Abstract
Background
Neuroinflammation is closely associated with functional outcome in subarachnoid hemorrhage (SAH) patients. Our recent study demonstrated that fluoxetine inhibited NLRP3 inflammasome activation and attenuated necrotic cell death in early brain injury after SAH, while the effects and potential mechanisms of fluoxetine on neuroinflammation after SAH have not been well-studied yet.
Methods
One hundred and fifty-three male SD rats were subjected to the endovascular perforation model of SAH. Fluoxetine (10 mg/kg) was administered intravenously at 6 h after SAH induction. TAK-242 (1.5 mg/kg), an exogenous TLR4 antagonist, was injected intraperitoneally 1 h after SAH. SAH grade, neurological scores, brain water content, Evans blue extravasation, immunofluorescence/TUNEL staining, quantitative real-time polymerase chain reaction (qRT-PCR), and western blot were performed.
Results
Fluoxetine administration attenuated BBB disruption, brain edema, and improved neurological function after SAH. In addition, fluoxetine alleviated the number of Iba-1-positive microglia/macrophages, neutrophil infiltration, and cell death. Moreover, fluoxetine reduced the levels of pro-inflammatory cytokines, downregulated the expression of TLR4 and MyD88, and promoted the nuclear translocation of NF-κB p65, which were also found in rats with TAK-242 administration. Combined administration of fluoxetine and TAK-242 did not enhance the neuroprotective effects of fluoxetine.
Conclusion
Fluoxetine attenuated neuroinflammation and improved neurological function in SAH rats. The potential mechanisms involved, at least in part, TLR4/MyD88/NF-κB signaling pathway.
Electronic supplementary material
The online version of this article (10.1186/s12974-018-1388-x) contains supplementary material, which is available to authorized users.
Keywords: Subarachnoid hemorrhage, Early brain injury, Neuroinflammation, Fluoxetine
Background
Subarachnoid hemorrhage (SAH) is a severe subtype of stroke with high mortality and morbidity; 25% of SAH patients died within 2 days, and cognitive and functional deficits present in about 20% of SAH survivors [1, 2]. In recent years, the importance of early brain injury has been concerned by researchers. Initial clinical severity after SAH has been shown to be the most important predictor for clinical outcomes in patients [3]. In addition, alleviating early brain injury has been reported to exert neuroprotective effects in SAH model [4–6].
Neuroinflammation is a well-recognized consequence of SAH and considered as an important contributor for early brain injury, cerebral vasospasm, and delayed brain injury after SAH [7, 8]. In addition, neuroinflammation is closely associated with functional outcome in SAH patients [9, 10]. Toll-like receptors (TLRs) belong to a large family of pattern recognition receptors that play a key role in inflammatory responses [11]. Of all the TLR family members, TLR4 is widely expressed in the central nervous system, including microglia, neurons, astrocytes, endothelial cells [12]. After stimulation of TLR4 with ligands, the MyD88-dependent pathway activates NF-κB, which produces pro-inflammatory cytokines mediators such as tumor necrosis factor (TNF-α) [13]. Thus, therapies target TLR4 signaling pathway, and subsequent neuroinflammation may offer potential treatment to protect against neuroinflammation after SAH.
Fluoxetine is one of the serotonin selective reuptake inhibitors. Since the least toxicity and side effects, fluoxetine has been widely prescribed depression and anxiety disorders [14]. Neuroprotective effects of fluoxetine have been demonstrated in different neurological diseases [15–18]. Fluoxetine has been shown to exert a capacity to regulate neuroinflammation [19, 20]. Our recent study also demonstrated that fluoxetine inhibited NLRP3 inflammasome activation and subsequent necrotic cell death in early brain injury after SAH [18]. Importantly, recent studies also demonstrated that fluoxetine inhibits TLR4 activation and subsequent NF-κB signaling pathway in vivo and in vitro [21–24]. In the present study, we investigated the effects of fluoxetine in neuroinflammation and the potential TLR4/MyD88/NF-κB signaling pathway in early brain injury after SAH.
Methods
Study design
Experiment 1
Male SD rats were randomly divided into four groups: sham, sham+fluoxetine, SAH+vehicle, and SAH+fluoxetine group. Fluoxetine was purchased from Selleck and dissolved in sterile 0.9% NaCl. Fluoxetine (10 mg/kg) or vehicle was injected intravenously at 6 h after SAH induction as previously described [18]. SAH grade and neurological scores were measured in all groups. Brain edema (n = 6/group), Evans blue extravasation (n = 6/group), immunofluorescence/TUNEL staining (n = 5/group), quantitative real-time polymerase chain reaction (qRT-PCR) (n = 5/group), and western blot (n = 6/group) were performed.
Experiment 2
Male SD rats were randomly divided into four groups: SAH+vehicle, SAH+TAK-242, and SAH+fluoxetine, SAH+fluoxetine+TAK-242 group. TAK-242 (1.5 mg/kg), an exogenous TLR4 antagonist, was injected intraperitoneally 1 h after SAH as previously described [25]. Neurological scores, Evans blue extravasation (n = 6/group), qRT-PCR (n = 5/group), and western blot (n = 6/group) were performed.
SAH model
Male Sprague-Dawley (SD) rats were purchased from SLAC Laboratory Animal Company (Shanghai, China) and housed in a controlled humidity and temperature conditions. The endovascular perforation model was performed to induce rat SAH as previously described [26]. Briefly, we isolated the left carotid artery and its branches under anesthesia of pentobarbital (50 mg/kg). Then, we divided the external carotid artery (ECA) and advanced a 4-0 monofilament suture until resistance was felt. Subsequently, we punctured the vessel and induced SAH. The sham rats underwent the same procedure without puncturing.
SAH grade and neurological scores
SAH grade and neurological scores were blindly assessed at 24 h after SAH as previously described [27]. Briefly, the basal cistern was divided into six segments. Each part was blindly obtained a grade from 0 to 3 judging by the amount of the blood clot in subarachnoid space. Then, the rats have received a total score ranging from 0 to 18. Neurological scores were blindly evaluated with a modification of the Garcia scoring system [28]. This scoring system has six parts as follow: spontaneous activity, spontaneous movements of all limbs, movement of forelimbs, climbing wall of the wire cage, reaction to touch on both side of trunk, and response to vibrissae touch. Then, the rats have received a total score ranging from 3 to 18. In this study, rats with SAH grade < 9 were excluded.
Brain edema and blood-brain barrier disruption
Brain edema and blood-brain barrier (BBB) disruption were evaluated at 24 h after SAH as previously described [29]. Brain water content and Evans blue leakage were used to assessing brain edema and BBB disruption. Briefly, under deep anesthesia, rats were sacrificed. Then, brains were removed and divided into the left hemisphere, right hemisphere, cerebellum, and brainstem. The left hemispheres were weighed immediately to get the wet weight and dried at 105 °C for 3 days to obtain dry weight. Brain water content was calculated as [(wet weight-dry weight)/wet weight] × 100%. Evans blue dye (2%, 5 ml/kg) was administrated via the left femoral vein and circulated for 1 h. Under deep anesthesia, rats were sacrificed by cardiac perfusion. Then, we removed and separated the brain to get the left hemispheres immediately. Subsequently, we weighted the brain samples and homogenized with 3 ml of 50% trichloroacetic acid, then centrifuged at 15000 g for 30 min. The supernatant was mixed with an equal volume of trichloroacetic acid with ethanol. After overnight incubation (4 °C), the samples were centrifuged again (15,000g, 30 min) and measured by spectrofluorophotometer (excitation wavelength 620 nm and emission wavelength 680 nm).
Quantitative real-time polymerase chain reaction
The left basal cortical specimen (about 50–100 mg) in the face of the blood clot was collected for PCR detection at 24 h after SAH (as shown in Additional file 1: Figure S1). The total mRNA was then extracted using TRIzolTM Plus RNA Purification Kit (#12183-555, Invitrogen, China). Then, we determined the quantity of the purified RNA using UV absorbance at 260 nm. Subsequently, 1 μg of purified RNA from each sample was reverse-transcribed to cDNA. Superscript™ III First-Stand Synthesis SuperMix for qRT-PCR (#11752-050) was used to synthesize cDNA. The specific sequence of primers used was described as follows: TNF-α: sense primer 5′-GGT CCC AAC AAG GAG GAG AAG TTC-3′, antisense primer 5′-CCG CTT GGT GGT TTG CTA CGA C-3′; IL-1β: sense primer 5′-CGT GGG ATG ATG ACG ACC TGC-3′, antisense primer 5′-GGA GAA TAC CAC TTG TTG GCT TAT-3′; IL-6: sense primer 5′-GAC AGC CAC TGC CTT CCC TAC TT-3′, antisense primer 5′-CAG AAT TGC CAT TGC ACA ACT CT-3′; CD86: sense primer 5′-CAT CTA AGC AAG GAT ACC CGA AAC-3′, and antisense primer 5′-GAG ATA GGC TGA TGG AGA CAC TGA A-3′. PCR amplification was performed with a program of 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, and 63 °C for 25 s. The relative mRNA level of each target gene was calculated using the 2-∇∇CT methods as previously described [30].
Immunofluorescence staining
Immunofluorescence staining was performed at 24 h after SAH as previously described [29]. Briefly, rats were deeply euthanized and perfused with 4% paraformaldehyde in 0.1-mM phosphate-buffered saline (PBS, PH7.4). Brain samples were immersed in 30% sucrose until sinking to the bottom; 18 um-thick slices were cut with a cryostat. The primary antibodies were polyclonal goat anti-Iba-1(1:500, ab5076, Abcam), monoclonal mouse anti-NeuN (1:500, ab104224, Abcam), and polyclonal rabbit anti-MPO (1:300, ab65871, Abcam). Alexa Fluor 594 donkey anti-goat IgG (1:500, Invitrogen) and donkey anti-mouse (1:500, Invitrogen) were used as secondary antibody. Terminal deoxynucleotide transferase-deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) was performed following the manufacturer’s protocol (Roche, Switzerland). Finally, the slices were covered by DAPI and observed under a fluorescence microscope. All procedures were conducted by two investigators blind to the experimental condition.
To quantify Iba-1-positive, MPO-positive, TUNEL-positive cells, we selected at least three sections per rats with similar areas of ipsilateral cortex (Additional file 1: Figure S1) and three fields with a magnification of × 200 or × 400 per section. For quantification of Iba-1-positive and MPO-positive cells, the numbers from these fields were averaged and expressed as positive cells per square millimeter for each mouse. For quantification of apoptotic neurons, the percentage of TUNEL-positive neurons was calculated as follows: (number of TUNEL-positive neurons/total number of neurons) × 100%. Tissue sections were analyzed by an observer who was blinded to the experimental cohorts.
Western blot
Western blot was performed at 24 h after SAH as previously described [26]. Under deep anesthesia, rats were sacrificed by cardiac perfusion with 0.1 M PBS, then brains were removed and the left basal cortical specimens in the face of the blood clot were obtained. The nuclear proteins were prepared by the NE-PER nuclear extraction reagents (Thermo, Rockford). Briefly, the ipsilateral cortex was homogenized and centrifuged for 10 min (1000g, 4 °C). Total protein was determined by BCA Protein Assay Kit (Beyotime, Shanghai, China). An equal amount of protein (60 μg) was suspended in loading buffer (denatured at 95 °C for 5 min) and loaded on an SDS-PAGE and transferred to nitrocellulose membranes. Then, we blocked the membranes with a nonfat dry milk buffer for 2 h, followed by incubation overnight with the following primary antibodies: polyclonal rabbit anti-NF-κB p65 (1:1000, ab16502, Abcam), monoclonal mouse anti-TLR4 (1:200, sc-293,072, Santa Cruz Biotechnology), polyclonal rabbit anti-MyD88 (1:1000, ab2064, Abcam), monoclonal rabbit anti-MMP9 (1:5000, ab137867, Abcam), polyclonal rabbit anti-ZO-1 (1:2000, ab96587, Abcam); polyclonal goat anti-claudin-5 (1:500, sc-17,668, Santa Cruz Biotechnology), monoclonal rabbit anti-occludin (1:50000, ab167161, Abcam). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Blot bands were detected by X-ray film and quantified using Image J software (NIH).
Statistical analysis
Data were presented as mean ± SD. For the data satisfying normal distribution, comparisons between groups were performed by one-way ANOVA as appropriate. For the data satisfying non-normal distribution, comparisons between groups were performed by Kruskal-Wallis test or Mann-Whitney test. Differences were considered significant at p < 0.05. All statistical analyses were performed using GraphPad Prism and SPSS software.
Results
Physiological parameters, mortality, and SAH grade
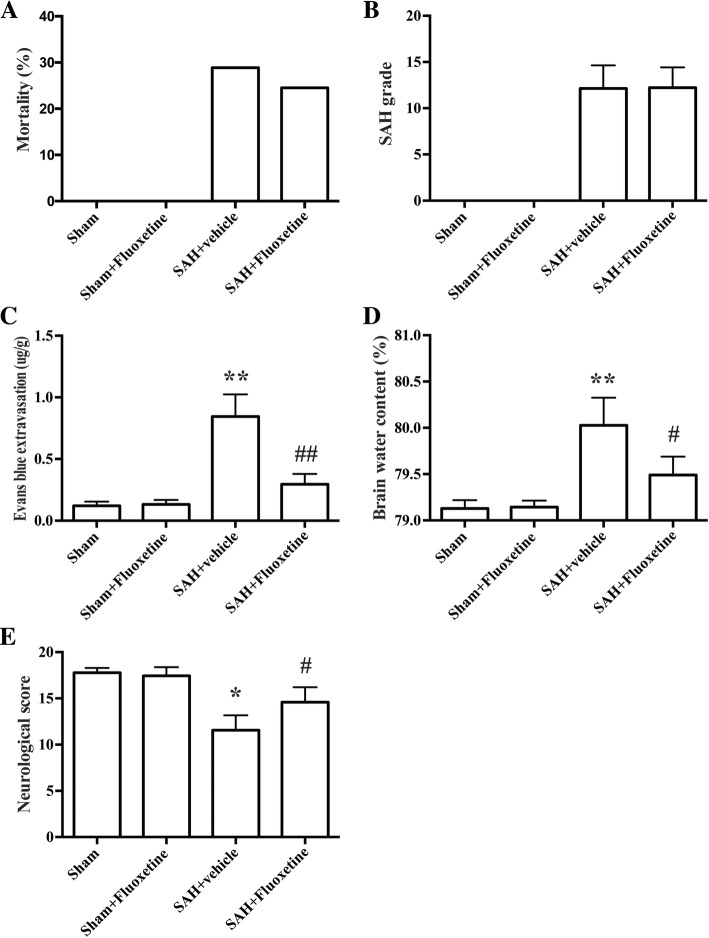
During the surgical procedures, no significant difference was observed in all physiological parameters including the mean arterial pressure, arterial PH, PO2, PCO2, and blood glucose levels (data not shown). None of the rats died in the sham and sham+fluoxetine group. In experiment 1, the mortality of SAH+vehicle and SAH+fluoxetine group were 28.2% (11/39, Fig. 1a) and 24.5% (9/37, Fig. 1a), respectively. In experiment 2, the mortality of SAH+vehicle, SAH+TAK-242, SAH+fluoxetine, and SAH+fluoxetine+TAK-242 group were 29.2% (7/24), 22.7% (5/22), 22.7% (5/22), and 19.0% (4/21), respectively. SAH grade was blindly evaluated in our study. However, there is no significant difference between SAH groups (p > 0.05, Fig. 1b).
Fig. 1.
The effects of fluoxetine on mortality, SAH grade, neurological scores, Evans blue extravasation, and brain edema. a The quantification of mortality. b The quantification of SAH grade. n = 28/group. c The quantification of Evans blue extravasation. *p < 0.05 vs sham. #p < 0.05 vs SAH+vehicle. n = 6/group. d The quantification of brain water content. **p < 0.01 vs sham. #p < 0.05 vs SAH+vehicle. n = 6/group. e The quantification of neurological score. *p < 0.05 vs sham. #p < 0.05 vs SAH+vehicle. n = 28/group
Fluoxetine attenuated BBB disruption, brain edema, and neurological deficits
SAH induction increased the amount of Evans blue extravasation, indicating the disruption of BBB (p < 0.01, Fig. 1c). Similarly, SAH also increased the ratio of brain water content, indicating brain edema (p < 0.01, Fig. 1d). In addition, neurological scores were lower in the SAH+vehicle group than in the sham rats, indicating neurological impairments (p < 0.01, Fig. 1e). The BBB disruption and brain edema were significantly attenuated by fluoxetine administration (p < 0.01, Fig. 1c and p < 0.05, Fig. 1d). Fluoxetine treatment also improved neurological function compared with the SAH+vehicle group (p < 0.05, Fig. 1e).
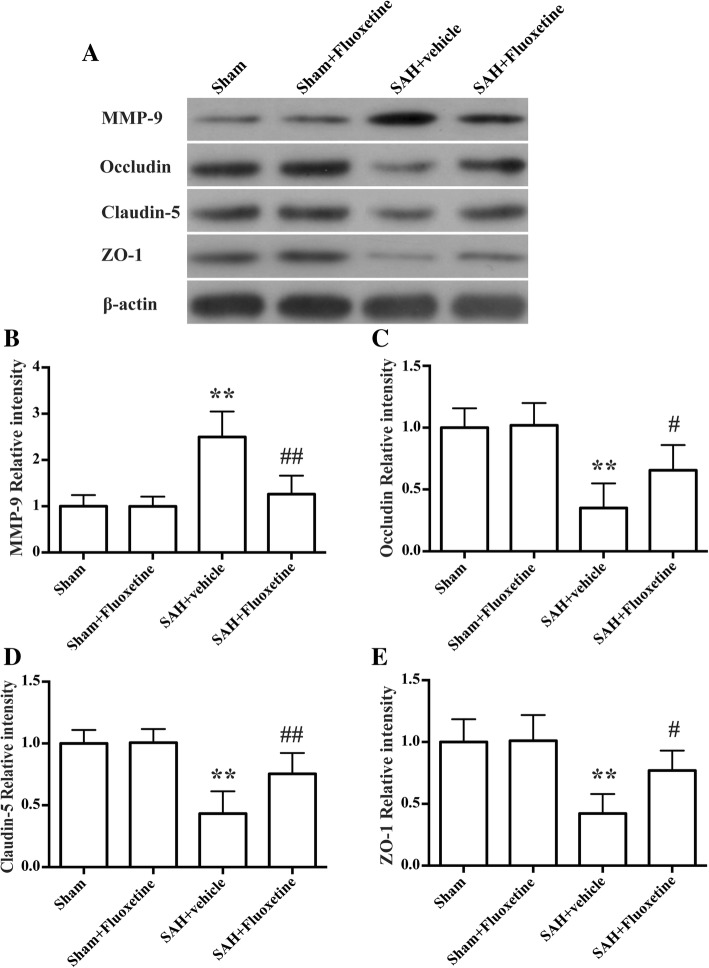
Fluoxetine downregulated MMP-9 expression and decreased the degradation of tight junction proteins
Rats in SAH+vehicle group had high levels of MMP-9 than control rats (p < 0.01, Fig. 2a, b), and rats post-treated with fluoxetine had a lower MMP-9 expression (p < 0.01, Fig. 2a, b). The protein levels of tight junction proteins, including occludin, claudin-5, and ZO-1, were significantly decreased in the SAH+vehicle group when compared with the sham group (p < 0.01, Fig. 2 a, c–e), whereas fluoxetine treatment significantly upregulated their protein levels (p < 0.05, Fig. 2c, e, and p < 0.01, Fig. 2d).
Fig. 2.
Fluoxetine downregulated MMP-9 expression and prevented degradation of the tight junction proteins in the ipsilateral cortex at 24 h after SAH. a Representative western blot bands of MMP-9, occludin, claudin-5, and ZO-1. b Densitometric quantification of MMP-9. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 6/group. c Densitometric quantification of occludin. **p < 0.01 vs sham. #p < 0.05 vs SAH+vehicle. n = 6/group. d Densitometric quantification of claudin-5. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 6/group. e Densitometric quantification of ZO-1. **p < 0.01 vs sham. #p < 0.05 vs SAH+vehicle. n = 6/group
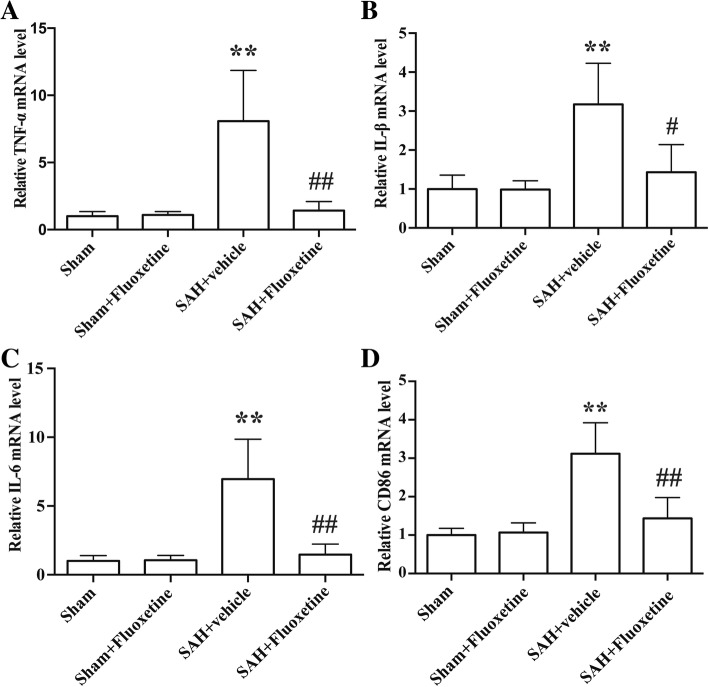
Fluoxetine decreased the expression of pro-inflammatory cytokines
qRT-PCR results showed the mRNA levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and CD86 were significantly increased in the SAH+vehicle group compared with the sham group (p < 0.01, Fig. 3a–d), while fluoxetine administration significantly reduced the mRNA levels of TNF-α (p < 0.01, Fig. 3a), IL-1β (p < 0.05, Fig. 3b), IL-6 (p < 0.01, Fig. 3c), and CD86 (p < 0.01, Fig. 3d).
Fig. 3.
Fluoxetine decreased the mRNA levels of pro-inflammatory cytokines. a The quantification of TNF-α mRNA levels. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 5/group. b The quantification of IL-1β mRNA levels. **p < 0.01 vs sham. #p < 0.05 vs SAH + vehicle. n = 5/group. c The quantification of IL-6 mRNA levels. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 5/group. d The quantification of CD86 mRNA levels. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 5/group
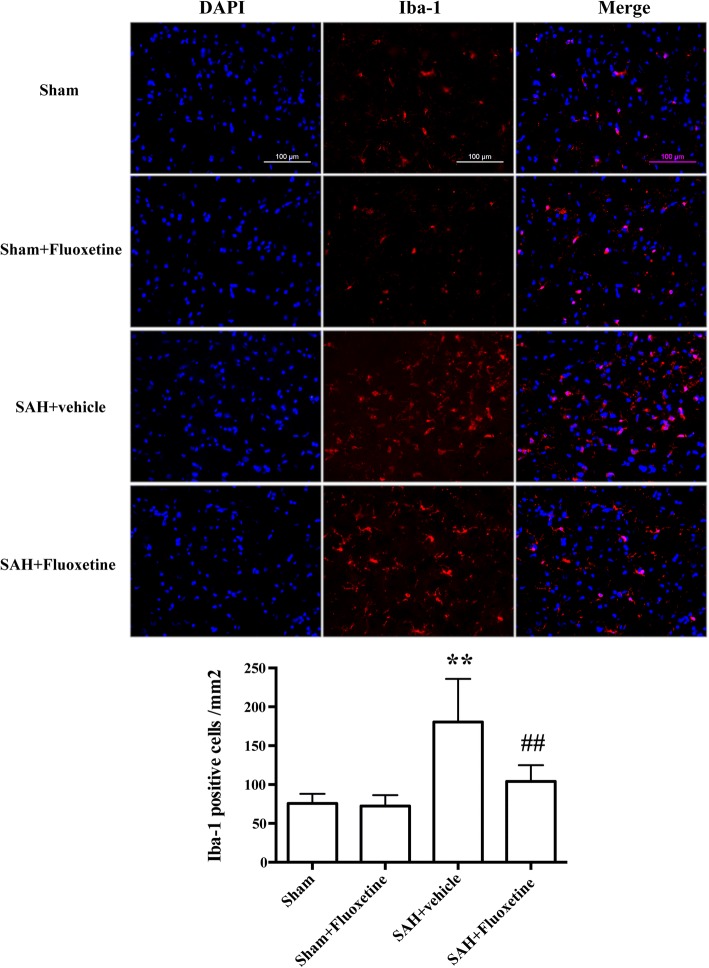
Fluoxetine reduced Iba-1-positive microglia/macrophages, neutrophil infiltration, and neuronal apoptosis
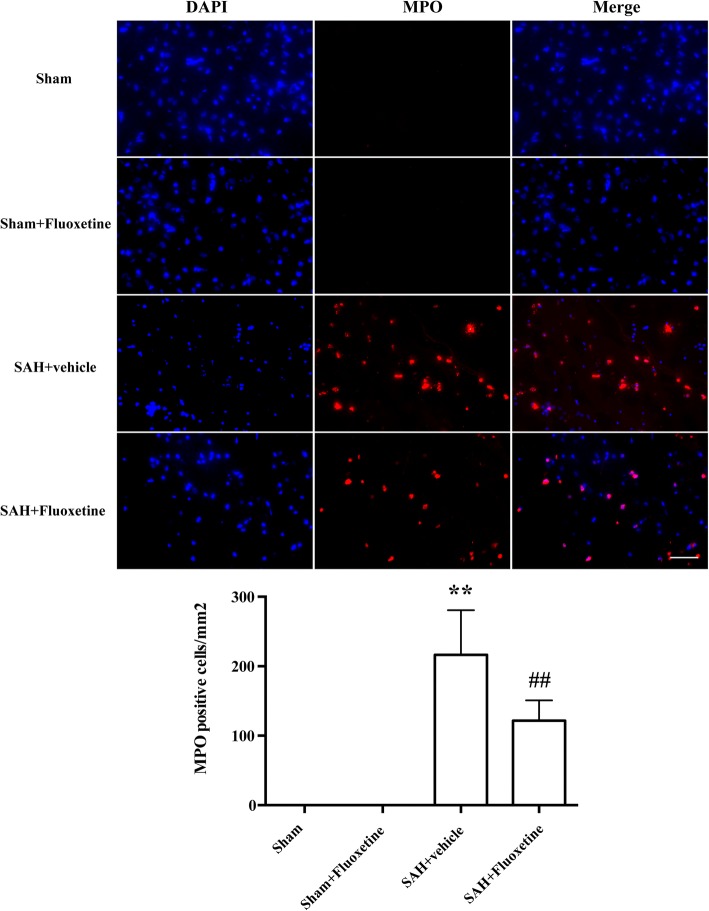
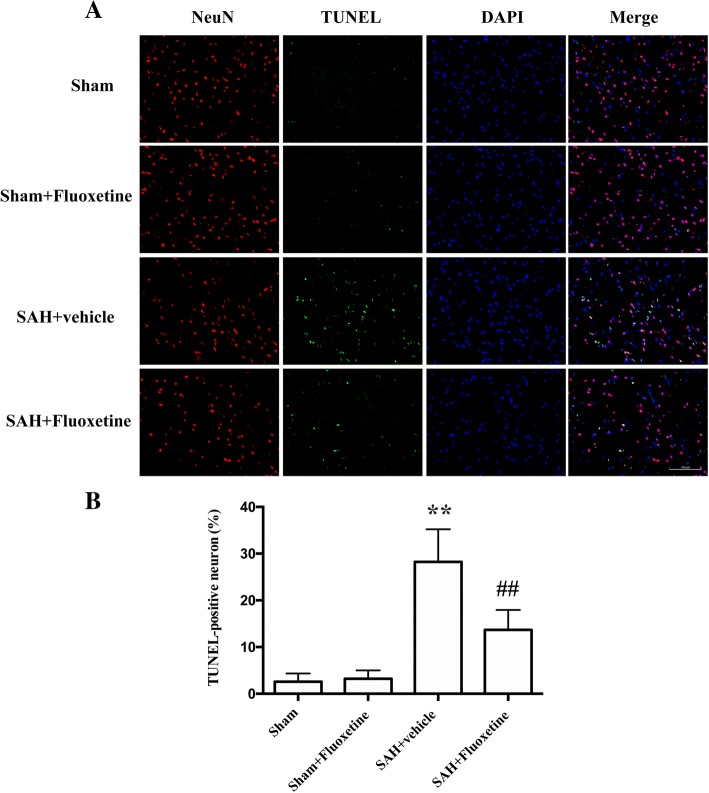
A significant increase of Iba-1positive cells was detected in SAH+vehicle group compared with the sham group (p < 0.01, Fig. 4a, b), whereas fluoxetine significantly decreased the number of Iba-1 positive cells (p < 0.01, Fig. 4a, b). MPO-positive cells markedly increased in the ipsilateral cortex after SAH when compared with the sham group (p < 0.01, Fig. 5a, b), while administration of fluoxetine significantly reduced the number of MPO-positive cells after SAH (p < 0.01, Fig. 5a, b). The percentage of TUNEL-positive neurons was significantly higher in the SAH+vehicle group than the control group (p < 0.01, Fig. 6a, b), whereas fluoxetine administration significantly decreased the percentage of TUNEL-positive neurons (p < 0.01, Fig. 6a, b).
Fig. 4.
Fluoxetine decreased the number of Iba-1-positive microglia/macrophages in the ipsilateral cortex at 24 h after SAH. a Representative Iba-1 staining. b The quantification of Iba-1-positive cells. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. Scale bar = 100 μm. n = 5/group
Fig. 5.
Fluoxetine inhibited neutrophil infiltration in the ipsilateral cortex at 24 h after SAH. a Representative MPO staining. b The quantification of MPO-positive cells. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. Scale bar = 50 μm. n = 5/group
Fig. 6.
Fluoxetine attenuated neuronal apoptosis in the ipsilateral cortex at 24 h after SAH. a Representative TUNEL staining. b The percentage of TUNEL-positive neurons. ***p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. Scale bar = 100 μm. n = 5/group
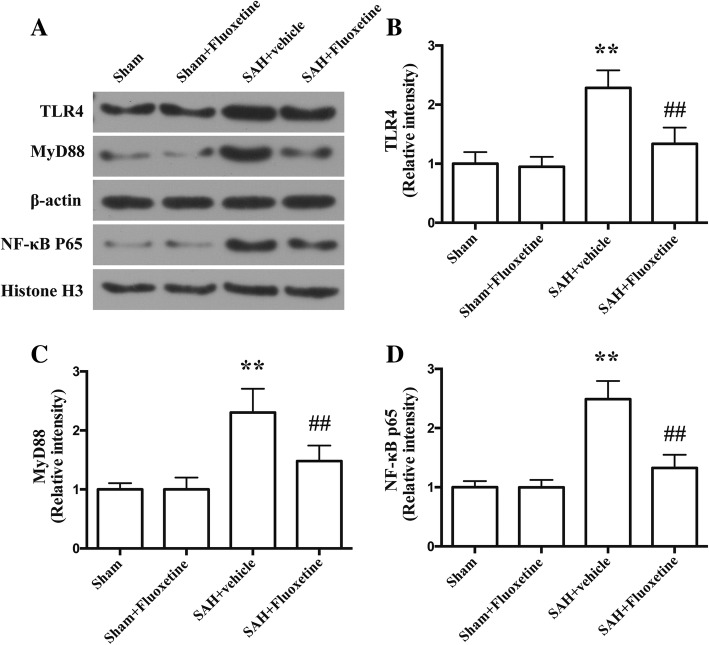
Fluoxetine attenuated TLR4 and MyD88 and reduced the nuclear translocation of NF-κB p65
The protein levels of TLR4 and MyD88 were significantly increased in the cortex in the SAH+vehicle group as compared with the sham group (p < 0.01, Fig. 7a–c). The protein levels of TLR4 and MyD88 in SAH+fluoxetine group were significantly lower than those of SAH+vehicle group (p < 0.01, Fig. 7a–c). We also examined the nuclear levels of NF-κB p65, an indicator of the activation of the NF-κB signaling pathway. The protein levels of NF-κB p65 were significantly increased in the SAH+vehicle group as compared with the sham group (p < 0.01, Fig. 7a, d). The protein levels of NF-κB p65 in SAH+fluoxetine group were significantly lower than those of SAH+vehicle group (p < 0.01, Fig. 7a, d).
Fig. 7.
Fluoxetine downregulated the protein levels of TLR4 and MyD88, but increased the nuclear protein levels of NF-κB p65 in the ipsilateral cortex at 24 h after SAH. a Representative western blot bands of TLR4, MyD88, and nuclear NF-κB p65. b Densitometric quantification of TLR4. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 6/group. c Densitometric quantification of MyD88. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 6/group. d Densitometric quantification of nuclear NF-κB p65. **p < 0.01 vs sham. ##p < 0.01 vs SAH+vehicle. n = 6/group
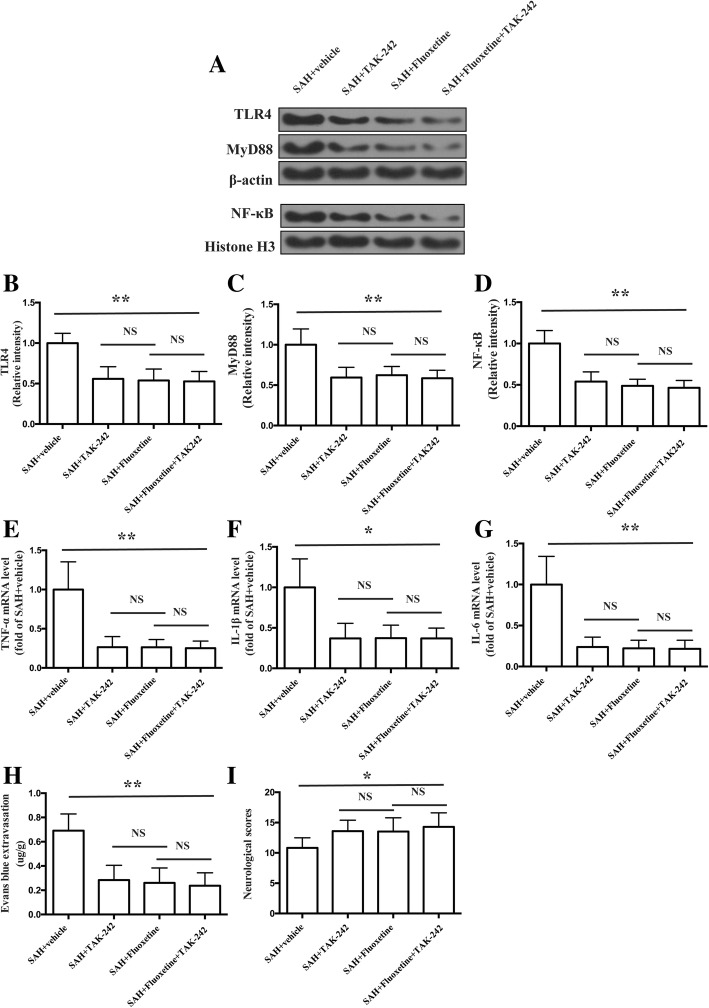
Combined administration of fluoxetine and TAK-242 did not enhance the effects of fluoxetine in the expression of TLR4, MyD88, nuclear NF-κB, and pro-inflammatory cytokines, BBB disruption, and neurological function
The protein levels of TLR4, MyD88, and nuclear NF-κB were significantly decreased in the cortex in the SAH+TAK242, SAH+fluoxetine, and SAH+fluoxetine+TAK-242 group as compared with the SAH+vehicle group (p < 0.01, Fig. 8a–d). There was no significant difference between SAH+fluoxetine and SAH+fluoxetine+TAK-242 in proteins level of TLR4, MyD88, and nuclear NF-κB (p > 0.05, Fig. 8a–d). The mRNA levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 were significantly decreased in the cortex in the SAH+TAK242, SAH+fluoxetine, and SAH+fluoxetine+TAK-242 group as compared with the SAH+vehicle group (p < 0.01, Fig. 8e, p < 0.05, Fig. 8f, and p < 0.01, Fig. 8g). No significant difference was observed in these mRNA levels between SAH+fluoxetine and SAH+fluoxetine+TAK-242 group (p > 0.05, Fig. 8e–g). Combined administration of fluoxetine and TAK-242 prevented the Evans blue extravasation (p < 0.01, Fig. 8h) and improved the neurological function (p < 0.05, Fig. 8i), which was similar with fluoxetine treatment (p > 0.05, Fig. 8h, i).
Fig. 8.
Combined administration of fluoxetine and TAK-242 did not enhance the effects of fluoxetine in the expression of TLR4, MyD88, nuclear NF-κB, and pro-inflammatory cytokines, BBB disruption, and neurological function. a Representative western blot bands of TLR4, MyD88, and nuclear NF-κB p65. b Densitometric quantification of TLR4. **p < 0.01 vs SAH+vehicle. n = 6/group. c Densitometric quantification of MyD88. **p < 0.01 vs SAH+vehicle. n = 6/group. d Densitometric quantification of nuclear NF-κB p65. **p < 0.01 vs SAH+vehicle. n = 6/group. e The quantification of TNF-α mRNA levels. **p < 0.01 vs SAH+vehicle. n = 5/group f The quantification of IL-1β mRNA levels. *p < 0.05 vs SAH+vehicle. n = 5/group. g The quantification of IL-6 mRNA levels. **p < 0.01 vs SAH+vehicle. n = 5/group. h The quantification of Evans blue extravasation. **p < 0.01vs SAH+vehicle. n = 6/group. i The quantification of neurological score. *p < 0.05 vs sham. #p < 0.05 vs SAH + vehicle. n = 17/group
Discussion
The current study presented several novel findings:(1) fluoxetine attenuated BBB disruption, brain edema, and improved neurological function after SAH. (2) Fluoxetine alleviated the number of Iba-1-positive microglia/macrophages, neutrophil infiltration, and cell death. (3) Fluoxetine reduced the levels of pro-inflammatory cytokines, and the underlying mechanisms, at least in part, involved the TLR4/MyD88/NF-κB signaling pathway (Fig. 9).
Fig. 9.
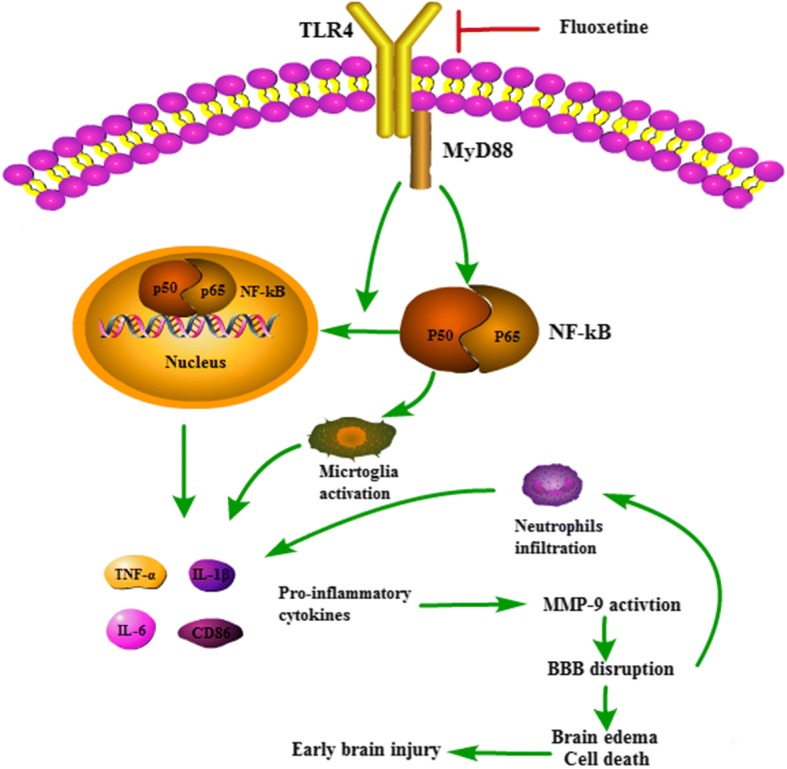
The potential molecular mechanisms of anti-inflammatory effects of fluoxetine through TLR4/MyD88/NF-κB signaling pathway
At present, no therapies are available to cure the neurological deficits in SAH patients [31]; however, an increasing number of studies show that inflammation contributes to early brain injury after SAH and inhibition of inflammation can ameliorate brain injury after SAH [10, 32–34]. One of the most important mediators in inflammation-induced brain injury after SAH is MMP-9. Notably, both clinical and basic studies have reported an elevation of MMP-9 in brain tissue, serum, and cerebrospinal fluid after SAH [35–37]. The MMP-9 elevation is responsible for the degradation of tight junction proteins, which are critical in the maintenance of BBB integrity. In the present study, we observed fluoxetine treatment alleviated MMP-9 expression and degradation of tight junction proteins (ZO-1, occludin, and claudin-5), attenuated BBB disruption and brain edema, and improved neurological function after SAH. The effects of fluoxetine on MMP-9 expression and subsequent BBB disruption were consistent with previous reports about fluoxetine treatment in experimental transient global ischemia and spinal cord injury [38, 39]. BBB disruption facilitates infiltration of peripheral inflammatory cells, including macrophage and neutrophils, which aggravate neuroinflammation by releasing a multitude of inflammatory factors [40]. In addition, BBB disruption also results in neuronal cell death [38]. In the current study, we found that fluoxetine inhibited the neutrophils infiltration and reduced neural cell death in early brain injury after injury.
Inflammatory cytokines are important regulators in MMP-9 activation and subsequent BBB disruption. A previous study demonstrated that the selective inhibitor of IL-1β blocked JNK-mediated MMP-9 activation and improved neurological function in SAH rats [41]. IL-6 induced MMP-9 expression through the JAK-mediated pathway in macrophages [42]. TNF-α was also reported as an upstream regulator for MMP-9 [43]. In our study, we found that fluoxetine downregulated these inflammatory cytokines, which is in accordance with previous studies [44, 45]. TLRs belong to a large family of pattern recognition receptors that play a key role in innate immunity and inflammatory responses. Of the TLR family members, TLR4 was of vital importance in this family. TLR4 is activated by many endogenous ligands such as heme, fibrinogen, heat shock proteins, all of which are produced after SAH [13, 46]. In fact, patients with aneurysmal SAH was reported to have higher TLR4 levels on peripheral blood mononuclear cells, which were associated with more massive SAH, occurrence of cerebral vasospasm, delayed cerebral infarction, and worse functional recovery [47]. TLR4 interacts with two distinct adaptor proteins, MyD88 and Toll-receptor-associated activator of interferon (TRIF), and activates two parallel signaling pathways to initiate activation of transcription factors that regulate expression of proinflammatory cytokines genes [12]. In addition, the TRIF-dependent pathway induces late phase activation of NF-κB, while the faster TLR4 route through MyD88 is the early activation of NF-κB. Many previous studies have demonstrated the anti-inflammatory effects of fluoxetine in other system disorders in vivo and in vitro [20–24, 48]. In these studies, TLR4 and downstream NF-κB were hotspots. However, limited studies focused on the effects of fluoxetine in the TLR4 signaling pathway after SAH. Therefore, we examined the TLR4-mediated MyD88 pathway in the current study. Our results showed that the protein levels of TLR4 and MyD88 were significantly increased in early brain injury after SAH and fluoxetine downregulated their expression. In addition, we found that fluoxetine also reduced the nuclear translocation of NF-κB p65, an indicator of NF-κB activation. What is more important is that we also used TAK-242, a small-molecule inhibitor of the TLR4 signaling pathway. We found that the combined administration of fluoxetine and TAK-242 reduced the expression of TLR4, MyD88, and nuclear NF-κB, decreased the mRNA levels of pro-inflammatory cytokines, prevented BBB disruption, and improved neurological function after SAH. These beneficial effects of combined administration were similar to alone fluoxetine treatment. Taken together, our study indicated that TLR4/MyD88/NF-κB signaling pathway was involved in anti-inflammatory effects of fluoxetine in early brain injury after SAH.
There are some limitations in our study. First, the present study aimed at investigating the effects and potential mechanism of fluoxetine in neuroinflammation in early brain injury after SAH, the long-term study of fluoxetine after SAH is still needed in the future. Second, previous studies showed anti-apoptotic effects of fluoxetine, which did not deeply evaluate in the current study.
Conclusions
The current study has demonstrated that fluoxetine attenuated neuroinflammation and improved neurological function after SAH. The potential mechanisms involved, at least in part, TLR4/MyD88/NF-κB signaling pathway.
Additional file
Figure S1. Representative pictures of brains in SAH group showing the sample region. (TIF 372 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (NO.81771246, 81571106, 81701152), Natural Science Foundation of Zhejiang province (LY17H090008, LY17H090007 and Y18H090019).
Availability of data and materials
All data supporting the conclusions of this article are included with this article.
Abbreviations
- BBB
Blood-brain barrier
- ECA
External carotid artery
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- MMP-9
Matrix metallopeptidase 9
- MPO
Myeloperoxidase
- MyD88
Myeloid differentiation primary response 88
- NF-κB
Nuclear factor kappa B
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SAH
Subarachnoid hemorrhage
- SD
Sprague-Dawley
- TLRs
Toll-like receptors
- TNF-α
Tumor necrosis factor-α
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Authors’ contributions
This work includes significant contributions from all authors. GC and FYL conceived the study and contributed to its experimental design. JC, CW, WR, HZP, GPG, and JRL carried out the laboratory experiments, CQ, JSC, GPG, and LW analyzed the data. FYL, JC, and GC wrote the manuscript. CW and GC contributed to the editing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University and carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fu-yi Liu, Email: liufuyi@zju.edu.cn.
Jing Cai, Email: ncudoctor@163.com.
Chun Wang, Email: wangchun@zju.edu.cn.
Wu Ruan, Email: rwhh@foxmail.com.
Guo-ping Guan, Email: guangp_neuro@163.com.
Hai-zhou Pan, Email: jackal5200@sina.com.
Jian-ru Li, Email: lijianru@zju.edu.cn.
Cong Qian, Email: congqian@zju.edu.cn.
Jing-sen Chen, Email: furycjs@126.com.
Lin Wang, Email: wanglin_77@hotmail.com.
Gao Chen, Phone: +86 057187784753, Email: d-chengao@zju.edu.cn.
References
- 1.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 2.Springer MV, Schmidt JM, Wartenberg KE, Frontera JA, Badjatia N, Mayer SA. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery. 2009;65:1043–1050. doi: 10.1227/01.NEU.0000359317.15269.20. [DOI] [PubMed] [Google Scholar]
- 3.Mocco J, Ransom ER, Komotar RJ, Schmidt JM, Sciacca RR, Mayer SA, Connolly ES., Jr Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery. 2006;59:529–538. doi: 10.1227/01.NEU.0000228680.22550.A2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W, Lv S, Zhou M, Zhang X, Hang C. Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of NLRP3 inflammasome activation. Front Neurosci. 2017;11:611. doi: 10.3389/fnins.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan R, Enkhjargal B, Camara R, Yan F, Gong L, ShengtaoYao TJ, Chen Y, Zhang JH. Critical role of EphA4 in early brain injury after subarachnoid hemorrhage in rat. Exp Neurol. 2017;296:41–48. doi: 10.1016/j.expneurol.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Kawakita F, Fujimoto M, Nakano F, Imanaka-Yoshida K, Yoshida T, Suzuki H. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2017;48:1108–1111. doi: 10.1161/STROKEAHA.117.016629. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kawakita F. Tenascin-C in aneurysmal subarachnoid hemorrhage: deleterious or protective? Neural Regen Res. 2016;11:230–231. doi: 10.4103/1673-5374.177721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider UC, Schiffler J, Hakiy N, Horn P, Vajkoczy P. Functional analysis of pro-inflammatory properties within the cerebrospinal fluid after subarachnoid hemorrhage in vivo and in vitro. J Neuroinflammation. 2012;9:28. doi: 10.1186/1742-2094-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa A, Osuka K, Nakura T, Matsuo N, Watabe T, Takayasu M. Interleukin-6, MCP-1, IP-10, and MIG are sequentially expressed in cerebrospinal fluid after subarachnoid hemorrhage. J Neuroinflammation. 2016;13:217. doi: 10.1186/s12974-016-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL, Simard JM. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. 2016;17:497. doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Nardo D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine. 2015;74:181–189. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakita F, Fujimoto M, Liu L, Nakano F, Nakatsuka Y, Suzuki H. Effects of toll-like receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2017;54:6624–6633. doi: 10.1007/s12035-016-0178-7. [DOI] [PubMed] [Google Scholar]
- 14.Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-O. [DOI] [PubMed] [Google Scholar]
- 15.Khodanovich M, Kisel A, Kudabaeva M, Chernysheva G, Smolyakova V, Krutenkova E, Wasserlauf I, Plotnikov M, Yarnykh V. Effects of fluoxetine on hippocampal neurogenesis and neuroprotection in the model of global cerebral ischemia in rats. Int J Mol Sci. 2018;19(1):162. [DOI] [PMC free article] [PubMed]
- 16.Lee JY, Choi HY, Yune TY. Fluoxetine and vitamin C synergistically inhibits blood-spinal cord barrier disruption and improves functional recovery after spinal cord injury. Neuropharmacology. 2016;109:78–87. doi: 10.1016/j.neuropharm.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Kang SR, Yune TY. Fluoxetine prevents oligodendrocyte cell death by inhibiting microglia activation after spinal cord injury. J Neurotrauma. 2015;32:633–644. doi: 10.1089/neu.2014.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JR, Xu HZ, Nie S, Peng YC, Fan LF, Wang ZJ, Wu C, Yan F, Chen JY, Gu C, et al. Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subarachnoid hemorrhage in rats. J Neuroinflammation. 2017;14:186. doi: 10.1186/s12974-017-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alboni S, Poggini S, Garofalo S, Milior G, El Hajj H, Lecours C, Girard I, Gagnon S, Boisjoly-Villeneuve S, Brunello N, et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun. 2016;58:261–271. doi: 10.1016/j.bbi.2016.07.155. [DOI] [PubMed] [Google Scholar]
- 20.Liechti FD, Grandgirard D, Leib SL. The antidepressant fluoxetine protects the hippocampus from brain damage in experimental pneumococcal meningitis. Neuroscience. 2015;297:89–94. doi: 10.1016/j.neuroscience.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Koh SJ, Kim JM, Kim IK, Kim N, Jung HC, Song IS, Kim JS. Fluoxetine inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G9–19. doi: 10.1152/ajpgi.00267.2010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Zhou H, Wilson BC, Shi JS, Hong JS, Gao HM. Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism Relat Disord. 2012;18(Suppl 1):S213–S217. doi: 10.1016/S1353-8020(11)70066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha E, Jung KH, Choe BK, Bae JH, Shin DH, Yim SV, Baik HH. Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neurosci Lett. 2006;397:185–189. doi: 10.1016/j.neulet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Waiskopf N, Ofek K, Gilboa-Geffen A, Bekenstein U, Bahat A, Bennett ER, Podoly E, Livnah O, Hartmann G, Soreq H. AChE and RACK1 promote the anti-inflammatory properties of fluoxetine. J Mol Neurosci. 2014;53:306–315. doi: 10.1007/s12031-013-0174-6. [DOI] [PubMed] [Google Scholar]
- 25.Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, Stein DG. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35:536–542. doi: 10.1038/jcbfm.2014.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Chen J, Mo H, Chen J, Qian C, Yan F, Gu C, Hu Q, Wang L, Chen G. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol. 2016;53:2668–2678. doi: 10.1007/s12035-015-9318-8. [DOI] [PubMed] [Google Scholar]
- 27.Ying GY, Jing CH, Li JR, Wu C, Yan F, Chen JY, Wang L, Dixon BJ, Chen G. Neuroprotective effects of valproic acid on blood-brain barrier disruption and apoptosis-related early brain injury in rats subjected to subarachnoid hemorrhage are modulated by heat shock protein 70/matrix metalloproteinases and heat shock protein 70/AKT pathways. Neurosurgery. 2016;79:286–295. doi: 10.1227/NEU.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W, Chen G. Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res. 2014;56:12–19. doi: 10.1111/jpi.12086. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Li J, Wang Z, Feng M, Shen Y, Cao S, Li T, Peng Y, Fan L, Chen J, et al. Methylene blue attenuates neuroinflammation after subarachnoid hemorrhage in rats through the Akt/GSK-3beta/MEF2D signaling pathway. Brain Behav Immun. 2017;65:125–139. doi: 10.1016/j.bbi.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Young AM, Karri SK, Helmy A, Budohoski KP, Kirollos RW, Bulters DO, Kirkpatrick PJ, Ogilvy CS, Trivedi RA. Pharmacologic management of subarachnoid hemorrhage. World Neurosurg. 2015;84:28–35. doi: 10.1016/j.wneu.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Savarraj JPJ, Parsha K, Hergenroeder GW, Zhu L, Bajgur SS, Ahn S, Lee K, Chang T, Kim DH, Liu Y, Choi HA. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology. 2017;88:1535–1545. doi: 10.1212/WNL.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort L. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care. 2017;26:48–57. doi: 10.1007/s12028-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You W, Wang Z, Li H, Shen H, Xu X, Jia G, Chen G. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J Neurol Sci. 2016;367:224–231. doi: 10.1016/j.jns.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Feiler S, Plesnila N, Thal SC, Zausinger S, Scholler K. Contribution of matrix metalloproteinase-9 to cerebral edema and functional outcome following experimental subarachnoid hemorrhage. Cerebrovasc Dis. 2011;32:289–295. doi: 10.1159/000328248. [DOI] [PubMed] [Google Scholar]
- 36.Fischer M, Dietmann A, Beer R, Broessner G, Helbok R, Pfausler B, Schmutzhard E, Lackner P. Differential regulation of matrix-metalloproteinases and their tissue inhibitors in patients with aneurysmal subarachnoid hemorrhage. PLoS One. 2013;8:e59952. doi: 10.1371/journal.pone.0059952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triglia T, Mezzapesa A, Martin JC, Verdier M, Lagier D, Dufour H, Bruder N, Alessi MC, Velly LJ. Early matrix metalloproteinase-9 concentration in the first 48 h after aneurysmal subarachnoid haemorrhage predicts delayed cerebral ischaemia: an observational study. Eur J Anaesthesiol. 2016;33:662–669. doi: 10.1097/EJA.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Lee HE, Kang SR, Choi HY, Ryu JH, Yune TY. Fluoxetine inhibits transient global ischemia-induced hippocampal neuronal death and memory impairment by preventing blood-brain barrier disruption. Neuropharmacology. 2014;79:161–171. doi: 10.1016/j.neuropharm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Kim HS, Choi HY, Oh TH, Yune TY. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain. 2012;135:2375–2389. doi: 10.1093/brain/aws171. [DOI] [PubMed] [Google Scholar]
- 40.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, Falcone DJ. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol. 2014;192:349–357. doi: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue H, Sun K, Xie W, Hu G, Kong H, Wang Q, Wang H. Etanercept attenuates short-term cigarette-smoke-exposure-induced pulmonary arterial remodelling in rats by suppressing the activation of TNF-a/NF-kB signal and the activities of MMP-2 and MMP-9. Pulm Pharmacol Ther. 2012;25:208–215. doi: 10.1016/j.pupt.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Aksu U, Guner I, Yaman OM, Erman H, Uzun D, Sengezer-Inceli M, Sahin A, Yelmen N, Gelisgen R, Uzun H, Sahin G. Fluoxetine ameliorates imbalance of redox homeostasis and inflammation in an acute kidney injury model. J Physiol Biochem. 2014;70:925–934. doi: 10.1007/s13105-014-0361-0. [DOI] [PubMed] [Google Scholar]
- 45.Yang JM, Rui BB, Chen C, Chen H, Xu TJ, Xu WP, Wei W. Acetylsalicylic acid enhances the anti-inflammatory effect of fluoxetine through inhibition of NF-kappaB, p38-MAPK and ERK1/2 activation in lipopolysaccharide-induced BV-2 microglia cells. Neuroscience. 2014;275:296–304. doi: 10.1016/j.neuroscience.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Fang H, Wang PF, Zhou Y, Wang YC, Yang QW. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation. 2013;10:27. doi: 10.1186/1742-2094-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma C, Zhou W, Yan Z, Qu M, Bu X. Toll-like receptor 4 (TLR4) is associated with cerebral vasospasm and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2015;55:878–884. doi: 10.2176/nmc.oa.2015-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habib M, Shaker S, El-Gayar N, Aboul-Fotouh S. The effects of antidepressants “fluoxetine and imipramine” on vascular abnormalities and Toll like receptor-4 expression in diabetic and non-diabetic rats exposed to chronic stress. PLoS One. 2015;10:e0120559. doi: 10.1371/journal.pone.0120559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative pictures of brains in SAH group showing the sample region. (TIF 372 kb)
Data Availability Statement
All data supporting the conclusions of this article are included with this article.