Abstract
The microbiome is composed of hundreds of interacting species that have co-evolved with the host and alterations in microbiome composition have been associated with health and disease. Insights from evolutionary ecology may aid efforts to ameliorate microbiome-associated diseases. One step toward this goal involves recognition that the idea of commensalism has been applied too broadly to human/microbe symbioses. Commensalism is most accurately viewed on a symbiosis continuum as a dividing line that separates a spectrum of mutualisms of decreasing positive interdependence from parasitisms of increasing severity. Insights into the evolution of the gut microbial symbiosis continuum will help distinguish between human actions that will advance or hinder health. Theory and research indicate that a major benefit of mutualistic microbes will be protection against pathogens. Mismatches between current and ancestral diets may disfavor mutualists, resulting in microbiome effects on health problems, including obesity, diabetes, autism, and childhood allergy. Evolutionary theory indicates that mutualisms will be favored when symbionts depend on resources that are not used by the host. These resources, which are referred to as human-inaccessible microbiota-accessible carbohydrates (HIMACs), can be supplied naturally through diet. Public health interventions need to consider the position of gut microbes on the mutualist-parasite continuum and the specific associations between prebiotics, such as HIMACs, and the mutualists they support. Otherwise interventions may fail to restore the match between human adaptations, diet, and microbiome function and may thereby fail to improve health and even inadvertently promote illness.
Keywords: evolution, microbiome, mutualism, parasitism, commensalism, breast feeding, pathogen
Introduction
Scientific understanding of microbiomes, the collections of microorganisms on or within a given organism, is rapidly expanding. Much work has concentrated on the human gut microbiome, which will be the focus of this paper, with implications for individual and public health. Natural selection has shaped host-microbiome interactions, but is dependent on the environmental context. Application of evolutionary and ecological principles may therefore provide essential insights into the composition, maintenance, and restoration of gut microbiomes and effects on human health [1-3].
In this paper we address three related ideas that are informed by evolutionary and ecological perspectives. The first proposes that interactions between humans and our microbiota can be better understood by considering commensalism to be a dividing line between parasitism and mutualism rather than a discrete category of symbioses. We propose further that microbiome enhancement needs to focus on the benefits provided by mutualisms, which will depend on the extent to which humans provide nutrients for microbes that are distinct from the nutrients that the microbes could gain if they exploited host tissues.
The second point is that illnesses associated with microbiome composition often result from dietary mismatches between the contemporary environments and those in which humans evolved. These illnesses may be prevented or treated by eliminating the mismatches.
Our third point is that these insights need to be applied integratively to public health actions to favor mutualists. For example, dietary interventions that favor particular mutualists must also ensure that the mutualists are present.
The Symbiosis Continuum
Definitions of Symbionts
In this paper we define symbionts as organisms that live intimately with other organisms, parasites as symbionts that have a net negative effect on their hosts, and mutualists as symbionts that have a net positive effect. In accordance with standard biological terminology, we define a commensal as a symbiont that has neither a harmful nor beneficial overall effect.
Microbiome researchers often refer to gut microbes as commensals when they confer no conspicuous harm (e.g., [4,5]). To qualify as a commensal, however, its net effect should be zero. From an evolutionary perspective this overall effect is most appropriately measured in units of evolutionary fitness. Insofar as any intimate symbiont will have at least slight effects on host fitness, commensalism is best considered a dividing line between parasitic and mutualistic associations. It is often considered a category because fitness effects are difficult to measure with sufficient accuracy to determine whether the overall effect is negative or positive.
The spectrum of intimate associations can be represented by a symbiosis continuum that reflects fitness effects on the host, ranging from obligate mutualisms on one end to lethal parasitisms on the other (Figure 1).
Figure 1.
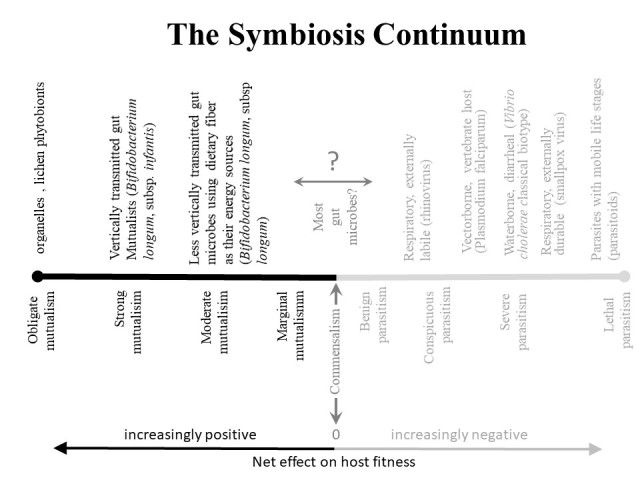
Waterborne, vectorborne, and durable respiratory pathogens, and parasites with mobile stages of development can be transmitted from very sick hosts and therefore have probably been molded by natural selection to cause severe disease [17,77] The most severe parasites are those with mobile life history stages (often referred to as parasitoids), which can exploit their hosts to death during their parasitic stage and still reach new hosts through their mobile stage. Microbes evolve to mutualists when they can provide fitness benefits to their hosts, particularly when they are vertically transmitted and when their hosts provide them with nutrients that are different from their own cellular components (e.g., dietary plant fiber or milk oligosaccharides that their own cells do not normally produce).
The use of evolutionary fitness as a criterion comes with caveats. First, evolutionary fitness is environment specific. Modern environments differ from historical and prehistorical environments. Additionally, because fitness effects are not precisely measurable there are uncertainties associated with the placement of any particular microbe on the continuum. Negative fitness effects arise from mortality, tissue damage, vitamin deficiency, and immunopathology. Positive effects involve protection against pathogens, and the provisioning of nutrients such as vitamins [6]. A broad spectrum of systemic effects may arise indirectly through the action of bacterial metabolites, such as short chain fatty acids (SCFAs) [7]. The difficulty lies in the zone straddling the dividing line of commensalism (Figure 1). We refer to the large number of microorganisms that must be placed in this zone as indeterminate symbionts. Of course, favoring indeterminate symbionts will have a positive effect on the host if they are mutualists, but a negative effect if they are parasites. If, instead, indeterminate symbionts are considered to be commensals, research and practice may overlook the best opportunities for improving health.
Coevolution of Mutualisms
There is always potential for a conflict of interest between microbe and host because the genetic makeup of the microbe differs from that of the host. Natural selection will favor microbes that act in their own genetic interest as circumstances permit. A microbe that, for example, produces a vitamin needed by its host could use the resources spent on that vitamin to enhance its own replication. Similarly, by invasion of the intestinal epithelium a microbe might gain resources for its own reproduction and survival at the host’s expense.
Strong mutualisms are distinguished from marginal mutualisms by the degree to which the symbiont enhances host fitness (Figure 1). Strong mutualisms therefore will tend to evolve when the biological aspects of the mutualism limit or prohibit the ability of the mutualist to cause damage to the host through its use of host resources.
Characteristics of strong gut mutualists include provisioning of specific nutrients that are needed by the host and are not simply waste products for the microbe, and release of compounds that specifically inhibit pathogens, together with an absence of negative effects on the host. Characteristics of marginal mutualists include suppression of pathogens that results simply from the mutualist’s use of resources in the gut lumen or interference with competing symbionts in ways that provide benefits directly to the mutualist and inadvertently to the host, together with negative effects on the host that are outweighed (marginally) by such benefits.
Bifidobacteria illustrate the value of distinguishing mutualists according to the strength of the mutualism. In discussions of the gut microbiota, bifidobacteria are generally considered commensals; however, present knowledge, though far from complete, indicates that bifidobacteria vary greatly in their overall effect on the host. Testing of bifidobacterial strains from infants, for example, found that two of 14 strains isolated from infants had antimicrobial effects on pathogenic bacteria [8]. Bifidobacterium longum, subsp. infantis (hereafter referred to as B. infantis), in particular, has characteristics of a strong mutualist. It releases antibacterial compounds that inhibit pathogenic bacteria [9], synthesizes vitamins [10], improves immunological responses, and protects premature infants against ulcerative colitis [11].
Knowledge about the composition of human breast milk suggests that the mutualistic association with B. infantis involves human adaptations that benefit the bacterium. About 30 percent of the caloric content of human milk is provided as a diverse spectrum of oligosaccharides that the infant cannot digest. B. infantis has evolved a corresponding array of genes that allow it to digest the human milk oligosaccharides (HMOs) [12,13]. It is more dependent on them and uses a greater variety than other bifidobacteria. The diversity of HMOs produced in milk appears to have coevolved for B. infantis more than for any other bacterium. HMOs are absent in the external environment; B. infantis is therefore not able to cheat on the mutualism by proliferating in the host to enhance its abundance and growth in the external environment.
The dependence of B. infantis on HMOs from human milk also creates a barrier to its exploitation of less cooperative options within the intestinal tract, such as deriving nutrients from the epithelial cells of the gut and its mucus layer, which protects against pathogens [14]. Unlike other bifidobacteria, B. infantis does not penetrate the mucus lining or use the mucus as an energy source [11]. Degradation of the mucus layer has been flagged as a characteristic of potentially damaging microbiota [6].
The threat posed by symbionts that invade or degrade the mucus layer is broadly relevant to understanding the effects of microbiota that have been considered commensals. Bacteroides thetaiotaomicron, for example,is referred to as a commensal that resides in the mucus layer and uses nutrients from mucus constituents [4,15]. Its net effect on its host, however, depends on the other microbes in its vicinity. When cohabiting with enterohemorrhagic E. coli, B. thetaiotaomicron exacerbates the damage caused by E. coli [4]. In this ecological setting B. thetaiotaomicron is therefore best considered parasitic. Considering that it apparently is mutualistic in other settings, by virtue of its ability to breakdown complex polysaccharides into usable sugars, we think that a new label is needed for it and for other parasites that can move from one side of the commensalism dividing line (Figure 1) to the other according to the environmental setting. We refer to such organisms as ambisymbionts (ambi- from Latin, meaning both ways). Ambisymbionts can be identified as on average parasites or mutualists, differentiating them from indeterminate symbionts for which the net effect is uncertain.
Routes of Transmission and the Symbiosis Continuum
Routes of transmission are central to an understanding of the symbiosis continuum. When transmission routes do not require hosts to be healthy, pathogens tend to be more severe than when transmission requires hosts to be sufficiently healthy to directly contact susceptible hosts (Figure 1). Vertical transmission of pathogens from parent to offspring should favor evolution toward benignity [16,17]. If vertical transmission is the only route, parasites should evolve into mutualists, because the success of the host determines the success of their transmission. Reciprocally, host attributes that favor vertical transmission of a mutualist will enhance host fitness. Cellular organelles and the algae of lichens represent the extreme end of the association between vertical transmission and mutualistic benefit.
The transmission of gut symbionts is still largely uncertain, but vertical transmission does seem to be very important for some bifidobacteria [5]. B. infantis is transmitted from mother to infant and is found in breast milk and the gastrointestinal tract of nursing mothers [5]. The dependence of B. infantis on HMOs suggests that persistent transmission cycles from baby to adult or adult to adult would be very restricted, as would perpetuation in the outside environment. Persistence in the baby through adulthood might occur in the gastrointestinal or genital tract, but competition with other organisms at these sites would seem to make persistence tenuous. Persistence within the breast (e.g., in ductal tissues), with latency prior to lactation seems more feasible. Exploration of the breast microbiome is in an early phase. One assessment found that the composition of the breast microbiome is variable from woman to woman [18]. This study identified bifidobacteria in some samples but did not distinguish bacteria to the species or subspecies levels; moreover, organisms may have been present but not detectable because the techniques used might fail to pick up latent phases in refugia. Persistence in the breast, activation during lactation, and transmission in mother’s milk could allow for persistence between pregnancies and from birth to childbearing ages. This possibility could be evaluated using sensitive tests for B. infantis nucleic acids in breast tissue before and after lactation and in milk before the presence of B. infantis in the neonate.
Mutualisms and Nutrients Inaccessible to Humans
This HMO/B. infantis model for explaining the evolution and maintenance of a strong gut mutualist raises a key question bearing on the presence of other strong mutualists in the gut microbiota: Are there components of the post-weaning diet that could similarly favor mutualists? The best candidates are probably dietary constituents that are accessible by microbes but not directly by humans. Recognizing the need to collectively consider carbohydrates that may influence human health through microbial processing Sonnenburg and Sonnenburg [6] coined the acronym MACs, which is short for microbiota-accessible carbohydrates. We suggest that evolution of strong mutualisms is favored by a subset of MACs, namely human-inaccessible-microbiota-accessible carbohydrates: HIMACs (pronounced high macks). By “human-inaccessible” we mean that the carbohydrate is not used by human cells or tissues for any normal physiological process. HMOs and dietary fiber are examples of HIMACs. Mucus components that are accessible to microbiota, such as mucin, however, would not be HIMACs even though they are MACs because mucin participates in the normal functioning of gut tissues. Similarly, glucose is a MAC but not a HIMAC, because it is used by normal host cells and gut microbes. The logic is that specialization on HIMACs decreases the microbe’s ability to use host carbohydrates, thus limiting its options for evolving toward parasitism.
Unlike the specialization on HMOs, however, specialization on HIMACs in the post-natal diet would allow the symbiont to be horizontally transmitted to other humans and would therefore lessen the intensity of the coevolutionary selection for a strong mutualism. The symbionts that digest post-natal HIMACs therefore are expected to be weaker mutualists than B. infantis. The conspecific B. longum subspecies longum, for example, has evolved a lesser tendency to use HMOs [13,19] and is less strongly mutualistic (see section on celiac disease).
The Symbiosis Continuum and the Hygiene Hypothesis
Writers on the subject of the microbiota sometimes equate the emphasis on diversity and species richness that is inherent in microbiome arguments with a goal of the Hygiene Hypothesis, namely to increase exposure to a variety of symbionts in order to tune immunological defenses [20,21]. A focus on the symbiosis continuum, however, suggests that decreased hygiene should be directed to the subset of organisms that have routes of transmission and/or use resources that favor mutualism (e.g., HIMACs).
Pervasiveness of Mutualisms
Although the conflict of interest between host and symbiont imposes constraints on the evolution of mutualisms, a large number of the microbes in the zone of commensalism should be mutualistic. The main reason is that the diversity of threats posed by pathogens is ever changing and unbounded. The immune system has adapted to counter a large portion of these threats by lymphocyte diversity, which is bounded by the make-up of each host’s genome. The reliance of somatic mutation of lymphocytes and the variation in MHC from one individual to another, however, suggest that the diversity of threats cannot be defended against by a single host genome relying on recombinatorial diversity. Mutualist gut microbes evolve to defend themselves against parasitic microbes and have short generation times with virtually unbounded evolutionary potential. It seems reasonable, therefore, to expect that they can offer a complementary defensive system that can respond to unpredictable changes in parasites in the gut lumen, which is relatively inaccessible to immunological defenses.
Evolutionary Mismatches and Disease
The Mismatch Concept
Evolutionary explanations of disease often invoke dietary mismatches between modern and ancestral environments (e.g., [22]). It is argued that adaptations for functioning in past environments leave humans vulnerable to current environmental causes of disease. Microbiota mismatches can result from the presence of microorganisms to which our ancestors were not exposed or dietary changes that alter the microbiota. The mismatch between formula feeding and breast feeding is a particular example pertaining to a dietary change because infant formula differs from breast milk. Many of the chronic diseases that affect modern populations may be influenced by mismatches between modern and ancestral diets. Some examples are provided below.
Celiac Disease
Celiac disease is characterized by gastrointestinal manifestations such as chronic diarrhea and malabsorption. It is an autoimmune disorder in which the body’s immune system produces self-destructive antibodies in response to gluten in the diet. Breast feeding is associated with a reduction in celiac disease [23], more so than delayed introduction of dietary gluten [24]. Onset of celiac disease is typically between 6 months and 2 years of age, suggesting that breast feeding might foster gut microbes that protect babies.
This hypothesis was tested by probiotic supplementation for babies from birth to 6 months of age [25]. The probiotics administered were Bifidobacterium breve and Propionibacterium freudenreichii subspecies shermanii. B. breve is used as a probiotic for various gastrointestinal disorders, and has been associated with protection against pathogens such as C. difficile [25], but net effects on health are uncertain; it can have severe negative effects on premature infants and exacerbate C. difficile infections in the presence of the prebiotic inulin [25,26]. These negative effects indicate that B. breve is most likely a marginal mutualist or ambisymbiont. P. freudenreichii is used in cheese preparation and ingested with palatable cheese; it is therefore assumed to have little if any negative effect on human health [27,28]. It may have a slightly beneficial effect due to a positive effect on bifidobacteria [27,28]. Babies receiving these probiotics did not have lower celiac disease rates.
In a separate study, Bifidobacterium longum subsp. longum was not associated with an improvement beyond that expected from the reduction in gluten that was imposed on the celiac disease patients [29,30]. In contrast, administration of B. infantis to adults was associated with improvement in celiac disease [30]. These findings accord with the idea that B. infantis is a stronger mutualist than the other species, although additional study is needed to control for ages of the patients.
Diabetes and Obesity
The pathogenesis of type 2 diabetes mellitus is still unclear. The classic view was that high carbohydrate diets, excess adipose tissue and genetic predispositions interact in uncertain ways to foster insulin resistance. More recently, the emphasis has been on insulin resistance resulting from inflammation associated with excessive adipose tissue [31]. A corollary of this presumption of chronicity is that the disease generation and therapeutic responses would tend to be gradual rather than sudden. Effects of gastric bypass surgery demand a rethinking of this paradigm. Improvements in insulin sensitivity in response to gastric bypass surgery occur rapidly, before weight loss, and are similar in obese and non-obese but overweight individuals [32-34]. The rapidly ameliorating effects of gastric bypass surgery are consistent with microbiome influences on diabetes, which could occur quickly as a result of the short generation times of bacteria. Gut microbiota are strongly affected by gastric bypass surgery [35]. Documentation of these effects have noted changes across a range of taxonomic scales from phyla to species, but effects are difficult to interpret and sometimes inconsistent [35]. Suggested mechanisms for the amelioration of diabetes have focused on direct positive effects on glucose metabolism or other physiological processes [35]. Balanced assessments, however, must also consider the possibility that the microbiota favored by the bypass surgery competitively inhibit pathogenic microbes that directly contribute to diabetes.
Changes in the microbiome are linked to both obesity and type 2 diabetes, the latter being ameliorated by increases in bacteria that digest HIMACS [6,36-39]. A metagenome-wide association study found a decrease in butyrate-producing bacteria and an increase in opportunistic pathogens in diabetics [40]. If gastric bypass surgery adversely affects causal pathogens, its beneficial effects on diabetes could be explained by a microbiome alteration. One hypothesis to explain these results proposes that dysbiosis causes damage to the gut lining, allowing entrance of bacterial lipopolysaccharide through compromised gut epithelium and consequent inflammation and bariatric surgery somehow ameliorates this dysbiosis [36]. Measurements of the microbiota composition, however, did not reveal any overall shift away from pro-inflammatory microbes in association with dietary amelioration of diabetes [36].
Alternatively, microbes suppressed by gastric bypass surgery may be acting by pathogenic mechanisms other than inflammation. Gut enterocytes have insulin receptors that regulate the uptake of glucose. Gut microbes might therefore increase their access to glucose by inhibiting glucose uptake. If they did so through a diffusible compound with a compromised mucosal barrier the effect could be systemic insulin resistance.
Staphylococcus aureus appears to be an example of such a microbe. It can infect the gut epithelium and is associated with diabetes [21], and its pathology depends on access to glucose [41]. S. aureus releases a peptide (eLtaS) that binds to insulin, blocking its function, thus increasing its access to glucose and favoring insulin resistance [42].
S. aureus may also help explain the association of diabetes with excess body fat, even if the excess fat is not the critical factor maintaining diabetes. In a study assessing the relationship between neonatal microbiota and obesity seven years later, S. aureus was found in the fecal samples that had been taken from obese 7-year-olds when they were neonates, whereas bifidobacteria dominated the neonatal feces of children who did not become obese [43]. Accordingly, breast feeding is associated with a substantially reduced probability of being overweight or obese during childhood [43,44]. This explanation suggests how a pathogen could contribute to diabetes and obesity when competitive inhibition by the gut microbiota is relaxed as a result of reductions in breast feeding.
E. coli can also interfere with insulin, in this case by secreting an insulin degrading enzyme, pitrilysin (protease III) [45]. As with S. aureus, competition for glucose may have selected for mechanisms that could result in reduced host cell uptake of this nutrient. In mice, feeding a western, high sugar, high fat diet reduced overall species richness and shifted the microbiome toward increased proteobacteria, including E. coli. It also increased susceptibility to pathogenic E. coli challenge in germ-free mice receiving fecal transplants from the Western diet fed mice [46]. Some studies have indicated that overweight and obese individuals have higher gut microbiome ratios of E. coli species than lean individuals [47,48], while others have not (for overview see [49]).
The importance of glucose to many gut bacteria suggests that other species may have evolved methods of interfering with insulin. If so, type 2 diabetes could be caused by a variety of pathogens. In the metagenome-wide association study mentioned above, E. coli was associated with diabetes, but S. aureus was not [40]. It would be useful to look at the other diabetes-associated gut bacteria identified by Qin et al. [40] to assess whether any of them similarly interfere with insulin.
Autism
Like obesity and diabetes, autism is linked to alterations in the microbiome [50]. An association between duration of breast feeding and likelihood of autism development was found in a multicenter study in Spain [51] and in a Danish Registry study [52] but not in a large US registry study [53]. A study evaluating over 6,000 teenagers with autism spectrum disorder (ASD) and 24,000 controls indicated a greater risk of developing type 2 diabetes in the individuals with ASD [54]. While it is early times in the investigation of the relationship between the microbiome and autism, probiotics have been shown to ameliorate autism symptoms [55]. Because children with autism often refuse varied diets the use of probiotic/prebiotic combinations, if proven effective, may be a particularly important intervention [50].
Allergies
Childhood food allergies were rare during and prior to the 1990s and are an increasing, and at times life threatening, problem according to newly gathered data [56]. Introduction of B. infantis ameliorated allergy in a mouse model [57]. Has the nearly worldwide shift to vast numbers of children being formula fed, a trend that is slowly being reversed in some countries, led to a reduction in the population level prevalence of important gut bacteria for the newborn? Several studies have associated breast feeding with a reduction in allergies, while others have failed to confirm this association [58]. One possible explanation for the discrepancies is that protective microbes are less prevalent in some individuals and populations.
Evolutionary Implications for Public Health
Public Health
The microbial community in the gut is composed of hundreds of different species [2] interacting with each other and with the host; thus, co-evolution and arms races dynamically shape the gut landscape. Many of the problems perceived as epidemic—obesity, type 2 diabetes, autism, childhood allergies—are linked to the microbiome. The evolutionary mismatch of a diet low in fiber and high in sugar and fat and the radical increase in formula feeding have led to near global disturbance of the human gut microbiome with shifts in species diversity and proportions. The spreading embrace of the Western diet [59] and the influence of companies such as Nestlé on formula feeding [60] help explain the long reach of microbiome disturbance and sequelae.
Breastfeeding
Global public health efforts are underway to revive breast feeding but likely much more will need to be done to counteract the marketing driven shift to formula feeding [61]. Low socioeconomic status is associated with lower rates of breastfeeding and increased rates of childhood obesity [62]. Given that there may be a link between breastfeeding, reduced rates of obesity [63], and other major health concerns, increased interventions and social policies that support initiation and maintenance of breastfeeding, particularly for low resource populations of women, are needed.
If B. infantis is largely vertically transmitted from mother to baby, its prevalence in infants can be expected to depend on its presence not only in the infant’s mother but also the mother’s mother. B. infantis may therefore have become extinct in family lineages in which a mother fed her infant solely using formula, and diminished in a population during a period when a high proportion of mothers used only formula. Supplementation of breast feeding could help remedy this problem for babies who are not colonized with B. infantis. Supplementing formula with B. infantis alone would not remedy the problem because the added B. infantis would be without the oligosaccharides on which they depend. The increased use of formula globally over the past century, has therefore undoubtedly led to a decline in B. infantis and any other microbial mutualists maintained by HMOs. These considerations represent a small scale application of the concern over microbiota extinctions due to diet [64].
Western Diet
The expanding adoption of the Western diet and the associated loss of diets composed of vegetables, grains, and legumes has created a global health crisis and much of this is likely mediated via the microbiome. The western low fiber, high sugar/fat diet influences gut microbial species composition, reduces diversity, and contributes to obesity and diabetes [6,65]. While even short-term dietary changes have been shown to alter the microbiome, possibly representing evolved flexibility for unpredictable food availability [66], the monotonous intake of the Western diet may ultimately lead to loss of HIMAC dependent microbial species within human populations [64]. Five days on a plant-based diet can shift the microbiome [66] and, for obese individuals, a dietary intervention enriched in complex carbohydrates and fiber can decrease diabetes markers [65]. The global epidemic of diabetes is following the spread of the Western diet [67]. Previously, the risk of diabetes in high income countries was greater among individuals of low socio-economic status (SES) while the reverse was true in low/middle income countries. Now obesity is becoming more common among low SES populations worldwide [67]. A diet rich in HIMACs is also more expensive than the processed Western diet, contributing to income related microbiome associated health disparities [68]. Governments serious about addressing obesity might consider subsidizing high-quality vegetables and fruits. The near-addictive qualities of high sugar/fat foods and the ease of preparation and long shelf life of many processed foods will also contribute to the complexity of designing interventions. Mexico, in response to rapidly increasing rates of obesity, has worked to improve school foods, and the World Health Organization is calling for regulations in the marketing of unhealthy foods; some countries have undertaken efforts to regulate marketing directly [59]. Many more small- and large-scale efforts to stem the health consequences of consuming the Western diet are no doubt underway and are certainly needed.
Probiotics
Starting with (and limiting to) the pool of microbes that have co-evolved as mutualists with their hosts seems a critical first step in selecting bacterial strains for probiotics. The use of the term commensal may inadvertently free companies in the business of selecting bacterial strains for probiotics to use almost anything that resides in or out of the host that has not been determined to be a full-time pathogen. Currently there are products that contain organisms that are not known to have lived in the human gut, for example, Bacillus licheniformis, a soil microbe attracting industrial interest for degrading bird feathers to produce animal feed [69]. Evolutionary logic would caution against the use of genetically modified probiotic strains because host-organism interactions have neither been tested over time nor shaped into mutualisms by natural selection. These considerations apply as well to interactions between introduced organisms and existing gut mutualists.
Furthermore, when selecting strains for probiotics it is important to consider the host ability to constrain the bacteria (the distinction between marginal and strong mutualisms applies here), the individual’s immune status, and the bacteria’s ability to accept virulence/antibiotic resistance genes from other microbes co-habiting the gut (see for example Enterococcus sps. [70-72]). The potential for the evolution of microbial virulence must be considered when selecting strains. Concentrated animal feeding operations (CAFOs) and hospitals are known for conditions that favor the acquisition of microbial virulence and antibiotic resistance, and resulting strains are not isolated from community populations [17].
Even a strong mutualist such as B. infantis can cause problems in the wrong time and place. The power of natural selection to shape the interaction between host and microbe may be lost in extreme novel conditions as exemplified by the current day survival of very premature infants. Necrotizing enterocolitis (NEC) represents a significant risk to prematurely born infants and use of probiotics containing B. infantis reduced NEC incidence [73]. In an NEC prevention study using the same brand of probiotic product as in the Lin intervention, no reduction in NEC was found, but it was determined that the composition of the probiotic had been changed from B. infantis to B. bifidum. Efforts to reach consensus on probiotic safety for use in premature infant populations are complicated by the findings that probiotics may be used to prevent or treat problems such as NEC or hospital acquired diarrhea but cause bacteremia in some of the exposed infants. This negative outcome has been documented for B. infantis [74,75]. Being aware that there are risks as well as substantial benefits to using probiotics may improve vigilance, allowing for rapid diagnosis and treatment for infection caused by probiotic strains.
Prebiotics
Favoring and disfavoring gut microbes through diet to improve health (consuming foods rich in appropriate prebiotics) may be effective if sufficient species diversity exists within the individual. Prebiotics as supplements may need to be constrained to the amount of food we would eat normally/evolutionarily rather than super concentrated into products. Large amounts of a given prebiotic such as inulin, a plant polysaccharide, might over favor certain bacterial populations and diminish mutualist richness. A study evaluating growth and toxin production of C. difficile by itself and in competition with bifidobacterial species showed increased toxin production when grown on inulin [25]. Early results from a study using 6 weeks of inulin supplementation for pre-diabetics while controlling all other aspects of diet showed no change in a metabolite associated with type 2 diabetes [76]. The study’s controlled diet, while calorie appropriate, resembles aspects of the Western diet including simple carbohydrates, fat, and low-fiber foods. Probiotics were excluded which would require that participants have appropriate inulin using bacteria in their microbiota. Further results from such studies will be necessary to see if efforts to enhance the Western diet with one or more prebiotics will be too limited to reverse or prevent microbiome associated disease. Inulin as well as other prebiotic supplements may be useful, but the gut microbiota encompasses a large number of interacting and competing species, and we evolved eating whole foods rather than discrete components; thus, it is hard to anticipate effects of interventions that introduce only prebiotics.
Public health efforts to counter negative effects of the Western diet, support breastfeeding, and assure access to high-fiber, low-sugar, and low-fat foods may have an outsized effect on seemingly unrelated widespread diseases such as diabetes, autism, and childhood allergies. The broad sweep of these effects suggests that health promotion by the microbiome may involve broad-based benefits such as the enhancement of immunological function and/or the protection by microbial mutualists against pathogens that would otherwise cause damage specifically to gut tissues as well as systemically. The evidence relative to diabetes may illustrate this breadth because the Western diet appears to shift the microbiome towards species that directly antagonize insulin in a pathological process that is preventable and treatable.
Acknowledgments
The authors thank Matthew Orr and an anonymous reviewer for helpful comments on the manuscript.
Glossary
- HIMACs
human-inaccessible microbiota-accessible carbohydrates
- MACs
microbiota-accessible carbohydrates
- SCFAs
short chain fatty acids
- HMOs
human milk oligosaccharides
- ASD
autism spectrum disorder
- SES
socio-economic status
- NEC
necrotizing enterocolitis
References
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336(6086):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biol. 2017;15(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MR, Kocurek KM, Young DL. Gut microbiota and human health: insights from ecological restoration. Q Rev Biol. 2018;93(2):3–90. [Google Scholar]
- Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. The gut commensal bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe. 2014;16(6):759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti S, Lugli GA, Mancabelli L, Armanini F, Turroni F, James K, et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome. 2017;5(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014;20(5):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, et al. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. 2000;47(5):646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77(4):412–20. [DOI] [PubMed] [Google Scholar]
- Kwak MJ, Kwon SK, Yoon JK, Song JY, Seo JG, Chung MJ, et al. Evolutionary architecture of the infant-adapted group of bifidobacterium species associated with the probiotic function. Syst Appl Microbiol. 2016;39(7):429–39. [DOI] [PubMed] [Google Scholar]
- Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77(1-2):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, et al. The genome sequence of bifidobacterium longum subsp. Infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105(48):18964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in bifidobacterium longum subsp. Infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76(22):7373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Patton S, Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol Neonate. 1998;74(2):143–62. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10(5):507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald PW. Transmission modes and evolution of the parasitism-mutualism continuum. Ann N Y Acad Sci. 1987;503:295–306. [DOI] [PubMed] [Google Scholar]
- Ewald PW. Evolution of infectious disease. New York: Oxford University Press; 1994. [Google Scholar]
- Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, et al. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80(10):3007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio RG, Ninonuevo MR, Kronewitter SR, Freeman SL, German JB, Lebrilla CB, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. 2009;2(3):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Arrieta MC, Finlay BB. A fresh look at the hygiene hypothesis: how intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol. 2013;25(5):378–87. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Shostak M, Konner M. The paleolithic prescription: A program of diet and exercise and a design for living. New York: HarperCollins, 1988. [Google Scholar]
- Falth-Magnusson K, Franzen L, Jansson G, Laurin P, Stenhammar L. Infant feeding history shows distinct differences between swedish celiac and reference children. Pediatr Allergy Immunol. 1996;7(1):1–5. [DOI] [PubMed] [Google Scholar]
- Peters U, Schneeweiss S, Trautwein EA, Erbersdobler HF. A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab. 2001;45(4):135–42. [DOI] [PubMed] [Google Scholar]
- Valdes-Varela L, Hernandez-Barranco AM, Ruas-Madiedo P, Gueimonde M. Effect of bifidobacterium upon clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front Microbiol. 2016;7:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Probiotics in Preterm Infants Study Collaborative G. Bifidobacterium breve bbg-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet. 2016;387(10019):649–60. [DOI] [PubMed] [Google Scholar]
- Falentin H, Deutsch SM, Jan G, Loux V, Thierry A, Parayre S, et al. The complete genome of propionibacterium freudenreichii cirm-bia1, a hardy actinobacterium with food and probiotic applications. PLoS One. 2010;5(7):e11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri C. Dairy propionibacteria as probiotics: recent evidences. World J Microbiol Biotechnol. 2016;32(10):172. [DOI] [PubMed] [Google Scholar]
- Olivares M, Castillejo G, Varea V, Sanz Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of bifidobacterium longum cect 7347 in children with newly diagnosed coeliac disease. Br J Nutr. 2014;112(1):30–40. [DOI] [PubMed] [Google Scholar]
- Smecuol E, Pinto-Sanchez MI, Bai JC. Understanding the role of probiotics in coeliac disease. Br J Nutr. 2015;113(10):1664–5. [DOI] [PubMed] [Google Scholar]
- Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38(3):183–91. [DOI] [PubMed] [Google Scholar]
- Yska JP, van Roon EN, de Boer A, Leufkens HG, Wilffert B, de Heide LJ, et al. Remission of type 2 diabetes mellitus in patients after different types of bariatric surgery: A population-based cohort study in the united kingdom. JAMA Surg. 2015;150(12):1126–33. [DOI] [PubMed] [Google Scholar]
- Rubino F. Surgery that shortens intestines gets rid of the illness, and new evidence shows the gut—not simply insulin—may be responsible. Sci Am. 2017;317(1):60–5. [Google Scholar]
- Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2018;61(2):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Geurts L, Matamoros S, Plovier H, Duparc T. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond. Diabetes Metab. 2014;40(4):246–57. [DOI] [PubMed] [Google Scholar]
- Caricilli AM, Saad MJ. The role of gut microbiota on insulin resistance. Nutrients. 2013;5(3):829–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5(1):3–17. [DOI] [PubMed] [Google Scholar]
- Utzschneider KM, Kratz M, Damman CJ, Hullar M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2016;101(4):1445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–6. [DOI] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- Vitko NP, Grosser MR, Khatri D, Lance TR, Richardson AR. Expanded glucose import capability affords staphylococcus aureus optimized glycolytic flux during infection. MBio. 2016;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu FJ, Guan ZC, Dong FT, Cheng JH, Gao YP, et al. The extracellular domain of staphylococcus aureus ltas binds insulin and induces insulin resistance during infection. Nat Microbiol. 2018;3(5):622–31. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–8. [DOI] [PubMed] [Google Scholar]
- Ortega-Garcia JA, Kloosterman N, Alvarez L, Tobarra-Sanchez E, Carceles-Alvarez A, Pastor-Valero R, et al. Full breastfeeding and obesity in children: A prospective study from birth to 6 years. Child Obes. 2018;14(5):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AB, Roth RA. Insulysin and pitrilysin: insulin-degrading enzymes of mammals and bacteria. Methods Enzymol. 1995;248:693–703. [DOI] [PubMed] [Google Scholar]
- Agus A, Denizot J, Thevenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive e. Coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. [DOI] [PubMed] [Google Scholar]
- Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. Obesity in school-aged children and its correlation with gut e.Coli and bifidobacteria: A case-control study. BMC Pediatr. 2015;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han Y, Dy AB, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Julvez J, Guxens M, Arranz E, Ibarluzea J, Sanchez de Miguel M, et al. Association between breastfeeding duration and cognitive development, autistic traits and adhd symptoms: A multicenter study in spain. Pediatr Res. 2017;81(3):434–42. [DOI] [PubMed] [Google Scholar]
- Lemcke S, Parner ET, Bjerrum M, Thomsen PH, Lauritsen MB. Early regulation in children who are later diagnosed with autism spectrum disorder. A longitudinal study within the danish national birth cohort. Infant Ment Health J. 2018;39(2):170–82. [DOI] [PubMed] [Google Scholar]
- Husk JS, Keim SA. Breastfeeding and autism spectrum disorder in the national survey of children’s health. Epidemiology. 2015;26(4):451–7. [DOI] [PubMed] [Google Scholar]
- Chen MH, Lan WH, Hsu JW, Huang KL, Su TP, Li CT, et al. Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: A nationwide longitudinal study. Diabetes Care. 2016;39(5):788–93. [DOI] [PubMed] [Google Scholar]
- Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HS, Saad K, et al. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr Neurosci. 2017:1–6. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn J, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Yang ZY, Dai WK, Huang JQ, Li YH, Zhang J, et al. Protective effect of bifidobacterium infantis cgmcc313-2 on ovalbumin-induced airway asthma and beta-lactoglobulin-induced intestinal food allergy mouse models. World J Gastroenterol. 2017;23(12):2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munblit D, Peroni DG, Boix-Amoros A, Hsu PS, Van’t Land B, Gay MC, et al. Human milk and allergic diseases: an unsolved puzzle. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg KD, Eastham CA, Kasehagen LJ, Sandoval AP. Marketing infant formula through hospitals: the impact of commercial hospital discharge packs on breastfeeding. Am J Public Health. 2008;98(2):290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwoz EG, Huffman SL. The impact of marketing of breast-milk substitutes on who-recommended breastfeeding practices. Food Nutr Bull. 2015;36(4):373–86. [DOI] [PubMed] [Google Scholar]
- Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9(2):135–46. [DOI] [PubMed] [Google Scholar]
- Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health. 2014;14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CA, Taren D. How poverty affects diet to shape the microbiota and chronic disease. Nat Rev Immunol. 2018;18(4):279–87. [DOI] [PubMed] [Google Scholar]
- Pedersen MB, Yu S, Plumstead P, Dalsgaard S. Comparison of four feed proteases for improvement of nutritive value of poultry feather meal. J Anim Sci. 2012;90 Suppl 4:350–2. [DOI] [PubMed] [Google Scholar]
- Lund B, Edlund C. Probiotic enterococcus faecium strain is a possible recipient of the vana gene cluster. Clin Infect Dis. 2001;32(9):1384–5. [DOI] [PubMed] [Google Scholar]
- Klare I, Konstabel C, Badstubner D, Werner G, Witte W. Occurrence and spread of antibiotic resistances in enterococcus faecium. Int J Food Microbiol. 2003;88(2-3):269–90. [DOI] [PubMed] [Google Scholar]
- Werner G, Coque TM, Franz CM, Grohmann E, Hegstad K, Jensen L, et al. Antibiotic resistant enterococci-tales of a drug resistance gene trafficker. Int J Med Microbiol. 2013;303(6-7):360–79. [DOI] [PubMed] [Google Scholar]
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115(1):1–4. [DOI] [PubMed] [Google Scholar]
- Bertelli C, Pillonel T, Torregrossa A, Prod’hom G, Fischer CJ, Greub G, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin Infect Dis. 2015;60(6):924–7. [DOI] [PubMed] [Google Scholar]
- Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Stoen R, Klingenberg C. Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerg Infect Dis. 2016;22(9):1664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh ME, Steele CN, Angiletta CJ, Mitchell CM, Neilson AP, Davy BM, et al. Inulin supplementation does not reduce plasma trimethylamine n-oxide concentrations in individuals at risk for type 2 diabetes. Nutrients. 2018;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther BA, Ewald PW. Pathogen survival in the external environment and the evolution of virulence. Biol Rev Camb Philos Soc. 2004;79(4):849–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


