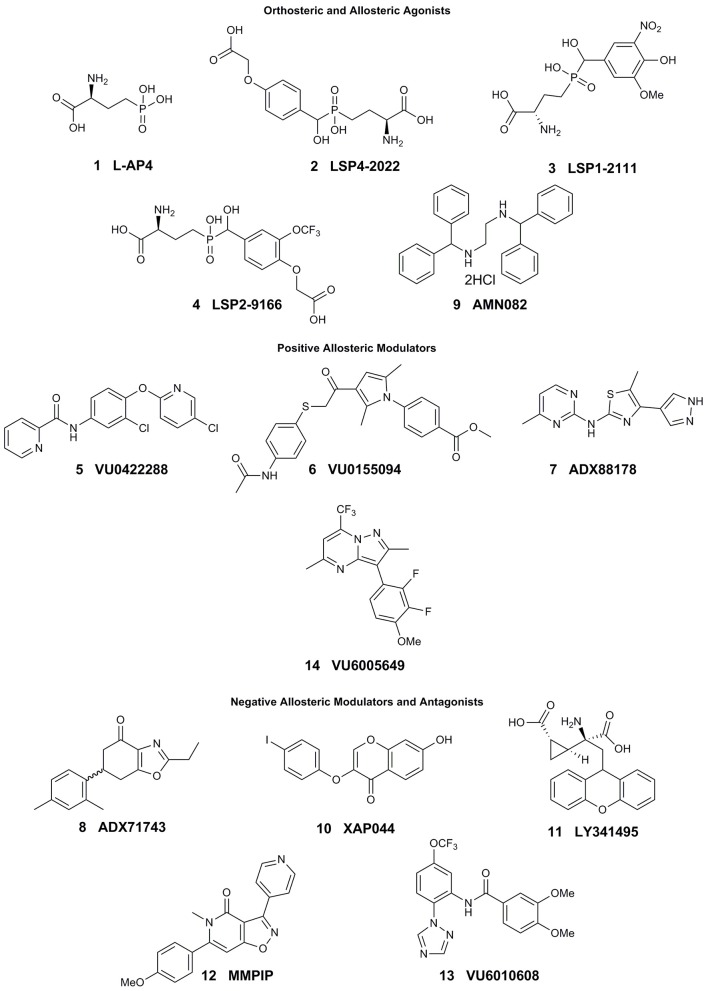
In the original article, there was a mistake in Figure 1 as published. The chirality of L-AP4 and LSP1-2111 was incorrect. pEC50 values have also been corrected for LSP1-2111 in Table 1. The authors apologize for these errors and state that they do not change the scientific conclusions of the article in any way. The original article has been updated.
Figure 1.
Current tool compounds used to study mGlu7.
Table 1.
Summary of current tool compounds used to study mGlu7.
| Name (#) | Type | mGlu7 pEC50/pIC50 | mGlu8 pEC50/pIC50 | mGlu4 pEC50/pIC50 | mGlu6 pEC50/pIC50 | Source |
|---|---|---|---|---|---|---|
| L-AP4 (1) | Orthosteric agonist | 3.47 (PIH) | 6.53 (PIH) | 7.00 (PIH) | 5.62 (PIH) | Acher et al., 2012; Selvam et al., 2018 |
| 3.61 (Ca2+) | 6.53 (Ca2+) | 6.89 (Ca2+) | 6.00 (Ca2+) | |||
| LSP4-2022 (2) | Orthosteric agonist | 4.34 (Ca2+) | 4.54 (Ca2+) | 6.96 (Ca2+) | 5.36 (Ca2+) | Acher et al., 2012; Goudet et al., 2012; Selvam et al., 2018 |
| LSP1-2111 (3) | Orthosteric agonist | 4.28 (PIH) | 4.18 (PIH) | 5.66 (PIH) | 5.77 (PIH) | Selvam et al., 2018 |
| 4.00 (Ca2+) | 4.71 (Ca2+) | 6.05 (Ca2+) | 5.49 (Ca2+) | |||
| LSP2-9166 (4) | Orthosteric agonist | 5.71 (Ca2+) | 4.25 (Ca2+) | 7.22 (Ca2+) | not reported | Acher et al., 2012 |
| VU0422288 (5) | Group III PAM | 6.85 (Ca2+) | 6.93 (Ca2+) | 6.98 (Ca2+) | not reported | Jalan-Sakrikar et al., 2014 |
| VU0155094 (6) | Group III PAM | 5.80 (Ca2+) | 6.07 (Ca2+) | 5.48 (Ca2+) | not reported | Jalan-Sakrikar et al., 2014 |
| ADX88178 (7) | mGlu4/8 PAM | >4.52 (Ca2+) | 5.66 (Ca2+) | 8.46 (Ca2+) | >5 | Le Poul et al., 2012 |
| ADX71743 (8) | mGlu7 NAM | 7.20 (human, Ca2+) | inactive | inactive | inactive | Kalinichev et al., 2014 |
| 7.06 (rat, Ca2+) | inactive | inactive | inactive | |||
| AMN082 (9) | Allosteric agonist | 6.59 (GTPγS) | >5 (GTPγS) | >5 (GTPγS) | >5 (GTPγS) | Mitsukawa et al., 2005 |
| XAP044 (10) | Antagonist | 5.26 (cAMP) | 4.48 (cAMP) | inactive | inactive | Gee et al., 2014 |
| 5.55 to 5.46 (GTPγS) | ||||||
| LY341495 (11) | Orthosteric antagonist | 6.00 (cAMP) | 6.76 (cAMP) | 4.66 (cAMP) | not reported | Kingston et al., 1998 |
| MMPIP (12) | mGlu7 NAM | 6.66 (cAMP) | >5 (cAMP) | >5 (cAMP) | not reported | Suzuki et al., 2007 |
| 7.15 (Ca2+) | Niswender et al., 2010 | |||||
| 6.14 (Thallium) | Niswender et al., 2010 | |||||
| VU6010608 (13) | mGlu7 NAM | 6.12 (Ca2+) | >5 (Ca2+) | >5 (Ca2+) | inactive (>5) | Reed et al., 2017 |
| VU6005649 (14) | mGlu7/8 PAM | 6.19 (Ca2+) | 5.59 (Ca2+) | >5 (Ca2+) | inactive | Abe et al., 2017 |
NAM, negative allosteric modulator; PAM, positive allosteric modulator; EC50, effective concentration 50; IC50, inhibitory concentration 50. Assay type is indicated in parentheses: PIH, Phosphatidylinositol hydrolysis; cAMP, cAMP accumulation; Ca2+, Calcium mobilization; GTPγS, GTPγS binding.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abe M., Seto M., Gogliotti R. G., Loch M. T., Bollinger K. A., Chang S., et al. (2017). Discovery of VU6005649, a CNS penetrant mGlu7/8 receptor PAM derived from a series of Pyrazolo[1,5-a]pyrimidines. ACS Med. Chem. Lett. 8, 1110–1115. 10.1021/acsmedchemlett.7b00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acher F., Pin J.-P., Goudet C., Eschalier A., Busserolles J., Rigault D., et al. (2012). Hypophosphorous Acid Derivatives Having Antihyperalgic Activity and Biological Applications Thereof . US Patent 9212196B2. Paris: Universite Paris Descartes. [Google Scholar]
- Gee C. E., Peterlik D., Neuhäuser C., Bouhelal R., Kaupmann K., Laue G., et al. (2014). Blocking metabotropic glutamate receptor subtype 7 (mGlu7) via the Venus flytrap domain (VFTD) inhibits amygdala plasticity, stress, and anxiety-related behavior. J. Biol. Chem. 289, 10975–10987. 10.1074/jbc.M113.542654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C., Vilar B., Courtiol T., Deltheil T., Bessiron T., Brabet I., et al. (2012). A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 26, 1682–1693. 10.1096/fj.11-195941 [DOI] [PubMed] [Google Scholar]
- Jalan-Sakrikar N., Field J. R., Klar R., Mattmann M. E., Gregory K. J., Zamorano R., et al. (2014). Identification of positive allosteric modulators VU0155094 (ML397) and VU0422288 (ML396) reveals new insights into the biology of metabotropic glutamate receptor 7. ACS Chem. Neurosci. 5, 1221–1237. 10.1021/cn500153z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M., Le Poul E., Boléa C., Girard F., Campo B., Fonsi M., et al. (2014). Characterization of the novel positive allosteric modulator of the metabotropic glutamate receptor 4 ADX88178 in rodent models of neuropsychiatric disorders. J. Pharmacol. Exp. Ther. 350, 495–505. 10.1124/jpet.114.214437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A. E., Ornstein P. L., Wright R. A., Johnson B. G., Mayne N. G., Burnett J. P., et al. (1998). LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37, 1–12. 10.1016/S0028-3908(97)00191-3 [DOI] [PubMed] [Google Scholar]
- Le Poul E., Boléa C., Girard F., Poli S., Charvin D., Campo B., et al. (2012). A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson's disease. J. Pharmacol. Exp. Ther. 343, 167–177. 10.1124/jpet.112.196063 [DOI] [PubMed] [Google Scholar]
- Mitsukawa K., Yamamoto R., Ofner S., Nozulak J., Pescott O., Lukic S., et al. (2005). A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc. Natl. Acad. Sci. U.S.A. 102, 18712–18717. 10.1073/pnas.0508063102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender C. M., Johnson K. A., Miller N. R., Ayala J. E., Luo Q., Williams R., et al. (2010). Context-dependent pharmacology exhibited by negative allosteric modulators of metabotropic glutamate receptor 7. Mol. Pharmacol. 77, 459–468. 10.1124/mol.109.058768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. W., McGowan K. M., Spearing P. K., Stansley B. J., Roenfanz H. F., Engers D. W., et al. (2017). VU6010608, a novel mGlu7 NAM from a series of N-(2-(1H-1,2,4-Triazol-1-yl)-5-(trifluoromethoxy)phenyl)benzamides. ACS Med. Chem. Lett. 8, 1326–1330. 10.1021/acsmedchemlett.7b00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvam C., Lemasson I. A., Brabet I., Oueslati N., Karaman B., Cabaye A., et al. (2018). Increased potency and selectivity for group III metabotropic glutamate receptor agonists binding at dual sites. J. Med. Chem. 61, 1969–1989. 10.1021/acs.jmedchem.7b01438 [DOI] [PubMed] [Google Scholar]
- Suzuki G., Tsukamoto N., Fushiki H., Kawagishi A., Nakamura M., Kurihara H., et al. (2007). In Vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J. Pharmacol. Exp. Ther. 323, 147–156. 10.1124/jpet.107.124701 [DOI] [PubMed] [Google Scholar]