Abstract
Background
Serum alkaline phosphatase (ALP) has been proved to be a negative prognostic factor for several malignancies, but its clinical significance in gastric cancer (GC) patients has not been sufficiently studied. In the present retrospective study, we investigated the effect of serum ALP on disease-free survival (DFS) after radical gastrectomy.
Material/Methods
We included 491 GC patients receiving radical gastrectomy at the Chinese People’s Liberation Army 309th Hospital. Univariate and multivariate analyses were performed to determine factors influencing serum ALP and DFS. The changes in serum ALP and its clinical relevance were also analyzed using the log-rank test and Cox proportional hazards model.
Results
There were 491 patients who met our inclusion and exclusion criteria. Pre-treatment serum ALP was elevated in 87 of these patients and was normal in the other 404 patients. Elevation of pre-treatment serum ALP was correlated with the tumor diameter (OR=2.642, P=0.017), TNM stage (OR=4.592, P=0.005), and T classification (OR=1.746, P=0.043). DFS was significantly different between patients with normal or elevated pre-treatment serum ALP (median 42.1 vs. 32.8 months, P=0.001) and multivariate analysis suggested pre-treatment serum ALP is an independent risk factor for poor DFS after radical gastrectomy (HR=2.035, P=0.021). In addition, removal of the primary tumor lesion led to an obvious decline in serum ALP activity (median 262 U/L vs. 152 U/L, P<0.001), and monitoring changes in serum ALP can help evaluate the risk of tumor relapse in GC patients (χ2=17.814, P<0.001).
Conclusions
Serum ALP is a good predictor of GC patient DFS after radical gastrectomy, and patients with elevated serum ALP have shorter relapse times.
MeSH Keywords: Alkaline Phosphatase, Disease-Free Survival, Gastrectomy, Stomach Neoplasms
Background
Gastric cancer (GC) is one of the most common as lethal malignancies around the world [1,2]. Despite numerous recent improvements in its diagnosis and therapy, approximately 50% of all GC patients after radical gastrectomy will develop recurrence and/or metastasis within 5 years [3]. The relapse of GC is a complex issue to cope with and the re-operation opportunity is usually minimal, so once there is evidence of tumor recurrence and/or metastasis, extremely poor prognosis is expected. Nevertheless, as an evaluable lesion is absent after radical gastrectomy, it is difficult to assess the therapeutic efficacy and predict the possible outcome of patients, which are pivotal for deciding further treatment strategy.
Serum alkaline phosphatase (ALP) is a routinely tested biochemical parameter in clinical work, which can be obtained conveniently and inexpensively at any time. Elevated serum ALP is usually a signal of liver or bone involvement due to inflammatory, traumatic, or metabolic diseases, but has also been associated with the pathogenesis, progression, and poor prognosis of certain carcinomas, including several digestive system malignancies, such as hepatoma [4], gallbladder cancer [5], colon cancer [6], and esophageal cancer [7]. Accordingly, we hypothesized that serum ALP was also a prognostic predictor for GC patients, and we performed the following research to test this hypothesis.
Material and Methods
Patients
We retrospectively studied the inpatient and follow-up data of 491 initially-treated GC patients receiving radical gastrectomy from January 2008 to December 2014 in the Department of General Surgery, PLA 309th Hospital, China. Patients’ characteristics are listed below in the results section. The sample size was calculated using EmpowerStats 2.17 to provide 90% power for survival analysis, and based on the ratio of patient numbers between groups to be 4: 1 according to the literature [8]. Inclusion criteria were: (1) age >18 years, KPS score >70; (2) TNM stage I–III (AJCC, 7th); and (3) Patients did not receive any anti-tumor treatment before admission. Exclusion criteria were: (1) Patients with distant metastasis; (2) Concomitant non-tumor diseases that could lead to serum ALP elevation; (3) Intact serum ALP or other required follow-up data could not be obtained; and (4) Severe postoperative complications lasting for ≥2 weeks. Besides operation, neoadjuvant or postoperative chemotherapy was applied alternatively according to the disease status of each specific patient. This study was approved by the Ethics Committee of the Chinese People’s Liberation Army 309th Hospital, Beijing, China, and complied with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all patients enrolled in the study.
Serum ALP
Fasting venous blood was extracted in the early morning and preserved in anticoagulant tubes. The phosphoric acid p-nitrophenol performance rate method was employed for serum ALP testing by the clinical laboratory of our hospital. Blind method was absent due to the retrospective nature of this study. The serum ALP value at the following 4 time points were extracted for further data analysis (Figure 1). (1) Pre-treatment: before operation or neo-adjuvant chemotherapy; (2) Post-operation: before the first cycle of postoperative chemotherapy, and for patients did not receive chemotherapy, before they were discharged; (3) Post-treatment: 6 months after all treatment had been completed; (4) Pre-terminal: the last result that could be obtained during the follow-up period. The normal reference range of serum ALP in our hospital was 40~150 U/L, and level >150 U/L was defined as elevated serum ALP.
Figure 1.
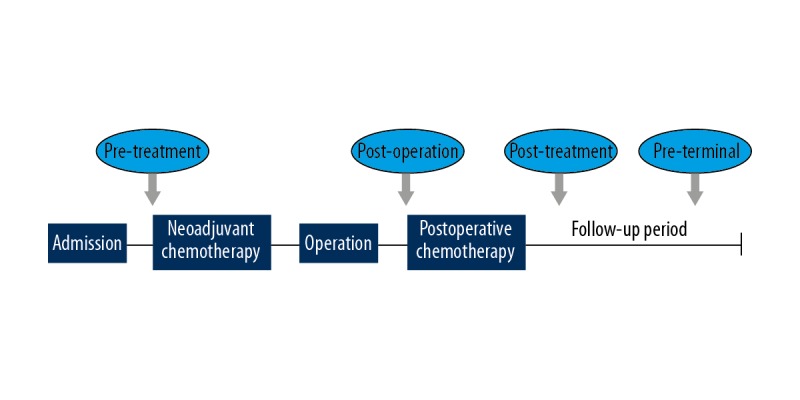
The 4 time points for extracting serum ALP data. Neoadjuvant or postoperative chemotherapy was applied alternatively according to the disease status of each specific patient.
Follow-up
Patients returned to our hospital for postoperative examination periodically. The items included routine test of blood, urine and stool, biochemistry, tumor marker, chest X-ray, ultrasonography, computed tomography, barium meal, and gastroscopy. DFS was defined as the time from operation to tumor recurrence, metastasis, or loss of follow-up. The DFS of those remaining tumor-free more than 60 months were recorded as 60 months. Patients were followed up every half year by telephone or mail. The median follow-up time was 41 months (range 13.2 to 60 months). There were 226 patients who developed recurrence or metastasis within the follow-up phase.
Statistics
Statistical analysis was performed on SPSS 13.0. Serum ALP value was used directly for data analysis without logarithmic conversion or other procedures. The alteration of serum ALP before and after the operation was processed through the Wilcoxon matched-pairs signed rank test. The association between serum ALP and patients’ clinical and pathological features was analyzed by χ2 test and logistic regression method (variable selection method: enter). The influence of serum ALP on patients’ DFS was determined using the log-rank test and Cox proportional hazards model (variable selection method: enter). Survival curves were plotted by Kaplan-Meier method. P<0.05 was considered to have statistical significance.
Results
Demographic characteristics
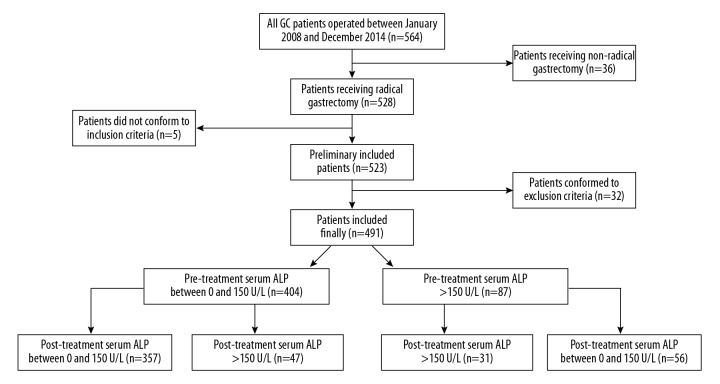
Figure 2 is a flow diagram showing the process of participant collection and analysis. There were 491 initially-treated GC patients who met our inclusion and exclusion criteria, among whom 333 were male and 158 were female; 112 were aged <60 years, 379 were aged ≥60; 57 were located in the fundus, 286 were in the corpora, and 148 were in the sinuses; 271 had a tumor diameter <3 cm, 220 had ≥3 cm; 91 were in TNM stage I, 206 were in stage II, and 194 were in stage III; 113 had a T1 classification, 206 had T2, 101 had T3, and 71 had T4; 111 had a N0 classification, 120 had N1, 144 had N2, and 116 had N3; 143 were poorly differentiated and 348 were moderately-highly differentiated; 419 had adeno carcinoma and 72 had signet ring cell carcinoma (SRCC); neoadjuvant chemotherapy was administered in 75 patients and was not administered in the other 416 patients; 410 patients received ≥4 cycles of postoperative chemotherapy and 81 received ≤3 cycles. Patients with missing values were excluded at participant enrollment.
Figure 2.
The flow diagram showing the process of participant collection and analysis.
The association between serum ALP and patients’ clinical and pathological features
The univariate χ2 test showed that tumor diameter (P=0.032), TNM stage (P=0.026), T classification (P=0.030), and N classification (P=0.020) were associated with the elevation of pre-treatment serum ALP. These above 4 factors were then subjected to multivariate logistic regression analysis and indicated that tumor diameter (P=0.017, OR=2.642), TNM stage (P=0.005, OR=4.592), and T classification (P=0.043, OR=1.746) were independent risk factors for elevated pre-treatment serum ALP (Table 1).
Table 1.
Univariate and multivariate analysis of factors influencing the pre-treatment serum ALP activity.
| Factors | Pre-treatment serum ALP | χ2-test P value | Logistic P value | OR (95%CI) | |
|---|---|---|---|---|---|
| Normal | Elevated | ||||
| Sex | 0.312 | ||||
| Male | 270 (81.1%) | 63 (18.9%) | |||
| Female | 134 (84.8%) | 24 (15.2%) | |||
| Age | 0.745 | ||||
| <60 | 91 (81.3%) | 21 (18.7%) | |||
| ≥60 | 313 (82.6%) | 66 (17.4%) | |||
| Tumor site | 0.296 | ||||
| Upper | 46 (70.7%) | 11 (19.3%) | |||
| Middle | 239 (83.7%) | 47 (16.3%) | |||
| Low | 119 (70.4%) | 29 (19.6%) | |||
| Tumor diameter | 0.032 | 0.017 | 2.642 (1.335~4.213) | ||
| <3 | 232 (85.6%) | 39 (14.4%) | |||
| ≥3 | 172 (78.2%) | 48 (21.8%) | |||
| T classification | 0.030 | 0.043 | 1.746 (1.069~2.758) | ||
| T1 | 102 (90.3%) | 11 (9.7%) | |||
| T2 | 170 (82.5%) | 36 (17.5%) | |||
| T3 | 79 (78.2%) | 22 (21.8%) | |||
| T4 | 53 (64.6%) | 18 (25.4%) | |||
| N classification | 0.020 | 0.065 | 1.878 (0.725~3.113) | ||
| N0 | 98 (88.3%) | 13 (11.7%) | |||
| N1 | 102 (85.0%) | 18 (15.0%) | |||
| N2 | 119 (82.6%) | 25 (17.4%) | |||
| N3 | 85 (73.3%) | 31 (26.7%) | |||
| TNM stage | 0.026 | 0.005 | 4.592 (2.238~7.851) | ||
| I | 82 (90.1%) | 9 (9.9%) | |||
| II | 172 (83.5%) | 34 (16.5%) | |||
| III | 150 (77.3%) | 44 (22.7%) | |||
| Differentiation | 0.930 | ||||
| Moderate and high | 286 (82.2%) | 62 (17.8%) | |||
| Poor | 118 (82.5%) | 25 (17.5%) | |||
| Pathological type | 0.156 | ||||
| Adenocarcinoma | 349 (83.3%) | 70 (16.7%) | |||
| Signet cell carcinoma | 55 (76.4%) | 17 (23.6%) | |||
The influence of pre-treatment serum ALP on GC patients’ DFS
Patients were stratified into 2 groups according to pre-treatment serum ALP, and Kaplan-Meier method was used to plot survival curves, which indicated that the DFS of 2 groups were significantly different (P=0.001, Figure 3). The univariate log-rank test showed that pre-treatment serum ALP (P=0.001), tumor diameter (P=0.025), TNM stage (P=0.027), T classification (P<0.001), N classification (P=0.003), differentiation (P=0.016), pathological type (P<0.001), and neoadjuvant chemotherapy (P=0.043) were associated with patients’ DFS. The above factors were subsequently included in the multivariate Cox proportional risk model, which indicated that pre-treatment serum ALP (P=0.021, HR=2.035), T classification (P=0.030, HR=1.615), TNM stage (P=0.017, HR=3.746), differentiation (P=0.008, HR=4.192), and pathological type (P<0.001, HR=6.804) were independent risk factors for poor DFS of GC patients (Table 2).
Figure 3.
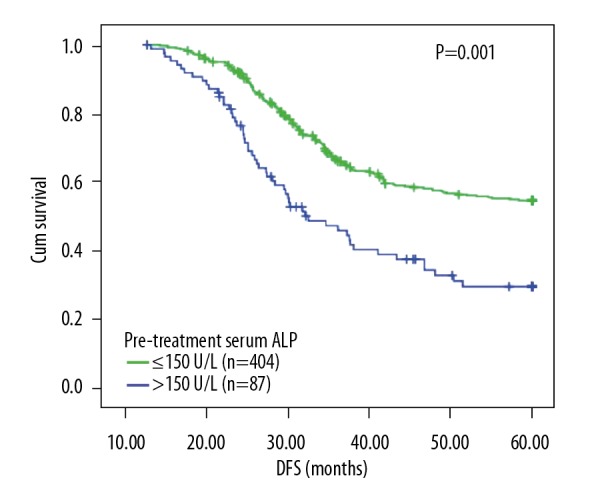
The survival curves of patients with normal or elevated pre-treatment serum ALP activity. Log-rank test shows that there was a statistically significant difference between the 2 groups with regard to patients’ DFS (P=0.001).
Table 2.
Univariate and multivariate analysis of factors influencing patients’ DFS.
| Factors | Median DFS (months) | Log-rank P value | Cox P value | HR (95%CI) |
|---|---|---|---|---|
| Sex | 0.636 | |||
| Male | 38.3 | |||
| Female | 36.7 | |||
| Age | 0.328 | |||
| <60 | 37.5 | |||
| ≥60 | 35.4 | |||
| Pre-treatment serum ALP | 0.001 | 0.021 | 2.035 (1.264~2.927) | |
| Normal | 42.1 | |||
| Elevated | 32.8 | |||
| Tumor site | 0.857 | |||
| Upper | 37.7 | |||
| Middle | 36.9 | |||
| Low | 38.2 | |||
| Tmuor diameter | 0.025 | 0.146 | 1.172 (0. 718~1.684) | |
| <3 | 39.6 | |||
| ≥3 | 34.3 | |||
| T classification | <0.001 | 0.030 | 1.615 (1.048~2.579) | |
| T1 | 48.7 | |||
| T2 | 41.4 | |||
| T3 | 33.8 | |||
| T4 | 31.5 | |||
| N classification | 0.003 | 0.086 | 1.368 (0.932~1.827) | |
| N0 | 47.9 | |||
| N1 | 42.6 | |||
| N2 | 36.2 | |||
| N3 | 30.3 | |||
| TNM stage | 0.027 | 0.017 | 3.746 (1.622~6.854) | |
| I | 48.4 | |||
| II | 39.7 | |||
| III | 32.5 | |||
| Differentiation | 0.016 | 0.008 | 4.192 (2.318~6.536) | |
| Moderate and high | 44.8 | |||
| Poor | 29.1 | |||
| Pathological type | <0.001 | <0.001 | 6.804 (3.573~10.495) | |
| Adenocarcinoma | 38.6 | |||
| Signet cell carcinoma | 25.2 | |||
| Neoadjuvant chemotherapy | 0.043 | 0.794 | 0.815 (0.572~1.358) | |
| Yes | 35.4 | |||
| No | 39.1 | |||
| Postoperative chemotherapy | 0.520 | |||
| ≤3 cycles | 37.3 | |||
| ≥4 cycles | 38.5 |
Changes in serum ALP level before and after gastrectomy
We analyzed the changes in serum ALP level in the 87 patients with elevated pre-treatment serum ALP. The medians of pre-treatment and post-operation serum ALP activity were 262 U/L (range, 166 U/L to 1132 U/L) and 152 U/L (range, 72U/L to 437U/L) respectively, which showed a significant decline after surgery (P<0.001, Figure 4), indicating that removal of the primary tumor lesion leads to a decrease in serum ALP level.
Figure 4.
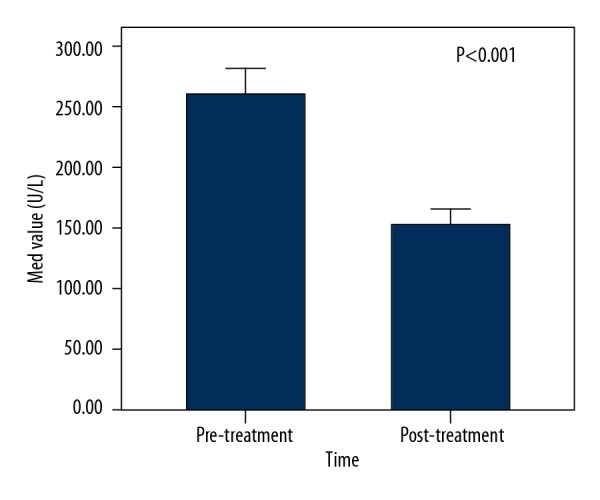
Comparison of the serum ALP activity before and after radical gastrectomy in the 87 patients with elevated pre-treatment serum ALP activity. The median of pre-treatment serum ALP activity was 262 U/L, which declined significantly to 152 U/L postopertaively (P<0.001).
The influence of serum ALP alteration on the DFS of GC patients
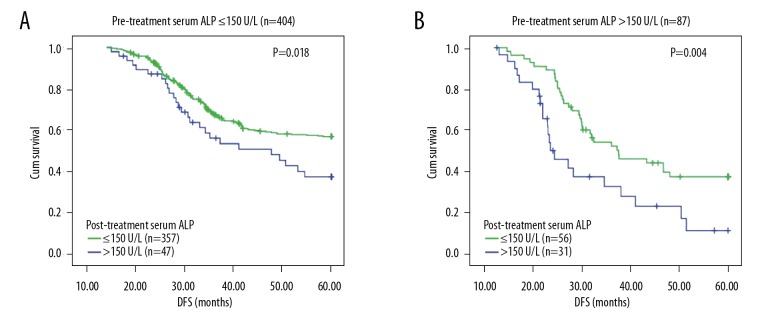
Patients were stratified into 2 groups according to pre-treatment serum ALP: group 1 contained 404 patients with a normal pre-treatment serum ALP, among whom the 47 patients with an elevated post-treatment serum ALP displayed significant poorer DFS than the other 357 patients keeping a normal post-treatment serum ALP (P=0.018, Figure 5A); group 2 contained 87 patients with an elevated pre-treatment serum ALP, among whom the 56 patients with a normal post-treatment serum ALP displayed significant better DFS than the other 31 patients keeping an elevated post-treatment serum ALP (P=0.004, Figure 5B).
Figure 5.
Alteration of serum ALP activity can predict the different prognoses of GC patients. (A) Among the 404 patients who had a pre-treatment serum ALP activity ≤150 U/L, 47 experienced an elevation of serum ALP after treatment and their DFS were significantly inferior than other 357 patients whose serum ALP activity were still ≤150 U/L, P=0.018. (B) Among the 87 patients who had a pre-treatment serum ALP activity >150 U/L, 56 experienced a decline of serum ALP after treatment and their DFS were superior than other 31 patients whose serum ALP activity were still >150 U/L, P=0.004.
The association between tumor recurrence/metastasis and serum ALP
Tumor recurrence/metastasis was observed in 226 patients during follow-up period, among whom the pre-terminal serum ALP activity of 31 patients was more than 150 U/L; the other 221 patients remained tumor-free during follow-up period, among whom the pre-terminal serum ALP activity of 6 patients was more than 150 U/L. The incidence of pre-terminal serum ALP elevation was significantly different between the 2 groups (χ2=17.814, P<0.001), indicating that serum ALP elevation may be an early indicator of tumor recurrence/metastasis.
Discussion
ALP catalyzes the dephosphorylation process of multiple molecule including nucleic acids, proteins, and alkaloids [5,9]. In humans, ALP is mainly located in the liver, bone, small intestine, and placenta. Under physiological conditions, serum ALP primarily comes from the liver, and in some specific populations it comes from bone or placenta (e.g., serum ALP activity of juveniles is significantly higher than that of adults because of skeletal growth, and it is also obviously elevated in pregnant women due to the increased production by placenta).
The primary or metastatic lesion in liver and/or bone is the most common cause of cancer-related serum ALP level elevation [10–12], but there have also been reports that tumor cells can generate and release ALP into blood directly. Chuang et al. [13] observed that serum ALP was elevated in 21.1% of renal cancer patients without liver or bone metastasis, indicating the possibility of aberrant ALP expression in some tumors. Previous studies have demonstrated that the ALP level in normal gastric mucosa is very low, but rises significantly in some GC cell lines and tumor tissues [8,14–16], and its expression in tumor tissues increases with tumor progression [17]. Consistent with these reports, we found that the tumor diameter and T classification, which can be viewed as representative of tumor progression, were independent risk factors for serum ALP elevation. We also found that, in patients with elevated pre-treatment serum ALP, the ALP value showed an obvious decrease after surgery, showing that the tumor itself is the origin of the elevated serum ALP in GC patients.
As verified by previous studies, serum ALP can provide survival information for patients with certain malignancies. Similarly, its prognostic value was also established in GC patients by the present study, which demonstrated that the DFS of patients with elevated pre-treatment serum ALP was significantly inferior compared with patients with normal pre-treatment serum ALP, and multivariate analysis showed that pre-treatment serum ALP was an independent factor influencing patients’ DFS. However, previous studies did not track the dynamic changes in serum ALP during patient follow-up phase so as to analyze their clinical significance. In the present study, we set 4 time points to monitor changes in serum ALP levels and found that in patients with elevated pre-treatment serum ALP, the normalization of post-treatment serum ALP was associated with a better DFS, while in patients with normal pre-treatment serum ALP, the elevation of post-treatment serum ALP was associated with a worse DFS. Furthermore, elevation of pre-terminal serum ALP was observed in 13.7% of tumor-recurrent patients, while in patients remaining tumor-free, it was only 2.7%. These findings indicate that besides the initial serum ALP level, its alteration thereafter has equal importance for evaluating GC patients’ prognosis.
Studies have proposed several possible mechanisms for the clinical relevance of serum ALP in tumor patients. First, patients with elevated serum ALP are considered to have higher tumor burden and heterogeneity, which agrees with our present results [7,9,18]. Second, elevated serum ALP seems to be associated with poor response to anti-tumor therapy. Maisano et al. retrospectively analyzed the data of 103 metastatic colorectal cancer (CRC) patients; 32 of them had a serum ALP activity ≥300 U/L (observation group), among whom there were only 3 cases (9.4%) of partial remission, while in the control group there were 3 complete remissions and 24 partial remission (41.5%, 27/65) [19]. Third, in a recent study published in 2017, tumor-derived ALP has been found to play pivotal roles in promoting the survival of prostate cancer cells and to participate in the processes of epithelial-to-mesenchymal transition (EMT) and bone metastasis [20], but whether it is the same in GC is currently unclear. Guo et al. discovered that aloe emodin can inhibit the proliferation and interrupt the cell cycle of MGC-803 cells, as well as decrease the activity of ALP [21], but they did not explore whether the former 2 effects were to some extent mediated by ALP suppression, so the exact significance of ALP expression in GC requires further investigation. Finally, the substrates of ALP are numerous, and some of their products theoretically may also participate in the development of cancer (e.g., adenosine (ADO) is a potent immunosuppressive and pro-tumor factor converted from adenosine triphosphate (ATP) by a series of enzymes including ALP [22]). Thus, future research should focus on these aspects at the molecular or cell level to elucidate the exact biological function ALP exerts in GC, as well as the underlying mechanisms.
Conclusions
Serum ALP is a valuable predictor of patients’ DFS after radical gastrectomy. Patients with elevated serum ALP have shorter relapse time, and may accordingly need more aggressive therapy [23,24], while in patients whose serum ALP increases or remains elevated after treatment, adjustment of the therapeutic strategy may be essential [13]. However, as this was a retrospective study, prospective studies are needed to verify our results.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Pisarska M, Pędziwiatr M, Major P, et al. Laparoscopic gastrectomy with enhanced recovery after surgery protocol: single-center experience. Med Sci Monit. 2017;23:1421–27. doi: 10.12659/MSM.898848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koç O, Şahiner İT, Ekiz F. a new method in laparoscopic sleeve gastrectomy: Reverse trendelenburg with right lateral tilt position prior to trocar entry. Med Sci Monit. 2017;23:4513–17. doi: 10.12659/MSM.906737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Wang R, Chen J, et al. miR-340 inhibits proliferation and induces apoptosis in gastric cancer cell line SGC-7901, possibly via the AKT pathway. Med Sci Monit. 2017;23:71–77. doi: 10.12659/MSM.898449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu XS, Wan Y, Song SD, et al. Model based on γ-glutamyltransferase and alkaline phosphatase for hepatocellular carcinoma prognosis. World J Gastroenterol. 2014;20(31):10944–52. doi: 10.3748/wjg.v20.i31.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu XS, Miao RC, Zhang LQ, et al. Model based on alkaline phosphatase and gamma- glutamyltransferase for gallbladder cancer prognosis. Asian Pac J Cancer Prev. 2015;16(15):6255–59. doi: 10.7314/apjcp.2015.16.15.6255. [DOI] [PubMed] [Google Scholar]
- 6.Aabo K, Pedersen H, Kjaer M. Carcinoembryonic antigen (CEA) and alkaline phosphatase in progressive colorectal cancer with special reference to patient survival. Eur J Cancer Clin Oncol. 1986;22(2):211–17. doi: 10.1016/0277-5379(86)90033-7. [DOI] [PubMed] [Google Scholar]
- 7.Wei XL, Zhang DS, He MM, et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37(2):1879–87. doi: 10.1007/s13277-015-3851-y. [DOI] [PubMed] [Google Scholar]
- 8.Su YH, Yang GL, Dong YM. [Light and electron microscopic histochemical studies of alkaline phosphatase (ALP) isoenzymes in gastric cancer]. Zhonghua Bing Li Xue Za Zhi. 1994;23(6):338–40. [in Chinese] [PubMed] [Google Scholar]
- 9.Jin Y, Yuan MQ, Chen JQ, et al. Serum alkaline phosphatase predicts survival outcomes in patients with skeletal metastatic nasopharyngeal carcinoma. Clinics (Sao Paulo) 2015;70(4):264–72. doi: 10.6061/clinics/2015(04)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Han KL, Wang ZY, et al. Value of C-telopeptide-cross-linked Type I collagen, osteocalcin, bone-specific alkaline phosphatase and procollagen Type I N-terminal propeptide in the diagnosis and prognosis of bone metastasis in patients with malignant tumors. Med Sci Monit. 2011;17:CR626–33. doi: 10.12659/MSM.882047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Yong B, Luo C, et al. High serum alkaline phosphatase cooperating with MMP-9 predicts metastasis and poor prognosis in patients with primary osteosarcoma in Southern China. World J Surg Oncol. 2012;10:37. doi: 10.1186/1477-7819-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han KS, Hong SJ. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J Cancer Res Clin Oncol. 2014;140(10):1769–76. doi: 10.1007/s00432-014-1710-7. [DOI] [PubMed] [Google Scholar]
- 13.Chuang YC, Lin AT, Chen KK, et al. Paraneoplastic elevation of serum alkaline phosphatase in renal cell carcinoma incidence and implication on prognosis. J Urol. 1997;158(5):1684–87. doi: 10.1016/s0022-5347(01)64095-3. [DOI] [PubMed] [Google Scholar]
- 14.Tokumitsu SI, Tokumitsu K, Kohnoe K, et al. Characterization of liver-type alkaline phosphatase from human gastric carcinoma cells (KMK-2) in vitro. Cancer Res. 1979;39(11):4732–38. [PubMed] [Google Scholar]
- 15.Aizawa K, Motoyama T, Watanabe H. Placental alkaline phosphatase-like isoenzymes produced by human gastric cancer cells. Acta Pathol Jpn. 1989;39(10):630–37. doi: 10.1111/j.1440-1827.1989.tb02409.x. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Tokuyama H, Ohta H, et al. Expression of placental alkaline phosphatase in gastric and colorectal cancers. An immunohistochemical study using the prepared monoclonal antibody. Cancer. 1990;66(12):2575–82. doi: 10.1002/1097-0142(19901215)66:12<2575::aid-cncr2820661221>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura W, Inada K, Hirano K, et al. Increased expression of sucrase and intestinal-type alkaline phosphatase in human gastric carcinomas with progression. Jpn J Cancer Res. 1998;89(2):186–91. doi: 10.1111/j.1349-7006.1998.tb00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Cai XY, Cai YC, et al. To build a prognositc score model containing indispensible tumour markers for metastatic nasopharyngeal carcinoma in an epidemic area. Eur J Cancer. 2012;48(6):882–88. doi: 10.1016/j.ejca.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Maisano R, Azzarello D, Del Medico P, et al. Alkaline phosphatase levels as a prognostic factor in metastatic colorectal cancer treated with the FOLFOX 4 regimen: a monoinstitutional retrospective study. Tumori. 2011;97(1):39–42. doi: 10.1177/030089161109700108. [DOI] [PubMed] [Google Scholar]
- 20.Rao SR, Snaith AE, Marino D, et al. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br J Cancer. 2017;116(2):227–36. doi: 10.1038/bjc.2016.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Xiao B, Zhang S, et al. Growth inhibitory effects of gastric cancer cells with an increase in S phase and alkaline phosphatase activity repression by aloe-emodin. Cancer Biol Ther. 2007;6(1):85–88. doi: 10.4161/cbt.6.1.3553. [DOI] [PubMed] [Google Scholar]
- 22.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16(3):177–92. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 23.Bacci G, Picci P, Ferrari S, et al. Prognostic significance of serum alkaline phosphatase measurements in patients with osteosarcoma treated with adjuvant or neoadjuvant chemotherapy. Cancer. 1993;71(4):1224–30. doi: 10.1002/1097-0142(19930215)71:4<1224::aid-cncr2820710409>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum alkaline phosphatase in osteosarcoma of the extremity treated with neoadjuvant chemotherapy recent experience at Rizzoli Institute. Oncol Rep. 2002;9(1):171–75. [PubMed] [Google Scholar]