Abstract
Stroke is the second-leading cause of death and a leading cause of serious long-term disability worldwide, with an increasing global burden due to the growing and aging population. However, strict eligibility criteria for current treatment opportunities make novel therapeutic approaches desirable. Oxi-dative stress plays a pivotal role during cerebral ischemia, eventually leading to neuronal injury and cell death. The significant correlation between redox imbalance and ischemic stroke has led to various treat-ment strategies targeting the endogenous antioxidant system in order to ameliorate the adverse prognosis in patients with cerebral infarction. One of the most extensively investigated cellular defense pathway in this regard is the Nrf2-heme oxygenase-1 (HO-1) axis. In this review, our aim is to focus on the poten-tial clinical relevance of targeting the HO-1 pathway in ischemic stroke.
Keywords: Ischemic stroke, oxidative stress, heme oxygenase-1, cerebral ischemia, cerebral infarction, antioxidant system
1. Introduction
Ischemic stroke is an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction [1]. Accounted for over 6 million deaths worldwide per year, stroke is the second-leading global cause of death and a leading cause of serious long-term disability [2]. Due to the growing and aging population, the global burden of cerebral infarction increases, in spite of a significant decline in age-standardized stroke rates [3, 4]. At the current state of the art, the only evidence-based acute treatment opportunities remain intravenous thrombolysis (within 4.5 hours after symptom onset) with alteplase and, in selected cases, endovascular intervention with mechanical thrombectomy [5]. However, due to the very narrow therapeutic time-window and various exclusion criteria, estimates of eligibility for systemic treatment within the population of ischemic stroke patients range only from 6% to 8% [6]. Furthermore, even if recanalization is achieved, the activation of a multi-step cascade, involving inflammatory immune responses, disruption of calcium homeostasis and enhanced production of Reactive Oxygen Species (ROS), can result in reperfusion injury [7]. Oxidative stress - with ROS causing damage to cellular components, including lipids, proteins and nucleic acids - plays a pivotal role during cerebral ischemia, eventually leading to neuronal injury and cell death [8-10]. The significant correlation between oxidative stress and ischemic stroke has led to various treatment strategies to ameliorate the adverse prognosis in patients.
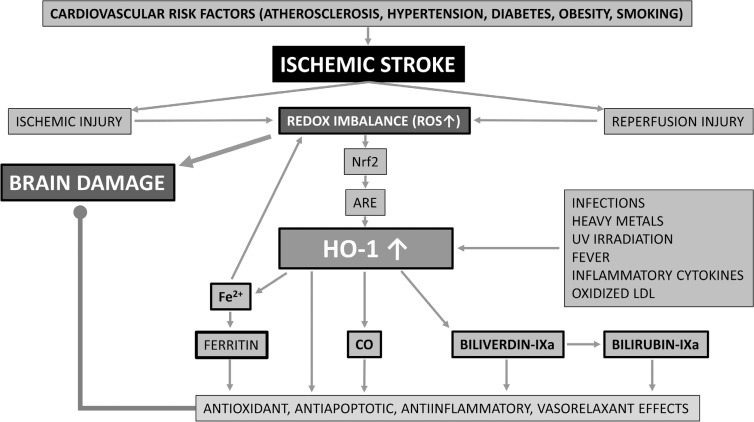
Among transcription factors, nuclear factor erythroid 2-related factor 2 (Nrf2) plays a central role in cellular defense against oxidative stress [11]. Under conditions of redox imbalance, Nrf2 activates various detoxifying and antioxidant enzymes, such as NAD(P)H:quinone oxidoreductase (NQO1), glutathione S-transferases (GSTs), heme oxygenase-1 (HO-1) and γ-glutamylcysteine synthetase (γ-GCS), by binding to antioxidant-response elements (AREs) and thereby inducing gene expression [12]. Among abovementioned phase 2 enzymes, HO-1 has been reported to have the most AREs on its promoter [13], making it a promising therapeutic target against brain injury in cerebral infarction. While HO-2 (expressed predominantly in the central nervous system) and the poorly characterized HO-3 are constitutive isoforms [14, 15], HO-1 - also referred to as heat shock protein 32 [16] - is the inducible type of heme oxygenase. It can be activated by various stimuli, including infections, heavy metals, ultraviolet irradiation, fever, inflammatory cytokines and oxidized Low-Density Lipoprotein (LDL) [17]. HO-1 catalyzes heme degradation, producing equimolar amounts of ferrous iron (Fe2+), Carbon Monoxide (CO) and biliverdin-IXa. Liberated free iron ultimately induces ferritin expression leading to Fe2+ sequestration and thereby limiting iron-mediated cell injury [18, 19], whereas biliverdin-IXa is rapidly converted to bilirubin-IXa by biliverdin reductase [20]. Among downstream products of HO-1, CO has emerged to have vasorelaxant, anti-inflammatory and antiapoptotic properties [21], while biliverdin and bilirubin are known as potent endogenous antioxidant and anti-inflammatory molecules [20]. Furthermore, ferritin has also been demonstrated to exert anti-inflammatory effects [22]. The protective role of the Nrf2-HO-1 pathway in ischemic stroke is summarized in Fig. 1.
Fig. (1).
The protective role of the Nrf2-HO-1 pathway in ischemic stroke. The redox imbalance in ischemic and reperfusion injury results in brain damage but also in an increase of HO-1. The increase in HO-1 together with ferritin, CO, biliverdin and bilirubin exert antioxidant, antiapoptitic, antiinflamatory and vasorelaxant effects, resulting in a protective effect against brain damage in ischemic stroke.
In this review, our aim is to focus on the potential clinical relevance of targeting the HO-1 pathway in ischemic stroke.
2. The HO-1 pathway and ischemic stroke: preclinical results
Among various animal stroke models, intra-arterial suture occlusion of the Middle Cerebral Artery (MCAO) has been used in more than 40% of the approximately 2,800 ischemic stroke experiments [23, 24]. The MCAO model is considered to be suitable for imitating human ischemic stroke with consecutive neuronal cell death, cerebral inflammation and blood-brain barrier damage [23, 24].
An early experiment using permanent MCAO in transgenic mice demonstrated that overexpression of HO-1 significantly reduces infarct volume [25]. On the other hand, HO-1 knockout mice showed significantly larger infarct size as compared to their wildtype counterparts [26].
In addition to genetically modified rodents, gene transfer vectors offer a further genetic strategy in preclinical experimental stroke. In a rat MCAO model using stereotaxic instrumentation, treatment with an adenoviral vector overexpressing HO-1 resulted in decreased infarct volume and attenuation of neurologic deficits [27]. Because of the deleterious side effects of viral vectors, such as immunogenicity and oncogenicity [28], non-viral gene carriers have been investigated and were shown to induce site-specific HO-1 gene expression and therapeutic effect when applied as stereotaxic injection into the rat ischemic brain [29]. HO-1 gene transfer into injured carotid arteries of ApoE-null mice led to earlier thrombolysis with decreased plasminogen activator-1 expression [30].
Inducers of HO-1 have also demonstrated promising results in preclinical studies. Numerous natural compounds that activate the Nrf2-HO-1 pathway, such as sulforaphane [31], the standardized Ginkgo biloba extract EGb761 [26], curcumin [32], polyphenols [33, 34] and triterpenoids [35, 36] exert neuroprotective effects against stroke in animal models. Among pharmaceutical inducers of the Nrf2-HO-1 pathway, dimethyl fumarate (DMF) - approved for clinical use in the treatment of patients with relapsing forms of multiple sclerosis [37] - showed dose-dependent neuroprotection in a mouse MCAO model resulting in significant reduction of infarct volume, neurological deficits, brain edema and cell death [38]. In another study in mice, DMF was able to prevent blood-brain barrier hyperpermeability during ischemic stroke if given as pretreatment before ischemia-reperfusion injury [39]. Other drugs on the market with the ability to increase HO-1 expression include aspirin [40], statins [41], candesartan [42] and metformin [43]. However, regarding statins, two recent human studies did not confirm HO-1-induction in subjects treated with clinically relevant doses of simvastatin and atorvastatin [44, 45] which could at least partially be explained by the discrepancy in concentrations used in experimental situations and those detected in humans [46].
In addition to genetic and pharmacologic strategies in order to enhance HO-1 expression or activity, direct application or HO-1 endproducts offers the additional potential to harness the therapeutic potential of the HO-1 pathway. In a permanent MCAO model, mice exposed to low-concentration CO after permanent ischemia had significantly reduced infarct size, an effect which was abolished in Nrf2-knockout species suggesting that CO-exposure after stroke can provide protection by activating the Nrf2 pathway [47]. Of note is that delaying CO-exposure resulted in weaker protection suggesting a therapeutic time window. In another mouse MCAO model, Zeylanov et al. showed that inhalation of CO attenuated infarct volume significantly, limited brain edema formation and improved neurological deficit scores [48]. In a further study, intravenously applied PEGylated COHb in a transient focal cerebral ischemia model in rats resulted in reduced infarct volumes and better neurologic deficit scores [49]. Besides CO, biliverdin treatment has also been shown to ameliorate oxidative injury on neurons and decrease infarct size in rat MCAO models [50, 51].
A novel therapeutic approach to cerebral infarction is the use of stem cells. In a rat model of Hypoxic-Ischemic Brain Damage (HIBD), where placenta-derived mesenchymal stem cells (PD-MSCs) were injected with a stereotaxic apparatus, subjects receiving PD-MSCs showed significant improvement in HIBD along with a pronounced elevation in HO-1 and Nrf2 levels [52]. In an in vitro study, neural stem cells (NSCs) were pretreated with the polyphenol resveratrol prior to oxygen-glucose deprivation/reoxygenation. The authors found that resveratrol markedly increased NSC survival and proliferation, whilst upregulating expression levels of Nrf2 and HO-1 [53].
3. The HO-1 pathway in humans - cardiovascular risk factors
3.1. HO-1 Gene Polymorphisms and Cardiovascular Risk
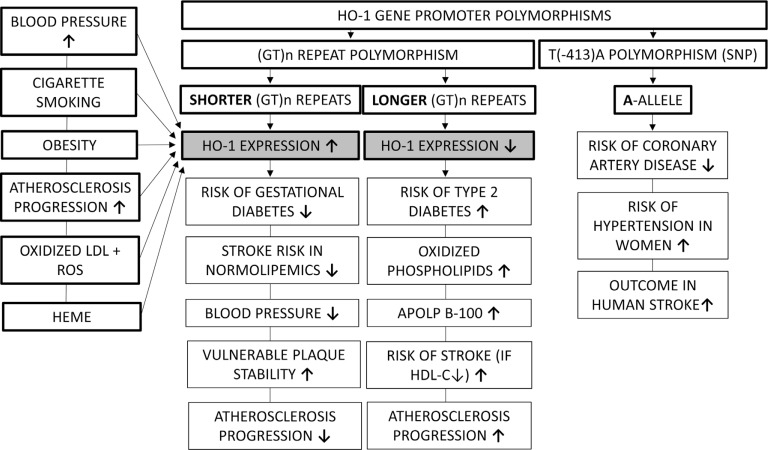
In humans, HO-1 expression shows a broad variability due to a highly polymorphic promoter, which has been correlated with cardiovascular risk factors. The most extensively studied variant is the length of the (GT)n-repeat region, which has been inversely related to HO-1 expression [54]. In a prospective, population-based survey, Pechlaner et al. found that subjects with longer (>32) repeats on both HO-1 alleles had increased cardiovascular disease risk, enhanced atherosclerosis progression, and a trend toward higher levels of oxidized phospholipids on apolipoprotein B-100 [55]. A recent systematic review of the epidemiological literature on HO-1 (GT)n-polymorphisms found that the short (<24-27) repeat SS genotype (higher HO-1 activity) was represented in a higher proportion among subjects without known cardiovascular disease. Interestingly, the review also found that racial disparities exist in the (GT)n-repeat length distribution with proportions of the protective SS genotype being 11% and 22% in Caucasian and Asian populations, respectively [56]. Among cardiovascular risk factors, a meta-analysis found that persons carrying the (GT)n L (long) allele had an increased odds ratio for type 2 diabetes as compared with those with S (short) allele [57]. Besides the abovementioned dinucletoide repeat polymorphism, one single nucleotide polymorphism (SNP) in the proximal promoter region of HO-1 (T[-413]A) has also been evaluated with the “A” allele promoter having higher activity than the “T” allele, however, data are scarce and conflicting. The AA genotype of the T(-413)A polymorphism has been associated with a lower incidence of coronary artery disease [58]. However, it has also been demonstrated that in women with AA genotype, the incidence of hypertension was increased [59].
3.2. Atherosclerosis
Oxidized low-density lipoproteins along with ROS play a central role in atherogenesis and can induce HO-1 [60, 61]. The role of HO-1 in the protection of the vascular wall from atherosclerosis was unraveled by the first reported HO-1 deficient patient, whose autopsy report revealed fatty streaks and fibrous plaques in the aorta at the age of six [62]. LDL isolated from the plasma of this child showed practically no oxidative resistance [63]. The presence of HO-1 was found in human atherosclerotic plaques with no HO-1 expression in normal arteries [64]. Also, expression increases with the severity of atherosclerosis [65]. The prooxidant environment in the advanced atheromatous lesion precipitates erythrocyte lysis and the oxidation of liberated hemoglobin to ferri- and ferrylhemoglobin, while the released heme and iron promote further oxidation of lipids [66]. These events amplify endothelial cell cytotoxicity of plaque components which is inhibited by HO-1. Induction of HO-1 was found to be a stabilizing factor of vulnerable plaques by reducing necrotic core size and intraplaque lipid accumulation, whereas increasing cap thickness and vascular smooth muscle cells [67]. In coronary arteries obtained from Japanese autopsy cases, the prevalence of HO-1 expression increased as the lesion type and grade of stenosis progressed and was significantly higher in diabetic patients [68]. A study examining carotid artery plaques removed during endarterectomy found a strong association between Helicobacter pylori infection and expression of HO-1, predominantly in specimens obtained from asymptomatic patients. The authors concluded that oxidative stress elicited by the Helicobacter pylori infection may have been inhibited by HO-1, resulting in the stabilization of the atherogenic process [69]. Among downstream products of HO-1, a reciprocal relationship between serum bilirubin levels and carotid atherosclerosis has already been reported in prior studies [70, 71]. Furthermore, in a meta-analysis of 11 studies, increased serum bilirubin levels were found to be a decreased risk for the development of atherosclerosis [72].
3.3. Hypertension
Data regarding the relationship between HO-1 expression and hypertension are based on animal experiments using spontaneously hypertensive rats. Using chronic angiotensin-II infusion in a hypertensive rat model, pressure overload upregulated HO-1 expression and activity in the aorta [73]. Furthermore, it has also been shown that transferring human HO-1 into spontaneously hypertensive rats resulted in attenuation of the development of hypertension attributed to the vasodilatory effects of CO [74]. In human studies, lung tissues of newborns suffering from congenital diaphragmatic hernia and pulmonary hypertension showed reduced expression of HO-1 [75]. The blood pressure lowering effect of a 3-month treatment with olmesartan in essential hypertension, was, in part, attributed to an increase in plasma HO-1 levels [76]. Among the catabolic metabolites of HO-1, concentrations of bilirubin have been shown to be significantly decreased in patients with untreated hypertension, whereas not differing between normotensive and treated hypertensive subjects [77]. Furthermore, in a 10-year health monitoring Korean study with normotensive subjects, serum bilirubin levels and the incidence of developing hypertension were found to be correlating negatively [78]. In women with gestational hypertension or pre-eclampsia, end-tidal breath CO levels were significantly lower than in healthy controls [79].
3.4. Diabetes Mellitus
HO-1 expression and diabetes have been shown to be reciprocally related in animal experiments, e.g. overexpressing HO-1 slowed the progression of diabetes in NOD mice or induction of HO-1 in Zucker diabetic rats improved insulin sensitivity [80, 81]. In humans, HO-1 expression was found to be reduced in peripheral blood mononuclear cells of diabetic patients [82], whereas high expression levels of HO-1 in early pregnancy has been associated with reduced risk of gestational diabetes [83]. Furthermore, elevated plasma HO-1 concentrations were affiliated with higher odds ratios for developing type II diabetes mellitus [84]. The HO-1 inducer Nrf2 has been shown to be lower in pre-diabetic and diabetic patients, suggesting that attenuated antioxidant mechanisms due to decreased Nrf2 might be involved in the development of diabetes [85]. Individuals with higher serum levels of bilirubin were associated with odds of having a lower incidence of diabetes mellitus [86]. In patients with Gilbert syndrome and diabetes, hyperbilirubinaemia is correlated with a lower prevalence of vascular complications compared with patients having diabetes alone [87]. Based on abovementioned results, atazanavir-induced experimental hyperbilirubinemia in type II diabetic patients over a 3-day treatment in a double-blind, placebo-controlled study, was associated with a significant improvement of endothelial function [88].
3.5. Obesity
Increased expression of HO-1 was observed in adipocytes obtained from patients with morbid obesity [89, 90]. Furthermore, nonsmoking bariatric patients were shown to have increased carboxyhemoglobin concentrations, as an indicator of HO-1 upregulation [91]. Data regarding HO-1, obesity and insulin resistance are controversial with one study indicating that diminished upregulation of HO-1 in visceral adipose tissue correlated with waist-to-hip ratio and insulin resistance in humans [89], whereas other authors demonstrated increased HO-1 expression in insulin-resistant obese patients with increased waist-hip-ratio when compared to insulin-sensitive obese controls [92]. A reverse correlation between bilirubin concentrations and abdominal obesity has been found in two studies [93, 94]. Moreover, Andersson et al. presented a linear reciprocity between weight loss and increase in serum bilirubin concentrations [95].
3.6. Cigarette Smoking
As a further risk factor for cardiovascular disease, cigarette smoking has been shown to increase HO-1 expression [96].
The relationship between cardiovascular risk factors and HO-1 expression is summarized in Fig. 2.
Fig. (2).
The relationship between cardiovascular risk factors and HO-1 expression. HO-1 expression is higher in patients with shorter (GT)n repeats, and higher levels of HO-1 are found in the presence of some stroke risk factors like hypertension, cigarette smoking, obesity and atherosclerosis. Higher HO-1 expression on the other hand decreases the risk of diabetes, decreases blood pressure, and protects against the progression of atherosclerosis.
4. The HO-1 pathway in patients with ischemic stroke
Current American acute ischemic stroke treatment guidelines do not recommend the use of any neuroprotective agents [5]. Nevertheless, edaravone (MCI-186), a free-radical scavenger implicated to exert neuroprotection through the HO-1 pathway [97], is recommended by the Japanese guidelines for acute ischemic stroke within 24 h from symptom-onset [98].
A prospective cohort study in an Asian population investigated the association between HO-1 gene T(-413)A polymorphism and clinical prognosis in patients with atherosclerotic stroke, concluding that patients with at least one A allele had significantly better outcomes than patients with TT genotype, possibly due to the high expression level of HO-1 [99]. Two studies in ischemic stroke patients evaluated (GT)n repeat-polymorphisms in the HO-1 promoter region. In the first one, authors found that carriers of short (<25) GT-repeats in the HO-1 gene promoter exhibited a reduced risk for stroke or TIA but only in the absence of elevated plasma lipids, implying that genetic polymorphisms have moderate effects compared to traditional risk factors like hyperlipidaemia [100]. The second study concluded that patients carrying longer (>26) GT-repeats may have greater susceptibility to develop ischemic stroke, but only in the presence of low HDL-C, implying that the insufficient antioxidant effect of low HDL-C levels may be compensated by shorter GT-repeats and thus, higher HO-1 induction [101]. It is of note, however, that the HO-1 inducer heme arginate was able to increase HO-1 in humans irrespective of the (GT)n phenotype [102]. A further study showed that stroke patients had higher serum levels of HO-1 compared to patients with transient ischemic attack (TIA), which was explained by the exposure to a high degree of oxidative stress caused by atherosclerosis, infection and hypertension leading to an upregulation of HO-1 [15].
Downstream metabolites of HO-1 may also exert protective functions in human ischemic stroke. The abovementioned study by Li et al. showed that stroke patients also had lower levels of total and direct bilirubin compared to patients with TIA [15]. A prospective Korean study suggested that elevated serum bilirubin might offer some protection against stroke risk in men [103]. In a large, community-based sample, however, higher exhaled CO was associated with a greater burden of subclinical cerebrovascular disease and with increased risk of stroke/TIA. It remains unknown, whether elevated endogenous CO concentration serves as a causative factor of cerebrovascular adverse events, or represents a result of oxidative stress and thus, upregulated HO-1 activity [104].
Alpha-lipoic acid, a thiol antioxidant has been shown to exert neuroprotective effects and promote recovery after ischemic stroke in animal studies by attenuating oxidative stress, partially mediated by the HO-1 pathway [105]. In a retrospective clinical study of patients with diabetes who were treated with thrombolysis due to acute ischemic stroke, among those who were using alpha-lipoic acid at the acute stage of stroke, favorable outcomes occurred at significantly higher rates both at 3 months and 1 year [106].
While neuroprotective agents are generally considered to be well-tolerated and safe, the phase III trial for the Nrf2/HO-1 inducer triterpenoid bardoxolone-methyl (BARD) designed for the treatment of patients with type II diabetes and stage 4 chronic kidney disease (BEACOM trial) has failed due to adverse cardiovascular events [107].
Conclusion
While the desire to find or develop effective neuroprotectants is high, to date, none of the numerous neuroprotective agents tested in preclinical research has yielded any positive outcomes in human studies [108, 109]. Main challenges to adapt experimental findings to human research include age-related issues, safety concerns, optimal dosage and delivery, and cell/tissue-specific expression. Based on abovementioned hurdles resulting in unsatisfactory translational power, the Stroke Therapy Academic Industry Roundtable (STAIR) has been established to improve the quality of preclinical stroke studies [110], thereby ultimately extending the currently narrow treatment palette of ischemic stroke and eventually improving the life quality of stroke patients.
Aknowledgements
Daniel Bereczki was supported by the Higher Education Institutional Excellence Program of the Ministry of Human Resources of the Government of Hungary.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Sacco R.L., Kasner S.E., Broderick J.P., et al. An updated definition of stroke for the 21st century. A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics - 2016 update. A report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Feigin V.L., Mensah G.A., Norrving B., et al. Atlas of the global burden of stroke (1990-2013): The GBD 2013 study. Neuroepidemiology. 2015;45:230–236. doi: 10.1159/000441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin V.L., Abajobir A.A., Abate K.H., et al. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers W.J., Rabinstein A.A., Ackerson T., et al. 2018 Guidelines for the early management of patients with acute ischemic stroke. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Damaerschalk B.M., Kleindorfer D.O., Adeoye O.M., et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke. A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 7.Pundik S., Xu K., Sundararajan S. Reperfusion brain injury. Focus on cellular bioenergetics. Neurology. 2012;79(Suppl. 1):S44–S51. doi: 10.1212/WNL.0b013e3182695a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Wang L., Zhang X., et al. The protection by octreotide against experimental ischemic stroke: up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80–87. doi: 10.1016/j.brainres.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi K., Nakano Y., Imai T., et al. A novel nuclear factor erythroid 2-related factor 2 (Nrf2) activator RS9 attentuates brain injury after ischemia reperfusion in mice. Neuroscience. 2016;333:302–310. doi: 10.1016/j.neuroscience.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Lv C., Maharjan S., Wang Q., et al. α-lipoic acid promotes neurological recovery after ischemic stroke by activating the Nrf2/HO-1 pathway to attenuate oxidative damage. Cell. Physiol. Biochem. 2017;43:1273–1287. doi: 10.1159/000481840. [DOI] [PubMed] [Google Scholar]
- 11.Ishii T., Itoh K., Takahashi S., et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi A., Ohta T., Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 13.Chumboatong W., Thummayot S., Govitrapong P., Tocharus C., Jittiwat J., Tocharus J. Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem. Int. 2017;102:114–122. doi: 10.1016/j.neuint.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Haines D.D., Lekli I., Teissier P., Bak I., Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. (Oxf.) 2012;204:487–501. doi: 10.1111/j.1748-1716.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Song G., Jin Y., et al. Higher level of heme oxygenase-1 in patients with stroke than TIA. J. Thorac. Dis. 2014;6:772–777. doi: 10.3978/j.issn.2072-1439.2014.06.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp F.R., Zhan X., Liu D.Z. Heat shock proteins in brain: role of Hsp70, Hsp 27 and HO-1 (Hsp32) and their therapeutic potential. Transl. Stroke Res. 2013;4:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ameriso S.F., Villamil A.R., Zedda C., et al. Heme oxygenase-1 is expressed in carotid atherosclerotic plaques infected by Helicobacter pylori and is more prevalent in asymptomatic subjects. Stroke. 2005;36:1896–1900. doi: 10.1161/01.STR.0000177494.43587.9e. [DOI] [PubMed] [Google Scholar]
- 18.Balla G., Jacob H.S., Balla J., et al. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 19.Balla J., Vercellotti G.M., Jeney V., et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid. Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 20.Fredenburgh L.E., Merz A.A., Cheng S. Haeme oxygenase signalling pathway: implications for cardiovascular disease. Eur. Heart J. 2015;36:1512–1518. doi: 10.1093/eurheartj/ehv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 22.Bolisetty S., Zarjou A., Hull T.D., et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 2015;88:95–108. doi: 10.1038/ki.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howells D.W., Porritt M.J., Rewell S.S., et al. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fluri F., Schuhmann M.K., Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Devel. Ther. 2015;9:3445–3454. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panahian N., Yoshiura M., Maines M.D. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J. Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 26.Shah Z.A., Nada S.E., Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao X.D., Ma Y.H., Luo P., et al. Up-regulation of heme oxygenase-1 attenuates brain damage after cerebral ischemia via simultaneous inhibition of superoxide production and preservation of NO bioavailability. Exp. Neurol. 2013;239:163–169. doi: 10.1016/j.expneurol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Kang H.C., Lee M., Bae Y.H. Polymeric gene carriers. Crit. Rev. Eukaryot. Gene Expr. 2005;15:317–342. doi: 10.1615/critreveukargeneexpr.v15.i4.30. [DOI] [PubMed] [Google Scholar]
- 29.Choi M., Oh J., Rhim T., Lee M. Delivery of hypoxia-inducible heme oxygenase-1 gene for site-specific gene therapy in the ischemic stroke animal model. Pharm. Res. 2016;33:2250–2258. doi: 10.1007/s11095-016-1962-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y.H., Tsai H.L., Chiang M.T., Chau L.Y. Carbon monoxide-induced early thrombolysis contributes to heme oxygenase-1-mediated inhibition of neointimal growth after vascular injury in hypercholesterolemic mice. J. Biomed. Sci. 2006;13:721–730. doi: 10.1007/s11373-006-9093-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J., Kobori N., Aronowski J., Dash P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 32.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Shah Z.A., Li R.C., Ahmad A.S., et al. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastianetto S., Ménard C., Quirion R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta. 2015;1852:1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F., Wang S., Zhang M., et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Y., Chen M., Wang M., Li Y., Wen A. Posttreatment with 11-keto-β-boswellic acid ameliorates cerebral ischemia-reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol. Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 37. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204063lbl.pdf
- 38.Yao Y., Miao W., Liu Z., et al. Dimethyl fumarate and monomethyl fumarate promote post-ischemic recovery in mice. Transl. Stroke Res. 2016;7:535–547. doi: 10.1007/s12975-016-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunze R., Urrutia A., Hoffmann A., et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp. Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Schrör K., Rauch B.H. Aspirin and lipid mediators in the cardiovascular system. Prostaglandins Other Lipid Mediat. 2015;121:17–23. doi: 10.1016/j.prostaglandins.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Dunn L.L., Midwinter R.G., Ni J., Hamid H.A., Parish C.R., Stocker R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014;20:1723–1742. doi: 10.1089/ars.2013.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shanab A.Y., Elshaer S.L., El-Azab M.F., et al. Candesartan stimulates reparative angiogenesis in ischemic retinopathy model: Role of hemeoxygenase-1 (HO-1). Angiogenesis. 2015;18:137–150. doi: 10.1007/s10456-014-9451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 44.Bharucha A.E., Choi K.M., Saw J.J., et al. Effects of aspirin & simvastatin and aspirin, simvastatin, & lipoic acid on heme oxygenase-1 in healthy human subjects. Neurogastroenterol. Motil. 2014;26:1437–1442. doi: 10.1111/nmo.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirjanic-Azaric B., Rizzo M., Jürgens G., et al. Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand. J. Clin. Lab. Invest. 2015;75:382–389. doi: 10.3109/00365513.2015.1031691. [DOI] [PubMed] [Google Scholar]
- 46.Björkhem-Bergman L., Lindh J.D., Bergman P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br. J. Clin. Pharmacol. 2011;72:164–165. doi: 10.1111/j.1365-2125.2011.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B., Cao W., Biswal S., Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeylanov E., Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox. Res. 2009;15:133–137. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klaus J.A., Kibler K.K., Abuchowski A., Koehler R.C. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif. Cells Blood Substit. Immobil. Biotechnol. 2010;38:223–229. doi: 10.3109/10731199.2010.488635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deguchi K., Hayashi T., Nagotani S., et al. Reduction of cerebral infarction in rats by biliverdin associated with amelioration of oxidative stress. Brain Res. 2008;1188:1–8. doi: 10.1016/j.brainres.2007.07.104. [DOI] [PubMed] [Google Scholar]
- 51.Li J.J., Zou Z.Y., Liu J., et al. Biliverdin administration ameliorates cerebral ischemia reperfusion injury in rats and is associated with proinflammatory factor downregulation. Exp. Ther. Med. 2017;14:671–679. doi: 10.3892/etm.2017.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding H.F., Zhang H., Ding H.F., et al. Therapeutic effect of placenta-derived mesenchymal stem cells on hypoxic-ischemic brain damage in rats. World J. Pediatr. 2015;11:74–82. doi: 10.1007/s12519-014-0531-8. [DOI] [PubMed] [Google Scholar]
- 53.Shen C., Cheng W., Yu P., et al. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016;14:3646–3654. doi: 10.3892/mmr.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y.H., Lin S.J., Lin M.W., et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum. Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 55.Pechlaner R., Willeit P., Summerer M., et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2015;35:229–236. doi: 10.1161/ATVBAHA.114.304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daenen K.E., Martens P., Bammens B. Association of HO-1 (GT)n promoter polymorphism and cardiovascular disease: A reanalysis of the literature. Can. J. Cardiol. 2016;32:160–168. doi: 10.1016/j.cjca.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Bao W., Song F., Li X., et al. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: A HuGE review and meta-analysis. Am. J. Epidemiol. 2010;172:631–636. doi: 10.1093/aje/kwq162. [DOI] [PubMed] [Google Scholar]
- 58.Ono K., Goto Y., Takagi S., et al. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Ono K., Mannami T., Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J. Hypertens. 2003;21:1497–1503. doi: 10.1097/00004872-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Agarwal A., Balla J., Balla G., Croatt A.J., Vercellotti G.M., Nath K.A. Renal tubular epithelial cells mimic endothelial cells upon exposure to oxidized LDL. Am. J. Physiol. 1996;271(4 Pt 2):F814–F823. doi: 10.1152/ajprenal.1996.271.4.F814. [DOI] [PubMed] [Google Scholar]
- 61.Anwar A.A., Li F.Y., Leake D.S., Ishii T., Mann G.E., Siow R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Yachie A., Niida Y., Wada T., et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeney V., Balla J., Yachie A., et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 64.Wang L.J., Lee T.S., Lee F.Y., Pai R.C., Chau L.Y. Expression of heme oxygenase-1 in atherosclerotic lesions. Am. J. Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 65.Morsi W.G., Shaker O.G., Ismail E.F., et al. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin. Biochem. 2006;39:1057–1062. doi: 10.1016/j.clinbiochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Nagy E., Eaton J.W., Jeney V., et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2010;30:1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng C., Noordeloos A.M., Jeney V., et al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–3027. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 68.Song J., Sumiyoshi S., Nakashima Y., et al. Overexpression of heme oxygenase-1 in coronary atherosclerosis of Japanese autopsies with diabetes mellitus: Hisayama study. Atherosclerosis. 2009;202:573–581. doi: 10.1016/j.atherosclerosis.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 69.Ameriso S.F., Villamil A.R., Zedda C., et al. Heme oxygenase-1 is expressed in carotid atherosclerotic plaques infected by Helicobacter pylori and is more prevalent in asymptomatic subjects. Stroke. 2005;36:1896–1900. doi: 10.1161/01.STR.0000177494.43587.9e. [DOI] [PubMed] [Google Scholar]
- 70.Ishizaka N., Ishizaka Y., Takahashi E., Yamakado M., Hashimoto H. High serum bilirubin level is inversely associated with the presence of carotid plaque. Stroke. 2001;32:580–583. doi: 10.1161/01.str.32.2.580-b. [DOI] [PubMed] [Google Scholar]
- 71.Vítek L., Novotný L., Sperl M., Holaj R., Spácil J. The inverse association of elevated serum bilirubin levels with subclinical carotid atherosclerosis. Cerebrovasc. Dis. 2006;21:408–414. doi: 10.1159/000091966. [DOI] [PubMed] [Google Scholar]
- 72.Novotný L., Vítek L. Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Exp. Biol. Med. (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 73.Ishizaka N., de León H., Laursen J.B., et al. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation. 1997;96:1923–1929. doi: 10.1161/01.cir.96.6.1923. [DOI] [PubMed] [Google Scholar]
- 74.Sabaawy H.E., Zhang F., Nguyen X., et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 75.Solari V., Piotrowska A.P., Puri P. Expression of heme oxygenase-1 and endothelial nitric oxide synthase in the lung of newborns with congenital diaphragmatic hernia and persistent pulmonary hypertension. J. Pediatr. Surg. 2003;38:808–813. doi: 10.1016/jpsu.2003.50172. [DOI] [PubMed] [Google Scholar]
- 76.Calò L.A., Maso L.D., Caielli P., et al. Effect of olmesartan on oxidative stress in hypertensive patients. Mechanistic support to clinical trials derived evidence. Blood Press. 2011;20:376–382. doi: 10.3109/08037051.2011.575570. [DOI] [PubMed] [Google Scholar]
- 77.Papadakis J.A., Ganotakis E.S., Jagroop I.A., Mikhailidis D.P., Winder A.F. Effect of hypertension and its treatment on lipid, lipoprotein(a), fibrinogen, and bilirubin levels in patients referred for dyslipidemia. Am. J. Hypertens. 1999;12:673–681. doi: 10.1016/s0895-7061(99)00049-7. [DOI] [PubMed] [Google Scholar]
- 78.Chin H.J., Song Y.R., Kim H.S., et al. The bilirubin level is negatively correlated with the incidence of hypertension in normotensive Korean population. J. Korean Med. Sci. 2009;24(Suppl.):S50–S56. doi: 10.3346/jkms.2009.24.S1.S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreiser D., Baum M., Seidman D.S., et al. End tidal carbon monoxide levels are lower in women with gestational hypertension and pre-eclampsia. J. Perinatol. 2004;24:213–217. doi: 10.1038/sj.jp.7211062. [DOI] [PubMed] [Google Scholar]
- 80.Hu C.M., Lin H.H., Chiang M.T., Chang P.F., Chau L.Y. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes. 2007;56:1240–1247. doi: 10.2337/db06-0495. [DOI] [PubMed] [Google Scholar]
- 81.Nicolai A., Li M., Kim D.H., et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song F., Li X., Zhang M., et al. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes in a Chinese population. Am. J. Epidemiol. 2009;170:747–756. doi: 10.1093/aje/kwp196. [DOI] [PubMed] [Google Scholar]
- 83.Qiu C., Hevner K., Enquobahrie D.A., Williams M.A. Maternal serum heme-oxygenase-1 (HO-1) concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. PLoS One. 2012;7:e48060. doi: 10.1371/journal.pone.0048060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bao W., Song F., Li X., et al. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One. 2010;5:e12371. doi: 10.1371/journal.pone.0012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiménez-Osorio A.S., Picazo A., González-Reyes S., Barrera-Oviedo D., Rodríguez-Arellano M.E., Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int. J. Mol. Sci. 2014;15:20290–20305. doi: 10.3390/ijms151120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheriyath P., Gorrepati V.S., Peters I., et al. High total bilirubin as a protective factor for diabetes mellitus: An analysis of NHANES data from 1999-2006. J. Clin. Med. Res. 2010;2:201–206. doi: 10.4021/jocmr425w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoguchi T., Sasaki S., Kobayashi K., Takayanagi R., Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298:1398–1400. doi: 10.1001/jama.298.12.1398-b. [DOI] [PubMed] [Google Scholar]
- 88.Dekker D., Dorresteijn M.J., Pijnenburg M., et al. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011;31:458–463. doi: 10.1161/ATVBAHA.110.211789. [DOI] [PubMed] [Google Scholar]
- 89.Shakeri-Manesch S., Zeyda M., Huber J., Ludvik B., Prager G., Stulnig T.M. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int. J. Obes. 2009;33:1257–1264. doi: 10.1038/ijo.2009.160. [DOI] [PubMed] [Google Scholar]
- 90.Lehr S, Hartwig S, Lamers D, et al. Identification and validation of novel adipokines released from primary human adipocytes. 2012. [DOI] [PMC free article] [PubMed]
- 91.Nielsen V.G., Galvani C.A., Boyle P.K., Steinbrenner E.B., Matika R.W. Bariatric patients have plasmatic hypercoagulability and systemic upregulation of heme oxygenase activity. Blood Coagul. Fibrinolysis. 2015;26:200–204. doi: 10.1097/MBC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 92.Jais A., Einwallner E., Sharif O., et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin L.Y., Kuo H.K., Hwang J.J., et al. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atheroscleosis. 2009;203:563–568. doi: 10.1016/j.atherosclerosis.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 94.Bhuiyan A.R., Srinivasan S.R., Chen W., Sultana A., Berenson G.S. Association of serum bilirubin with pulsatile arterial function in asymptomatic young adults: The Bogalusa Heart Study. Metabolism. 2008;57:612–616. doi: 10.1016/j.metabol.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Andersson C., Weeke P., Fosbøl E.L., et al. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: An analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism. 2009;58:1109–1115. doi: 10.1016/j.metabol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Favatier F., Polla B.S. Tobacco-smoke-inducible human haem oxygenase-1 gene expression: role of distinct transcription factors and reactive oxygen intermediates. Biochem. J. 2001;353:475–482. doi: 10.1042/0264-6021:3530475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang D., Xiao Y., Lv P., et al. Edaravone attenuates oxidative stress induced by chronic cerebral hypoperfusion injury: Role of ERK/Nrf2/HO-1 signaling pathway. Neurol. Res. 2018;40:1–10. doi: 10.1080/01616412.2017.1376457. [DOI] [PubMed] [Google Scholar]
- 98.Shinohara Y., Yamaguchi T. Outline of the Japanese Guidelines for the Management of Stroke 2004 and subsequent revision. Int. J. Stroke. 2008;3:55–62. doi: 10.1111/j.1747-4949.2008.00178.x. [DOI] [PubMed] [Google Scholar]
- 99.Cao L., Zhang Z., Cai B., et al. Association of heme oxygenase-1 gene rs2071746 polymorphism with vascular outcomes in patients with atherosclerotic stroke. J. Neurol. Sci. 2014;344:154–157. doi: 10.1016/j.jns.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 100.Funk M., Endler G., Schillinger M., et al. The effect of a promoter polymorphism in the heme oxygenase-1 gene on the risk of ischaemic cerebrovascular events: the influence of other vascular risk factors. Thromb. Res. 2004;113:217–223. doi: 10.1016/j.thromres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Bai C.H., Chen J.R., Chiu H.C., Chou C.C., Chau L.Y., Pan W.H. Shorter GT repeat polymorphism in the heme oxygenase-1 gene promoter has protective effect on ischemic stroke in dyslipidemia patients. J. Biomed. Sci. 2010;17:12. doi: 10.1186/1423-0127-17-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doberer D., Haschemi A., Andreas M., et al. Haem arginate infusion stimulates haem oxygenase-1 expression in healthy subjects. Br. J. Pharmacol. 2010;161:1751–1762. doi: 10.1111/j.1476-5381.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimm H., Yun J.E., Jo J., Jee S.H. Low serum bilirubin level as an independent predictor of stroke incidence: A prospective study in Korean men and women. Stroke. 2009;40:3422–3427. doi: 10.1161/STROKEAHA.109.560649. [DOI] [PubMed] [Google Scholar]
- 104.Nayor M., Enserro D.M., Beiser A.S., et al. Association of exhaled carbon monoxide with stroke incidence and subclinical vascular brain injury. Stroke. 2016;47:383–389. doi: 10.1161/STROKEAHA.115.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv C., Maharjan S., Wang Q., et al. α-Lipoic acid promotes neurological recovery after ischemic stroke by activating the Nrf2/HO-1 pathway to attenuate oxidative damage. Cell. Physiol. Biochem. 2017;43:1273–1287. doi: 10.1159/000481840. [DOI] [PubMed] [Google Scholar]
- 106.Choi K.H., Park M.S., Kim J.T., et al. Lipoic acid use and functional outcomes after thrombolysis in patients with acute ischemic stroke and diabetes. PLoS One. 2016;11:e0163484. doi: 10.1371/journal.pone.0163484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Zeeuw D., Akizawa T., Audhya P., et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., van der Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 109.O’Collins V.E., Macleod M.R., Cox S.F., et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J. Cereb. Blood Flow Metab. 2011;31:962–975. doi: 10.1038/jcbfm.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fisher M., Feuerstein G., Howells D.W., et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]