To the Editor: With the advent of antibiotics application, intracranial complications of paranasal sinusitis, including meningitis, intracranial abscess, subdural empyema (SDE), epidural abscess, cavernous sinus thrombosis, and thrombosis of other dural sinuses, have become uncommon.[1] However, SDE is still a life-threatening disease entity. It is defined as a purulent collection between dura mater and arachnoid. The most common cause of SDE is sinusitis. Other causes include meningitis, otitis media, operative infection, head trauma, and bacteremic seeding of previous subdural hematoma.[1,2] Here, we present a rapidly progressive case of interhemispheric SDE following sinusitis. Clinicians should be aware of SDE when the patient presents with headache and fever, and neuroimaging manifests as a dilated subdural space especially in adolescent with sinusitis.
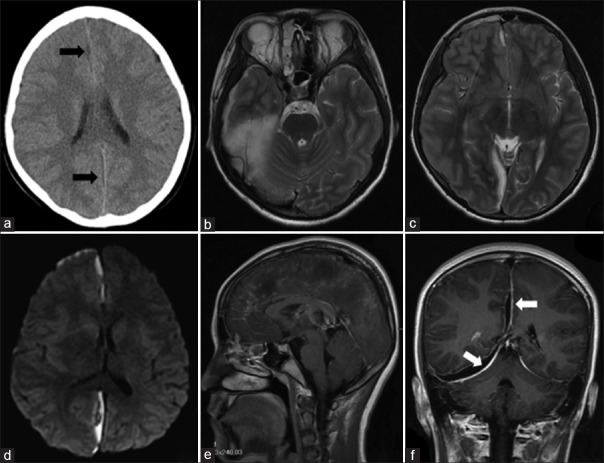
A 13-year-old female patient presented with right-sided orbital pain, high-grade fever, and drowsiness for 1 week. Five days before presentation, she experienced continuously frontal headache and intermittent nausea and vomiting. Her medical history was unremarkable other than a recent history of acute sinusitis without treatment. On admission, she was alert and oriented. Her vital signs were a temperature of 39°C, a heart rate of 76 beats/min, and blood pressure of 103/66 mmHg. Neurological examination revealed a sign of meningeal irritation and otherwise unremarkable. Serological examination yielded increased leukocytosis (12.5 × 109/L) with segmented neutrophils (90.0%), elevatesd C-reactive protein (98.47 mg/L), and accelerate erythrocyte sedimentation rate (50 mm/h). A noncontrast computed tomography (CT) of the head showed slightly dilated interhemispheric space [Figure 1]. As the imaging change was not obvious on initial CT scanning, our physician did not pay more attention to it. Cerebrospinal fluid analysis (CSF) showed elevated presure (290 mmH2O), increased protein (74 mg/dl), and polynuclear leukocytes (257 per view). No organisms could be identified in cultures of the CSF. Tuberculin and fungal tests were also negative. She was initially administrated with broad-spectrum antibiotics, 20% mannitol, and supportive care. On day 2 after admission, she developed left hemiparesis, and her consciousness level deteriorated to lethargy. Magnetic resonance imaging (MRI) of the brain demonstrated a collection of fluid along the right falx cerebri and cerebellar tentorium and shift of brain parenchyma to the left [Figure 1]. In addition, right-sided pan sinusitis was noted with opacification of maxillary, ethmoid, and frontal sinuses. These imaging findings together with clinical presentation suggested a possible diagnosis of SDE. When family members were informed to transfer to the operating room for urgent craniotomy, the patient had unequal pupils, and neurological status deteriorated to coma. Unfortunately, she was unable to receive operation and discharged according to the patient's request.
Figure 1.
The radiological findings of the patient. Axial CT showed slightly broadened subdual space (black arrows) (a). Brain MRI demonstrated a collection of empyema along right falx cerebri and cerebellar tentorium with hyperintense on T2-weighted images (b and c) and restricted diffusion (d). T1-weighted with gadolinium MRI showed enhancement connecting from ethmoidal sinus to the bottom of frontal lobe (e) as well as enhanced dura mater involving cerebral falx and bilateral tentorium of cerebellum (white arrows) (f). CT: Computed tomography; MRI: Magnetic resonance imaging.
The most common cause of SDE is sinusitis, which accounts for 15% of the total number of hospitalized patients.[3] This entity is more commonly seen in males and predominantly occurs in the second decade of life.[1] When sinusitis is the cause, the pus may directly evade the leptomeninges by eroding the bony walls of the sinuses or organism disseminate to the leptomeninges through bridging veins.[4] Once the pus invades subdural space from adjacent sinuses, it may accumulate in potential spaces and can rapidly grow to a large size resulting in mass effect on surrounding structures. In addition, the purulent material can trigger a cascade of severe inflammatory reaction within the surrounding tissues. Those above-mentioned pathophysiological mechanisms can lead to a series of neurological complications such as encephalitis, cortical vein thrombosis, vasospasm of cerebral arteries, or hydrocephalus. With increased intracranial pressure and enlarged mass effect on neighboring tissues, the most serious and life-threatening complication of brain herniation may happen eventually. Clinical presentations of SDE are sometimes difficult to differentiate from meningitis because the most common symptoms of SDE are headache, fever, and neck stiffness. Altered mental status, focal neurological deficits or cranial nerve palsies, hemiparesis, papilloedema, vomiting, and septic shock are hallmarks of clinical deterioration.[1] Radiographic imaging should be performed in all patients in whom SDE is suspected. CT imaging reveals a hypointense subdural collection, generally crescent shaped. Conventional MRI sequences have been showed to be sensitive to SDE. Subdural collection demonstrates hypointense on T1 and hyperintensity on T2 with peripheral contrast enhancement and diffusion restriction on diffusion-weighted imaging (DWI). Isolated medical treatment has not been successful to manage SDE. A broad-spectrum antibiotic against aerobic and anaerobic cocci and bacilli, such as a third-generation cephalosporin plus metronidazole, is recommended for at least 2 weeks of intravenous therapy. Surgical intervention should be performed as early as possible before clinical deterioration. There is no consensus on which is better for surgical intervention-burr hole or craniotomy. Previous publications suggested that craniotomy is superior to burr holes for SDE.[3,5] Burr-hole drainage has a high recurrence rate and may cause damage to friable hyperemic cortex from the catheter, wash solution, or antibiotics. Moreover, it is not effective when a severe mass effect and/or a thick membrane are noted on neuroimaging findings.[5] Anyhow, the determination of which procedure to do should be made in consultation with the neurosurgeon.
In our case, the clinical presentation, including headache, fever, drowsiness, and meningeal irritation, was nonspecific; and neuroimaging finding was unconspicuous on initial CT scanning. Hence, our physicians failed to assess the severity of the disease, accurately. When a second neuroimaging of the brain MRI was conducted on the next day, empyema located in interhemispheric rapidly increased to a large size within a relatively short time. Unfortunately, the most serious complication of the brain herniation was subsequently happened. This case emphasizes that when patients present with headache and fever and neuroradiological findings manifest as a broadened dura mater, a diagnosis of SDE should be taken into consideration. In addition, MRI may be more sensitive to identify empyema than a nonenhanced CT especially on DWI. On contrast-enhanced MRI, the enhancement was confined from ethmoidal sinus to the bottom of frontal lobe, which suggests the empyema stems from sinus and flows along the space of interhemispheric and infratentorial subdural.
In conclusion, interhemispheric SDE is a life-threatening complication secondary to sinusitis. The empyema may rapidly increase in size and eventually lead to brain herniation. Our physicians should maintain a high level of awareness of interhemispheric SDE, which requires immediate medical and surgical intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s)/patient's guardians has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the article. The patients/patient's guardians understand that their names and initials will not be published and due efforts will be made to conceal the identity of the patient, although anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Osborn MK, Steinberg JP. Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis. 2007;7:62–7. doi: 10.1016/S1473-3099(06)70688-0. doi: 10.1016/S1473-3099(06)70688-0. [DOI] [PubMed] [Google Scholar]
- 2.Mattogno PP, LA Rocca G, Signorelli F, Visocchi M. Intracranial subdural empyema: Diagnosis and treatment update. J Neurourg Sci. 2017 doi: 10.23736/S0390-5616.16.03825-X. [Epub ahead of print]. doi: 10.23736/S0390-5616.16.03825-X. [DOI] [PubMed] [Google Scholar]
- 3.Nathoo N, Nadvi SS, Gouws E, van Dellen JR. Craniotomy improves outcomes for cranial subdural empyemas: Computed tomography-era experience with 699 patients. Neurosurgery. 2001;49:872–7. doi: 10.1097/00006123-200110000-00017. doi: 10.1097/00006123-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Holland AA, Morriss M, Glasier PC, Stavinoha PL. Complicated subdural empyema in an adolescent. Arch Clin Neuropsychol. 2013;28:81–91. doi: 10.1093/arclin/acs104. doi: 10.1093/arclin/acs104. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Joo SP, Song DJ, Kim SH, Kim TS. Delayed intracranial subdural empyema following burr hole drainage: Case series and literature review. Medicine (Baltimore) 2018;97:e0664. doi: 10.1097/MD.0000000000010664. doi: 10.1097/MD.0000000000010664. [DOI] [PMC free article] [PubMed] [Google Scholar]