Abstract
My scientific career has focused on understanding the mechanisms underlying insulin resistance with the goal of developing new strategies to prevent and treat type 2 diabetes. My early studies focused on understanding how insulin promotes glucose transport into adipocytes, a classic model of highly insulin-responsive target cells. When we found changes in adipocyte glucose transport in altered metabolic states, we were highly motivated to understand the consequences of this on whole-body glucose homeostasis. In the late 1980s, when GLUT4, the major insulin-regulated glucose transporter, was identified, my lab observed that it was downregulated in adipocytes but not in skeletal muscle in insulin-resistant states, such as obesity and type 2 diabetes, in humans and rodents. We investigated the role of GLUT4 in adipose tissue and muscle in whole-body insulin sensitivity, making tissue-specific GLUT4-overexpressing and GLUT4 knockout mice. These studies led to the discovery that adipocytes, and specifically glucose transport into adipocytes, regulate whole-body glucose homeostasis. As adipocytes take up relatively little glucose, we investigated the underlying mechanisms. In the 1990s, we performed DNA microarrays on adipose tissue from adipose-specific GLUT4-overexpressing and GLUT4 knockout mice to find reciprocally regulated genes, and we identified several molecules that were not previously known to regulate systemic insulin sensitivity and/or energy balance. More recently, with Alan Saghatelian’s lab, we discovered a novel class of lipids with antidiabetes and anti-inflammatory effects. We also investigated the effects of the adipose-secreted hormone, leptin, on insulin sensitivity. We found that the AMP-activated protein kinase (AMPK) pathway mediates leptin’s effects on fatty acid oxidation in muscle and also plays a role in leptin’s anorexigenic effects in the hypothalamus. These studies transformed AMPK from a “fuel gauge” that regulates energy supply at the cellular level to a sensing and signaling pathway that regulates organismal energy balance. Overall, these studies have expanded our understanding of the multifaceted role of adipose tissue in metabolic health and how adipose dysfunction increases the risk for type 2 diabetes.
Friends and colleagues, I am honored to accept the 2016 Banting Medal on behalf of the talented postdoctoral fellows, junior faculty, students, and collaborators with whom I have had the privilege of working over the last 26 years. This award reflects their ingenuity, creativity, and dedication, and I thank them for the important roles they have played in galvanizing the discoveries in my lab.
I want to thank my mentors: Sam Cushman, for taking me into his hard-core cell biology lab at the National Institutes of Health when I had no basic science experience and for patiently guiding me as I made the transition from physician to physician-scientist, and Jeff Flier, for setting an example with his love of scientific discovery and his ability to “think outside the box” and for his invaluable colleagueship and support in the formative years of my scientific career.
Early in my medical training as a physician who was taking care of patients with diabetes, I was determined to learn everything I could about how insulin works and how blood glucose is regulated.
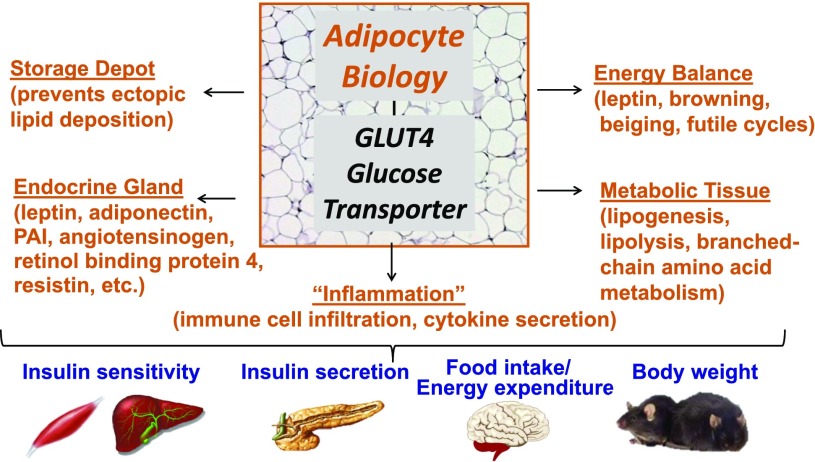
My lab has been interested in the question of what links obesity to insulin resistance and type 2 diabetes—since these are states in which insulin action is impaired. We have approached this from the standpoint of white adipose tissue and how it regulates whole-body metabolism. When we started our studies nearly three decades ago, little was known about adipocyte biology (Fig. 1). We knew that adipose tissue was a storage depot, but we did not even completely appreciate that this storage function prevented ectopic lipid deposition in muscle, liver, heart, and other tissues, which can cause insulin resistance and other metabolic problems including hepatic steatohepatitis. Only since we have started our studies has it become clear that the adipocyte is an endocrine gland, secreting many hormones, cytokines, and vasoactive substances (Fig. 1). In addition, white adipose tissue plays a role in energy balance, not just by secreting hormones like leptin but also by browning and beiging and the futile cycles that burn energy even in white adipose tissue.
Figure 1.
White adipose tissue regulates whole-body metabolism. The adipocyte is not only a storage depot but has many other functions including 1) as an endocrine gland, secreting many hormones, cytokines, and vasoactive substances; 2) having a role in energy balance, by secreting hormones such as leptin and also by browning and beiging and the futile cycles that burn energy; and 3) as a metabolic tissue that performs lipogenesis, lipolysis, and metabolism of branched-chain amino acids and other molecules. Adipose inflammation, characterized by immune cell infiltration and cytokine secretion, occurs in the setting of obesity and insulin resistance. All of these processes contribute to regulation of insulin sensitivity, insulin secretion, food intake and energy expenditure, and ultimately body weight. PAI, plasminogen activator inhibitor.
We have known that adipose tissue is a metabolic tissue that performs lipogenesis and lipolysis. Over the past couple decades, the work of members of my lab has revealed that it is also an important metabolizer of branched-chain amino acids (1) and other molecules not previously known to be metabolized by white fat cells. Adipose inflammation, characterized by immune cell infiltration and cytokine secretion, occurs in the setting of obesity and insulin resistance. All of these functions of the adipocyte contribute to insulin sensitivity, insulin secretion, food intake and energy expenditure, and ultimately regulation of body weight (Fig. 1).
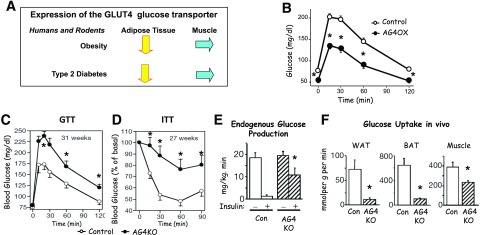
Our studies started with the GLUT4 glucose transporter, which is the major insulin-responsive glucose transporter. After eating, insulin levels rise and GLUT4 moves to the cell surface to rapidly increase glucose uptake into fat cells and muscle. We studied the expression of GLUT4 in adipose tissue and muscle in humans and rodents with obesity and type 2 diabetes. We found that GLUT4 is downregulated in adipose tissue but not in muscle (Fig. 2A). This was a surprise as adipose tissue takes up very little glucose, and muscle is the major site for glucose uptake in the presence of insulin. We were very intrigued as to the possible role this downregulation of GLUT4 in adipose tissue had on whole-body glucose metabolism and the risk for type 2 diabetes.
Figure 2.
Role of GLUT4 in regulating whole-body glucose tolerance and insulin sensitivity. GLUT4 protein levels are decreased in white adipose tissue but not in skeletal muscle, of insulin-resistant humans and rodents (A). Adipose-specific overexpression of GLUT4 (AG4OX) lowers fasting glycemia and improves whole-body glucose tolerance (B), whereas adipose-specific GLUT4 deletion (AG4KO) causes whole-body glucose intolerance (C) and insulin resistance (D–F). Adipose-specific GLUT4 deletion causes liver insulin resistance (E) and decreases glucose uptake in adipose tissue and skeletal muscle (F). For experimental details for panel B, see Carvalho et al. (4). For experimental details for panels C–F, see Abel et al. (5). Panel B reprinted from Carvalho et al. (4). Panels C–F reprinted from Abel et al. (5). *Panels B–D and F: P < 0.05 vs. control; panel E: P < 0.05 vs. control + insulin. BAT, brown adipose tissue; Con, control; GTT, glucose tolerance test; ITT, insulin tolerance test; WAT, white adipose tissue.
In 1990, Peter Shepherd, my first fellow, came to the lab, and we decided to overexpress GLUT4 selectively in adipocytes to see if it would have effects on glucose homeostasis and whole-body insulin sensitivity. This was in the early days of tissue-specific transgenics and, actually, no one had done a study overexpressing a metabolic gene in adipose tissue. We thought it would work because Bruce Spiegelman, who kindly provided the plasmid containing the adipose-specific promoter, was overexpressing diphtheria toxin in adipocytes in mice at that time (2). So Peter made the adipose-specific GLUT4 overexpressor mice, which were characterized by high GLUT4 expression in fat, normal GLUT4 expression in muscle, and no ectopic expression (3). Two years later, in 1992, Peter, working with Effie Tozzo and Luigi Gnudi, who had joined the lab, got an amazing glucose tolerance test result (Fig. 2B). Overexpression of GLUT4 selectively in adipocytes lowers fasting glycemia and improves whole-body glucose tolerance (3,4). It was a wild idea we had, and it worked.
Several years later, Dale Abel came to the lab, and at that point, Cre-lox technology was just emerging. Again, no one had done an adipose-specific knockout of a gene. In fact, no one had done a tissue-specific knockout in any tissue to study insulin action or diabetes. We thought it would be important to see whether knocking out GLUT4 only in fat, recapitulating the low levels in people with obesity and type 2 diabetes, would be sufficient to cause insulin resistance. Dale made an adipose-specific GLUT4 knockout mouse and, together with Odile Peroni, showed that the mice had normal adiposity but whole-body glucose intolerance and insulin resistance (5) (Fig. 2C and D). Liver and muscle became insulin-resistant secondarily (Fig. 2E and F), and these mice had a markedly increased risk of diabetes.
In collaboration with Ulf Smith, we found that GLUT4 protein levels in adipose tissue in humans correlated highly with insulin sensitivity measured by glucose infusion rate during a clamp. In addition, Ulf showed that decreased GLUT4 protein levels in adipose tissue are an early predictor of type 2 diabetes in people (6).
So we created the adipose-GLUT4 knockout mice that were insulin resistant with an increased risk of type 2 diabetes (5) and the adipose-GLUT4 overexpressors that had enhanced glucose tolerance and lower fasting glycemia (3). When we studied the tissues in the animals and then ex vivo, we had evidence for systemic effects on muscle and liver. At that time, this result was somewhat surprising—that a defined genetic alteration in adipose tissue could affect muscle and liver. But it seemed more plausible when leptin was discovered in 1994 by Jeff Friedman and colleagues (7).
Leptin is an adipocyte-secreted hormone that regulates food intake and body weight. We wanted to investigate how leptin works to better understand how adipocytes regulate whole-body energy balance. When Juleen Zierath was a fellow in my lab, she showed that leptin did not directly stimulate glucose transport in adipocytes or muscle. Then, Young-Bum Kim studied leptin’s effects on signal transduction, and he showed that leptin activates signal transduction directly in insulin-sensitive tissues through overlapping but distinct pathways from insulin (8).
New findings were coming out about the role of other signaling pathways, and I became very interested in the potential role of AMP-activated protein kinase (AMPK) in leptin action and the regulation of energy balance. This seemed plausible because when the energy levels in the cell go down, AMPK activity is increased, stimulating pathways that generate energy at the cellular level, such as fatty acid oxidation. Could AMPK regulate systemic energy balance in a whole mouse or human? When Yasuhiko Minokoshi came to the lab, I asked him if he could determine whether leptin activated AMPK in muscle and whether this accounted for some of its metabolic effects.
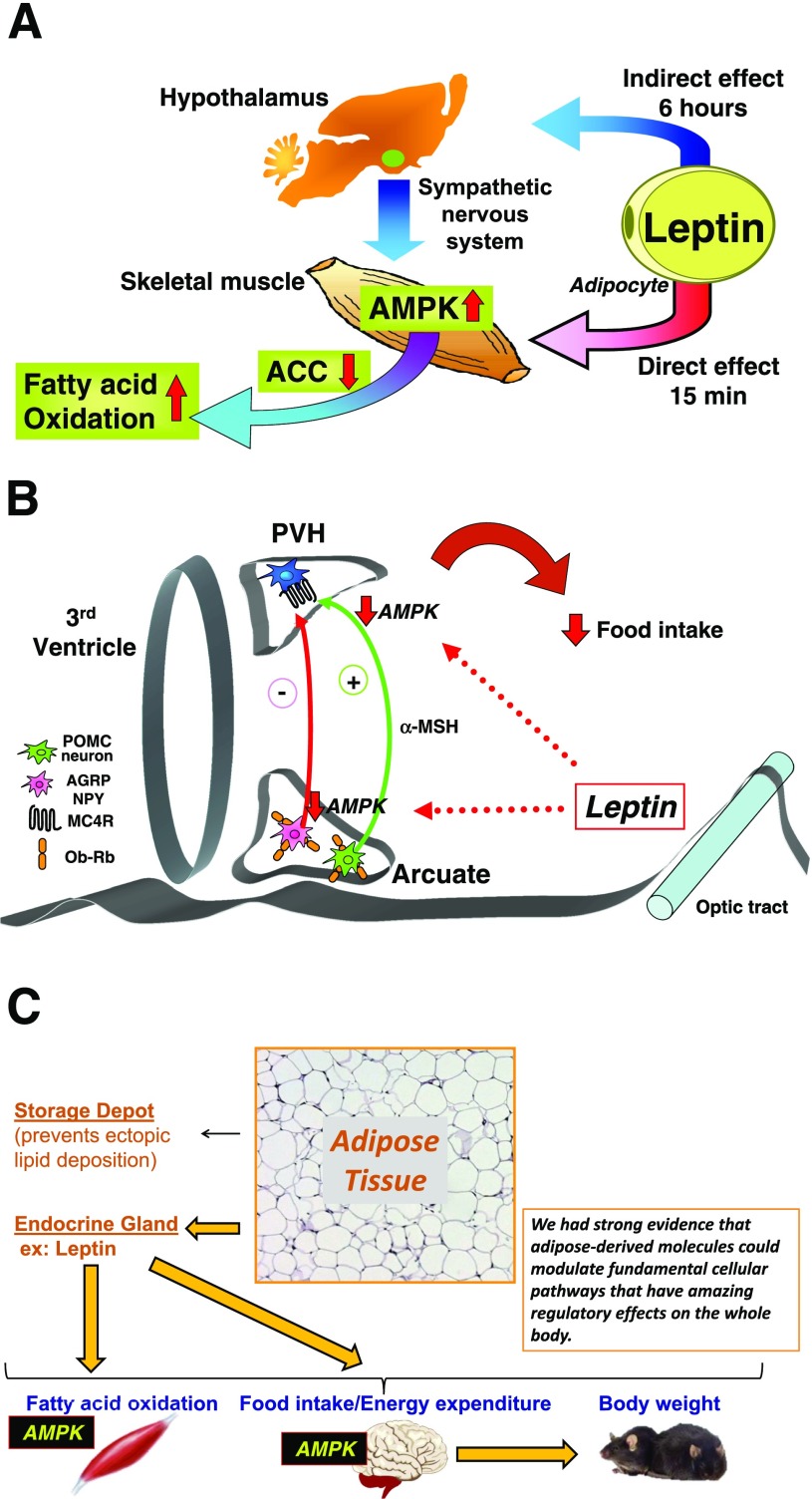
At that point Deb Muoio had shown in vitro that leptin could stimulate fatty acid oxidation in muscle (9). Yasuhiko showed that leptin has a direct effect within 15 min in muscle to stimulate AMPK, which inhibits acetyl-coA carboxylase and results in stimulation of fatty acid oxidation (10) (Fig. 3A). Surprisingly, he also found there is a second, indirect effect, which takes 6 h and is mediated through the hypothalamus and the sympathetic nervous system, that also activates AMPK and fatty acid oxidation (10) (Fig. 3A).
Figure 3.
Leptin regulates fatty acid oxidation in muscle by activating AMPK. A: Leptin activates α2-AMPK in red skeletal muscle through two distinct mechanisms. Early activation of AMPK after intravenous leptin administration appears to be direct, whereas later activation depends on the hypothalamic–sympathetic nervous system axis. Leptin’s effect to activate AMPK is associated with suppression of acetyl-CoA carboxylase (ACC) activity and results in stimulation of fatty acid oxidation. Panel A reprinted from Minokoshi et al. (10). B: Leptin inhibits α2-AMPK activity in the arcuate and paraventricular hypothalamus (PVH), and this is necessary to decrease food intake in response to leptin. Panel B reprinted from Minokoshi et al. (11). C: Schematic of how white adipose tissue regulates whole-body metabolism. Through its endocrine actions—in this case, leptin—white adipose tissue can modulate AMPK signaling in other tissues. This is fundamental to maintaining energy supplies at the cellular level, and this pathway stimulates fatty acid oxidation in muscle. In addition, leptin secreted from white adipose tissue inhibits the AMPK pathway in the hypothalamus to regulate food intake and energy expenditure and ultimately body weight. Our early work with leptin and AMPK provided strong evidence that adipose-derived molecules could modulate fundamental cellular pathways that have regulatory effects on the whole body.
Once we saw that AMPK could mediate these effects of leptin, we wondered whether AMPK also mediates the effects of leptin on food intake and body weight. Around that time a number of groups were revealing the neurocircuits that regulate food intake. What these groups were showing was that there are specialized neurons in the arcuate nucleus of the hypothalamus, POMC and NPY/AgRP neurons, which project to the paraventricular hypothalamus and also to the lateral hypothalamus, and that integration of these neural circuits is critical to regulate food intake. Yasuhiko went on to show that leptin inhibits AMPK in the arcuate nucleus (Fig. 3B) and in the paraventricular nucleus and that this inhibition of AMPK is necessary to decrease food intake in response to leptin (11) (Fig. 3B).
We and others proceeded to study AMPK in other metabolic states, and it turned out that this is a fundamental mechanism for regulating food intake in response to many physiologic changes (12). We found that AMPK activity was decreased in many anorexigenic states. And in orexigenic states, AMPK activity was increased in the hypothalamus. We went on to show that these changes in AMPK activity are necessary for the changes in food intake (12).
Thierry Alquier in the lab also showed that AMPK activation in discrete hypothalamic areas is necessary for the counterregulatory hormone response to hypoglycemia, and, in the state of hypoglycemic unawareness, AMPK activation in the hypothalamus is impaired (13). Rury McCrimmon and Bob Sherwin also made major contributions in this area (14).
These studies demonstrated that adipose tissue through its endocrine actions—in this case leptin—could activate the AMPK signaling pathway that is fundamental to maintaining energy supplies at the cellular level, and this pathway could stimulate fatty acid oxidation in muscle (Fig. 3A and C). In addition, leptin could engage the AMPK pathway in the hypothalamus to regulate food intake and energy expenditure and that ultimately regulated body weight (Fig. 3C). We had strong evidence then that adipose-derived molecules could modulate fundamental cellular pathways that have amazing regulatory effects on the whole body.
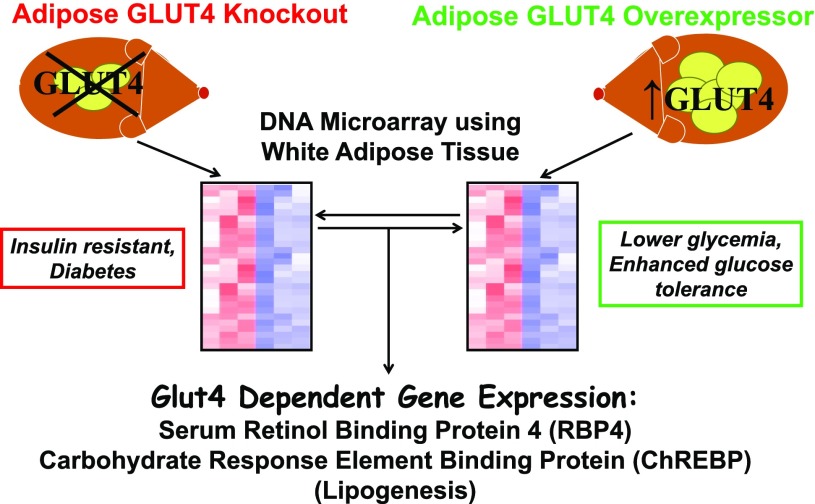
Then we went back to our adipose-specific GLUT4 mouse models and we asked “What are the molecules that mediate the effects of altered glucose transport in adipocytes on systemic insulin sensitivity?” To answer that, we took the adipose tissue from the adipose GLUT4 knockout and adipose GLUT4 overexpressors and subjected it to DNA array analysis of 16,000 genes (Fig. 4). We were looking for GLUT4-dependent genes, and in the beginning, we wanted to find genes that encoded secreted proteins that could have distal effects on other tissues such as muscle and liver.
Figure 4.
Microarray analysis of adipose tissue from adipose-specific GLUT4 knockout and adipose-specific GLUT4 overexpressor mice. White adipose tissue from these genetically modified mice was subjected to Affymetrix DNA array analysis. This led to the identification of GLUT4-dependent genes, which have systemic effects on insulin sensitivity. Examples are RBP4, the major protein transporting retinol in the blood, and ChREBP, a transcription factor that regulates lipogenesis and glycolysis.
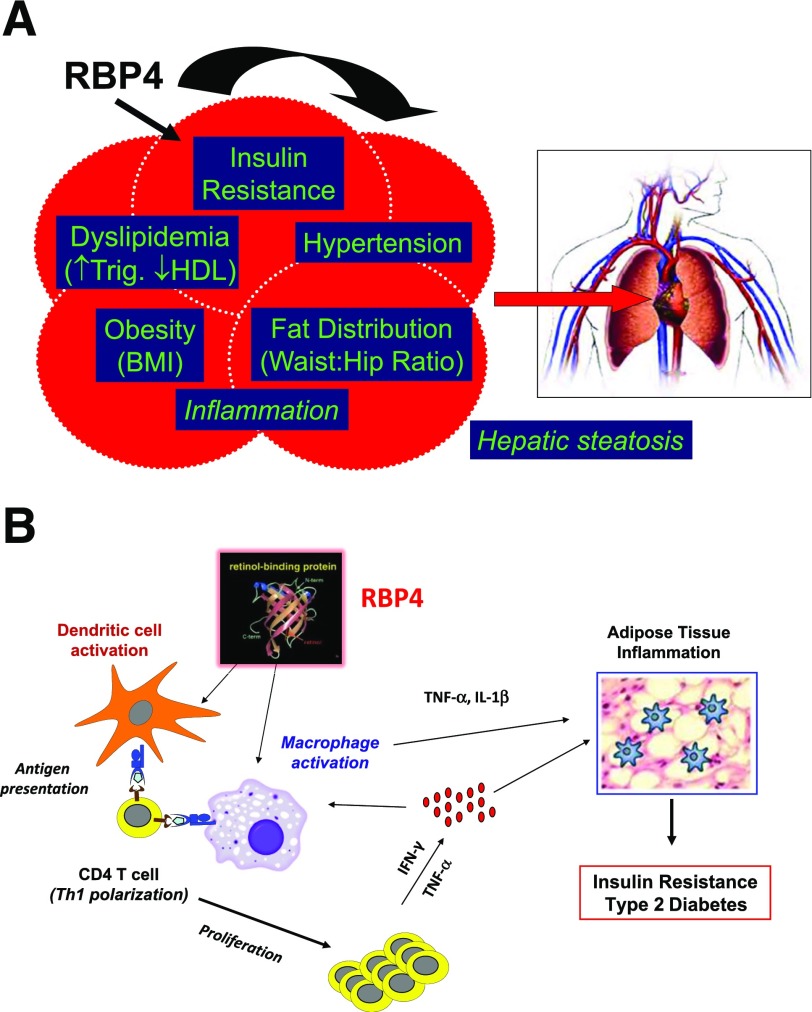
Out of this analysis came serum retinol binding protein 4 (RBP4). Retinol is vitamin A, and RBP4 is in the lipocalin family of binding proteins that carry fatty acids, steroids, and bilins in the blood. Qin Yang and Tim Graham led this project in the lab. We knew at the time that RBP4 was a retinol carrier protein, but nothing was known about its potential effects on glucose homeostasis. With our invaluable collaborators, Qin and Tim showed that RBP4 is elevated in serum in many insulin-resistant states in humans (15). Many other groups also studied this, and now there are hundreds of papers showing that RBP4 is elevated in association with insulin resistance and components of the metabolic syndrome (Fig. 5A), including not only visceral-type obesity and type 2 diabetes but also impaired glucose tolerance, dyslipidemia, coronary artery disease, hypertension, polycystic ovarian disease, hepatic steatosis, and HIV lipodystrophy—many different insulin resistant states. Furthermore, RBP4 levels are elevated before disease is apparent in family members of people with type 2 diabetes who are lean and do not have diabetes (15).
Figure 5.
Role of RBP4 in regulating whole-body insulin sensitivity. A: Serum RBP4 levels correlate with multiple components of the metabolic syndrome. B: RBP4-induced activation of antigen-presenting cells in adipose tissue is sufficient to cause adipose “inflammation” and systemic insulin resistance. Panel B reprinted from Moraes-Vieira et al. (25). Trig., triglycerides.
Many therapies that improve insulin sensitivity, such as dietary weight loss, bariatric surgery, exercise, and insulin-sensitizing drugs, also lower serum RBP4 levels. We became very interested in whether RBP4 is a marker or a cause of insulin resistance and diabetes. At this point, we expanded the team working on RBP4 to address this question in animal models, which we obtained from Bill Blaner at Columbia. We found that when we increase RBP4, either by transgenic overexpression or by injection of purified RBP4 into normal mice, it causes insulin resistance (16), whereas when we decrease RBP4 by knocking it out genetically or by treating obese mice with an agent that lowers serum RBP4, insulin sensitivity is conferred (16). Lowering serum RBP4 is an effective strategy for reducing insulin resistance and preventing or treating type 2 diabetes.
In humans, we continued to find a very strong inverse correlation between serum RBP4 levels and glucose disposal rate by clamp (15). The higher the RBP4, the lower the glucose disposal rate and the more insulin resistant people are. We went on to find that RBP4 is highly associated not only with insulin resistance, but also with many different components of the metabolic syndrome (15,17) (Fig. 5A).
When Bettina Kraus came to the lab, she showed that elevation of RBP4 directly causes hypertension and lowering RBP4 lowers blood pressure (18). Other groups showed that elevated RBP4 is highly associated with hepatic steatosis. We were very interested in the possibility that RBP4 was at least a strong biomarker for metabolic syndrome. We were happy to see a paper from the Chinese Academy of Sciences, which was a healthy aging study in over 3,000 participants (19). Each participant received a metabolic syndrome score based on five criteria. With each increase in metabolic syndrome components, there is an increase in serum RBP4 levels (19). This indicated that RBP4 is a good biomarker for metabolic syndrome. This result has been replicated in other ethnic groups and in younger subjects.
Elevated RBP4 is also a predictor of prediabetes and of myocardial infarction. Could elevated RBP4 cause insulin resistance in humans? The best way to approach that question is with genetic studies. I think the strongest study is the Rotterdam study. Again, a healthy aging study, this time with over 6,500 people (20). These investigators studied a naturally occurring polymorphism in the RBP4 promoter that is a gain-of-function mutation that increases RBP4 expression in adipose tissue (21). They showed that the people who had this allele had an 80% increased risk of type 2 diabetes, demonstrating that, at least in some genetic populations, elevated RBP4 in fat can be a risk factor for type 2 diabetes (20). Seeing these studies in humans, we really wanted to know what the mechanisms for RBP4-induced insulin resistance are.
We studied potential retinol-dependent and retinol-independent mechanisms. We found that RBP4 causes adipose tissue inflammation (Fig. 5B), which plays a causative role in the insulin resistance, and the effect is retinol independent (22). We started these studies with the hypothesis that RBP4 may cause insulin resistance by inducing inflammation in white adipose tissue. This was based on observations in humans that showed that elevated RBP4 correlates highly with the elevation of inflammatory markers in serum and adipose tissue (23,24). We found that RBP4 levels in adipose tissue increase in insulin-resistant states, which causes adipose inflammation by causing adipose cells to secrete chemoattractant factors such as MCP1. Those factors attract macrophages to infiltrate the fat and secrete proinflammatory cytokines, which then interfere with insulin signaling, block AKT phosphorylation, and block glucose transport (22). We know this is the case because when we use neutralizing antibodies to the cytokines, they block RBP4-induced insulin resistance (22). The neutralizing antibodies restore insulin signaling and insulin-stimulated glucose transport.
What kind of inflammation is this? Is this the kind of inflammation that might occur as a result of certain gut microbiota, or even sepsis, when the microorganisms secrete endotoxin that binds to the Toll-like receptor 4 complex and elicits signaling through the NF-κB pathway that causes the release of these proinflammatory cytokines that are known to cause insulin resistance?
We hypothesized that increased RBP4 in obesity might activate this same receptor complex and the same classic signaling pathway, resulting in cytokine production and insulin resistance. To test that, Tetsuya Hosooka, Mark Yore, and others in the lab knocked out TLR4 from macrophages and showed marked decreases in proinflammatory signaling, cytokine production, and insulin resistance (22). Thus, the effects of RBP4 engage classic inflammatory pathways in immune cells. We wanted to know whether the proinflammatory effect of RBP4 is sufficient to cause insulin resistance in vivo. We had shown that RBP4-overexpressing mice have glucose intolerance and insulin resistance (16). But does this result from RBP4-induced inflammation? To approach that, Pedro Vieira did a very intriguing experiment. He took dendritic cells from normal mice and treated them with either RBP4, which caused immune activation, or with control solution, which did not cause activation. Then he transferred these cells into normal mice. The question was: Do these RBP4-activated dendritic cells cause adipose tissue inflammation and systemic insulin resistance? The answer is yes. The mice that received the RBP4-activated dendritic cells developed insulin resistance compared with mice that received nonactivated dendritic cells or no dendritic cells (25).
Furthermore, the RBP-activated dendritic cell transfer was accompanied by proinflammatory effects on the adipose tissue macrophages and T cells and proinflammatory cytokine production. This showed us that RBP4 can activate dendritic cells or macrophages, which causes antigen presentation and T-cell polarization (Fig. 5B) (25). The T cells then proliferate and release cytokines, which cause adipose tissue inflammation and insulin resistance. The cytokines produced by the T cells further activate the macrophages, eliciting more proinflammatory cytokine production and more insulin resistance, which increases the risk for type 2 diabetes (Fig. 5B).
Can blockade of this antigen presentation improve adipose tissue inflammation and insulin resistance in the setting of elevated RBP4? To answer that, Pedro used a drug that is used to treat rheumatoid arthritis and other immune diseases in people, CTLA4-Ig. With this drug, he blocked antigen presentation in response to elevated RBP4 in mice. This completely blocked the T-cell proliferation and the cytokine production and reduced the adipose inflammation and restored insulin sensitivity (26). Thus, antigen presentation and inflammation in response to RBP4 are sufficient to cause insulin resistance in otherwise normal mice. As RBP4 is elevated in many insulin-resistant states in humans, antigen presentation is likely to be a factor in at least aggravating the insulin resistance.
Very recently we had the opportunity to collaborate with Seung-Ah Lee and Bill Blaner at Columbia, who made adipose-specific RBP4-overexpressing mice. On a chow diet, these mice have normal circulating RBP4 levels but elevated RBP4 levels in adipocytes (27). We showed this is sufficient to cause glucose intolerance, even without elevated circulating RBP4 (27). The elevation of RBP4 selectively in the adipocytes results in hepatic steatosis, even in mice on a chow diet.
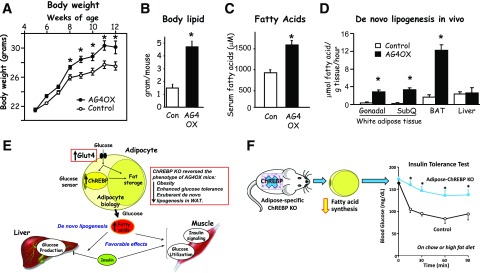
With striking effects like these, we started to wonder, how does the fat cell actually sense glucose? In obesity and type 2 diabetes, GLUT4 is downregulated and somehow that is sensed by the fat cell. But what in the fat cell responds to that and then how does that affect adipocyte biology such as the release of adipokines and RBP4, which then have effects on glucose production by the liver and insulin signaling and glucose utilization in the muscle? To address this, we went back to the DNA arrays. Fortunately, Mark Herman came to the lab, and he decided to apply gene set enrichment analysis. Instead of looking for a needle in a haystack, we were looking for pathways. Mark found that the fatty acid synthesis pathway was most highly upregulated in the adipose GLUT4 overexpressors and that was driven by carbohydrate response element binding protein (ChREBP), a transcription factor that regulates lipogenesis and glycolysis (28). This was really interesting because we had seen earlier that the adipose GLUT4 overexpressor mice were heavier (Fig. 6A) and all that extra weight was body lipid (Fig. 6B), but they had exquisitely improved glucose tolerance (Fig. 2B). In addition, they had elevated serum fatty acid levels (Fig. 6C), which clearly is not the usual picture of insulin sensitivity. We became very interested in this, and Mark set up the assay to measure substrate incorporation into new fatty acids, or de novo lipogenesis, in adipose tissue in awake mice. De novo lipogenesis is increased in all of the fat depots in the adipose GLUT4 overexpressor mice but not in the liver (Fig. 6D) (28). This model dissociates obesity from insulin resistance and glucose intolerance, and we felt we could really learn a lot from it.
Figure 6.
Role of GLUT4 in the regulation of adiposity and lipid metabolism. Adipose-specific overexpression of GLUT4 (AG4OX) results in increased body weight (A), adiposity (B), serum free fatty acids (C), and de novo lipogenesis in white and brown adipose depots but not in liver (D). Panels A and B reprinted from Shepherd et al. (3). Panel C adapted from Table 2 in Carvalho et al. (4). In panel D, some data are adapted from Herman et al. (28). E: Increasing glucose transport in adipocytes enhances systemic insulin sensitivity by channeling glucose into fatty acid synthesis. When GLUT4 protein levels increase, ChREBP senses the increased glucose transport, leading to alterations in adipocyte biology, including driving more glucose into fatty acid synthesis (also known as de novo lipogenesis). That process has favorable effects on insulin action to suppress hepatic glucose production and on insulin signaling and glucose utilization in muscle. Knocking out (KO) ChREBP reverses the increased fatty acid synthesis and the beneficial effects of increased GLUT4 expression in adipocytes (see box insert). M.A. Herman contributed to the original design of panel E. F: Adipose-specific KO of ChREBP in otherwise normal mice reduces fatty acid synthesis and causes systemic insulin resistance. Data in panel F was reprinted from Vijayakumar et al. (29). For experimental details for panels A and B, see Shepherd et al. (3); for panel C, see Carvalho et al. (4); for panel D, see Herman et al. (28); and for panel F, see Vijayakumar et al. (29). BAT, brown adipose tissue; Con, control; SubQ, subcutaneous adipose tissue. *Panels A–D and F: P < 0.05 vs. control.
So, when GLUT4 goes up, that is sensed by ChREBP and that alters adipocyte biology and drives more glucose into fatty acid synthesis or de novo lipogenesis (Fig. 6E), which has favorable effects on insulin action to suppress hepatic glucose production and on insulin signaling and glucose utilization in muscle. If ChREBP is really critical for this, then knocking it out should reverse the increased fatty acid synthesis and the beneficial effects. Mark bred the ChREBP knockout mice to the adipose GLUT4 overexpressors and that reversed the obesity, but it also reversed the enhanced glucose tolerance and the exuberant de novo lipogenesis in fat (28) (Fig. 6E). So ChREBP and fatty acid synthesis from glucose in adipocytes have a profound effect on glucose homeostasis.
How do these ChREBP data apply to human obesity and insulin resistance—states in which GLUT4 expression downregulates in fat? We collaborated with Sam Klein and Nada Abumrad at Washington University in St. Louis and measured ChREBP expression in human adipose tissue and analyzed the correlation with insulin sensitivity measured by clamp in the same people. There is a strong correlation between adipose ChREBP and insulin sensitivity in people (28). Other groups had shown that expression of de novo lipogenic enzymes in subcutaneous adipose tissue in humans over a range of BMI correlates highly with insulin sensitivity.
These correlations are great, but does reduction of ChREBP in normal mice or humans actually cause insulin resistance? To answer that, recently Archana Vijayakumar in the lab made an adipose-specific ChREBP knockout mouse. The mouse is otherwise normal and does not overexpress GLUT4 in adipocytes. She showed the decreased ChREBP expression decreased fatty acid synthesis from glucose in adipocytes and the adipose-specific ChREBP knockout was sufficient to cause insulin resistance and adipose tissue inflammation on chow or high-fat diet (29) (Fig. 6F). This seems to be a critical pathway for insulin sensitivity, and decreasing ChREBP in adipocytes in otherwise normal mice is sufficient to cause systemic insulin resistance. These studies show us that when we increase glucose transport into the fat cell, it is associated with increased insulin sensitivity. It is critical that the glucose is channeled into increased fatty acid synthesis, which is regulated, at least largely, by ChREBP.
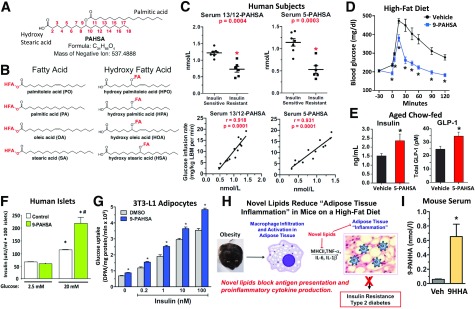
At this point, we were very interested in determining the identity of these fatty acids with beneficial metabolic effects. We were fortunate to collaborate with Alan Saghatelian and his student, Edwin Homan, when they were at Harvard University. We did untargeted lipidomic analysis of adipose tissue from the adipose GLUT4-overexpressing mice. Several ions were 16- to 18-fold elevated. I asked Alan if he could identify those ions, and he said they were not in any of the publicly available databases. It was clear that we had found a novel class of lipids. Alan and Edwin went on to solve their structure. These lipids consist of a fatty acid group, such as palmitic acid, and a hydroxy fatty acid group, and they are joined by an ester bond at any of the carbons except the ones near the ends of the chain (30) (Fig. 7A). There are multiple isomers of palmitic acid hydroxy stearic acid (PAHSA) defined by the position of the ester bond. In addition, we found that four different fatty acids and their hydroxy fatty acids could be combined in any combination. Initially, we thought there were a total of 16 different family members (Fig. 7B), but now we, and other laboratories, have found even more. For a number of reasons, we decided to study the PAHSA family of isomers. PAHSAs are one family of the fatty acid hydroxy fatty acid (FAHFA) class of lipids.
Figure 7.
FAHFAs are a novel class of lipids with beneficial metabolic and anti-inflammatory effects. A: FAHFAs consist of a fatty acid group, such as palmitic acid, and a hydroxy fatty acid group joined by an ester bond (PAHSA). B: FAHFAs can consist of multiple different fatty acids and their hydroxy fatty acids in various combinations. Examples of some constituent fatty acids are shown. C: PAHSA levels in serum are lower in insulin-resistant people than in the insulin-sensitive people and serum levels correlate highly with insulin sensitivity determined by glucose infusion rate during a euglycemic-hyperinsulinemic clamp. A single oral dose of PAHSA improves glucose tolerance of high-fat diet–fed mice (D) and augments insulin and GLP1 secretion in aged, insulin-resistant chow-fed mice (E). These secretory effects are not seen in high-fat diet–fed mice. PAHSAs enhance glucose-stimulated insulin secretion directly in human islets (F) and enhance insulin-stimulated glucose transport in cultured adipocytes (G). H: Anti-inflammatory effects of PAHSAs include blocking antigen presentation, proinflammatory cytokine production, and costimulatory molecule production. This leads to decreased adipose tissue inflammation and insulin resistance. I: Administration of 9HHA, a nonnatural hydroxy fatty acid, results in the synthesis of 9PAHHA, proving that PAHSA can be synthesized in vivo. For experimental details for panels A–G and I, see Yore et al. (30). Panels A–G and I reprinted from Yore et al. (30). *Panel C: P < 0.0005 vs. insulin-sensitive subjects; panels D, E, and I: P < 0.05 vs. vehicle (Veh); panel F: P < 0.05 vs. 2.5 mmol/L glucose, same treatment; panel G: P < 0.05 vs. DMSO, same insulin concentration. #P < 0.05 vs. control, same glucose concentration. DPM, disintegrations per minute; LBM, lean body mass.
What is the tissue distribution of the novel lipids? They are in every tissue that we have studied, with highest levels in white and brown fat. There is a significantly lower level in liver, which is also a highly lipogenic tissue. Are the novel lipids regulated in altered physiologic states? Are they regulated with changes in glucose flux?
In the adipose GLUT4-overexpressing mice, all of the isomers of the lipids are upregulated in fat, whereas they are decreased in the liver (30). This indicates the opportunity for tissue-specific regulation. Adipose tissue also has many more isomers than liver. Are these novel lipids regulated in insulin-resistant people? To study that, we collaborated with Ulf Smith in Gothenberg, Sweden, and we studied relatively young people. They were only modestly obese but significantly insulin resistant by clamp. They all had normal hemoglobin A1c, so none had diabetes. We showed that the novel lipid levels in serum are lower in the insulin-resistant people than in the insulin-sensitive people (Fig. 7C). The levels of these novel lipids in serum correlated very highly with insulin sensitivity by clamp (30). Ulf also sent us adipose biopsies from the same people, and the levels of the lipids were also low in the fat from insulin-resistant people. The levels correlated with insulin sensitivity, although the correlations were stronger for the serum levels. This motivated us to ask what the biologic effects of these novel lipids are.
We started with a glucose tolerance test. With a single oral dose of the lipids, we markedly improved glucose tolerance (Fig. 7D). This rapid effect suggested to us that they might be stimulating insulin secretion. We measured insulin secretion as well as GLP1 secretion at 5 min after giving glucose. The novel lipids given orally augmented insulin and GLP1 secretion in aged, insulin-resistant chow-fed mice (but not in high-fat diet–fed mice) (30) (Fig. 7E). We wanted to know whether those were direct effects, so we studied the lipid effects in human islets. At 2.5 mmol/L glucose, the lipids have no effect on insulin secretion, which is good. The normal glucose-stimulated insulin secretion is markedly enhanced with the novel lipid treatment. The lipids have a direct effect to enhance glucose-stimulated insulin secretion in human islets (Fig. 7F). We also wanted to know if the GLP1 secretion was direct, so we studied that in enteroendocrine cells from the gut. There was a nice dose-response stimulation of GLP1 secretion from these gut cells reaching the levels of a positive control.
Recently, we have shown that chronic treatment with the 5 or 9 isomer of PAHSAs also improves insulin sensitivity in mice (31,32), and we became very interested in the mechanisms. Do they augment insulin action on glucose transport into fat cells? We studied this in cultured adipocytes and at every insulin concentration the PAHSAs enhance insulin-stimulated glucose transport (Fig. 7G). Do the PAHSAs exert their biologic effects through specific receptors? A lot of literature is emerging about lipid-sensing G protein–coupled receptors (GPCRs), which have multiple roles in glucose homeostasis and diabetes. The data show that GPR-120 is present in gut enteroendocrine cells, and it is involved in GLP1 secretion. GPR-120 is also in macrophages and has anti-inflammatory effects. GPR-120 is also in adipocytes and can affect differentiation and glucose uptake, whereas other GPCRs are present in pancreatic β-cells and mediate insulin secretion. Because of the data on GPR-120, we decided to study that GPCR. Mark Yore then went back to the adipocytes and knocked down GPR-120. In the control cells without PAHSAs, GPR-120 knockdown had no effect on glucose transport in response to vehicle or insulin. However, the augmentation of glucose transport with PAHSAs is completely abolished when we knock down GPR-120 (30), indicating that GPR-120 mediates at least this effect of the novel lipids.
Our more recent data suggest that there are other GPCRs that are also involved in other effects of PAHSAs (31,33). Some data suggest that GPR-120 may also mediate anti-inflammatory effects so we asked whether PAHSAs are anti-inflammatory. Pedro showed that the novel lipids block antigen presentation and proinflammatory cytokine production. They block the costimulatory molecules and the proinflammatory cytokines and that decreases adipose tissue inflammation and insulin resistance (30,33) (Fig. 7H).
Are FAHFAs made in our bodies? This is an important question because this could uncover new pathways that could be targeted to restore levels of the novel lipids in insulin-resistant states and prevent or treat type 2 diabetes. For example, we might be able to increase the synthesis or decrease the degradation of the lipids. To answer the question about synthesis, Alan actually synthesized a form of hydroxy fatty acid that is found only at very low levels in nature that we can use as a sort of label. We gave it orally to mice, and subsequently analyzed the serum and tissues by mass spectrometry to see if we could find that unnatural lipid incorporated into a full-length FAHFA.
The mouse is able to make a FAHFA out of that labeled lipid that Alan had synthesized (Fig. 7I). Thus, FAHFAs are definitely synthesized in our bodies, and when we studied other tissues including human fat, we found synthesis in those tissues as well. Biosynthetic pathways could provide targets to restore or increase FAHFA levels in insulin-resistant people and potentially improve their insulin sensitivity.
What do we know about how FAHFAs are broken down in our bodies? Increased degradation, also referred to as hydrolysis, by specific enzymes would lead to decreased levels of the novel lipids which would lead to decreased beneficial effects. We have recently found several enzymes that can hydrolyze FAHFAs (34,35). I want to tell you about carboxyl ester lipase (CEL), which we just recently found hydrolyzes FAHFAs. This is important because a mutation in this enzyme causes maturity-onset diabetes of the young type 8 (MODY8) (36). CEL has a number of names. It is also called bile salt–stimulated lipase, cholesterol esterase, and lysophospholipase (37). Much is known about the protein (38). Importantly, the MODY8 mutation in the CEL gene results in impaired insulin secretion and pancreatic exocrine dysfunction (36). CEL knockout mice do not have diabetes. It turns out that the MODY8 mutation is actually a gain-of-function mutation, so a knock-in of the mutant is needed.
We next studied how active that mutant is in hydrolyzing FAHFAs. The hydrolysis assay works by starting with the intact PAHSA and then studying the rate of cleavage of the ester bond (hydrolysis) resulting in the products, palmitic acid and hydroxy stearic acid. When we looked at the activity of the wild-type CEL or the MODY8, there was a greater than twofold increase in the hydrolysis rate with the mutant (35). We compared hydrolysis in multiple tissues in vitro and found that the rate was 10 times higher in the pancreas than in any other tissue. Recently, we have shown that CEL is the major hydrolytic enzyme for FAHFAs in the pancreas. Furthermore, if we treat wild-type mice with a CEL inhibitor, it markedly decreases the hydrolytic activity (35). The notion is that the MODY8 gain-of-function mutation would increase hydrolysis of FAHFAs in the pancreas, which would decrease the levels of these beneficial lipids and that would impair insulin secretion.
To investigate the anti-inflammatory effects of FAHFAs, we started with ulcerative colitis, another inflammatory disease affecting many people in Western society. The integrity of the gut depends in large part on an intact gut mucosa and breakdown of the mucosa is fundamental to the etiology of the disease. The same types of immune cells—macrophages, dendritic cells, and T cells—that we have been studying in the adipose tissue in obesity and insulin resistance are present in the gut mucosa.
To summarize the colitis results, Jennifer Lee and Pedro found that PAHSA treatment reduces the clinical colitis score and markedly reduces gut inflammation (33). These anti-inflammatory effects apply to other diseases, including possibly type 1 diabetes. In colitis, dendritic cells present antigens to CD4 T cells, stimulating them to secrete IL-12, which causes CD4 T-cell activation, polarization, and clonal expansion. That increases interferon-γ secretion, which then causes gut inflammation. Pedro showed that PAHSAs decrease antigen presentation, which decreases the polarization and clonal expansion of T cells and the cytokine production and markedly decreases the gut inflammation (33). Could these anti-inflammatory effects be beneficial for type 1 diabetes? In very recent studies, Ismail Syed and Pedro have shown that in an NOD mouse model of autoimmune type 1 diabetes, once daily oral treatment with PAHSAs delays the onset of type 1 diabetes and markedly reduces the incidence (39).
Many agents can improve the risk for diabetes in NOD mice, so we are very excited about trying to take this into human studies. But in the meantime, we have done some direct studies regarding islet viability. When we expose normal islets in culture to cytokine stress, there is cell death but when we incubate them with PAHSAs and the cytokine, we can prevent this to a high degree. Apoptosis and necrosis are both increased with the cytomix, and PAHSA treatment markedly reduces this. β-Cell proliferation is markedly impaired with the cytomix and is improved with PAHSA treatment. We are very excited about the possibility that PAHSAs could help prevent or even treat early-stage type 1 diabetes.
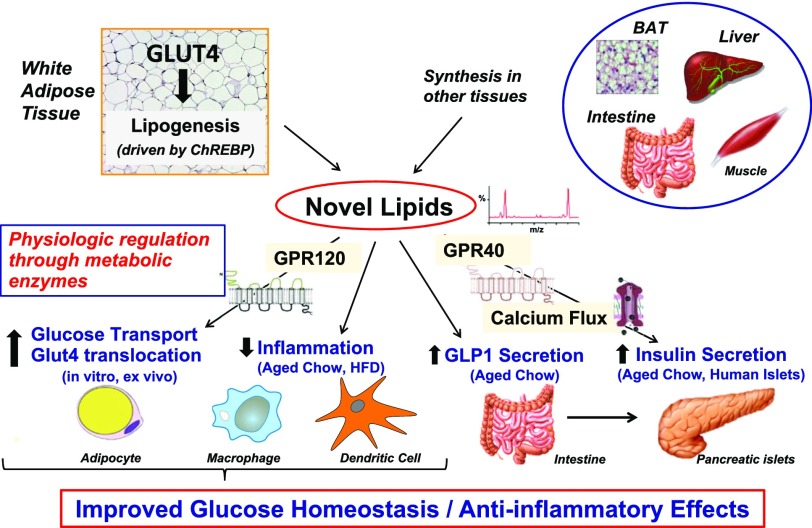
In conclusion, I have described that with increased GLUT4, we increase lipogenesis driven by ChREBP in adipocytes (Fig. 8). This results in the production of FAHFAs, which act through GPCRs to augment glucose transport and GLUT4 translocation. These lipids decrease inflammatory responses in macrophages and dendritic cells. These anti-inflammatory effects may be protective in type 1 diabetes, a state in which GLUT4 is also downregulated in adipocytes. In aged, insulin-resistant chow-fed mice, PAHSAs, one family of FAHFAs, also increase glucose-stimulated GLP1 secretion and insulin secretion. They directly increase insulin secretion in human pancreatic β-cells, which we think is through GPR-40 (31). But we do not see these secretory effects of PAHSAs in mice on a high-fat diet, suggesting that increased insulin sensitivity plays a major role in the beneficial effects in high-fat diet–fed mice. FAHFAs/PAHSAs are present in many other tissues, which undoubtedly contribute to the beneficial effects to improve glucose homeostasis and reduce inflammation. Most recently, we have shown that there is physiologic regulation through specific metabolic enzymes that could be targeted to increase the levels of FAHFAs in insulin-resistant states and potentially prevent or treat type 2 diabetes. Returning to adipocyte biology, over the past several decades we have learned more about the adipocyte as an endocrine gland, for example, its effects through secretion of RBP4 and how it regulates energy balance through molecules that affect AMPK, through its role in de novo lipogenesis and the role of adipose inflammation in the setting of many of these other changes.
Figure 8.
Summary of biologic activities of FAHFAs. Increased GLUT4 protein levels in adipocytes results in increased de novo lipogenesis driven by ChREBP. This results in the production of FAHFAs, which act through GPCRs (GPR-120) to augment glucose transport and GLUT4 translocation. These lipids also decrease inflammatory responses in macrophages and dendritic cells. In aged, insulin-resistant chow-fed mice (but not in high-fat diet [HFD]–fed mice), these lipids increase glucose-stimulated GLP1 secretion and insulin secretion. FAHFAs directly increase insulin secretion in human pancreatic β-cells, which involves GPR-40. As we do not see these secretory effects of the novel lipids in mice on an HFD but find increased insulin sensitivity with chronic PAHSA treatment, it is likely that insulin-sensitizing effects of the PAHSAs play a major role in their beneficial effects in HFD-fed mice. Adapted from Yore et al. (30). BAT, brown adipose tissue.
I want to thank the postdoctoral fellows, students, research assistants, and associates and my long-standing collaborators, some of whom I have known since I was a fellow. We continue to have vibrant collaborations. I want to thank the Endocrine Division—both clinical and research faculty—at Beth Israel Deaconess Medical Center. I want to thank my family, many of whom are here today, especially my three brothers, my father who always believed in me, and my mother who, in the 10th decade of her life, continues to be an inspiration. As a scientist, I thank you for your discoveries that have moved the field forward. As a physician, I thank you for your compassionate care of our patients. And as a person who has been deeply touched by friends and family members with diabetes, I hope you share my hope for the future driven by the power of scientific discovery.
Article Information
Acknowledgments. The author again expresses her deep gratitude to the postdoctoral fellows, junior faculty, and students who have made this work possible. She also thanks Odile D. Peroni and Suzanne Ruscitti, Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center, for assistance with the manuscript.
Funding. Work in the laboratory of B.B.K. has been supported by grants from the National Institutes of Health (R01 DK43051, R01 DK106210, R01 DK098002, and P30 DK57521), the American Diabetes Association, and the JPB Foundation.
Duality of Interest. B.B.K. has served as an advisor or consultant within the past 12 months to Janssen Pharmaceuticals and Ironwood Pharmaceuticals and previously received research support from Janssen Pharmaceuticals. Neither B.B.K. nor her family members hold stock directly or indirectly in any of these companies. B.B.K. is an inventor on patents related to RBP4 and FAHFAs. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The Banting Medal for Scientific Achievement Lecture was presented at the American Diabetes Association’s 76th Scientific Sessions in New Orleans, LA, on Sunday, 12 June 2016.
References
- 1.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 2010;285:11348–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross SR, Graves RA, Spiegelman BM. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev 1993;7:1318–1324 [DOI] [PubMed] [Google Scholar]
- 3.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 1993;268:22243–22246 [PubMed] [Google Scholar]
- 4.Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 2005;289:E551–E561 [DOI] [PubMed] [Google Scholar]
- 5.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001;409:729–733 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J 2001;15:1101–1103 [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 8.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 2000;141:2328–2339 [DOI] [PubMed] [Google Scholar]
- 9.Muoio DM, Dohm GL, Fiedorek FT Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle [published correction appears inDiabetes 1997;46:1663]. Diabetes 1997;46:1360–1363 [DOI] [PubMed] [Google Scholar]
- 10.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339–343 [DOI] [PubMed] [Google Scholar]
- 11.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 12.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 13.Alquier T, Kawashima J, Tsuji Y, Kahn BB. Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 2007;148:1367–1375 [DOI] [PubMed] [Google Scholar]
- 14.McCrimmon RJ, Shaw M, Fan X, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008;57:444–450 [DOI] [PubMed] [Google Scholar]
- 15.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362 [DOI] [PubMed] [Google Scholar]
- 17.Klöting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 2007;6:79–87 [DOI] [PubMed] [Google Scholar]
- 18.Kraus BJ, Sartoretto JL, Polak P, et al. Novel role for retinol-binding protein 4 in the regulation of blood pressure. FASEB J 2015;29:3133–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi Q, Yu Z, Ye X, et al. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab 2007;92:4827–4834 [DOI] [PubMed] [Google Scholar]
- 20.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 2008;51:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munkhtulga L, Nagashima S, Nakayama K, et al. Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity (Silver Spring) 2010;18:1006–1014 [DOI] [PubMed] [Google Scholar]
- 22.Norseen J, Hosooka T, Hammarstedt A, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and Toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 2007;92:1971–1974 [DOI] [PubMed] [Google Scholar]
- 24.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 2007;92:2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab 2014;19:512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moraes-Vieira PM, Castoldi A, Aryal P, Wellenstein K, Peroni OD, Kahn BB. Antigen presentation and T-cell activation are critical for RBP4-induced insulin resistance. Diabetes 2016;65:1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SA, Yuen JJ, Jiang H, Kahn BB, Blaner WS. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 2016;64:1534–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012;484:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayakumar A, Aryal P, Wen J, et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Reports 2017;21:1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yore MM, Syed I, Moraes-Vieira PM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014;159:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed I, Lee J, Moraes-Vieira PM, et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab 2018;27:419–427.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoro A, Zhou P, Peroni OD, et al. Palmitic acid esters of hydroxy stearic acids are hepatic insulin sensitizers in chow and high-fat diet (HFD)-fed mice. Diabetes 2018;67(Suppl. 1):A488 [Google Scholar]
- 33.Lee J, Moraes-Vieira PM, Castoldi A, et al. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) protect against colitis by regulating gut innate and adaptive immune responses. J Biol Chem 2016;291:22207–22217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons WH, Kolar MJ, Kamat SS, et al. AIG1 and ADTRP are atypical integral membrane hydrolases that degrade bioactive FAHFAs. Nat Chem Biol 2016;12:367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolar MJ, Kamat SS, Parsons WH, et al. Branched fatty acid esters of hydroxy fatty acids are preferred substrates of the MODY8 protein carboxyl ester lipase. Biochemistry 2016;55:4636–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raeder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet 2006;38:54–62 [DOI] [PubMed] [Google Scholar]
- 37.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem 1996;271:7196–7202 [DOI] [PubMed] [Google Scholar]
- 38.Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J Lipid Res 2002;43:2017–2030 [DOI] [PubMed] [Google Scholar]
- 39.Syed I, Mohan JF, Moraes-Vieira PM, et al. PAHSAs reduce type 1 diabetes incidence in nonobese diabetic (NOD) mice through anti-inflammatory effects and direct protection against cytokine-induced islet β-cell death. Diabetes 2017;66(Suppl. 1):A87 [Google Scholar]