Abstract
Background:
Plasminogen activator inhibitor 1 (PAI-1) was previously established to impact several phenotypes in many kinds of cancer, including pancreatic cancer. However, its prognostic significance in pancreatic ductal adenocarcinoma (PDAC) needs support of further evidence. This study was designed to address the issue.
Methods:
PAI-1 expression was detected by tissue microarray-based immunohistochemical staining in formalin-fixed paraffin-embedded specimens from 93 PDAC patients with surgical resection from September 2004 to December 2008. Its relationships with clinicopathologic variables and tumor-specific survival (TSS) were further evaluated using Chi-square, Kaplan-Meier, log-rank, as well as Cox regression analyses.
Results:
Expression of PAI-1 was much higher in tumor than that in nontumor tissues, based on comparison of all samples and 74 matched ones (95 [47.5, 180] vs. 80 [45, 95], Z = −2.439, P = 0.015 and 100 [46.9, 182.5] vs. 80 [45, 95], Z = −2.594, P = 0.009, respectively). In addition, tumoral PAI-1 expression was positively associated with N stage (22/35 for N1 vs. 21/51 for N0, χ2 = 3.903, P = 0.048). Univariate analyses showed that TSS of patients with high PAI-1 tumors was significantly poorer than that of those with low PAI-1 tumors (log rank value = 19.00, P < 0.0001). In multivariate Cox regression test, PAI-1 expression was identified as an independent predictor for long-term prognosis of resectable PDAC (hazard ratio = 2.559, 95% confidence interval = 1.499–4.367, P = 0.001).
Conclusion:
These results suggest that expression of PAI-1 is upregulated in PDAC and might serve as a poor prognostic indicator.
Keywords: Pancreatic Ductal Adenocarcinoma, Plasminogen Activator Inhibitor 1, Prognosis
摘要
背景:
之前研究发现纤溶酶元激活物抑制物1(PAI-1)在多种癌症,包括胰腺癌中影响许多表型。然而,其在胰腺导管腺癌(PDAC)中的预后意义需要进一步证据支持。本研究意在阐述这个问题。
方法:
采用基于组织芯片的免疫组化染色检测2004年9月至2008年12月93例行外科切除的胰腺导管腺癌福尔马林固定石蜡包 埋标本中PAI-1的表达。其与临床病理参数和肿瘤特异性生存(TSS)的关系以卡方检验、Kaplan-Meier和Log-rank检验以及 Cox回归进行评价。
结果:
在所有标本和74例配对标本中,癌组织PAI-1表达显著高于非癌组织(95 [47.5, 180] vs 80 [45, 95] ,Z=-2.439,P=0.015 和100 [46.875, 182.5] vs 80 [45, 95],Z=-2.594,P=0.009)。而且,肿瘤组织PAI-1表达与N分期呈正相关(N1组22/35比 N0组21/51,χ2=3.903,P=0.048)。单因素分析显示癌组织PAI-1高表达患者肿瘤特异性生存显著差于低表达者(Log-rank 值=19.00,P<0.0001)。在多因素Cox回归中,PAI-1表达被鉴定为可切除胰腺导管腺癌长期预后的独立预测因素(相对危险 度[HR]=2.559,95%可信区间[CI]=1.499–4.367,P=0.001)。
结论:
这些结果提示胰腺导管腺癌中PAI-1表达上调,并可能作为不良预后的指标。
INTRODUCTION
Pancreatic cancer (PC), especially its main histological type, pancreatic ductal adenocarcinoma (PDAC), has long been well known as one of the most lethal malignant neoplasms worldwide.[1] In China, its incidence and mortality rate have also been reported to be remarkably raised in recent years.[2] Up to now, its overall long-term prognosis remains dismal, although curative resection and adjuvant therapy have achieved some favorable effects in highly selected patients.[3,4,5] Therefore, survival-associated variables in patients with this malignancy caught much attention. The main identified ones were crucial clinicopathologic and surgical factors, including lymph node metastasis, tumor histology grade, peri-neural invasion, and resection margin.[6,7,8,9] On the other hand, data on prognostic biomarkers in PC, particularly for resectable patients, were also accumulated.[10,11] However, further candidates need to be supplemented.
In view of the fact that approximately 70% of PDAC patients died from extensive metastatic disease,[1] invasion-related molecules in this malignancy, such as those previously reported,[12,13,14] were of particular importance. Plasminogen activator inhibitor-1 (PAI-1), encoded by SERPINE1, is a specific inhibitory member of plasminogen activation (PA) system.[15] Except for its key role in acute thrombotic events, PAI-1 was also expected to prevent cancer invasion and metastasis through its inhibition of urokinase-type plasminogen activator, which was demonstrated to be involved in malignant dissemination.[15] Indeed, there were some published articles showing this metastasis inhibitory effect.[16,17,18] However, more articles suggested its pro-oncogenic roles.[19,20,21,22,23,24] In PC/PDAC, different in vitro and in vivo experiments also got inconsistent, even opposite results.[17,19,23] However, those for its prognostic significance in PDAC seem to be more consistent. It was shown that PAI-1 mRNA/protein overexpression tended to predict poor patient survival, but not being statistically significant.[25,26] The smaller sample size and incomplete staining evaluation criteria (without staining intensity) might be the main limits of these investigations.
The aim of this study was to discover the clinicopathologic and prognostic implications of PAI-1 expression in PDAC, through improvements of aforementioned limits.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking Union Medical College Hospital (No. JS1178). Being a retrospective study and since data analysis was performed anonymously, this study was exempted from the informed consent from patients.
Patients
This study enrolled 93 resected patients with PDAC from September 2004 to December 2008. The inclusion criteria included: (1) without chemo-/chemo-radiation therapy before surgery; (2) underwent curative resection; and (3) histologically confirmed. Fifty-eight patients were male and 35 were female. The age ranged from 34 to 85 years (median: 62 years). The main clinical and pathologic variables of patients are summarized in Table 1.
Table 1.
Relationships between PAI-1 expression and clinicopathologic variables of resectable PDAC
| Variables | n | PAI-1 expression, n | χ2 | P | |
|---|---|---|---|---|---|
| High | Low | ||||
| Gender | |||||
| Male | 58 | 30 | 28 | 0.001 | 0.978 |
| Female | 35 | 17 | 18 | ||
| Age (years) | |||||
| ≥62 | 47 | 24 | 23 | 0.011 | 0.915 |
| <62 | 46 | 24 | 22 | ||
| Tumor site | |||||
| Head | 57 | 30 | 27 | 0.061 | 0.805 |
| Nonhead | 36 | 18 | 18 | ||
| Tumor size (cm) | |||||
| >4 | 54 | 27 | 27 | 0.016 | 0.899 |
| ≤4 | 37 | 19 | 18 | ||
| Histological grade | |||||
| G1–G2 | 64 | 33 | 31 | <0.001 | 0.988 |
| G3–G4 | 29 | 15 | 14 | ||
| T stage | |||||
| T1–T2 | 71 | 39 | 32 | 2.479 | 0.115 |
| T3 | 20 | 7 | 13 | ||
| N stage | |||||
| N0 | 51 | 21 | 30 | 3.903 | 0.048 |
| N1 | 35 | 22 | 13 | ||
Partial data were not available, and statistics were based on available data. P values were derived from the Pearson’s Chi-square test (two tailed). PAI-1: Plasminogen activator inhibitor 1; PDAC: Pancreatic ductal adenocarcinoma; G1: Well differentiated; G2: Moderately differentiated; G3: Poorly differentiated; G4: Undifferentiated; T: Tumor; N: Lymph node.
Tissue microarray construction
The tissue microarray was constructed using formalin-fixed paraffin-embedded blocks. The construction method was same as our previous report.[27]
Immunohistochemistry
A mouse monoclonal antibody for human PAI-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and a two-step staining kit (EnVision ™ plus kit, Dako, Denmark) were used for staining. First, 4-μm-thick slides were mounted, deparaffinized, and rehydrated. After antigen retrieval in an autoclave, sections were incubated with 3% hydrogen peroxide (10 min) to block endogenous peroxidase. Then, slides were sequentially incubated with the primary antibody (dilution: 1: 50) overnight at 4°C and horseradish peroxidase-labeled secondary antibody (30 min). Diaminobenzidine was applied as a chromogen. Finally, those were counterstained with hematoxylin. Nonimmune mouse serum at the same dilution was used as the negative control.
Staining evaluation
Two experienced pathologists who had no clinicopathologic and follow-up information evaluated the sections, according to the H-score,[28] a widely used immunostaining evaluation criterion.[29,30] Similar to a previous study,[31] the H-score with the largest Youden index (YI) within the receiver operating characteristic (ROC) curve for survival status was adopted as the cutoff value.
Follow-up
All patients accepted the postsurgical follow-up (range: 2–87 months; median: 11 months). A total of 61 patients (65.6%) died, and the other 32 (34.4%) were alive.
Statistical analysis
The H-scores of PAI-1 in tumor and nontumor tissues were compared using Mann-Whitney U-test. Chi-square test was applied to determine the relationships between PAI-1 expression and clinicopathologic variables. The prognostic significance of PAI-1 was explored by Kaplan-Meier method and log rank test. Univariate and multivariate Cox regression (proportional hazard model) analysis was adopted for prognostic factor identification. Statistical software package Statistical Package for the Social Sciences version 11.5 (SPSS Inc., Chicago, IL, USA) was employed for all the analyses. A statistical significance was defined when P < 0.05.
RESULTS
Expression pattern of plasminogen activator inhibitor 1 in resectable pancreatic ductal adenocarcinoma
As shown in Figure 1a and 1b, the positive staining of PAI-1 was mainly located in the cytoplasm of both tumor and nontumor tissues. In all patients and 74 with matched tumor and nontumor samples, the H-scores in tumor tissues were much higher than those in nontumor ones (95 [47.5, 180] vs. 80 [45, 95], Z = −2.439, P = 0.015 and 100 [46.9, 182.5] vs. 80 [45, 95], Z = −2.594, P = 0.009, respectively) [Figure 1c and 1d].
Figure 1.
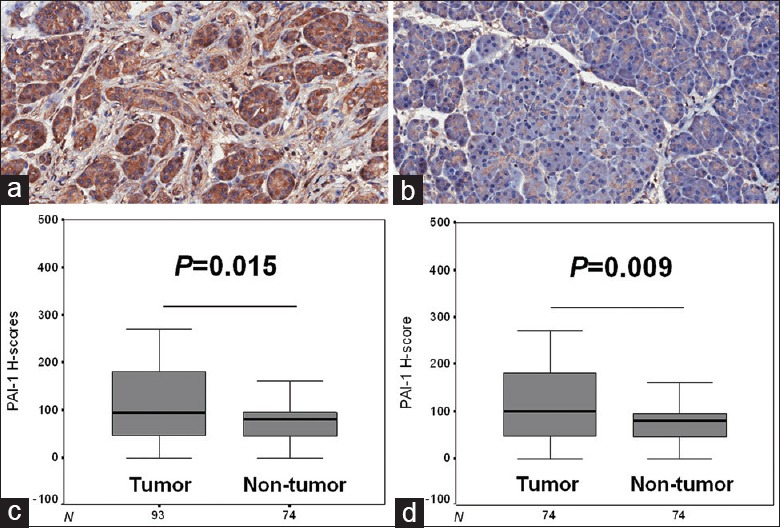
PAI-1 expression in resectable PDAC. (a) High expression in tumor tissue (immunohistochemistry, ×200); (b) Low expression in nontumor tissue (immunohistochemistry, ×200); (c) Comparison of PAI-1 H-scores between all tumor and nontumor tissues (Mann-Whitney U-test; Z = −2.439, P = 0.015); (d) Comparison of PAI-1 H-scores between matched tumor and nontumor tissues (Mann-Whitney U-test; Z = −2.594, P = 0.009). PAI-1: Plasminogen activator inhibitor 1; PDAC: Pancreatic ductal adenocarcinoma.
Prognostic value of plasminogen activator inhibitor 1 in resectable pancreatic ductal adenocarcinoma
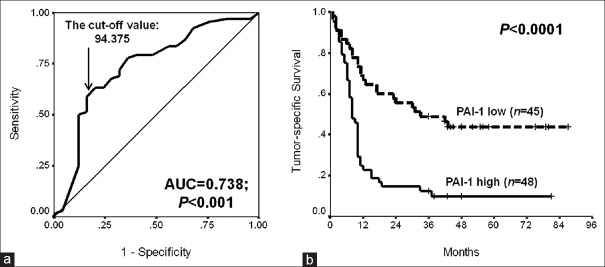
In the first, the H-score value of PAI-1 (cutoff value: 94.375) with the largest YI in the ROC curve for survival status was selected as the cutoff one [Figure 2a]. In contrast to patients’ low tumoral PAI-1 expression, those with high PAI-1 expression carried significantly poorer tumor-specific survival (TSS) [log rank value = 19.00, P < 0.0001, Figure 2b].
Figure 2.
Prognostic value of PAI-1 in resectable PDAC. (a) The cutoff value determination using the ROC curve of tumoral PAI-1 H-scores for tumor-specific survival status; (b) Tumor-specific survival curves of patients with high or low tumoral PAI-1 expression (log rank value = 19.00, P < 0.0001). PAI-1: Plasminogen activator inhibitor 1; PDAC: Pancreatic ductal adenocarcinoma; ROC: Receiver operating characteristic.
Relationships of plasminogen activator inhibitor 1 with clinicopathologic variables of resectable pancreatic ductal adenocarcinoma
Using Chi-square analysis, high tumoral PAI-1 expression, based on the same cutoff value, was associated with N1 stage [22/35 for N1 vs. 21/51 for N0, χ2 = 3.903, P = 0.048, Table 1]. No significant associations between PAI-1 expression and other clinicopathologic parameters were found [P > 0.05, Table 1].
Identification of prognostic indicators in resectable pancreatic ductal adenocarcinoma
It was revealed by univariate Cox regression analysis that histological grade, N stage, and PAI-1 expression were predictors of TSS [P < 0.05, Table 2]. Multivariately, these factors remained to be significant, thus being independent prognostic determinants [P < 0.05, Table 2].
Table 2.
Univariate and multivariate analyses for prognostic indicators of resectable PDAC
| Variables | n | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | |||||||
| Male | 58 | 1.182 | 0.718–1.945 | 0.510 | |||
| Female | 35 | 1 | |||||
| Age (years) | |||||||
| ≥62 | 47 | 1.341 | 0.832–2.159 | 0.228 | |||
| <62 | 46 | 1 | |||||
| Tumor site | |||||||
| Head | 57 | 1 | 0.980 | ||||
| Nonhead | 36 | 0.994 | 0.611–1.616 | ||||
| Tumor size (cm) | |||||||
| >4 | 54 | 0.807 | 0.496–1.313 | 0.387 | |||
| ≤4 | 37 | 1 | |||||
| Histological grade | |||||||
| G1–G2 | 64 | 1 | 0.018 | 1 | 0.001 | ||
| G3–G4 | 29 | 1.824 | 1.106–3.005 | 2725 | 1.495–4.968 | ||
| T stage | |||||||
| T1–T2 | 71 | 1 | 0.941 | ||||
| T3 | 20 | 0.978 | 0.541–1.766 | ||||
| N stage | |||||||
| N0 | 51 | 1 | 0.010 | 1 | 0.004 | ||
| N1 | 35 | 1.940 | 1.169–3.221 | 2.372 | 1.327–4.241 | ||
| PAI-1 expression | |||||||
| High | 48 | 2.874 | 1.727–4.782 | <0.001 | 2.559 | 1.499–4.367 | 0.001 |
| Low | 45 | 1 | 1 | ||||
Partial data were not available, and statistics were based on available data. P values were derived from the univariate and multivariate Cox regression analyses. PDAC: Pancreatic ductal adenocarcinoma; HR: Hazard ratio; CI: Confidence interval; G1: Well differentiated; G2: Moderately differentiated; G3: Poorly differentiated; G4: Undifferentiated; T: Tumor; N: Lymph node; PAI-1: Plasminogen activator inhibitor 1.
DISCUSSION
It was long unexpectedly found that PAI-1 promoted many malignant behaviors of several kinds of cancer cells,[19,20,21,22,23,24] although it is one of the specific inhibitory members of PA system and was thus thought to inhibit cancer invasion/metastasis.[16,17,18] Therefore, its biological roles in cancer might vary largely. In PC/PDAC, previous articles revealed that high PAI-1 expression at mRNA and protein levels all tended to be associated with poor patient survival,[25,26] unlike inconsistent data from in vitro and in vivo experiments.[17,19,23] The possible reasons of imperfect statistical results might be attributed to smaller sample size (46 cases)[26] and defective staining evaluation criteria (absence of staining intensity).[25] In the present study, we included relatively more patients and used the H-score that considered both positive cell ratio and staining intensity[28] and wished to make it more comprehensive and reliable than aforementioned ones.[25,26] Our data showed that PAI-1 expression was remarkably higher in tumor than that in nontumor tissues of PDAC, based on the comparison of all samples and 74 matched ones. Moreover, PAI-1 expression positively correlated with N stage, an important factor that reflects tumor cell dissemination and predicts dismal prognosis in PC,[6,7] similar with evidence from other cancers.[32,33] Thus, the data preliminarily provide the histological evidence of PAI-1 as a proto-oncogene in PDAC. However, detailed mechanistic explorations remain to be in need.
More importantly, the prognostic value of PAI-1 expression remains to be elucidated in PDAC, on the basis of previous clues.[25,26] Using H-score and reasonable cutoff value determination method,[25,28] we showed that high tumoral PAI-1 expression was significantly related to poor TSS [Figure 2b]. Furthermore, PAI-1 expression plus the key clinicopathologic parameters of PDAC, histological grade and N stage, were significant prognostic factors, estimated by univariate Cox regression analysis. In view of its positive correlation with N stage, its influence on patient survival might easily be understood. Subsequent multivariate Cox regression analysis identified PAI-1 expression as one of the independent predictors of postsurgical survival of PDAC. These findings, which are largely consistent with those from other tumor types,[32,33,34,35,36,37,38,39] suggested that PAI-1 might be a strong biomarker for long-term prognosis of PDAC. All our data support its promoting role in PDAC invasion.[19,23] In the future, combined evaluation of this molecule and other variables might be of interest. On the other hand, relative molecular mechanisms are also worth further investigation.
However, this work has some limitations, such as its retrospective design and single detection method. Therefore, subsequent prospective and more comprehensive validations might be necessary.
In conclusion, our data indicate that PAI-1 expression is upregulated in PDAC and might serve as a poor prognostic marker.
Financial support and sponsorship
This work was supported by a grant from the National Natural Science Foundation (No. 81402027).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–49. doi: 10.1056/NEJMra1404198. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Mitchem JB, Hamilton N, Gao F, Hawkins WG, Linehan DC, Strasberg SM. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure. J Am Coll Surg. 2012;214:46–52. doi: 10.1016/j.jamcollsurg.2011.10.008. doi: 10.1016/j.jamcollsurg.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet. 2016;388:248–57. doi: 10.1016/S0140-6736(16)30583-9. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 5.Picozzi VJ, Oh SY, Edwards A, Mandelson MT, Dorer R, Rocha FG, et al. Five-year actual overall survival in resected pancreatic cancer: A contemporary single-institution experience from a multidisciplinary perspective. Ann Surg Oncol. 2017;24:1722–30. doi: 10.1245/s10434-016-5716-z. doi: 10.1245/s10434-016-5716-z. [DOI] [PubMed] [Google Scholar]
- 6.Kim R, Tsao R, Tan A, Byrne M, Almhanna K, Lazaryan A, et al. Asingle institution review of adjuvant therapy outcomes for resectable pancreatic adenocarcinoma: Outcome and prognostic indicators. J Gastrointest Surg. 2010;14:1159–69. doi: 10.1007/s11605-010-1213-z. doi: 10.1007/s11605-010-1213-z. [DOI] [PubMed] [Google Scholar]
- 7.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: Better prediction of outcome. Ann Surg. 2011;254:311–9. doi: 10.1097/SLA.0b013e31821fd334. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee D, Katz MH, Rashid A, Wang H, Iuga AC, Varadhachary GR, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–17. doi: 10.1097/PAS.0b013e31824104c5. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Frampton AE, Cohen P, Kyriakides C, Bong JJ, Habib NA, et al. Tumor infiltration in the medial resection margin predicts survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2012;17:1875–82. doi: 10.1007/s11605-012-1985-4. doi: 10.1007/s11605-012-1985-4. [DOI] [PubMed] [Google Scholar]
- 10.Ansari D, Rosendahl A, Elebro J, Andersson R. Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. Br J Surg. 2011;98:1041–55. doi: 10.1002/bjs.7574. doi: 10.1002/bjs.7574. [DOI] [PubMed] [Google Scholar]
- 11.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22. doi: 10.1002/jso.23192. doi: 10.1002/jso.23192. [DOI] [PubMed] [Google Scholar]
- 12.Whittle MC, Izeradjene K, Rani PG, Feng L, Carlson MA, DelGiorno KE, et al. RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell. 2015;161:1345–60. doi: 10.1016/j.cell.2015.04.048. doi: 10.1016/j.cell.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Liu Z, You L, Hou P, Ren X, Jiao T, et al. Pancreatic cancer progression relies upon mutant p53-induced oncogenic signaling mediated by NOP14. Cancer Res. 2017;77:2661–73. doi: 10.1158/0008-5472.CAN-16-2339. doi: 10.1158/0008-5472.CAN-16-2339. [DOI] [PubMed] [Google Scholar]
- 14.Siret C, Dobric A, Martirosyan A, Terciolo C, Germain S, Bonier R, et al. Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma. Br J Cancer. 2018;118:546–57. doi: 10.1038/bjc.2017.411. doi: 10.1038/bjc.2017.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Wei X, He J, Tian X, Yuan S, Sun L. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother. 2018;105:83–94. doi: 10.1016/j.biopha.2018.05.119. doi: 10.1016/j.biopha.2018.05.119. [DOI] [PubMed] [Google Scholar]
- 16.Hjortland GO, Bjørnland K, Pettersen S, Garman-Vik SS, Emilsen E, Nesland JM, et al. Modulation of glioma cell invasion and motility by adenoviral gene transfer of PAI-1. Clin Exp Metastasis. 2003;20:301–9. doi: 10.1023/a:1024040718238. [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Sawada T, Uchima Y, Kimura K, Nishihara T, Tanaka H, et al. Plasminogen activator inhibitor-1 (PAI-1) gene transfection inhibits the liver metastasis of pancreatic cancer by preventing angiogenesis. Oncol Rep. 2005;14:1445–51. [PubMed] [Google Scholar]
- 18.Rubina KA, Sysoeva VY, Zagorujko EI, Tsokolaeva ZI, Kurdina MI, Parfyonova YV, et al. Increased expression of uPA, uPAR, and PAI-1 in psoriatic skin and in basal cell carcinomas. Arch Dermatol Res. 2017;309:433–42. doi: 10.1007/s00403-017-1738-z. doi: 10.1007/s00403-017-1738-z. [DOI] [PubMed] [Google Scholar]
- 19.Lupu-Meiri M, Geras-Raaka E, Lupu R, Shapira H, Sandbank J, Segal L, et al. Knock-down of plasminogen-activator inhibitor-1 enhances expression of E-cadherin and promotes epithelial differentiation of human pancreatic adenocarcinoma cells. J Cell Physiol. 2012;227:3621–8. doi: 10.1002/jcp.24068. doi: 10.1002/jcp.24068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geis T, Döring C, Popp R, Grossmann N, Fleming I, Hansmann ML, et al. HIF-2alpha-dependent PAI-1 induction contributes to angiogenesis in hepatocellular carcinoma. Exp Cell Res. 2015;331:46–57. doi: 10.1016/j.yexcr.2014.11.018. doi: 10.1016/j.yexcr.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Pavón MA, Arroyo-Solera I, Téllez-Gabriel M, León X, Virós D, López M, et al. Enhanced cell migration and apoptosis resistance may underlie the association between high SERPINE1 expression and poor outcome in head and neck carcinoma patients. Oncotarget. 2015;6:29016–33. doi: 10.18632/oncotarget.5032. doi: 10.18632/oncotarget.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirahata M, Osaki M, Kanda Y, Sugimoto Y, Yoshioka Y, Kosaka N, et al. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med. 2016;5:892–902. doi: 10.1002/cam4.651. doi: 10.1002/cam4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botla SK, Savant S, Jandaghi P, Bauer AS, Mücke O, Moskalev EA, et al. Early epigenetic downregulation of microRNA-192 expression promotes pancreatic cancer progression. Cancer Res. 2016;76:4149–59. doi: 10.1158/0008-5472.CAN-15-0390. doi: 10.1158/0008-5472.CAN-15-0390. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Lin BW, Chen XL, Zhang BL, Xiao XJ, Shi JS, et al. PAI-1/PIAS3/Stat3/miR-34a forms a positive feedback loop to promote EMT-mediated metastasis through Stat3 signaling in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;493:1464–70. doi: 10.1016/j.bbrc.2017.10.014. doi: 10.1016/j.bbrc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi Y, Nakao A, Harada A, Nonami T, Fukatsu T, Takagi H. Expression of plasminogen activators and their inhibitors in human pancreatic carcinoma: Immunohistochemical study. Am J Gastroenterol. 1993;88:1928–33. [PubMed] [Google Scholar]
- 26.Smith R, Xue A, Gill A, Scarlett C, Saxby A, Clarkson A, et al. High expression of plasminogen activator inhibitor-2 (PAI-2) is a predictor of improved survival in patients with pancreatic adenocarcinoma. World J Surg. 2007;31:493–502. doi: 10.1007/s00268-006-0289-9. doi: 10.1007/s00268-006-0289-9. [DOI] [PubMed] [Google Scholar]
- 27.Liu WJ, Zhou L, Liang ZY, Zhou WX, You L, Zhang TP, et al. High expression of GRK3 is associated with favorable prognosis in pancreatic ductal adenocarcinoma. Pathol Res Pract. 2018;214:228–32. doi: 10.1016/j.prp.2017.11.013. doi: 10.1016/j.prp.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 28.McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, et al. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50:3545–50. [PubMed] [Google Scholar]
- 29.Righi L, Papotti MG, Ceppi P, Billè A, Bacillo E, Molinaro L, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010;28:1534–9. doi: 10.1200/JCO.2009.25.9275. doi: 10.1200/JCO.2009.25.9275. [DOI] [PubMed] [Google Scholar]
- 30.Ortolan E, Arisio R, Morone S, Bovino P, Lo-Buono N, Nacci G, et al. Functional role and prognostic significance of CD157 in ovarian carcinoma. J Natl Cancer Inst. 2010;102:1160–77. doi: 10.1093/jnci/djq256. doi: 10.1093/jnci/djq256. [DOI] [PubMed] [Google Scholar]
- 31.Stanaway FF, Gnjidic D, Blyth FM, Le Couteur DG, Naganathan V, Waite L, et al. How fast does the grim reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ. 2011;343:d7679. doi: 10.1136/bmj.d7679. doi: 10.1136/bmj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angenete E, Langenskiöld M, Palmgren I, Falk P, Oresland T, Ivarsson ML. UPA and PAI-1 in rectal cancer – Relationship to radiotherapy and clinical outcome. J Surg Res. 2009;153:46–53. doi: 10.1016/j.jss.2008.02.043. doi: 10.1016/j.jss.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Becker M, Szarvas T, Wittschier M, vom Dorp F, Tötsch M, Schmid KW, et al. Prognostic impact of plasminogen activator inhibitor type 1 expression in bladder cancer. Cancer. 2010;116:4502–12. doi: 10.1002/cncr.25326. doi: 10.1002/cncr.25326. [DOI] [PubMed] [Google Scholar]
- 34.Steiner E, Pollow K, Hasenclever D, Schormann W, Hermes M, Schmidt M, et al. Role of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1) for prognosis in endometrial cancer. Gynecol Oncol. 2008;108:569–76. doi: 10.1016/j.ygyno.2007.11.025. doi: 10.1016/j.ygyno.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Colin C, Voutsinos-Porche B, Nanni I, Fina F, Metellus P, Intagliata D, et al. High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol. 2009;118:745–54. doi: 10.1007/s00401-009-0592-2. doi: 10.1007/s00401-009-0592-2. [DOI] [PubMed] [Google Scholar]
- 36.Zubac DP, Wentzel-Larsen T, Seidal T, Bostad L. Type 1 plasminogen activator inhibitor (PAI-1) in clear cell renal cell carcinoma (CCRCC) and its impact on angiogenesis, progression and patient survival after radical nephrectomy. BMC Urol. 2010;10:20. doi: 10.1186/1471-2490-10-20. doi: 10.1186/1471-2490-10- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelisavac-Cosic S, Sirotkovic-Skerlev M, Kulic A, Jakic-Razumovic J, Kovac Z, Vrbanec D. Prognostic significance of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor (PAI-1) in patients with primary invasive ductal breast carcinoma – A 7.5-year follow-up study. Tumori. 2011;97:532–9. doi: 10.1177/030089161109700419. doi: 10.1700/950.10409. [DOI] [PubMed] [Google Scholar]
- 38.Horvatic Herceg G, Herceg D, Kralik M, Kulic A, Bence-Zigman Z, Tomic-Brzac H, et al. Urokinase plasminogen activator and its inhibitor type-1 as prognostic factors in differentiated thyroid carcinoma patients. Otolaryngol Head Neck Surg. 2013;149:533–40. doi: 10.1177/0194599813496374. doi: 10.1177/0194599813496374. [DOI] [PubMed] [Google Scholar]
- 39.Ostheimer C, Evers C, Bache M, Reese T, Vordermark D. Prognostic implications of the co-detection of the urokinase plasminogen activator system and osteopontin in patients with non-small-cell lung cancer undergoing radiotherapy and correlation with gross tumor volume. Strahlenther Onkol. 2018;194:539–51. doi: 10.1007/s00066-017-1255-1. doi: 10.1007/s00066-017-1255-1. [DOI] [PubMed] [Google Scholar]