Abstract
Background
Diabetic nephropathy was one of the most serious and harmful diabetic complications, characterized by progressive loss of renal function and renal fibrosis. Aerobic exercise training is an important non-pharmacologic method to prevent and treat diabetes mellitus and diabetic complications.
Material/Methods
Intraperitoneal (i.p.) injection of streptozocin (STZ) was used to construct a type 1 diabetic mouse model. Renal function and mitochondrial function were measured by urinary protein level, Masson staining and ATP, superoxide production, and membrane potential, respectively. The purpose of the research was to explore the effect of aerobic exercise training on renal and renal mitochondrial function, as well as the expression of Sirt1and PGC1α in type-1 diabetic mice.
Results
Sedentary diabetic mice exhibited increased urinary protein level, blood glucose, and collagen deposition in renal tissues compared with sedentary control mice, which were significantly mitigated by aerobic exercise training. Diabetic mice displayed renal tissue mitochondrial dysfunction (decreased mitochondrial ATP production and membrane potential), as well as increased mitochondrial superoxide production, which were reversed by aerobic exercise. By using Western blot analysis, we identified the decreased expression of Sirt1 and PGC1α in the renal tissue of diabetic mice, which were partly reversed by aerobic exercise training. Data showed that silencing of Sirt1 abrogated the beneficial effect of aerobic exercise training against diabetes-induced mitochondrial abnormalities and renal damage in mice.
Conclusions
Aerobic exercise training alleviates diabetes-induced renal injury by improving mitochondrial function.
MeSH Keywords: Diabetic Nephropathies; Exercise; Mitochondrial Diseases; PPAR gamma; Renal Insufficiency, Chronic; Sirtuin 1
Background
Diabetic nephropathy has become the main cause of end-stage renal disease (ESRD) worldwide and is one of the most serious and harmful chronic complications of diabetes mellitus, featuring progressive renal dysfunction and renal fibrosis and leading to a dramatic increase in morbidity and mortality in both type1 and type 2 diabetic patients [1].
In diabetic patients, persistent hyperglycemia induces increased generation of reactive oxygen species (ROS) and oxidative stress, which may mediate alteration of multiple cellular pathways and ultimately lead to the pathogenesis of diabetic nephropathy [2]. Mitochondrial electron transport is considered to be the main source of ROS in the kidneys. Hyperglycemia leads to vast production of detrimental mitochondrial ROS, which provokes oxidative damage in mitochondria and then results in structural and functional changes in mitochondria, such as impaired mitochondrial respiratory function and membrane potential, increased mitochondrial apoptosis, and posttranslational modification of mitochondrial proteins in diabetic kidneys [3–6]. The resulting mitochondrial dysfunction contributes to diabetic renal malfunction.
Among many genes influencing mitochondrial function, studies have emphasized the essential role of Sirt1 and PGC1α. Sirt1-PGC1α is a major regulator of mitochondrial function, and Sirt1 and PGC1α expression disorder-mediated mitochondrial oxidative stress plays a critical role in numerous diseases [7,8].
It is generally accepted that exercise training is an important non-pharmacologic method to prevent and treat diabetes mellitus and diabetic complications. Regular physical exercise training has proved to prevent diabetes mellitus development, perhaps through its antioxidant and anti-inflammatory properties. Moreover, exercise training can alleviate diabetic renal injury through reducing renal oxidative stress and inflammation [9]. However, whether aerobic exercise training alleviates diabetic renal injury through alteration mitochondrial function remains unknown.
In our study, we explored the effect of aerobic exercise training on diabetes-induced renal injury in a mouse model and examined the mitochondrial function and the change of Sirt1 and PGC1α in kidney tissues to determine whether regulation of mitochondrial function is involved in the beneficial effect of aerobic exercise training against diabetic renal injury.
Material and Methods
Animals
Male 8-week-old C57BL/6 mice (18–20 g) were obtained from Shanghai Sippr-BK Laboratory Animal Co. (Shanghai, China). Animals were raised in a comfortable environment with 23±1°C and a natural 12 h/12 h light/dark cycle (lights on at 8: 00; lights off at 20: 00), and were supplied with water and standard diet. Animal protocols were approved by the Ethics Committee on Experimental Animals of the Second Military Medical University.
Construction of the type 1 diabetic mouse model
The type 1 diabetic mouse model was constructed by administration of STZ (100 mg/kg, i.p.) in citrate buffer (pH 4.5), for 2 consecutive days, after fasting for 12 h. The diabetic condition was measured 1 week after a second STZ injection and the model was regarded as being successfully established if the blood glucose level was above 16.7 mmol/L. Control or type1 diabetic mice were subjected to sedentary activity or regular treadmill exercise conditions after 4 weeks.
Aerobic training protocol
As shown in Figure 1, in the first experiment, animals were assigned to 4 random groups: Sedentary control mice were sedentary (n=7), aerobic exercise training control mice underwent treadmill exercise (n=7), sedentary diabetic mice were sedentary (n=7), and aerobic exercise training diabetic mice underwent treadmill exercise. The aerobic exercise training group mice underwent 7 weeks of regular treadmill exercise (6 days per week). The first week was an adaptative phase with mild exercise for 30 min (4, 5, 6, 8, 10, and 12 m/min, no slope, 5 min/stage). Then, mice ran for 1 h/day (12 m/min) during the following 6 weeks without exhaustion [10].
Figure 1.
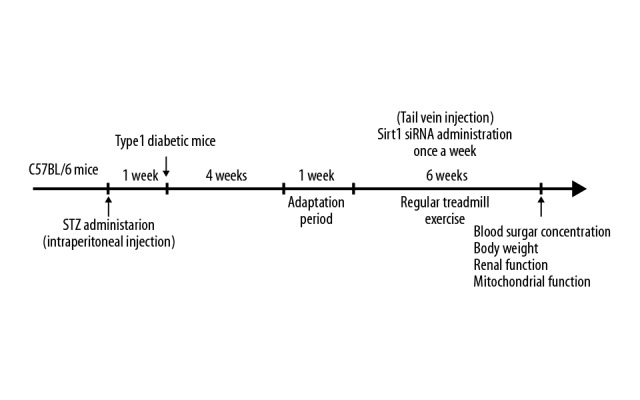
Schematic diagram of the experiment. Mice were subjected to STZ to construct a type1 diabetic mouse model. One week later, blood glucose levels were assessed, and then the mice were subjected to sedentary conditions or aerobic exercise training.
The second experiment explored whether Sirt1 siRNA could eliminate the protective impact of aerobic exercise training on the kidneys. Control and type 1 diabetic mice were randomly divided into 6 groups: sedentary control mice who received only vehicle (n=7), sedentary diabetic control mice who received only vehicle (n=7), aerobic exercise training diabetic mice who received only vehicle (n=7), sedentary control mice who received Sirt1 siRNA (n=7), sedentary diabetic mice who received Sirt1 siRNA (n=7), and aerobic exercise training diabetic mice who received Sirt1 siRNA (n=7).
Sirt1 siRNA treatment
For the Sirt1-siRNA-treated group, 50 ug of Sirt1 siRNA was dissolved in a 100-μl mixture of equal in vivo-jetPEI™ and 10% glucose injected into the tail vein once a week for 6 weeks after adjustment to exercise. The Control-siRNA-treated group received same volume of control siRNA. Design and synthetization of siRNAs were performed by GenePharma Corporation (Shanghai, China). The Sirt1 siRNA sequence was: 5′-GAUGAAGUUGACCUCCUCATT-3′ (sense), 5′-UGAGGAGGUCAACUUCAUCTT-3′ (antisense); negative control siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense), and 5′-ACGUGACACGUUCGGAGAATT-3′ (antisense) [11].
Mitochondria isolation of renal tissue
The Tissue Mitochondria Isolation Kit (Beyotime) was used to isolate renal mitochondria. Briefly, renal tissue was removed and washed once in chilled PBS. Tissue was cut into pieces and placed into mitochondrial isolation reagent A for homogenates. The homogenate composition was centrifuged at 600 g for 5 min and the supernatant was subjected to centrifugation at 11 000 g for 10 min. The remaining sediment consisted of mitochondria and was suspended in storage buffer.
Measurement of superoxide production
MitoSOX™ Red Mitochondrial Superoxide Indicator (Thermo Fisher) is a novel fluorescent dye that specifically targets mitochondria and can be rapidly oxidized by superoxide rather than other reactive oxygen species (ROS), and then becomes fluorescent. MitoSOX™ was dissolved in DMSO, forming 5 mM of solution and was diluted into 5 uM of working buffer with HBSS/Ca/Mg, and then applied to isolated renal tissue mitochondria. The mixed liquor was incubated for 10 min at 37°C in the dark and then detected at 510/580 nm (excitation/emission light) by a Synergy TM fluorescence plate reader (Bio-Tek Instruments).
Measurement of membrane potential in mitochondria
JC-1 is a fluorescent probe (Beyotime) that can detect changes in membrane potential in mitochondria. Red fluorescence is generated by JC-1 polymer in mitochondrial matrix when mitochondria have high membrane potential. In contrast, green fluorescence is generated by JC-1 monomer outside of the matrix when mitochondria have low membrane potential. The ratio of red to green fluorescence was assessed to indicate the mitochondrial depolarization state. Loading was performed by incubating isolated renal mitochondria (2 μM/20 min), after which red and green fluorescent signals were detected at 490/530 nm and 488/530 nm excitation/emission, respectively, by a multimode reader.
Assessment of mitochondrial ATP production
Measurement of renal tissue ATP concentration was performed using the ATP Assay Kit (Beyotime). Briefly, renal tissues were homogenized in ATP Detection Lysis Buffer and then subjected to centrifugation at 12 000 g for 5 min and the supernatant was used to measure ATP concentration by luminometer (Tecan).
Masson staining
The fixed renal tissues were dehydrated in graded alcohol and then embedded in paraffin. Paraffin-embedded sections (5-μm) were subjected to Masson staining to measure the level of collagen deposition.
Detection of urine protein
Mice were put in metabolic cages to collect urine for 24 h. The urinary protein level was assessed using the BCA assay kit and the level of proteinuria was measured as the ratio of urinary protein to serum creatinine.
Western blot analysis
Mouse renal tissue protein was lysed with chilled RIPA (Beyotime) with 1% Protease Inhibitor Cocktail (Sigma-Aldrich). We used 10% SDS-PAGE to separate protein, which was then transferred to NC membranes. The membrane was blocked with 5% skim milk powder in Tris-buffered saline containing 0.05% Tween 20 (TBST), then incubated with primary antibodies against PGC1α (Abcam), Sirt1 (Santa Cruz), or GAPDH (Santa Cruz), then incubated with secondary antibodies in milk. Chemiluminescence was performed with a Western blotting detection system (Millipore). The chemiluminescent signal from the membranes was quantified by a GeneGnome HR scanner using GeneTools software (SynGene).
Statistical analysis
Data are presented as means ±SEM. When comparing multiple groups, one-way ANOVA was performed and, when significant (p<0.05), comparisons between groups were conducted using the Student-Newman-Keuls test. Data analyses were performed with SPSS 13.0. P<0.05 was set as the level of statistical significance.
Results
Aerobic exercise training mitigates diabetes-induced renal injury in STZ-induced type1 diabetic mice
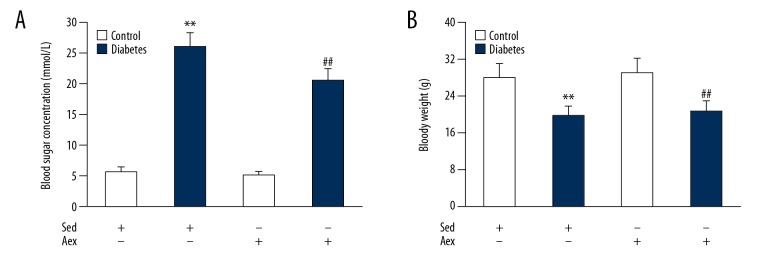
We first examined the body weight and blood sugar concentration of STZ-induced type 1 diabetic mice. As shown in Figure 2, type 1 diabetic mice exhibited significant loss of body weight and increased blood sugar concentration compared with the sedentary control. Aerobic exercise training led to a sharp decrease in blood sugar concentration in the diabetic mice but had no influence on body weight.
Figure 2.
Impact of aerobic exercise training on blood glucose concentration and body weight in lean and diabetic mice. The blood glucose concentration (A) and body weight (B) were measured in control and diabetic mice in Aex or Sed. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes.
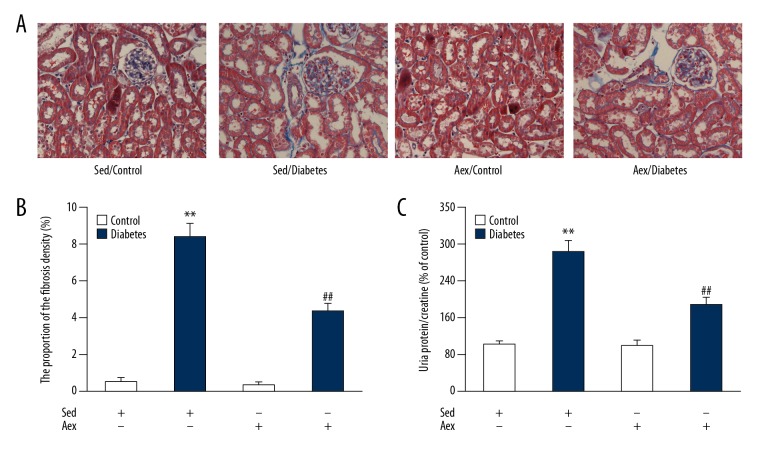
To assess the impact of aerobic exercise training on diabetes-induced renal injury, we assessed the level of proteinuria and Masson’s trichrome staining was performed. As shown in Figure 3, levels of proteinuria and collagen deposition in renal tissue were notably increased in diabetic mice compared to sedentary control mice, and this was dramatically reversed by aerobic exercise training, indicating that aerobic exercise training mitigates diabetes-induced renal injury.
Figure 3.
(A) Effect of aerobic exercise training on renal fibrosis and urinary protein level in lean and diabetic mice. The proportion of the fibrosis density (%) (B) and the level of urinary protein/creatine (C) were measured in control and diabetic mice in Aex or Sed. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes.
Aerobic exercise training attenuates diabetes-induced renal mitochondrial dysfunction
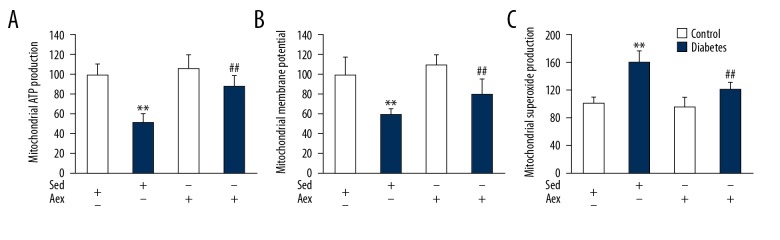
Previous studies indicated that mitochondrial dysfunction takes part in the progression of diabetic nephropathy [12]; therefore, we measured the impact of aerobic exercise training on diabetes-induced renal mitochondrial dysfunctions, including mitochondrial ATP production, membrane potential, and superoxide production. As displayed in Figure 4, diabetic mice renal mitochondria had higher mitochondrial superoxide production but lower ATP production and membrane potential when compared with lean control mice. Renal mitochondria from diabetic mice subjected to aerobic exercise training had significantly decreased mitochondrial superoxide production, as well as increased mitochondrial membrane potential and ATP production, when compared with lean diabetic mice, indicating that aerobic exercise training mitigates diabetes-induced renal mitochondrial dysfunction.
Figure 4.
Effect of aerobic exercise training on mitochondrial function in sedentary and diabetic mice kidney. The ATP production (A), membrane potential (B), and superoxide production (C) in mitochondria were measured in control and diabetic mice in Aex or Sed. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; # P<0.05, ## P<0.01 vs. Sed-Diabetes.
Aerobic exercise training protects against diabetes-associated mitochondrial dysfunction in kidney via Sirt1
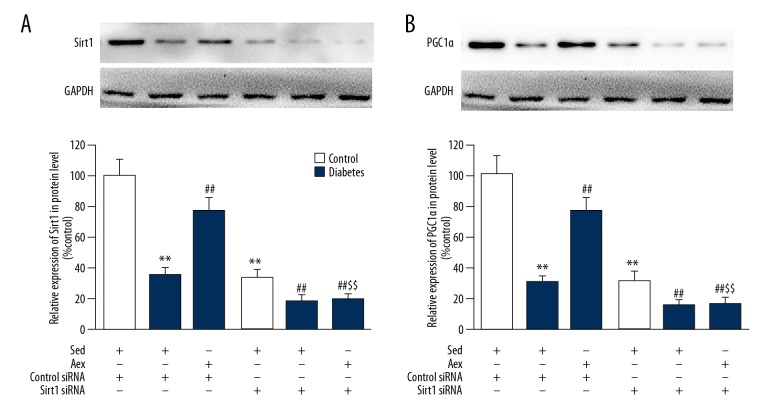
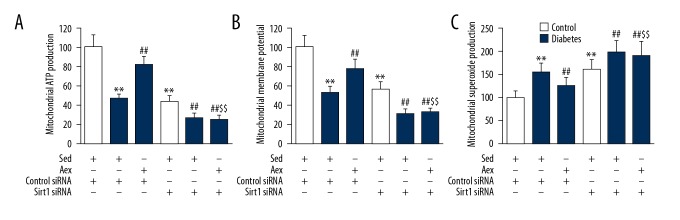
A number of researchers have demonstrated that Sirt1 and PGC1α exerts vital action on mitochondrial function. As displayed in Figure 5, protein levels of Sirt1 and PGC1α were dramatically reduced in diabetic kidneys, which was reversed by aerobic exercise training. To examine the function of Sirt1 in renal injury, siRNA was used to knockdown Sirt1 expression in the kidneys, showing that Sirt1 siRNA led to an appropriately 80% reduction in renal Sirt1 expression. Furthermore, Sirt1 siRNA blocked aerobic exercise training-induced upregulation of diabetic renal Sirt1 expression. Notably, it was found that aerobic exercise training-induced PGC1α expression in diabetic kidneys was dramatically suppressed by Sirt1 siRNA. As displayed in Figure 6, Sirt1 siRNA blocked the beneficial action of aerobic exercise training against diabetes-induced mitochondrial impairment, proving the increased superoxide generation in mitochondria, distinctly decreased ATP generation, and mitochondrial membrane potential in diabetic kidneys.
Figure 5.
Silencing of Sirt1 mitigated the positive effect of aerobic exercise training on diabetes-associated lower expression of Sirt1 and PGC1α in diabetic kidneys. Western blot analysis was used to measure expression of Sirt1 (A) and PGC1α (B) in protein level. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes; $$ P<0.01 vs. Aex-Diabetes.
Figure 6.
Silencing of Sirt1 mitigates the beneficial impact of aerobic exercise training on diabetic mitochondrial dysfunction in diabetic kidney. The mitochondrial function was measured by mitochondrial ATP generation (A), membrane potential (B), and superoxide production (C) in mitochondria. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes; $$ P<0.01 vs. Aex-Diabetes.
Aerobic exercise training against diabetes-associated renal injury in kidneys via Sirt1
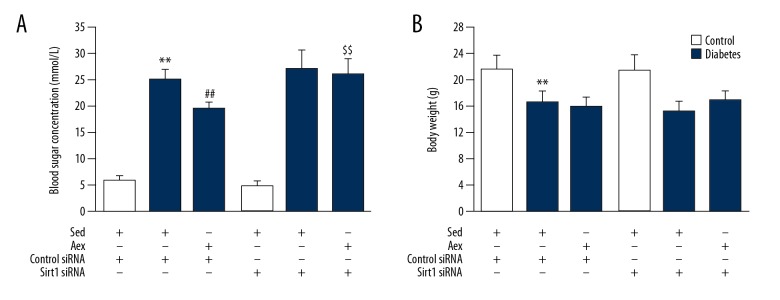
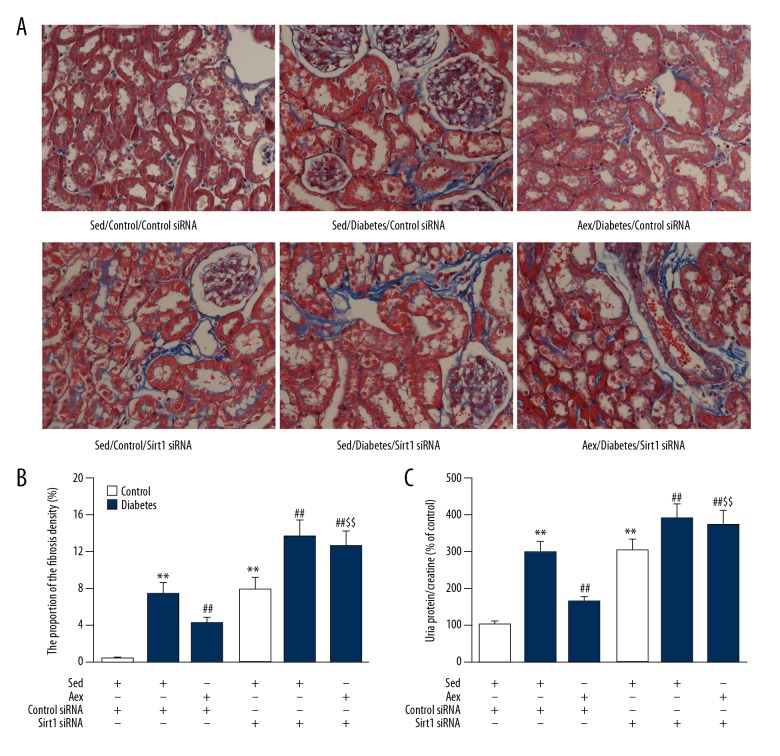
As displayed in Figure 7, Sirt1 siRNA also blocked the beneficial action of aerobic exercise training against diabetes-induced renal injury, proving the increased level of proteinuria and collagen deposition in kidneys. Notably, as displayed in Figure 8, Sirt1 siRNA blocked the decreased blood glucose concentration induced by aerobic exercise training; however, it had no influence on animal body weight.
Figure 7.
Effect of Silencing of Sirt1 on blood sugar concentration and body weight in sed-control, sed-diabetes, and aex-diabetes mice. The blood glucose concentration (A) and body weight (B) were measured in sed-control, sed-diabetes, and aex-diabetes mice in control siRNA or Sirt1 siRNA conditions. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes; $$ P<0.01 vs. Aex-Diabetes.
Figure 8.
(A) Silencing of Sirt1 mitigated the beneficial impact of aerobic exercise training against diabetes-associated renal damage in diabetic mice. The proportion of the fibrosis density (%) (B) and the level of urinary protein/creatine (C) were measured in sed-control, sed-diabetes, and aex-diabetes mice in control siRNA or Sirt1 siRNA. Bar charts represent means ±SEM (n=7). ** P<0.01 vs. Sed-Control; ## P<0.01 vs. Sed-Diabetes; $$ P<0.01 vs. Aex-Diabetes.
Discussion
It is well known that exercise training exerts renal-protective effects in numerous animal models, including hypertension, chronic renal failure, ischemia-reperfusion injury, and diabetes mellitus [13–16]. Amaral et al. showed that regular aerobic exercise training improved metabolic control and had a renoprotective effect on diabetic kidneys, as well as preventing diabetes-associated renal structural abnormality in STZ-induced diabetic female rats, which was partly attributed to the decreased expression of TGF-beta1 [13]. Consistent with the above results, our data show that 6 weeks of aerobic exercise training alleviated diabetes-induced renal injury, as evidenced by significantly decreased levels of proteinuria and renal fibrosis in diabetic mice. TGF-beta is the primary factor that drives fibrosis in most forms of chronic kidney disease. Previous studies documented that inhibition of TGF-beta1 or its downstream signaling pathways dramatically preserves renal fibrosis in a number of disease models, but overexpression of TGF-beta1induces renal fibrosis [17–19]. Decreased expression TGF-beta1 may be the mechanism underlying the effect of aerobic exercise training in alleviating diabetes-associated renal fibrosis, but this needs to be confirmed by further research.
Exercise training is also considered to be an important basic therapeutic measure to counteract mitochondrial oxidative stress and dysfunction in multiple diseases, including aging, obesity, and Parkinson’s disease [20–22]. Many studies have indicated that exercise training improves mitochondrial function and clinical symptoms of subcutaneous adipose tissue, skeletal muscle, and myocardium in diabetic subjects [23–25]. Qi et al. found that aerobic exercise training exerted a renoprotective effect by protecting mitochondrial function and abating renal oxidative stress injury in spontaneously hypertensive rats [26]. Consistent with the above results, our data provide experimental evidence that exercise training significantly alleviates diabetes-associated mitochondrial dysfunction, as indicated by reductions in mitochondrial ATP production and membrane potential, as well as increased mitochondrial ROS generation in STZ-induced type 1 diabetic mice.
Previous studies have demonstrated the critical role of Sirt1 and PGC1α in regulating mitochondrial function [27]. Moreover, PGC1α is a pivotal regulatory factor in mitochondrial function by activating respiratory function, biogenesis, and energy metabolism. Increasing evidence demonstrates that Sirt1 together with PGC-1α participate in mediating diabetic nephropathy [28,29]. Kim et al. demonstrated that db/db mice exhibited renal mesangial cell AMPK-Sirt1-PGC1α axis expression dysfunction with resveratrol treatment [28]. Hou et al. also reported that db/db nephropathy model mice showed inactivation of Sirt1/PGC-1α signaling in their kidneys [29]. In our study, we found that decreased protein expression of Sirt1/PGC-1α in diabetic kidney as well, which was reversed by aerobic exercise training. Notably, Sirt1 knockdown abolished aerobic exercise training-induced PGC1α expression in diabetic kidneys. Moreover, the protective effect of aerobic exercise training against diabetes-associated mitochondrial compromise and renal damage in diabetic kidney was also blocked by Sirt1 siRNA. Taken together, the evidence suggests that PGC1α is concurrently modulated by 2 post-transcriptional pathways: mitochondrial oxidative stress-dependent suppression and Sirt1-dependent activation. In kidneys of type 1 diabetic mice subjected to aerobic exercise training, loss of mitochondrial oxidative stress-dependent suppression and upregulation of Sirt1 led to increased expression of PGC1α. Therefore, Sirt1 appears to be the critical regulator involved in the aerobic exercise training-induced protection against diabetes-induced renal injury.
Conclusions
Aerobic exercise training increases renal expression of Sirt1 and PGC1α, thus contributing to mitochondrial function improvement and suppression of renal injury in type 1 diabetic mice.
Footnotes
Source of support: This work was supported by the New Young Teachers Funding Scheme to Tang Lin-xia in the Sports Department (A1-U1720500474)
References
- 1.Salvatore SP, Reddi AS, Chandran CB, et al. Collapsing glomerulopathy superimposed on diabetic nephropathy: insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol Dial Transplant. 2014;29:392–99. doi: 10.1093/ndt/gft408. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A, Morocutti A, Mercuri F, et al. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49:2170–77. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- 3.Czajka A, Malik AN. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016;10:100–7. doi: 10.1016/j.redox.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock F, Shahzad K, Wang H, et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci USA. 2013;110:648–53. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–58. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 6.Rosca MG, Mustata TG, Kinter MT, et al. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol. 2005;289:F420–30. doi: 10.1152/ajprenal.00415.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bu X, Wu, Lu X, et al. Role of SIRT1/PGC-1α in mitochondrial oxidative stress in autistic spectrum disorder. Neuropsychiatr Dis Treat. 2017;13:1633–45. doi: 10.2147/NDT.S129081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa Y, Gohda T, Tanimoto M, et al. Effect of exercise on kidney function, oxidative stress, and inflammation in type 2 diabetic KK-A(y) mice. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/702948. 702948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du SF, Yu Q, Chuan K, et al. In obese mice, exercise training increases 11β-HSD1 expression, contributing to glucocorticoid activation and suppression of pulmonary inflammation. J Appl Physiol (1985) 2017;123(4):717–27. doi: 10.1152/japplphysiol.00652.2016. [DOI] [PubMed] [Google Scholar]
- 11.Hu N, Long H, Zhao M, et al. Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice. Scand J Rheumatol. 2009;38:464–71. doi: 10.3109/03009740902895750. [DOI] [PubMed] [Google Scholar]
- 12.Czajka A, Ajaz S, Gnudi L, et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine. 2015;2:499–512. doi: 10.1016/j.ebiom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amaral LS, Silva FA, Correia VB, et al. Beneficial effects of previous exercise training on renal changes in streptozotocin-induced diabetic female rats. Exp Biol Med (Maywood) 2016;241:437–45. doi: 10.1177/1535370215609696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Sun XQ, Cao JM, et al. Protective effect of epimedium combined with oligomeric proanthocyanidins on exercise-induced renal ischemia-reperfusion injury of rats. Int J Clin Exp Med. 2014;7:5730–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa Neto O, Abate DT, Marocolo M, Junior, et al. Exercise training improves cardiovascular autonomic activity and attenuates renal damage in spontaneously hypertensive rats. J Sports Sci Med. 2013;12:52–59. [PMC free article] [PubMed] [Google Scholar]
- 16.Kohzuki M, Kamimoto M, Wu XM, et al. Renal protective effects of chronic exercise and antihypertensive therapy in hypertensive rats with chronic renal failure. J Hypertens. 2001;19:1877–82. doi: 10.1097/00004872-200110000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Haberstroh U, Zahner G, Disser M, et al. TGF-beta stimulates rat mesangial cell proliferation in culture: Role of PDGF beta-receptor expression. Am J Physiol. 1993;264:F199–205. doi: 10.1152/ajprenal.1993.264.2.F199. [DOI] [PubMed] [Google Scholar]
- 18.Wilson HM, Reid FJ, Brown PA, et al. Effect of transforming growth factor-beta 1 on plasminogen activators and plasminogen activator inhibitor-1 in renal glomerular cells. Exp Nephrol. 1993;1:343–50. [PubMed] [Google Scholar]
- 19.Lopez-Hernandez FJ, Lopez-Novoa JM. Role of TGF-β in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347:141–54. doi: 10.1007/s00441-011-1275-6. [DOI] [PubMed] [Google Scholar]
- 20.Chandwaney R, Leichtweis S, Leeuwenburgh C, Ji LL. Oxidative stress and mitochondrial function in skeletal muscle: Effects of aging and exercise training. Age (Omaha) 1998;21:109–17. doi: 10.1007/s11357-998-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo JW, No MH, Park DH, et al. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J Physiol Pharmacol. 2017;21:567–77. doi: 10.4196/kjpp.2017.21.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendricks S, Ojuka E, Kellaway LA, et al. Effect of maternal separation on mitochondrial function and role of exercise in a rat model of Parkinson’s disease. Metab Brain Dis. 2012;27:387–92. doi: 10.1007/s11011-012-9305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59:572–79. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midaoui A, Tancrède G, Nadeau A. Effect of physical training on mitochondrial function in skeletal muscle of normal and diabetic rats. Metabolism. 1996;45:810–16. doi: 10.1016/s0026-0495(96)90151-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Bei Y, Lu Y, et al. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell Physiol Biochem. 2015;35:2159–68. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 26.Gu Q, Zhao L, Ma YP, Liu JD. Contribution of mitochondrial function to exercise-induced attenuation of renal dysfunction in spontaneously hypertensive rats. Mol Cell Biochem. 2015;406:217–25. doi: 10.1007/s11010-015-2439-6. [DOI] [PubMed] [Google Scholar]
- 27.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MY, Lim JH, Youn HH, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56:204–17. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 29.Hou S, Zhang T, Li Y, et al. Glycyrrhizic acid prevents diabetic nephropathy by activating AMPK/SIRT1/PGC-1α signaling in db/db mice. J Diabetes Res. 2017;2017 doi: 10.1155/2017/2865912. 2865912. [DOI] [PMC free article] [PubMed] [Google Scholar]