with) environmental stimuli that themselves normally change with 24-hour periodicity.
In mammals, the hypothalamic suprachiasmatic nucleus (SCN) acts as ‘master pacemaker’, coordinating the circadian organisation of behaviour, physiology and cellular functions by neuronal and hormonal means [11, 12, 13, 14, 15, 16, 17, 18]. However, it has been clear for more than 10 years that the fundamental unit of circadian timekeeping is the cell, since circadian regulation of gene expression and cellular activity persists ex vivo in almost all mammalian cell types tested [19, 20, 21, 22]. Data from mouse models show that homozygous mutations that impair cell-autonomous circadian regulation of transcription in cultured cells also impair circadian organisation at higher levels of organismal biology [23,24,25•,26]. This suggests that circadian organisation of rest-activity cycles and overt physiology is driven by the rhythmic regulation of gene expression on a cellular level.
Cell-autonomous circadian timekeeping also extends to the green and fungal eukaryotic lineages [27]. Thus, whilst the translational and economic consequences of circadian rhythms and their disruption have been primarily studied in multicellular organisms, it is necessary to look towards the molecular biology of the cell when considering the fundamental mechanisms that drive this 24-hour regulation at multiple levels of biological scale. This review will therefore focus on the molecular determinants of cell-autonomous circadian timekeeping.
Transcription/translation feedback facilitates rhythmic gene expression
In healthy young wild type mice, 43% of protein-encoding genes in the genome are subject to circadian regulation in at least one tissue [28], with available data from humans suggesting that similar proportions of our own genomes are subject to diurnal regulation in healthy individuals [29•]. Circadian regulation of gene expression in mammalian and other eukaryotic cells has been proposed to rely upon delayed transcriptional feedback repression at a handful of genomic loci (so-called ‘clock genes’), which auto-regulate their own expression, as well as that of many ‘clock-controlled genes’ that vary between tissues [27,30,31]. From the context in which it is most often used, the term ‘clock gene’ generally refers to a gene whose expression contributes to the fidelity of circadian timekeeping, and whose gene product directly or indirectly represses its own activity to elicit a ∼24 hour oscillation in its abundance and/or activity [32]. The clock gene circuitry that facilitates these delayed transcriptional-translational feedback loops (TTFLs) has been successfully delineated for several model organisms within each eukaryotic kingdom [27,33], with current understanding of the mammalian TTFL summarised in an excellent recent review [34•]. Whilst in general there exists a fairly poor correlation between the identities, relative amplitude and waveform of rhythmically abundant mRNA transcripts compared with their encoded proteins [35, 36, 37, 38, 39, 40], daily oscillations in clock gene transcription occur in all mammalian tissues in vivo [28]. Combined with the strong phenotype of clock gene loss or gain-of-function mutations therefore, there exists overwhelming evidence for the idea that temporal coordination of gene expression programs by the cycling activity of clock proteins is the ultimate means by which cellular clocks organise most daily biological functions [41]. To provide context to the non-transcriptional aspects of circadian timekeeping, we will briefly describe the main TTFL features that facilitate circadian gene expression rhythms in mammalian cells.
The transcription factors BMAL1 and CLOCK are heterodimeric partners, belonging to the basic helix-loop-helix (bHLH) – PER-ARNT-SIM (PAS) transcription factor family [42, 43, 44]. Whilst CLOCK functions semi-redundantly with NPAS2, the recruitment of BMAL1-containing complexes to promoter/enhancer regions of the Period genes (encoding transcriptional repressors PER1, 2, 3) and Cryptochrome genes (encoding obligate co-repressors CRY1, 2) appears to be essential for their differential expression over the daily cycle. PER and CRY proteins assemble into large macromolecular complexes to mediate the repressive limb of the feedback loop, rhythmically binding to and inhibiting the transcriptional activity of BMAL1-containing complexes [45•,46,47,48]. This represses transcription from their own genomic loci as well as many clock-controlled genes [49,50]. In recent years it has become clear that this model is much simpler than the reality, in that each ‘clock gene’ has many additional functions beyond circadian regulation [51,52•,53,54, 55, 56, 57, 58]. Moreover the activating and repressive transcriptional complexes do not operate in isolation, rather they function in tandem with enhancer elements to effect the differential recruitment of assorted histone modifying and chromatin remodelling complexes, resulting in large-scale tissue-specific changes in chromatin compaction and accessibility [34•,50,59,60•,61•].
The broader rhythmic regulation of gene expression programs is elaborated by several auxiliary feedback mechanisms, whose relative contribution to cellular clock function likely varies between tissues [26,62]. For example, CLOCK/BMAL1 complexes activate the transcription of nuclear receptors REV-ERBα (Nr1d1) and REV-ERBβ (Nr1d2), which mediate feedback repression of Bmal1 and other targets via ROR-binding promoter elements [63]. A further transcriptional feedback loop involves the activating transcription factor DBP (D-box binding protein), whose rhythmic transcription is dependent on CLOCK/BMAL1 activity [64,65]. DBP binds to D-box promoter elements, antagonising the repressor NFIL3 (nuclear factor, interleukin-3 regulated), which is regulated by REV-ERB-ROR loop activity [66,67]. These auxiliary loops integrate with the central TTFL to fine-tune the amplitude and phase of downstream clock-controlled gene expression [68], but they are not essential for rhythms of the central TTFL circuit [26]. Finally, as we will discuss later, the activities of many rhythmically regulated cellular processes (outputs) also feedback to modulate the fidelity of circadian timekeeping, further increasing the apparent complexity of the cellular clockwork (Figure 1a).
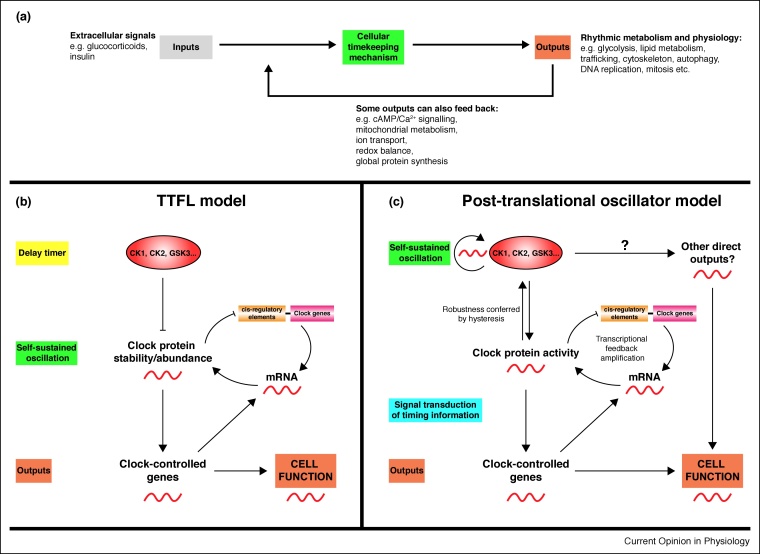
Figure 1.
Models for circadian rhythm generation.
(a) Biological clocks consist of a timekeeping mechanism whose phase, period and amplitude may be regulated by various inputs. Outputs of the clock control rhythmic metabolism, physiology and behaviour of organisms, and some of these may in turn feedback to modulate the timekeeping mechanism itself, thus also acting as inputs. Rhythmic input regulation is not essential for circadian rhythm generation however. (b) TTFL-based model for cellular circadian timekeeping: the TTFL itself can sustain oscillations, with the 24-hour period conferred by a post-translational delay-timer. The clock-controlled genes are direct outputs of the TTFL, and result in rhythmic cell function. Some of these outputs can feed back to regulate TTFL function. (c) Post-translational model for cellular circadian timekeeping: a self-sustained post-translational timekeeping mechanism is sufficient to sustain ∼24h rhythms in enzyme activity. The TTFL acts as a signal transducer, receiving timing information from this biochemical oscillation through post-translational modification of TTFL components, to differentially regulate transcription of clock and clock-controlled genes. The TTFL may confer robustness upon the post-translational oscillation by amplifying timing information and also by differentially regulating the activity of the enzymes that post-translationally modify clock proteins.
The explicit molecular mechanism that determines the 24-hour periodicity of clock gene expression cycles in mammalian cells is not understood. TTFLs are common in cell biology, and represent a common means by which cells terminate a signalling process and return to baseline. These work by the negative feedback of immediate-early gene products that induce a change in gene expression whilst simultaneously sowing the seeds for their own destruction [69]. Such gene expression feedback loops endow cell-autonomous oscillations upon the ERK signalling pathway (2–3 hour) [70,71], NF-κB pathway (3–4 hour) [72], and the developmental segmentation clock (∼1–2 hour) [73,74], with period lengths reflecting the time taken for RNA processing and translation, and oscillations that damp out after several cycles (unlike circadian oscillations) [20,75]. In the context of the TTFL whose components co-ordinate mammalian circadian gene expression rhythms, the summed time constants associated with transcriptional feedback are insufficient to account for the day-long interval between transcriptional activation of the Per and Cry genes and the disassembly of their repressive complexes. For this reason, and supported by strong genetic evidence [76, 77, 78, 79, 80, 81, 82], post-translational1 regulation of clock protein activity by casein kinases and other enzymes is invariably invoked to accommodate the lengthy delay that occurs with each circadian cycle (Figure 1b).
Layers of complexity beyond transcriptional feedback loops
In mouse tissues in vivo [37,39,40,83,84], in SCN slices ex vivo [85] and in cultured fibroblasts in vitro [35], roughly 15% of detected proteins exhibited significant circadian abundance rhythms. Such hypothesis-free proteomic studies have revealed a surprising discrepancy between cycling proteins and their mRNAs. Almost half of cycling proteins in mouse tissues do not have a corresponding cycling transcript, as shown in liver and SCN by a two-dimensional difference gel electrophoresis (2D-DIGE) approach [39,85], and recapitulated in cultured Drosophila Schneider 2 (S2) cells where only 7% of detected rhythmic proteins had a corresponding cycling mRNA [86]. This indicates the major role that post-transcriptional processes must play in the regulation of proteins whose abundance oscillates over circadian timescales.
Along these lines, Robles et al. revealed that half of the cycling proteome was delayed by more than 6 hours with respect to the corresponding mRNA profile, and that 20% of the cycling proteome did not have correspondingly cycling transcripts [40], with others reporting similar findings under diurnal conditions [37]. Moreover, recent technical innovations in mass spectrometry have permitted the detection of time-of day variations in the post-translational modifications of purified Neurospora FREQUENCY (FRQ) protein [87], and in mouse liver phosphoproteome and acetylome [88•,89], with as great as 25% of the 20,000 detected phospho-sites showing circadian rhythms. In many contexts, reversible allosteric and post-translational regulation of protein activity allows more efficient regulation of cellular function than do changes in gene expression. For example, glycolytic enzymes have such high expression levels and turnover numbers that their abundance is rarely rate-limiting; and so metabolic flux is regulated primarily allosterically and by covalent modification [90,91]. Therefore, these innovative new circadian post-translational “-omics” studies all point towards a critical participation of post-translational regulation in the circadian control of cell function. Put simply, circadian transcriptional rhythms may be insufficient to fully account for the daily regulation of cellular processes.
Discrepancies between the TTFL model and experimental observations
Indeed, it is not clear that rhythmic expression of central TTFL components is actually essential for circadian timekeeping to persist at the cellular level. For gene expression rhythms to be observed in cultured mammalian cells, all that seems to be absolutely required is that the activity of each central TTFL component BMAL1 (or paralog BMAL2), CLOCK (or paralog NPAS2), PER1 or PER2, and CRY1 or CRY2 reside within a permissive range [92,93,94,95•,96,97]. Consistent with this, circadian transcriptional rhythms are not observed in cells cultured from mice that are deficient for Bmal1, or both Cry genes, or both Per1 and Per2. Critically though, in complementation assays, constitutive expression of BMAL1 or CRY1 or PER2 in their respective single or double knockout backgrounds is sufficient to rescue circadian gene expression rhythms [26,97,98,99•,100]. When the repressor clock proteins CRY1 or PER2 are instead-over expressed under strong constitutive promoters, in Cry- or Per-deficient backgrounds respectively, rhythmic transcription is again severely impaired [97,101]. Similarly, constitutive expression of the essential repressors (per or tim) of the central TTFL of the clock in Drosophila melanogaster is sufficient to facilitate circadian rhythms, whilst overexpression or knockout of either gene abolishes rhythms in behaviour [102]. In addition, recent experiments using the fungal clock model, Neurospora crassa, showed that circadian rhythms in the activity of FRQ (the major TTFL repressor protein in Neurospora, functionally equivalent to PER) could be decoupled from its stability by deletion of the F-box protein (FWD1) that normally facilitates its degradation, thus allowing FRQ to accumulate to constant levels but with persistent activity rhythms [103]. These lines of evidence support the possibility that rhythmic clock protein activity is the key determinant of circadian transcriptional oscillations, with transcriptional feedback reinforcing clock protein activity rhythms rather than generating them. Certainly, the elegant reverse genetics findings described above suggest that the critical transcription factors of circadian TTFLs simply need to be expressed within an appropriate concentration range in order for activity rhythms to be observed.
Post-translational models for circadian rhythm generation
Following from this, if rhythmic clock protein activity, rather than abundance, is the essential prerequisite for circadian recruitment of downstream transcriptional programs, it is plausible that loss-of-function mutations in central and auxiliary TTFL components are simply epistatic to the expression of most cellular circadian rhythms [58,104,105]. In this case, an alternative model for the causal relationships underlying circadian regulation of gene expression suggests itself: that post-translational modifications of clock proteins are necessary and sufficient to confer circadian regulation to their activity (Figure 1c). Since most clock proteins auto-regulate their own production, and because any mRNA or protein with a circadian rhythm of production will exhibit rhythmic abundance if its half-life is less than 6 h [106], rhythmic clock protein activity will inevitably generate rhythms in the transcript and protein levels of most clock genes (e.g. Per2 and PER2 half-lives are <3 hour) [107, 108, 109]. In this alternative model, rather than determining its period of oscillation, the utility of transcriptional feedback repression in the cellular circadian clock is primarily to amplify post-translationally derived oscillations in clock protein activity through oscillations in clock protein abundance, effectively increasing the ‘gain’ of the transcriptional output (Figure 1c). Amplifying rhythms in clock protein activity through rhythms in clock protein abundance confers the intrinsic advantage of increased scope for phase-dependent differential recruitment of downstream clock-controlled gene expression programmes. In other words, TTFLs are required for high amplitude clock-controlled gene expression, rather than circadian rhythm generation itself. In turn, this predicts that amino acid substitutions that affect the activity of clock proteins will have more marked effects on the amplitude and period of gene expression rhythms than those which affect their abundance, until this latter exceeds or falls below their permissive concentration range [25•,103].
Extending this argument, if circadian-regulated transcription factors, such as PER, are considered as executive signal transducers rather than rhythm generators (Figure 1c), the critical advantage of a post-translational timekeeping mechanism would be the scope for hysteresis. Hysteresis describes the phenomenon whereby the activity of regulatory complexes is a function of not only their current condition (e.g. phosphorylation status) but also their history due to changes in constituent protein abundance (i.e. due to changes in transcription/translation/degradation) [110]. This requires bi-directional regulation between TTFL and post-translational timekeeping mechanisms, and indeed the reciprocal regulation of activity between PER2 and one of its post-translational regulators, CK1ε, was recently reported [111].
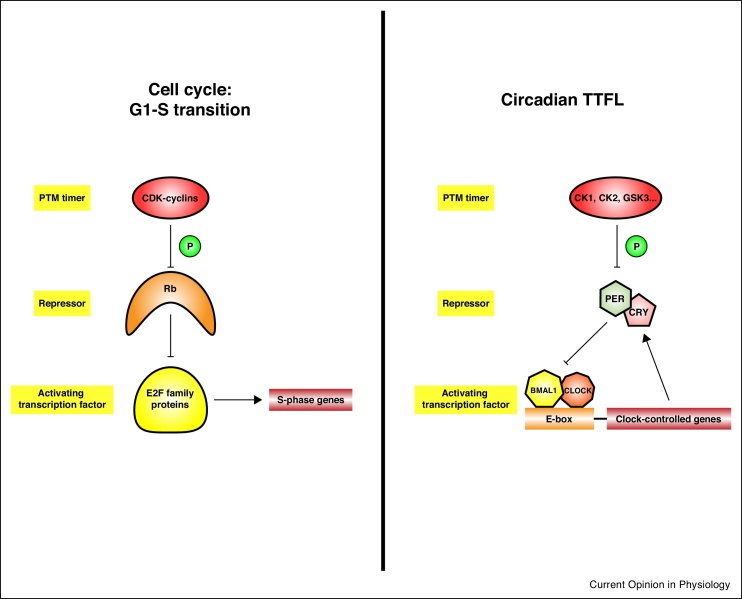
This post-translational view of the cellular circadian cycle has obvious parallels with the cell division cycle transition between G1 (growth phase) and S phase (DNA replication). This transition is known as the ‘restriction point’ [112], because it is thought to be the point of no return for cell cycle entry (Figure 2). Double negative feedback regulation of transcriptional control factors (activating E2F and repressor Rb proteins) imparts bistability around the G1-S phase transition, such that each steady state is robustly buffered against extrinsic noise as well as intrinsically stochastic fluctuations in gene expression [113]. In the cell division cycle, the timing of this transition is governed by the activity of cyclin-dependent kinases (CDKs), whose activity is rate-limiting for Rb inactivation. Hence, in both cell division and circadian cycles, stability is conferred by feedback loops whose components are the target for the activity of kinases that determine the rate of progression.
Figure 2.
Comparing the circadian cycle to the cell cycle G1/S transition.
A critical point for both the cell cycle and the circadian cycle is the activation of key transcription factors. This is achieved in both cases by the relief of repression by a negative regulator. The mechanism for this is phosphorylation: of Rb by cyclin-dependent kinases for the cell cycle, and of PER by CK1δ/ε for the circadian cycle. Hence the de-repression of crucial activating transcription factors via kinase activity is a common network motif for the temporal regulation of cellular processes.
As will be discussed below, both post-translational and TTFL-based models for circadian rhythm generation are consistent with the vast majority of published observations, but importantly, they can be distinguished experimentally. In either case, the key processes that determine periodicity occur post-translationally, either as a delay timer (canonical TTFL model, Figure 1b) or as a self-sustained enzymatic oscillation (post-translational model, Figure 1c). In the next section, we will consider the range of cellular processes that contribute to circadian period determination.
What is an essential post-translational clock mechanism?
From acetylation to sumoylation, from glycosylation to cysteine oxidation, all the major classes of post-translational protein modification have been implicated as regulating and/or being regulated by circadian timekeeping [114, 115, 116, 117, 118, 119]. Moreover, the activity of many essential cellular systems, such as cAMP/Ca2+ signalling [120], the actin cytoskeleton [35], mitochondrial metabolism [121], ion transport [122•], redox balance [32,107], and even global protein synthesis rates [38,123], exhibit circadian regulation in vivo and in vitro, with several also feeding back to affect the amplitude or phase of clock gene expression rhythms, and in some cases even the period of oscillation. If we consider each such system as being an essential timekeeping mechanism, then the cell itself becomes the simplest level of abstraction at which circadian rhythm generation can be understood. This complexity highlights an obstacle for the complete understanding of the mammalian cellular clock, due to inherent difficulties of distinguishing cause from effect in a cycle where oscillations of gene expression are the principal experimental reporter of rhythmicity.
For simplicity’s sake, we will consider redox balance as a point in case, specifically the major cellular redox couples NADH:NAD+, NADPH:NADP+ and GSH:GSSG. Flux through the oxidative pentose phosphate pathway generates NADPH, providing the primary source of cellular reducing equivalents (NADPH:NADP+ usually >100:1 in cytosol) for biosynthesis and maintenance of redox homeostasis (GSH:GSSG usually >100:1 in cytosol); whereas glycolytic activity generates NADH and along with lactate dehydrogenase, is the principle determinant of cytosolic NADH:NAD+ ratio (usually <1:100) [124,125]. Glucose catabolism and mitochondrial respiration are circadian regulated in many different contexts in vivo and in vitro [107,121,126, 127, 128, 129]. In such cases the rate at which cytosolic reducing equivalents (NADPH, NADH) are produced must necessarily also be circadian. If the rate of cytosolic NADH or NADPH oxidation does not match the rate of NAD+ or NADP+ reduction over the circadian cycle, or if the rate of de novo NAD+/NADP+ synthesis approaches the rate of redox recycling, this must inevitably generate a circadian rhythm in the respective steady state redox couple; and this latter has also been observed in several contexts [121,130, 131, 132, 133]. Similarly, if the rate at which cellular H2O2 oxidises cytosolic cysteine residues exceeds the rate at which they are reduced by the cell’s antioxidant systems over the daily cycle, then inter- and intramolecular disulphides will form and the cytosolic GSH:GSSG couple will inevitably change over the 24 hour cycle [32,134,135].
Separately, there are multiple established mechanisms by which acute changes in each of these three redox couples have been demonstrated to affect protein function, including transcription factor activity [32,136]. Moreover, there exists ample evidence that acute changes in each of these redox couples are competent to affect the phase and amplitude of circadian gene expression cycles [107,134,137•,138,139, 140, 141]. If oscillations in redox balance can be a rhythmic output, and can also regulate circadian gene expression rhythms, it is reasonable to suggest that dynamic regulation of redox balance constitutes a central timekeeping mechanism [134,141, 142, 143]. However, when cells are subject to chronic oxidative or reductive stresses, or when cytosolic NADH or NADPH production are pharmacologically inhibited, so long as cells remain viable then circadian rhythms of clock protein production are sustained — with reduced amplitude but with quite modest effects (<10%) on the period of oscillation [107]. Even the activity of important and ubiquitous antioxidant proteins such as peroxiredoxins is not essential for circadian rhythms [82]. This suggests that whilst any circadian regulation of redox balance may well feedback to affect the phase and amplitude of clock gene expression under physiological conditions, it cannot be an essential timekeeping mechanism [107]. Most likely, the balance of any given redox couple simply needs to reside within some permissive range for timekeeping to persist.
Extending this line of argument, it seems likely that acute or sustained genetic or pharmacological perturbation of very many different cellular systems can elicit effects upon the fidelity of circadian timekeeping, because their activity may be permissive for circadian rhythms to be observed, without necessarily meaning that they play any physiological role in period determination or rhythm generation. With reference to beautiful experiments performed with Neurospora crassa [144], an essential timekeeping mechanism may be described as a cellular process whose activity contributes to period determination and whose rhythmic regulation is absolutely required for any other circadian rhythm to be observed. Testing essentiality therefore, simply requires that activity be clamped at what would be its average level over the course of a normal circadian cycle. This type of experiment has demonstrated that rhythmic regulation of BMAL1 abundance is not essential for timekeeping at the cellular level, for example [26,145]. Similarly, such experiments allow us to discount the possibility that TOR-mediated rhythmic regulation of global protein synthesis rates constitutes an essential timekeeping mechanism; clearly rhythmic TOR activity is likely to be of enormous physiological relevance and does appear to contribute to circadian timekeeping in healthy cells, but when circadian regulation of TOR activity is abolished, either genetically or pharmacologically, circadian rhythms in clock protein activity persist, albeit with reduced amplitude and modestly lengthened period [146,147•,146–148].
Candidates for the post-translational clock mechanism
Applying the above criterion to the many post-translational mechanisms that have been implicated in regulation of circadian rhythms in mammalian cells, we are left with very few plausible candidates for essential post-translational clock mechanisms: casein kinase I (CK1δ/ε) [77,149, 150, 151, 152, 153], casein kinase II (CK2α/α’ catalytic subunit, CK2β regulatory subunit) [154,155], glycogen synthase kinase (GSK3α/β) [156, 157, 158, 159, 160, 161], protein phosphatase 1 (PP1CA/PP1CB/PP1CC catalytic subunits, many regulatory subunits) [149], as well as the ubiquitin-mediated proteasomal degradation system [64,76,169, 170, 171,80,162, 163, 164, 165, 166, 167, 168]. This list is unlikely to be comprehensive, as many post-translational processes have not been fully scrutinised, nor is the evidence for each equally strong. However, each candidate has been implicated independently and multiple times, by both genetic and pharmacological approaches, and all of them act directly to determine the activity of multiple clock protein transcription factors [149,164,168,169]. The functional contribution to cellular timekeeping made by the ubiquitous enzymes mentioned above has largely been interpreted in the context of net increases in site-specific clock protein phosphorylation occurring synergistically over the circadian cycle, with some phosphorylation events promoting nuclear entry [156,172, 173, 174], others regulating transcriptional or translational activity [98,175, 176, 177, 178, 179, 180, 181], and others promoting protein hyperphosphorylation which licenses ubiquitin-mediated proteasomal degradation [167,171,182]. In this context, many other enzymes that post-translationally modify clock proteins, such as AMPK [183,184], O-GlcNAc transferase [185,186], and PKA [120,187,188], appear to modulate clock protein activity to communicate temporal change in the local metabolic or extracellular environment but are non-essential for timekeeping function. It is noteworthy that, whilst the activity of each enzyme system listed above is encoded by 2 or more paralogs, overall the activity of each is essential for cellular viability [189, 190, 191].
Evolutionary implications
In positing a post-translational model for circadian rhythm generation, it is informative to consider the implications for the evolutionary origin of 24-hour timekeeping. Each of the essential post-translational timekeeping mechanisms discussed above is strongly implicated in the regulation of circadian rhythms in the fungal and green lineages, functioning similarly to determine the activity of the clock proteins in other eukaryotic kingdoms despite their transcription factor substrates not being thought to share a common origin [30,192•]. Thus, a primarily post-translational cellular clock mechanism suggests a divergent evolutionary history because critical post-translational determinants of 24-hour periodicity are highly conserved (proteasomes, casein kinases, glycogen synthase kinases and protein phosphatases), whilst the identities of TTFL components are poorly conserved. Again, there are clear parallels between post-translational models of circadian timekeeping and the cell cycle. Whilst the CDK enzymatic substrates vary between kingdoms of eukaryotes, the central dogma of eukaryotic cell division is that cell cycle progression is controlled by the activity of the CDKs [110,193], reflecting their common origin in an early eukaryotic progenitor. In contrast, current TTFL-based models for circadian rhythm generation suggest that daily timekeeping has arisen multiple times, convergently, because sequence homology is so poor between the central transcriptional components of the metazoan, fungal and plant circadian clocks [30].
Circadian timekeeping in the absence of transcription
In TTFL-based models of circadian rhythms, the period of oscillation should be highly sensitive to the rate and timing of transcriptional activation. However, the period of circadian rhythms in mammalian cells is surprisingly resilient against inhibition of RNA polymerase II [194] and, under these conditions, remains compensated against changes in temperature. Moreover, both transcription and translation are temperature-dependent processes [195, 196, 197, 198], whereas circadian period determination is not. A potential solution to this quandary is vested in some recent work where the activity of CK1 against its clock protein substrates was shown to be intrinsically temperature-compensated [199,200•], suggesting that since temperature compensation of circadian period occurs post-translationally, then perhaps so too does period determination.
This notion is supported by the precedent set by the entirely post-translational circadian clock encoded by the KaiBC and KaiA operons in the cyanobacterium Synechococcus elongatus. Here the temperature-compensated ∼24 hour rhythm of KaiC protein phosphorylation and complex formation with KaiA/B that occurs in living cells, and normally interacts reciprocally with genome-wide transcriptional regulation, can be reconstituted in vitro using just the three recombinant proteins (KaiA, B & C) with Mg.ATP [201]. The remarkable elucidation of the cyanobacterial circadian clock over the last two decades has been reviewed extensively elsewhere [202, 203, 204], and so just a few key similarities with mammalian post-translational timekeeping mechanisms will be considered here. Firstly, KaiC is a slow and inefficient Ser/Thr phosphotransferase, with turnover rates (kcat) of the order of h−1 [205]; this has some similarity with CK1δ in that the initial phosphorylation of PER peptide substrates has a surprisingly slow kcat (order of m−1, [206]) compared with most cellular kinases (usually order of ≥10 s−1, [207, 208, 209]). Secondly, the TTFL in cyanobacteria is not sufficient to generate circadian rhythms of gene expression, whereas the post-translational KaiC-based oscillation is [210]. Critically though, when the capacity for transcriptional feedback repression is removed, cellular oscillations in gene expression become less robust, that is the post-translational oscillation is less effective when transcriptional feedback is removed [210]. Finally, KaiC exists primarily in large macromolecular protein complexes with several substrate effectors [211] where dynamic subunit exchange is critical to timekeeping competence [212,213]. This is also mirrored by CK1δ/ε, which has multiple cellular substrates and functions, but consistently co-purifies from the cytosol with the PER and CRY proteins in large (∼1 M Da) macromolecular protein complexes [45•].
The successful delineation of the post-translational mechanisms that drive this prokaryotic clockwork raises the question of whether there is evidence for circadian rhythms in the absence of transcription in mammalian and other eukaryotic cells. This question is particularly pertinent given the evolutionary conservation of post-translational timekeeping mechanisms in eukaryotes. The idea of a eukaryotic circadian post-translational oscillator is rendered more plausible by comparison to an analogous mechanism regulating the cell cycle. As mentioned above, the pan-eukaryotic cyclin-dependent kinases (CDKs) are key post-translational enzymes regulating the timing of the G1-S transition of the cell cycle [110,193,214]. Early embryonic cells have a simplified system that alternates between S phase and mitosis, and the activity of CDK1-cyclin complex (originally named in Xenopus laevis as maturation promoting factor, MPF) determines the timing of progression to M-phase [215]. Oscillations in MPF were observed in enucleated Xenopus laevis eggs, hence driven independently of transcription, and this is now known to result from a negative feedback loop where anaphase-promoting complex (APC/C), acts in concert with kinase/phosphatase positive feedback loops that contribute to stability [216, 217, 218, 219, 220]. The purpose of this analogy is to show that eukaryotic systems can harness post-translational mechanisms to drive oscillations that coordinate diverse cellular processes, using phosphorylation to convey temporal information to many downstream targets including transcription factors, whose change in activity also feeds back to affect the activity of components that drive the oscillation for example by changes in cyclin levels.
To our knowledge, there exist five unrelated experimental observations, most having been independently reproduced, demonstrating that a eukaryotic cellular circadian timekeeping mechanism continues to function in the absence of transcriptional feedback repression. Firstly, in the macroscopic alga Acetabularia mediterranea, circadian rhythms of chloroplast migration persist under constant conditions over many days when the cell nucleus is removed, thus rendering nascent nuclear mRNA production impossible [221,222]. Second, in the eukaryotic red alga, Cyanidioschyzon merolae, circadian rhythms of protein phosphorylation persist in the absence of translation [223]. Third, an RNA-Seq study revealed circadian rhythms of bioluminescence and photosynthesis in Lingulodinium polyedrum do not require rhythmic changes in RNA abundance [224]. Fourth, in the picoeukaryotic alga Ostreococcus tauri, rhythms in ion transport and oxidised peroxiredoxin proteins (PRX-SO2/3) oscillate with ∼24 hour period under constant darkness — when all RNA production ceases [122•,225,226]. Finally, entrainable temperature-compensated ∼24 hour rhythms of NAD(P)H concentration, PRX-SO2/3 abundance and membrane physiology have been observed in isolated mammalian red blood cells which, lacking nuclei and all other organelles, lack the capacity for TTFL-dependent circadian timekeeping [132,227,228•].
Because the biological oscillators that drive these diverse non-transcriptional rhythms are not known, it is currently unclear whether these examples represent atypical cellular specialisations that allow circadian rhythms to persist in the absence of transcription, or whether they result from the same post-translational mechanism that confers circadian periodicity upon clock protein activity in other eukaryotic cellular contexts. Given the evolutionarily conserved role of CK1 in determining circadian period and its putative role in temperature compensation, it would be informative to learn whether period and temperature compensation of these non-transcriptional circadian rhythms depend on the activity of CK1, as well as CK2, GSK3, and other conserved post-translational mechanisms that are already strongly implicated in rhythm generation and period determination where transcriptional cycles are present.
Experimental testing of models for circadian rhythm generation
In light of all the evidence of which we are aware, there exist two plausible models for circadian rhythm generation:
-
1)
Convergence: Circadian rhythms have evolved independently at least three times in eukaryotes. In each case these have converged upon a timekeeping mechanism based on transcriptional feedback repression that has recruited the same set of ubiquitous post-translational regulatory mechanisms which function as delay timers to fine-tune gene expression rhythms to a 24 hour period. Cellular circadian rhythms that do not involve cycles of transcriptional repression reflect recent adaptions to specific environmental or physiological niches. The relative timing of clock gene transcriptional activation and repression is the ultimate arbiter of period determination and rhythm generation in eukaryotic organisms.
-
2)
Divergence: A post-translational biological oscillation, involving ubiquitous and essential enzymes such as CK1, was present in the last eukaryotic common ancestor. Its targets and function subsequently diverged, recruiting lineage-specific transcriptional effectors (‘clock proteins’) as different kingdoms and phyla arose. Transcriptional feedback repression facilitates hysteresis, thus providing an adaptive advantage that was selected for as it confers rhythmic robustness. Post-translational mechanisms retain executive control of period determination and rhythm generation in modern organisms, but their activity is modulated by their expression level and interacting partners.
There are several experiments that would distinguish between these two models:
-
1)
Constitutive expression of clock genes: if the mRNAs of all transcription factors implicated as being essential to circadian regulation were expressed constitutively around the mean of their habitual level of oscillation then no circadian rhythm in any cellular process would be observed according to the convergence model.
-
2)
Constitutive activation of post-translational regulators: if the activity of the evolutionarily conserved kinases that determine the activity of clock proteins were clamped around the mean of their habitual level (e.g. by mutation of the auto-phosphorylated regulatory motif of CK1), this would abolish circadian rhythms according to the divergence model
-
3)
Reconstitution: if the minimal set of enzymatic components that confer circadian periodicity upon clock protein activity in mammalian cells were known, then an oscillation could be reconstituted in solution according to the divergence model. Conversely the mammalian TTFL will produce circadian rhythms when introduced to an appropriate cellular system that does not normally exhibit circadian timekeeping according to the convergence model.
Should these experiments produce ambiguous results, a third model must be considered: that circadian rhythms are an emergent property of mammalian cells, and whilst the molecular mechanisms of individual components can be dissected, the biological oscillation itself cannot be meaningfully engaged with at any simpler level of abstraction than the entire cell.
Conclusion
The biological significance of mammalian circadian rhythms can only be properly understood in an organismal context, where the clock in every cell is synchronised with its neighbours, other tissues, and the outside world by myriad humoral timing cues. To fully realise and exploit the potential translational consequences of our innate clockwork it is imperative that we also understand how our cells keep time. Circadian timekeeping occurs cell-autonomously and so, as with the cell division cycle, there is clear utility in the application of cell culture and comparative models for delineating the biochemical mechanisms that allow individual cells to maintain this cell-autonomous oscillation, removed from the continual barrage of extracellular cues that cells experience in vivo. Whilst many important components of the mammalian cellular circadian timekeeping machinery have been identified, as well as their interacting partners and functional consequences, the fundamental molecular mechanisms generating the daily oscillation in most cells of the human body are not understood at the same level of detail that allow us to describe the timing of the eukaryotic cell division cycle or the cyanobacterial circadian clock. In the latter two cases, timing function ultimately resides post-translationally, vested in the activity of evolutionarily ancient serine/threonine kinases. Time will tell whether the same is true for eukaryotic circadian clocks.
Conflict of interest statement
Nothing declared.
Funding
JSO is supported by the Medical Research Council (MC_UP_1201/4). DCSW is supported by the MRC Doctoral Training Programme and the Frank Edward Elmore Fund.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
Acknowledgments
We would like to thank Alessandra Stangherlin, Priya Crosby, Marrit Putker, Andrew Beale, Estere Seinkmane and David Tourigny for valuable discussion.
Footnotes
We note several excellent studies revealing that post-transcriptional regulation of RNA processing and small interfering RNA-mediated RNA silencing have also been strongly implicated in circadian control, as well as both global and specific regulation of protein synthesis rates [38,98,229, 230, 231, 232, 233, 234, 235, 236]. Whilst perturbation of these essential cellular processes clearly affects circadian gene expression rhythms, to our knowledge it has not been demonstrated that their rhythmic regulation is essential for timekeeping competence and we thus direct the interested reader to these excellent reviews [237, 238, 239].
References
- 1.Woelfle M.A., Ouyang Y., Phanvijhitsiri K., Johnson C.H. The adaptive value of circadian clocks: an experimental assessment in Cyanobacteria. Curr Biol. 2004;14:1481–1486. doi: 10.1016/j.cub.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Ma P., Woelfle M.A., Johnson C.H. An evolutionary fitness enhancement conferred by the circadian system in Cyanobacteria. Chaos Solitons Fractals. 2013;50:65–74. doi: 10.1016/j.chaos.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roenneberg T., Merrow M. What watch?… such much!” complexity and evolution of circadian clocks. Cell Tissue Res. 2002;309:3–9. doi: 10.1007/s00441-002-0568-1. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang Y., Andersson C.R., Kondo T., Golden S.S., Johnson C.H. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd A.N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J.M., Millar A.J., Webb A.A.R. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 6.Klarsfeld A., Rouyer F. Effects of circadian mutations and LD periodicity on the life span of Drosophila melanogaster. J Biol Rhythms. 1998;13:471–478. doi: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- 7.Spoelstra K., Wikelski M., Daan S., Loudon A.S.I., Hau M. Natural selection against a circadian clock gene mutation in mice. Proc Natl Acad Sci U S A. 2016;113:686–691. doi: 10.1073/pnas.1516442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittendrigh C.S. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 9.Dunlap J.C., Loros J.J., DeCoursey P.J. Sinauer Associates; 2004. Chronobiology: biological timekeeping. [Google Scholar]
- 10.Pittendrigh C.S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Hastings M.H., Reddy A.B., Maywood E.S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 12.Welsh D.K., Takahashi J.S., Kay S.A. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 14.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 17.Patton A.P., Hastings M.H. The suprachiasmatic nucleus. Curr Biol. 2018;28:R816–R822. doi: 10.1016/j.cub.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 18.Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018 doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A., Damiola F., Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 20.Welsh D.K., Yoo S.-H., Liu A.C., Takahashi J.S., Kay S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo S.-H., Yamazaki S., Lowrey P.L., Shimomura K., Ko C.H., Buhr E.D., Siepka S.M., Hong H.-K., Oh W.J., Yoo O.J. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulimeno P., Mannic T., Sage D., Giovannoni L., Salmon P., Lemeille S., Giry-Laterriere M., Unser M., Bosco D., Bauer C. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56:497–507. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Horst G.T., Muijtjens M., Kobayashi K., Takano R., Kanno S., Takao M., de Wit J., Verkerk A., Eker a P., van Leenen D. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 24.Bunger M.K., Wilsbacher L.D., Moran S.M., Clendenin C., Radcliffe L.A., Hogenesch J.B., Simon M.C., Takahashi J.S., Bradfield C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Ode K.L., Ukai H., Susaki E.A., Narumi R., Matsumoto K., Hara J., Koide N., Abe T., Kanemaki M.T., Kiyonari H. Knockout-rescue embryonic stem cell-derived mouse reveals circadian-period control by quality and quantity of CRY1. Mol Cell. 2017;65:176–190. doi: 10.1016/j.molcel.2016.11.022. [DOI] [PubMed] [Google Scholar]; A very impressive study in mice and mouse cells suggesting that CRY1 determines circadian period through both degradation-dependent and -independent pathways, extending previous findings in Neurospora [103].
- 26.Liu A.C., Tran H.G., Zhang E.E., Priest A.A., Welsh D.K., Kay S.A. Redundant function of REV-ERBα and β and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000023. e1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlap J.C. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Anafi R.C., Francey L.J., Hogenesch J.B., Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A. 2017;114:5312–5317. doi: 10.1073/pnas.1619320114. [DOI] [PMC free article] [PubMed] [Google Scholar]; This landmark paper bridges the gap between mouse models and daily co-ordination of the transcriptome in human tissues.
- 30.Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000062. e1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardin P.E., Hall J.C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 32.Putker M., O’Neill J.S. Reciprocal control of the circadian clock and cellular redox state - a critical appraisal. Mol Cells. 2016;39:6–19. doi: 10.14348/molcells.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown S.A., Kowalska E., Dallmann R. (Re)inventing the circadian feedback loop. Dev Cell. 2012;22:477–487. doi: 10.1016/j.devcel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34•.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2016;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]; A highly recommended review on the current state of understanding of the mammalian TTFL and its global control of cellular processes.
- 35.Hoyle N.P., Seinkmane E., Putker M., Feeney K.A., Krogager T.P., Chesham J.E., Bray L.K., Thomas J.M., Dunn K., Blaikley J. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal2774. eaal2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes M.E., DiTacchio L., Hayes K.R., Vollmers C., Pulivarthy S., Baggs J.E., Panda S., Hogenesch J.B. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauvoisin D., Wang J., Jouffe C., Martin E., Atger F., Waridel P., Quadroni M., Gachon F., Naef F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc Natl Acad Sci U S A. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang C., Lahens N.F., Hogenesch J.B., Sehgal A. Ribosome profiling reveals an important role for translational control in circadian gene expression. Genome Res. 2015;25:1836–1847. doi: 10.1101/gr.191296.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy A.B., Karp N.A., Maywood E.S., Sage E.A., Deery M., O’Neill J.S., Wong G.K.Y., Chesham J., Odell M., Lilley K.S. Circadian Orchestration of the Hepatic Proteome. Curr Biol. 2006;16:1107–1115. doi: 10.1016/j.cub.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Robles M.S., Cox J., Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ripperger J.A., Jud C., Albrecht U. The daily rhythm of mice. FEBS Lett. 2011;585:1384–1392. doi: 10.1016/j.febslet.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., Weitz C.J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 43.King D.P., Zhao Y., Sangoram A.M., Wilsbacher L.D., Tanaka M., Antoch M.P., Steeves T.D., Vitaterna M.H., Kornhauser J.M., Lowrey P.L. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogenesch J.B., Chan W.K., Jackiw V.H., Brown R.C., Gu Y.Z., Pray-Grant M., Perdew G.H., Bradfield C.A. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272:8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 45•.Aryal R.P., Kwak P.B., Tamayo A.G., Gebert M., Chiu P., Walz T., Weitz C.J. Macromolecular assemblies of the mammalian circadian clock. Mol Cell. 2017;67:770–782. doi: 10.1016/j.molcel.2017.07.017. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; A fantastic biochemical characterisation of PER/CRY/CK1 complexes purified from mouse liver.
- 46.Kim J.Y., Kwak P.B., Weitz C.J. Specificity in circadian clock feedback from targeted reconstitution of the NuRD corepressor. Mol Cell. 2014;56:738–748. doi: 10.1016/j.molcel.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.Y., Kwak P.B., Gebert M., Duong H.A., Weitz C.J. Purification and analysis of PERIOD protein complexes of the mammalian circadian clock. Methods Enzymol. 2015;551:197–210. doi: 10.1016/bs.mie.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Brown S.A., Ripperger J., Kadener S., Fleury-Olela F., Vilbois F., Rosbash M., Schibler U. PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science (80-) 2005;308:693–696. doi: 10.1126/science.1107373. [DOI] [PubMed] [Google Scholar]
- 49.Partch C.L., Green C.B., Takahashi J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koike N., Yoo S.-H., Huang H.-C., Kumar V., Lee C., Kim T.-K., Takahashi J.S., Lowrey P.L., Takahashi J.S., Bass J. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamia K.A., Papp S.J., Yu RT Barish G.D., Uhlenhaut N.H., Jonker J.W., Downes M., Evans R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Huber A.L., Papp S.J., Chan A.B., Henriksson E., Jordan S.D., Kriebs A., Nguyen M., Wallace M., Li Z., Metallo C.M. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Mol Cell. 2016;64:774–789. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent study characterising one of the many additional functions that clock proteins perform.
- 53.Zhang E.E., Liu Y., Dentin R., Pongsawakul P.Y., Liu A.C., Hirota T., Nusinow D.A., Sun X., Landais S., Kodama Y. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondratov R.V. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Bunger M.K., Walisser J.A., Sullivan R., Manley P.A., Moran S.M., Kalscheur V.L., Colman R.J., Bradfield C.A. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 56.Mullenders J., Fabius A.W.M., Madiredjo M., Bernards R., Beijersbergen R.L. A Large Scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One. 2009;4:e4798. doi: 10.1371/journal.pone.0004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su X., Chen D., Yang K., Zhao Q., Zhao D., Lv X., Ao Y. The circadian clock gene PER2 plays an important role in tumor suppression through regulating tumor-associated genes in human oral squamous cell carcinoma. Oncol Rep. 2017;38:472–480. doi: 10.3892/or.2017.5653. [DOI] [PubMed] [Google Scholar]
- 58.Musiek E.S., Lim M.M., Yang G., Bauer A.Q., Qi L., Lee Y., Roh J.H., Ortiz-Gonzalez X., Dearborn J.T., Culver J.P. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y.H., Marhon S.A., Zhang Y., Steger D.J., Won K.-J., Lazar M.A. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359:1274–1277. doi: 10.1126/science.aao6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Mermet J., Yeung J., Hurni C., Mauvoisin D., Gustafson K., Jouffe C., Nicolas D., Emmenegger Y., Gobet C., Franken P. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes Dev. 2018;32:347–358. doi: 10.1101/gad.312397.118. [DOI] [PMC free article] [PubMed] [Google Scholar]; See below
- 61•.Yeung J., Mermet J., Jouffe C., Marquis J., Charpagne A., Gachon F., Naef F. Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res. 2018;28:182–191. doi: 10.1101/gr.222430.117. [DOI] [PMC free article] [PubMed] [Google Scholar]; These two groundbreaking papers provide a mechanistic framework for understanding tissue-specific circadian co-ordination of gene expression.
- 62.Kumaki Y., Ukai-Tadenuma M., Uno K.D., Nishio J., Masumoto K., Nagano M., Komori T., Shigeyoshi Y., Hogenesch J.B., Ueda H.R. Analysis and synthesis of high-amplitude Cis-elements in the mammalian circadian clock. Proc Natl Acad Sci U S A. 2008;105:14946–14951. doi: 10.1073/pnas.0802636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Fang B., Emmett M.J., Damle M., Sun Z., Feng D., Armour S.M., Remsberg J.R., Jager J., Soccio R.E. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stratmann M., Suter D.M., Molina N., Naef F., Schibler U. Circadian Dbp transcription relies on highly dynamic BMAL1-CLOCK interaction with E boxes and requires the proteasome. Mol Cell. 2012;48:277–287. doi: 10.1016/j.molcel.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Ripperger J.A., Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 66.Ueda H.R., Hayashi S., Chen W., Sano M., Machida M., Shigeyoshi Y., Iino M., Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 67.Mitsui S., Yamaguchi S., Matsuo T., Ishida Y., Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. 2001;15:995–1006. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ukai-Tadenuma M., Kasukawa T., Ueda H.R. Proof-by-synthesis of the transcriptional logic of mammalian circadian clocks. Nat Cell Biol. 2008;10:1154–1163. doi: 10.1038/ncb1775. [DOI] [PubMed] [Google Scholar]
- 69.Kholodenko B.N., Hancock J.F., Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin S.-Y., Rath O., Choo S.-M., Fee F., McFerran B., Kolch W., K-H Cho. Positive- and negative-feedback regulations coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal transduction pathway. J Cell Sci. 2009;122:425–435. doi: 10.1242/jcs.036319. [DOI] [PubMed] [Google Scholar]
- 71.Nakayama K., Satoh T., Igari A., Kageyama R., Nishida E. FGF induces oscillations of Hes1 expression and Ras/ERK activation. Curr Biol. 2008;18:R332–4. doi: 10.1016/j.cub.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Ashall L., Horton C.A., Nelson D.E., Paszek P., Harper C.V., Sillitoe K., Ryan S., Spiller D.G., Unitt J.F., Broomhead D.S. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibb S., Maroto M., Dale J.K. The segmentation clock mechanism moves up a notch. Trends Cell Biol. 2010;20:593–600. doi: 10.1016/j.tcb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmeirim I., Henrique D., Ish-Horowicz D., Pourquié O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 75.Leise T.L., Wang C.W., Gitis P.J., Welsh D.K. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2::LUC bioluminescence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lowrey P.L., Shimomura K., Antoch M.P., Yamazaki S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kloss B., Price J.L., Saez L., Blau J., Rothenfluh A., Wesley C.S., Young M.W. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 78.Fang Y., Sathyanarayanan S., Sehgal A. Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1) Genes Dev. 2007;21:1506–1518. doi: 10.1101/gad.1541607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nawathean P., Rosbash M. The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol Cell. 2004;13:213–223. doi: 10.1016/s1097-2765(03)00503-3. [DOI] [PubMed] [Google Scholar]
- 80.Martinek S., Inonog S., Manoukian A.S., Young M.W. A Role for the Segment Polarity Gene shaggy/GSK-3 in the Drosophila Circadian Clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- 81.Ko H.W., Jiang J., Edery I. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature. 2002;420:673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 82.Causton H.C., Feeney K.A., Ziegler C.A., O’Neill J.S. Metabolic cycles in yeast share features conserved among circadian rhythms. Curr Biol. 2015;25:1056–1062. doi: 10.1016/j.cub.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mauvoisin D., Dayon L., Gachon F., Kussmann M. Proteomics and circadian rhythms: It’s all about signaling! Proteomics. 2015;15:310–317. doi: 10.1002/pmic.201400187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robles Maria S., Mann M. Proteomic approaches in circadian biology. Handb Exp Pharmacol. 2013;217:29–44. doi: 10.1007/978-3-642-25950-0_17. [DOI] [PubMed] [Google Scholar]
- 85.Deery M.J., Maywood E.S., Chesham J.E., Sládek M., Karp N.A., Green E.W., Charles P.D., Reddy A.B., Kyriacou C.P., Lilley K.S. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr Biol. 2009;19:2031–2036. doi: 10.1016/j.cub.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 86.Rey G., Milev N.B., Valekunja U.K., Ch R., Ray S., Santos M.S., Dos, Nagy A.D., Antrobus R., MacRae J.I., Reddy A.B. Metabolic oscillations on the circadian time scale in Drosophila cells lacking clock genes. Mol Syst Biol. 2018;14:e8376. doi: 10.15252/msb.20188376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker C.L., Kettenbach A.N., Loros J.J., Gerber S.A., Dunlap J.C. Quantitative proteomics reveals a dynamic interactome and phase-specific phosphorylation in the Neurospora circadian clock. Mol Cell. 2009;34:354–363. doi: 10.1016/j.molcel.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Robles M.S., Humphrey S.J., Mann M., Altelaar A.F., Munoz J., Heck A.J., Baker C.L., Kettenbach A.N., Loros J.J., Gerber S.A. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2016;0:35–48. doi: 10.1016/j.cmet.2016.10.004. [DOI] [PubMed] [Google Scholar]; An exciting study exploring the circadian phosphoproteome in mouse liver.
- 89.Mauvoisin D., Atger F., Dayon L., Núñez Galindo A., Wang J., Martin E., Da Silva L., Montoliu I., Collino S., Martin F.-P. Circadian and feeding rhythms orchestrate the diurnal liver Acetylome. Cell Rep. 2017;20:1729–1743. doi: 10.1016/j.celrep.2017.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wis̈niewski J.R., Gizak A., Rakus D. Integrating proteomics and enzyme kinetics reveals tissue-specific types of the glycolytic and gluconeogenic pathways. J Proteome Res. 2015;14:3263–3273. doi: 10.1021/acs.jproteome.5b00276. [DOI] [PubMed] [Google Scholar]
- 91.Wegner A., Meiser J., Weindl D., Hiller K. How metabolites modulate metabolic flux. Curr Opin Biotechnol. 2015;34:16–22. doi: 10.1016/j.copbio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Baggs J.E., Price T.S., DiTacchio L., Panda S., FitzGerald G.A., Hogenesch J.B. Network features of the mammalian circadian clock. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi S., Hida A., McGuinness O.P., Wasserman D.H., Yamazaki S., Johnson C.H. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fan Y., Hida A., Anderson D.A., Izumo M., Johnson C.H. Cycling of CRYPTOCHROME proteins is not necessary for circadian-clock function in mammalian fibroblasts. Curr Biol. 2007;17:1091–1100. doi: 10.1016/j.cub.2007.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Landgraf D., Wang L.L., Diemer T., Welsh D.K. NPAS2 compensates for loss of CLOCK in peripheral circadian oscillators. PLOS Genet. 2016;12 doi: 10.1371/journal.pgen.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]; This controversial study strongly challenges the prior paradigm that the CLOCK gene is essential for circadian coordination of transcription in peripheral tissues.
- 96.Liu A.C., Welsh D.K., Ko C.H., Tran H.G., Zhang E.E., Priest A.A., Buhr E.D., Singer O., Meeker K., Verma I.M. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Alessandro M., Beesley S., Kim J.K., Chen R., Abich E., Cheng W., Yi P., Takahashi J.S., Lee C. A tunable artificial circadian clock in clock-defective mice. Nat Commun. 2015;6:1–11. doi: 10.1038/ncomms9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lipton J.O., Yuan E.D., Boyle L.M., Ebrahimi-Fakhari D., Kwiatkowski E., Nathan A., Güttler T., Davis F., Asara J.M., Sahin M. The Circadian Protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell. 2015;161:1138–1151. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99•.Gustafson C.L., Parsley N.C., Asimgil H., Lee H.-W., Ahlbach C., Michael A.K., Xu H., Williams O.L., Davis T.L., Liu A.C. A slow conformational switch in the BMAL1 transactivation domain modulates circadian rhythms. Mol Cell. 2017 doi: 10.1016/j.molcel.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; A superb example of the importance of post-translational regulation of clock protein activity.
- 100.Nangle S.N., Rosensweig C., Koike N., Tei H., Takahashi J.S., Green C.B., Zheng N., Adams P., Afonine P., Bunkoczi G. Molecular assembly of the period-cryptochrome circadian transcriptional repressor complex. Elife. 2014;3 doi: 10.7554/eLife.03674. e03674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edwards M.D., Brancaccio M., Chesham J.E., Maywood E.S., Hastings M.H. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc Natl Acad Sci U S A. 2016;113:2732–2737. doi: 10.1073/pnas.1519044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Z., Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 103.Larrondo L.F., Olivares-Yañez C., Baker C.L., Loros J.J., Dunlap J.C. Circadian rhythms. Decoupling circadian clock protein turnover from circadian period determination. Science. 2015;347 doi: 10.1126/science.1257277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ko C.H., Yamada Y.R., Welsh D.K., Buhr E.D., Liu A.C., Zhang E.E., Ralph M.R., Kay S.A., Forger D.B., Takahashi J.S. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maywood E.S., Chesham J.E., O’Brien J.A., Hastings M.H. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacobshagen S., Kessler B., Rinehart C.A. At least four distinct circadian regulatory mechanisms are required for all phases of rhythms in mRNA amount. J Biol Rhythms. 2008;23:511–524. doi: 10.1177/0748730408325753. [DOI] [PubMed] [Google Scholar]
- 107.Putker M., Crosby P., Feeney K.A., Hoyle N.P., da Costa A.S.H., Gaude E., Frezza C., O’Neill J.S. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid Redox Signal. 2017;4523 doi: 10.1089/ars.2016.6911. ars.2016.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gotoh T., Kim J.K., Liu J., Vila-Caballer M., Stauffer P.E., Tyson J.J., Finkielstein C.V. Model-driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2. Proc Natl Acad Sci U S A. 2016;113:13516–13521. doi: 10.1073/pnas.1607984113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maywood E.S., Chesham J.E., Meng Q.-J., Nolan P.M., Loudon A.S.I., Hastings M.H. Tuning the period of the mammalian circadian clock: additive and independent effects of CK1εTau and Fbxl3Afh mutations on mouse circadian behavior and molecular pacemaking. J Neurosci. 2011;31:1539–1544. doi: 10.1523/JNEUROSCI.4107-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harashima H., Dissmeyer N., Schnittger A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013;23:345–356. doi: 10.1016/j.tcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 111.Qin X., Mori T., Zhang Y., Johnson C.H. PER2 differentially regulates clock phosphorylation versus transcription by reciprocal switching of CK1 activity. J Biol Rhythms. 2015;30:206–216. doi: 10.1177/0748730415582127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pardee A.B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao G., Lee T.J., Mori S., Nevins J.R., You L. A bistable Rb–E2F switch underlies the restriction point. Nat Cell Biol. 2008;10:476–482. doi: 10.1038/ncb1711. [DOI] [PubMed] [Google Scholar]
- 114.Doi M., Hirayama J., Sassone-Corsi P. Circadian regulator CLOCK is a histone Acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 115.Durgan D.J., Pat B.M., Laczy B., Bradley J.A., Tsai J.-Y., Grenett M.H., Ratcliffe W.F., Brewer R.A., Nagendran J., Villegas-Montoya C. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gupta N., Ragsdale S.W. Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erb{beta} J Biol Chem. 2011;286:4392–4403. doi: 10.1074/jbc.M110.193466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee C., Etchegaray J.-P., Cagampang F.R.A., Loudon A.S.I., Reppert S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 118.Lee J., Lee Y., Lee M.J., Park E., Kang S.H., Chung C.H., Lee K.H., Kim K. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol. 2008;28:6056–6065. doi: 10.1128/MCB.00583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mehra A., Baker C.L., Loros J.J., Dunlap J.C. Post-translational modifications in circadian rhythms. Trends Biochem Sci. 2009;34:483–490. doi: 10.1016/j.tibs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Neill J.S., Maywood E.S., Chesham J.E., Takahashi J.S., Hastings M.H. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peek C.B., Affinati A.H., Ramsey K.M., Kuo H.-Y., Yu W., Sena L.A., Ilkayeva O., Marcheva B., Kobayashi Y., Omura C. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342 doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122•.Feeney K.A., Hansen L.L., Putker M., Olivares-Yañez C., Day J., Eades L.J., Larrondo L.F., Hoyle N.P., O’Neill J.S., van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of many examples of how a cellular circadian output (cytosolic Mg2+) can feedback to affect timekeeping competence i.e. appropriate pertubation of Mg2+ is sufficient to determine the period, amplitude and phase of clock gene expression rhythms.
- 123.Hastings J.W. The Gonyaulax clock at 50: Translational control of circadian expression. Cold Spring Harb Symp Quant Biol. 2007;72:141–144. doi: 10.1101/sqb.2007.72.026. [DOI] [PubMed] [Google Scholar]
- 124.Xiao W., Wang R.-S., Handy D.E., Loscalzo J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal. 2018;28:251–272. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.López-Mirabal H.R., Winther J.R. Redox characteristics of the eukaryotic cytosol. Biochim Biophys Acta - Mol Cell Res. 2008;1783:629–640. doi: 10.1016/j.bbamcr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 126.Kil I.S., Ryu K.W., Lee S.K., Kim J.Y., Chu S.Y., Kim J.H., Park S., Rhee S.G. Circadian oscillation of Sulfiredoxin in the mitochondria. Mol Cell. 2015;59:651–663. doi: 10.1016/j.molcel.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 127.Manella G., Asher G. The circadian nature of mitochondrial biology. Front Endocrinol (Lausanne) 2016;7:162. doi: 10.3389/fendo.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masri S., Patel V.R., Eckel-Mahan K.L., Peleg S., Forne I., Ladurner A.G., Baldi P., Imhof A., Sassone-Corsi P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc Natl Acad Sci U S A. 2013;110:3339–3344. doi: 10.1073/pnas.1217632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science. 2010;220:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramsey K.M., Yoshino J., Brace C.S., Abrassart D., Kobayashi Y., Marcheva B., Hong H.-K., Chong J.L., Buhr E.D., Lee C. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science (80-) 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asher G., Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 132.O’Neill J., Reddy A. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stringari C., Wang H., Geyfman M., Crosignani V., Kumar V., Takahashi J.S., Andersen B., Gratton E. In vivo single-cell detection of metabolic oscillations in stem cells. Cell Rep. 2015;10:1–7. doi: 10.1016/j.celrep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang T.A., Yu Y.V., Govindaiah G., Ye X., Artinian L., Coleman T.P., Sweedler J.V., Cox C.L., Gillette M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337:839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rhee S.G., Kil I.S. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: Linking mitochondrial function to circadian rhythm. Free Radic Biol Med. 2016;100:73–80. doi: 10.1016/j.freeradbiomed.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Yoshii K., Ishijima S., Sagami I. Effects of NAD(P)H and its derivatives on the DNA-binding activity of NPAS2, a mammalian circadian transcription factor. Biochem Biophys Res Commun. 2013;437:386–391. doi: 10.1016/j.bbrc.2013.06.086. [DOI] [PubMed] [Google Scholar]
- 137•.Wible R.S., Ramanathan C., Sutter C.H., Olesen K.M., Kensler T.W., Liu A.C., Sutter T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife. 2018;7 doi: 10.7554/eLife.31656. e31656. [DOI] [PMC free article] [PubMed] [Google Scholar]; This careful study demonstrates that NRF2, an important antioxidant regulator, is clock-regulated and can affect timekeeping in some, but not all, cellular contexts: another example of a circadian output that’s also a conditional input.
- 138.Rey G., Valekunja U.K., Feeney K.A., Velagapudi V., Neill J.S.O., Reddy A.B., Rey G., Valekunja U.K., Feeney K.A., Wulund L. The pentose phosphate pathway regulates the circadian clock. Cell Metab. 2016;24:462–473. doi: 10.1016/j.cmet.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou M., Wang W., Karapetyan S., Mwimba M., Marqués J., Buchler N.E., Dong X. Redox rhythm reinforces the circadian clock to gate immune response. Nature. 2015;523:472–476. doi: 10.1038/nature14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshida Y., Iigusa H., Wang N., Hasunuma K. Cross-Talk between the cellular redox state and the circadian system in Neurospora. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rutter J., Reick M., Wu L.C., McKnight S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 142.Wu L., Reddy A.B. Rethinking the clockwork: redox cycles and non-transcriptional control of circadian rhythms. Biochem Soc Trans. 2014;42:1–10. doi: 10.1042/BST20130169. [DOI] [PubMed] [Google Scholar]
- 143.Stangherlin A., Reddy A.B. Regulation of circadian clocks by redox homeostasis. J Biol Chem. 2013;288:26505–26511. doi: 10.1074/jbc.R113.457564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aronson B.D., Johnson K.A., Loros J.J., Dunlap J.C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 145.Xu H., Gustafson C.L., Sammons P.J., Khan S.K., Parsley N.C., Ramanathan C., Lee H.-W., Liu A.C., Partch C.L. Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus. Nat Struct Mol Biol. 2015;22:476–484. doi: 10.1038/nsmb.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lipton J.O., Boyle L.M., Yuan E.D., Hochstrasser K.J., Chifamba F.F., Nathan A., Tsai P.T., Davis F., Sahin M. Aberrant proteostasis of BMAL1 Underlies Circadian Abnormalities in a Paradigmatic mTOR-opathy. Cell Rep. 2017;20:868–880. doi: 10.1016/j.celrep.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147•.Ramanathan C., Kathale N.D., Liu D., Lee C., Freeman D.A., Hogenesch J.B., Cao R., Liu A.C. mTOR signaling regulates central and peripheral circadian clock function. PLOS Genet. 2018;14 doi: 10.1371/journal.pgen.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of several studies demonstrating that mTOR activity can modulate circadian gene expression, but is not essential for cellular timekeeping.
- 148.Walton Z.E., Patel C.H., Brooks R.C., Yu Y., Ibrahim-Hashim A., Riddle M., Porcu A., Jiang T., Ecker B.L., Tameire F. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell. 2018 doi: 10.1016/j.cell.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee H., Chen R., Kim H., Etchegaray J.-P., Weaver D.R., Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci U S A. 2011;108:16451–16456. doi: 10.1073/pnas.1107178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Etchegaray J.-P., Yu EA Indic P., Dallmann R., Weaver D.R. Casein kinase 1 delta (CK1δ) regulates period length of the mouse suprachiasmatic circadian clock in vitro. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meng Q.-J., Maywood E.S., Bechtold D.A., Lu W.-Q., Li J., Gibbs J.E., Dupré S.M., Chesham J.E., Rajamohan F., Knafels J. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci U S A. 2010;107:15240–15245. doi: 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reischl S., Vanselow K., Westermark P.O., Thierfelder N., Maier B., Herzel H., Kramer A. β-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J Biol Rhythms. 2007;22:375–386. doi: 10.1177/0748730407303926. [DOI] [PubMed] [Google Scholar]
- 153.Hirota T., Lee J.W., Lewis W.G., Zhang E.E., Breton G., Liu X., Garcia M., Peters E.C., Etchegaray J.-P., Traver D. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maier B., Wendt S., Vanselow J.T., Wallach T., Reischl S., Oehmke S., Schlosser A., Kramer A. A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev. 2009;23:708–718. doi: 10.1101/gad.512209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsuchiya Y., Akashi M., Matsuda M., Goto K., Miyata Y., Node K., Nishida E. Involvement of the protein kinase CK2 in the regulation of mammalian circadian rhythms. Sci Signal. 2009;2:ra26. doi: 10.1126/scisignal.2000305. [DOI] [PubMed] [Google Scholar]
- 156.Iitaka C., Miyazaki K., Akaike T., Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 157.Yin L., Wang J., Klein P.S., Lazar M.A. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 158.Hirota T., Lewis W.G., Liu A.C., Lee J.W., Schultz P.G., Kay S.A. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci U S A. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurabayashi N., Hirota T., Sakai M., Sanada K., Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol Cell Biol. 2010;30:1757–1768. doi: 10.1128/MCB.01047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sahar S., Zocchi L., Kinoshita C., Borrelli E., Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PLoS One. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spengler M.L., Kuropatwinski K.K., Schumer M., Antoch M.P. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle. 2009;8:4138–4146. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O’Neill J.S., Maywood E.S., Hastings M.H. Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. Handb Exp Pharmacol. 2013 doi: 10.1007/978-3-642-25950-0_4. [DOI] [PubMed] [Google Scholar]
- 163.Hastings M.H., Maywood E.S., O’Neill J.S. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol. 2008;18:R805–R815. doi: 10.1016/j.cub.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 164.Godinho S.I.H., Maywood E.S., Shaw L., Tucci V., Barnard A.R., Busino L., Pagano M., Kendall R., Quwailid M.M., Romero M.R. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 165.Siepka S.M., Yoo S.-H., Park J., Song W., Kumar V., Hu Y., Lee C., Takahashi J.S. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Ooijen G., Dixon L.E., Troein C., Millar A.J. Proteasome function is required for biological timing throughout the twenty-four hour cycle. Curr Biol. 2011;21:869–875. doi: 10.1016/j.cub.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gallego M., Virshup D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 168.Smith E.M., Lin J.-M., Meissner R.-A., Allada R. Dominant-negative CK2α induces potent effects on circadian rhythmicity. PLoS Genet. 2008;4:e12. doi: 10.1371/journal.pgen.0040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Merrow M., Mazzotta G., Chen Z., Roenneberg T. The right place at the right time: regulation of daily timing by phosphorylation. Genes Dev. 2006;20:2629–2633. doi: 10.1101/gad.1479706. [DOI] [PubMed] [Google Scholar]
- 170.He Q., Liu Y. Degradation of the Neurospora circadian clock protein FREQUENCY through the ubiquitin-proteasome pathway. Biochem Soc Trans. 2005;33:953–956. doi: 10.1042/BST20050953. [DOI] [PubMed] [Google Scholar]
- 171.Virshup D.M., Eide E.J., Forger D.B., Gallego M., Harnish E.V. Reversible protein phosphorylation regulates circadian rhythms. Cold Spring Harb Symp Quant Biol. 2007;72:413–420. doi: 10.1101/sqb.2007.72.048. [DOI] [PubMed] [Google Scholar]
- 172.Takano A., Shimizu K., Kani S., Buijs R.M., Okada M., Nagai K. Cloning and characterization of rat casein kinase 1ε. FEBS Lett. 2000;477:106–112. doi: 10.1016/s0014-5793(00)01755-5. [DOI] [PubMed] [Google Scholar]
- 173.Vielhaber E., Eide E., Rivers A., Gao Z.H., Virshup D.M. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akashi M., Tsuchiya Y., Yoshino T., Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol Cell Biol. 2002;22:1693–1703. doi: 10.1128/MCB.22.6.1693-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eide E.J., Vielhaber E.L., Hinz W.A., Virshup D.M. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gallego M., Eide E.J., Woolf M.F., Virshup D.M., Forger D.B. An opposite role for tau in circadian rhythms revealed by mathematical modeling. Proc Natl Acad Sci U S A. 2006;103:10618–10623. doi: 10.1073/pnas.0604511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sanada K., Okano T., Fukada Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J Biol Chem. 2002;277:267–271. doi: 10.1074/jbc.M107850200. [DOI] [PubMed] [Google Scholar]
- 178.Yu W., Zheng H., Houl J.H., Dauwalder B., Hardin P.E. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev. 2006;20:723–733. doi: 10.1101/gad.1404406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim E.Y., Edery I. Balance between DBT/CKIepsilon kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc Natl Acad Sci U S A. 2006;103:6178–6183. doi: 10.1073/pnas.0511215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schafmeier T., Haase A., Káldi K., Scholz J., Fuchs M., Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 181.He Q., Cha J., He Q., Lee H.-C., Yang Y., Liu Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 2006;20:2552–2565. doi: 10.1101/gad.1463506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eide E.J., Woolf M.F., Kang H., Woolf P., Hurst W., Camacho F., Vielhaber E.L., Giovanni A., Virshup D.M. Control of Mammalian Circadian Rhythm by CKI ε -Regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lamia K.A., Sachdeva U.M., DiTacchio L., Williams E.C., Alvarez J.G., Egan D.F., Vasquez D.S., Juguilon H., Panda S., Shaw R.J. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Um J.H., Yang S., Yamazaki S., Kang H., Viollet B., Foretz M., Chung J.H. Activation of 5’-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 185.Li M.-D., Ruan H.-B., Hughes M.E., Lee J.-S., Singh J.P., Jones S.P., Nitabach M.N., Yang X. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17:303–310. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim E.Y., Jeong E.H., Park S., Jeong H.-J., Edery I., Cho J.W. A role for O-GlcNAcylation in setting circadian clock speed. Genes Dev. 2012;26:490–502. doi: 10.1101/gad.182378.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu X., Li H., Liu Q., Niu Y., Hu Q., Deng H., Cha J., Wang Y., Liu Y., He Q. Role for protein kinase A in the Neurospora circadian clock by regulating white collar-independent frequency transcription through phosphorylation of RCM-1. Mol Cell Biol. 2015;35:2088–2102. doi: 10.1128/MCB.00709-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weber F., Hung H.-C., Maurer C., Kay S.A. Second messenger and Ras/MAPK signalling pathways regulate CLOCK/CYCLE-dependent transcription. J Neurochem. 2006;98:248–257. doi: 10.1111/j.1471-4159.2006.03865.x. [DOI] [PubMed] [Google Scholar]
- 189.Jeon S.-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48 doi: 10.1038/emm.2016.81. e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Levine Z.G., Walker S. The Biochemistry of O-GlcNAc Transferase: which functions make it essential in mammalian cells? Annu Rev Biochem. 2016;85:631–657. doi: 10.1146/annurev-biochem-060713-035344. [DOI] [PubMed] [Google Scholar]
- 191.Cho-Chung Y.S., Pepe S., Clair T., Budillon A., Nesterova M. cAMP-dependent protein kinase: role in normal and malignant growth. Crit Rev Oncol Hematol. 1995;21:33–61. doi: 10.1016/1040-8428(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 192•.Dunlap J.C., Loros J.J. Just-so stories and origin myths: phosphorylation and structural disorder in circadian clock proteins. Mol Cell. 2017 doi: 10.1016/j.molcel.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; This highly insightful and amusing review is strongly recommended.
- 193.Schafer K.A. The cell cycle: a review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 194.Dibner C., Sage D., Unser M., Bauer C., D’ eysmond T., Naef F., Schibler U. Circadian gene expression is resilient to large fluctuations in overall transcription rates. EMBO J. 2009;28262:123–134. doi: 10.1038/emboj.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oliveira S.M.D., Häkkinen A., Lloyd-Price J., Tran H., Kandavalli V., Ribeiro A.S. Temperature-dependent model of multi-step transcription initiation in Escherichia coli based on live single-cell measurements. PLOS Comput Biol. 2016;12 doi: 10.1371/journal.pcbi.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Abbondanzieri E.A., Shaevitz J.W., Block S.M. Picocalorimetry of Transcription by RNA Polymerase. Biophys J. 2005;89:L61–L63. doi: 10.1529/biophysj.105.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Farewell A., Neidhardt F.C. Effect of temperature on in vivo protein synthetic capacity in Escherichia coli. J Bacteriol. 1998;180:4704–4710. doi: 10.1128/jb.180.17.4704-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Craig N. Effect of reduced temperatures on protein synthesis in mouse L cells. Cell. 1975;4:329–335. doi: 10.1016/0092-8674(75)90153-1. [DOI] [PubMed] [Google Scholar]
- 199.Isojima Y., Nakajima M., Ukai H., Fujishima H., Yamada R.G., Masumoto K.-h., Kiuchi R., Ishida M., Ukai-Tadenuma M., Minami Y. CKI / -dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci. 2009;106:15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200•.Shinohara Y., Koyama Y.M., Ukai-tadenuma M., Hirokawa T., Kikuchi M., Yamada R.G., Ukai H., Fujishima H., Umehara T., Tainaka K. Temperature-sensitive substrate and product binding underlie temperature-compensated phosphorylation in the clock. Mol Cell. 2017;67:783–798. doi: 10.1016/j.molcel.2017.08.009. e20. [DOI] [PubMed] [Google Scholar]; This paper presents detailed biochemical and molecular evidence that temperature compensation is intrinsic to casein kinase 1.
- 201.Nakajima M., Imai K., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 202.Egli M. Intricate protein-protein interactions in the cyanobacterial circadian clock. J Biol Chem. 2014;289:21267–21275. doi: 10.1074/jbc.R114.579607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cohen S.E., Golden S.S. Circadian rhythms in cyanobacteria. Microbiol Mol Biol Rev. 2015;79:373–385. doi: 10.1128/MMBR.00036-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kondo T. A cyanobacterial circadian clock based on the kai oscillator. Cold Spring Harb Symp Quant Biol. 2007;72:47–55. doi: 10.1101/sqb.2007.72.029. [DOI] [PubMed] [Google Scholar]
- 205.Abe J., Hiyama T.B., Mukaiyama A., Son S., Mori T., Saito S., Osako M., Wolanin J., Yamashita E., Kondo T. Atomic-scale origins of slowness in the cyanobacterial circadian clock. Science. 2015;349:312–316. doi: 10.1126/science.1261040. [DOI] [PubMed] [Google Scholar]
- 206.Ode K.L., Ueda H.R. Design principles of phosphorylation-dependent timekeeping in eukaryotic circadian clocks. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moore M.J., Adams J.A., Taylor S.S. Structural basis for peptide binding in protein kinase A. Role of glutamic acid 203 and tyrosine 204 in the peptide-positioning loop. J Biol Chem. 2003;278:10613–10618. doi: 10.1074/jbc.M210807200. [DOI] [PubMed] [Google Scholar]
- 208.Bar-Even A., Noor E., Savir Y., Liebermeister W., Davidi D., Tawfik D.S., Milo R. The moderately efficient enzyme: Evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry. 2011;50:4402–4410. doi: 10.1021/bi2002289. [DOI] [PubMed] [Google Scholar]
- 209.Gandy S., Czernik A.J., Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988;85:6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teng S.-W., Mukherji S., Moffitt J.R., de Buyl S., O’Shea E.K., Harmer S.L., Panda S., Kay S.A., Dunlap J.C., Mihalcescu I. Robust circadian oscillations in growing cyanobacteria require transcriptional feedback. Science. 2013;340:737–740. doi: 10.1126/science.1230996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gutu A., O’Shea E.K. Two antagonistic clock-regulated histidine kinases time the activation of circadian gene expression. Mol Cell. 2013;50:288–294. doi: 10.1016/j.molcel.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kitayama Y., Nishiwaki-Ohkawa T., Sugisawa Y., Kondo T. KaiC intersubunit communication facilitates robustness of circadian rhythms in cyanobacteria. Nat Commun. 2013;4:2897. doi: 10.1038/ncomms3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Egli M. Architecture and mechanism of the central gear in an ancient molecular timer. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2016.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morgan D.O. CYCLIN-DEPENDENT KINASES: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 215.Miake-Lye R., Kirschner M.W. Induction of early mitotic events in a cell-free system. Cell. 1985;41:165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- 216.Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hara K., Tydeman P., Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc Natl Acad Sci U S A. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dabauvalle M.C., Doree M., Bravo R., Karsenti E. Role of nuclear material in the early cell cycle of xenopus embryos. Cell. 1988;52:525–533. doi: 10.1016/0092-8674(88)90465-5. [DOI] [PubMed] [Google Scholar]
- 219.Pomerening J.R., Kim S.Y., Ferrell J.E. Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell. 2005;122:565–578. doi: 10.1016/j.cell.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 220.Pomerening J.R., Sontag E.D., Ferrell J.E. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 221.Woolum J.C. A re-examination of the role of the nucleus in generating the circadian rhythm in Acetabularia. J Biol Rhythms. 1991;6:129–136. doi: 10.1177/074873049100600203. [DOI] [PubMed] [Google Scholar]
- 222.Sweeney B.M., Haxo F.T. Persistence of a photosynthetic rhythm in enucleated Acetabularia. Science. 1961;134:1361–1363. doi: 10.1126/science.134.3487.1361. [DOI] [PubMed] [Google Scholar]
- 223.Miyagishima S., Fujiwara T., Sumiya N., Hirooka S., Nakano A., Kabeya Y., Nakamura M. Translation-independent circadian control of the cell cycle in a unicellular photosynthetic eukaryote. Nat Commun. 2014;5:3807. doi: 10.1038/ncomms4807. [DOI] [PubMed] [Google Scholar]
- 224.Roy S., Beauchemin M., Dagenais-Bellefeuille S., Letourneau L., Cappadocia M., Morse D. The Lingulodinium circadian system lacks rhythmic changes in transcript abundance. BMC Biol. 2014;12:107. doi: 10.1186/s12915-014-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.O’Neill J.S., van Ooijen G., Dixon L.E., Troein C., Corellou F., Bouget F.-Y., Reddy A.B., Millar A.J. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bouget F.-Y., Lefranc M., Thommen Q., Pfeuty B., Lozano J.-C., Schatt P., Botebol H., Vergé V. Transcriptional versus non-transcriptional clocks: a case study in Ostreococcus. Mar Genomics. 2014;14:17–22. doi: 10.1016/j.margen.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 227.Cho C.-S., Yoon H.J., Kim J.Y., Woo H.A., Rhee S.G. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc Natl Acad Sci. 2014;111:12043–12048. doi: 10.1073/pnas.1401100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228•.Henslee E.A., Crosby P., Kitcatt S.J., Parry J.S.W., Bernardini A., Abdallat R.G., Braun G., Fatoyinbo H.O., Harrison E.J., Edgar R.S. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat Commun. 2017;8:1978. doi: 10.1038/s41467-017-02161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper extends knowledge of circadian rhythms in isolated human red blood cells to encompass membrane physiology and ion transport.
- 229.Fustin J.-M., Doi M., Yamaguchi Y., Hida H., Nishimura S., Yoshida M., Isagawa T., Morioka M.S., Kakeya H., Manabe I. RNA-Methylation-Dependent RNA Processing Controls the Speed of the Circadian Clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 230.Woo K.-C., Ha D.-C., Lee K.-H., Kim D.-Y., Kim T.-D., K-T Kim. Circadian amplitude of cryptochrome 1 is modulated by mRNA stability regulation via cytoplasmic hnRNP D oscillation. Mol Cell Biol. 2010;30:197–205. doi: 10.1128/MCB.01154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Castelo-Szekely V., Arpat A.B., Janich P., Gatfield D. Translational contributions to tissue specificity in rhythmic and constitutive gene expression. Genome Biol. 2017;18:116. doi: 10.1186/s13059-017-1222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Atger F., Gobet C., Marquis J., Martin E., Wang J., Weger B., Lefebvre G., Descombes P., Naef F., Gachon F. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci. 2015;112:E6579–E6588. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kojima S., Sher-Chen E.L., Green C.B. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev. 2012;26:2724–2736. doi: 10.1101/gad.208306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kojima S., Gendreau K.L., Sher-Chen E.L., Gao P., Green C.B. Changes in poly(A) tail length dynamics from the loss of the circadian deadenylase Nocturnin. Sci Rep. 2015;5:17059. doi: 10.1038/srep17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Preußner M., Wilhelmi I., Schultz A.-S., Finkernagel F., Michel M., Möröy T., Heyd F. Rhythmic U2af26 alternative splicing controls PERIOD1 stability and the circadian clock in mice. Mol Cell. 2014;54:651–662. doi: 10.1016/j.molcel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 236.Woo K.-C., Kim T.-D., Lee K.-H., Kim D.-Y., Kim W., Lee K.-Y., K-T Kim. Mouse period 2 mRNA circadian oscillation is modulated by PTB–mediated rhythmic mRNA degradation. Nucleic Acids Res. 2009;37:26–37. doi: 10.1093/nar/gkn893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Green C.B. Circadian posttranscriptional regulatory mechanisms in mammals. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Benegiamo G., Brown S.A., Panda S. RNA Dynamics in the Control of Circadian Rhythm. Adv Exp Med Biol. 2016;907 doi: 10.1007/978-3-319-29073-7_5. [DOI] [PubMed] [Google Scholar]
- 239.Green C.B. Circadian Post-transcriptional Control of Metabolism. A Time Metab Horm. 2016 [PubMed] [Google Scholar]