Abstract
2-Amino-4H-chromene derivatives possess anticancer property proved on different in vivo and in vitro models of malignancies such breast, nasopharyngeal, bladder, ovary carcinomas, astrocytoma, and osteosarcoma. We assumed it might be effective to apply one of the derivatives as promising approach to lung carcinoma treatment. to evaluate how novel 4-aryl substituted 2-amino-4H-chromene derivative AX-554 impacts tumor growth and progression, as well as possible mechanisms for anticancer effect development on in vivo patient-derived heterotopic xenograft model of lung carcinoma in mice. This was an experimental in vivo study. 40 nu/nu BALB/c female mice were randomly allocated into four equal groups: Intact, control, reference, and main group. Animals of three latter groups were ingrafted with human-derived lung adenocarcinoma. Antitumor and antimetastatic action of AX-554 novel aminochromone derivative as a substance were studied. Mice survival was registered. Kinase of anaplastic lymphoma (ALK), tubulin Beta-3 (TUBB3), and c-mesenchymal-epithelial transition (MET) concentrations in the prime tumor nodes homogenates were determined by quantitative enzyme-linked immunosorbent assay. Dannet's parametric criterion and the nonparametric exact Fisher test were used. The normality of the distribution was determined using ANOVA. The survival curve was analyzed using Gehan's criterion with the Yates's correction. Aminochromone derivative possesses an inhibitory effect on human lung adenocarcinoma transplanted into nu/nu BALB/c female mice, as well as significant antimetastatic activity. About 50 mg/kg/day AX-554 intragastric course increases animals’ life expectancy of more than 3.3 times when compared with the control and induces remission in 60% of cases. The anticancer effect of the derivative is due to anti-ALK-mediated activation of tumor cells apoptosis and suppression TUBB3-dependent cell proliferation.
Keywords: Aminochromone derivative, apoptosis, lung cancer, receptor tyrosine kinases, tubulin
INTRODUCTION
Nonsmall cell lung cancer is the most common form of the disease, accounting for at least 80% of all malignant lung processes, with adenocarcinoma having the leading position (approximately 40% of all cases of human cancers). Even though chemotherapeutic treatment is widely used in oncology, pharmacological approaches to lung cancer have a number of restraints, leading among which is the difficulty of creating drug appropriate concentrations in the lung tissue, even when administered at high doses.[1] In recent years, due to advances in molecular biology, genetics, and immunology, a variety of molecular changes, mutations, and gene transformations have been discovered responsible for the development of tumor tissue, and therefore, determining the prognosis of the disease.[1,2,3] Thus, a number of intracellular signaling pathways have been identified that can play a crucial role in the treatment of lung cancer. Among these factors, tubulin (TUB), receptor tyrosine kinases have a special prognostic role.[2,3] Pharmacotherapeutic approaches, based on specific molecular pathways of tumor development, determine the essence of directed, targeted, or personalized therapy. Similar chemotherapeutic protocols affect specific biological targets and significantly increase survival, especially in patients without surgical experience.[4,5] In vitro studies have shown promising results for novel aminochromone derivatives in suppressing cancer cell growth, proliferation, and invasiveness of different malignant cell lines. Thus, Patil et al. showed high nanomolar cytotoxicity of five substituted 4H-chromenes against human glioma, melanoma, and prostate cancer cells. All the compounds demonstrated affinity to beta-TUB colchicine-binding site.[6] A novel series of 2-amino-4-(3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes was identified as potent apoptosis inducers in in vivo study on xenograft models. One of the agents, MX-116407b was active in the human lung tumor xenograft through tumor cells apoptosis activation.[7] Kinase-inhibitory effect of 4-aryl substituted derivatives of 2-amino-4H-chromene was shown on colon and lung cancer cell lines.[8] Among them 2-aminium-7-(diethylamino)-4-(4-metoxybenzo[d][1,3]dioxol-5-yl)-4H-chromene carbonitrile was one of the more potent. That was why the derivative has been chosen for further anticancer investigation.
The aim of this study was to evaluate how novel 4-aryl substituted 2-amino-4H-chromene derivative AX-554 impacts tumor growth and progression, as well as possible mechanisms for anticancer effect development on in vivo patient-derived heterotopic xenograft model of lung carcinoma in mice.
SUBJECTS AND METHODS
The study has met all requirements of GLP and European Convention for the Protection of Vertebrates Animals used for Experimental and Other Scientific Purposes regulations. The study protocols have passed an ethical review at the Local Ethics Committee of Ogarev National Research Mordovia State University (Saransk, Russia) meeting November 20, 2017, No. of Approval 11 (Reg. No. 11/12-2017).
Animals
Forty athymic 6–8-week-old female nu/nu BALB/c mice were obtained from the SFP Animals Breeding Facility of Institute of Bioorganic Chemistry of Russian Academy of Sciences (Pushchino, Russia). Animals were raised in the University Animals facilities and housed in the macrolon cages. Mice were kept under specific pathogen-free condition on natural daylight cycles. Autoclaved standard food and water were provided ad libitum and room temperature (25°C ± 2°C) as well as humidity (60% ± 10%) were maintained. Animals were randomly divided into four groups of 10 mice in each: Intact mice, control, reference group, main group.
Aminochromone derivative and reference drug
Novel Aminochromone derivative AX-554, 2-aminium-7-(diethylamino)-4-(4-metoxybenzo[d][1,3]dioxol-5-yl)-4H-chromene carbonitrile as substance (purity 99,25%) was synthetized at All-Union Scientific Center for Biological Active Substances Safety (Staraja Kupavna, Russia) according to Bardashov et al. one-pot method in N-acetylamino-ethanoate form.[9] The substance was administered intragastrically daily at 50 mg/kg dose, which was 1% of the LD50 value determined for this route of administration, in 0.5 ml of 2% starch gel, 7 days after tumor transplantation for 7 days as an effective regimen on in vivo syngeneic model of Lewis lung carcinoma in mice.[10] AX-544 starch formulation was prepared ex tempore. As a reference, cyclophosphamide (Sigma-Aldrich, Germany, purity 99%, 75%) was used because of its high bioavailability with enteral administration and efficacy in tumors of epithelial origin.[11] The route, regimen, duration of administration, as well as calculation of the administered dose fully corresponded to those of AX-554 substance. Mice of control group received the same volume of starch gel.
Xenograft model and antitumor action assessment
The pharmacological activity of the substance was studied on a heterotopic tumor model using xenograft of nonsmall cell lung cancer (histologically confirmed adenocarcinoma) received intraoperatively at Oncology Clinic of I.M. Sechenov First Moscow State Medical University from 63-year-old female patient who gave voluntary informed consent and received no chemotherapeutic treatment before. The tumor model was reproduced by sequential three-stage transplantation of tumor tissue particles to animals’ left hind leg subcutaneously according to Morton and Houghton and Chijiwa et al.[12,13] On day 22 after the cessation of treatment, half of the animals randomly selected in each group were withdrawn from the experiment under ether anesthesia. The antitumor effect of the substance and its antimetastatic activity were evaluated in accordance with the current guidelines by measuring the size of the primary tumor node, its weight (at the end of the experiment) and calculation of Tumor Growth Inhibition Index (TGII). The number of superficial metastases in the brain was counted using a binocular microscope with 8 × 2 magnification. The remaining animals in each group were followed up to 90 days from the point of tumor transplantation to record survival and remission rates.
Enzyme-linked immunosorbent assay
In the primary tumor nodes homogenates (lungs in intact animals), the quantitative determination of the mesenchymal–epithelial transition factor receptor (c-MET/HGF enzyme-linked immunosorbent assay [ELISA] Kit manufactured by Cusabio [USA], Cat. No. CSB-E13492 m), TUB beta-3 chain (TUBB3 ELISA Kit, Cusabio [USA], Cat. No. CSB-E14123 m), and the receptor tyrosine kinase of anaplastic lymphoma (ALK Tyrosine Kinase Receptor [ALK] ELISA Kit, Abbexa [USA], Cat. No. abx515274) was performed by ELISA using an automatic reader StatFax4200 (USA).
Statistical analysis
Statistical processing of the obtained data was carried out using SPSS version 16.0 (IBM, USA).[14] Dannet's parametric criterion and nonparametric exact Fisher test were used. The normality of the distribution was determined using ANOVA. The survival curve as a line indicated deceased animals at different time points after cessation of treatment was analyzed using Gehan's criterion with the Yates's correction.
RESULTS
Intragastric administration of 50 mg/kg/day AX-554 for 7 days from day 7 after transplantation of tumor tissue to animals was accompanied by statistically significant differences in the tumor node volume when compared with control group and the group of animals receiving 18.0 mg/kg cyclophosphamide on days 14 and 21 of observation. At the same time intervals, TGII was also statistically significant [Table 1]. The course of the test substance administration decreased the incidence of tumor brain metastasis to 40% [Table 1]. Antimetastatic effect development was accompanied by a decrease in the number of visible surface metastases, both in comparison with the control group, and with the group of animals receiving the reference drug. A proportional increase in the metastatic inhibition index was noted.
Table 1.
Antitumor and antimetastatic action of intragastrically administered AX-554 in nu/nu BALB/c mice with lung adenocarcinoma heterotopic xenograft
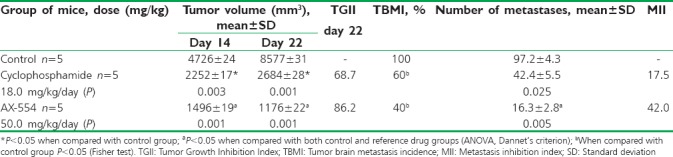
Results of experimental animals’ survival depended on the mice group assignment and summarized in Figure 1. The onset of mice death in control group was recorded on the 26th day after the abolition of an intragastric placebo with the highest lethality between 30 and 40 days of observation. The average life expectancy in the group was 31 ± 1 days. Course of cyclophosphamide intragastric administration prolonged the animals’ lifespan to 85 ± 2 days (P = 0.001 when compared with the control); in 2 out of 5 mice, a stable remission was noted in the group. Aminochromone derivative AX-554 induced tumor remission in 3 animals out of 5 formed the main group, with the mean life expectancy being predicted to be 103 ± 2 days (P = 0.001 when compared with the control and P= 0.03 when compared with cyclophosphamide).
Figure 1.

Experimental mice survival curves
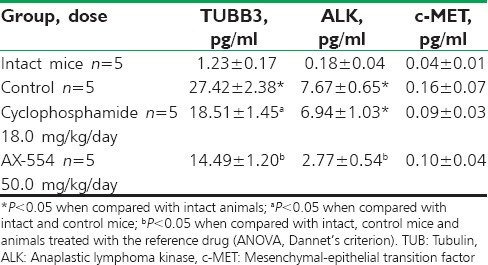
On day 22 after subcutaneous transplantation of tumor tissue, significant growth of both TUBB3 and ALK concentration was registered in the primary tumor node homogenates of control mice in comparison with the markers content in homogenated lungs of intact animals [Table 2]. At the same time, the concentration of c-MET, although it showed a tendency to increase, remained within the borders of reference values. Seven-day intragastric AX-554 administration was accompanied by a significant decrease in the tissue TUBB3 and ALK concentration in comparison with the control group and the group of animals treated with cyclophosphamide. It should be noted that in cyclophosphamide group of mice no changes in the quantitative ALK content were found. The substance and the reference drug had no impact on c-MET tissue concentration in the primary tumor node.
Table 2.
Oncomarkers’ concentration (mean±standard deviation) in the primary tumor node homogenates of female nu/nu BALB/c mice treated with AX-554
DISCUSSION
Thus, analyzing the results obtained, it can be noted that the course (within 7 days) of intragastric administration of AX-554 substance, by chemical nature being a representative of 4-alkyl substituted 2-amino-4H-chromenes, lead to the formation of antitumor and anti-metastatic effect superior in rate to the reference anticancer alkylating drug cyclophosphamide. Our observation is appropriate to previously obtained data of high anticancer activity of some 2-amino-4H-chromenes.[15] At the same time, AX-554 prolongs the lifespan of xenograft tumor carriers and causes remission in 60% of cases. Gourdeau et al. reported both remission rate and survival time increasing in all 10 mice with human-derived lung carcinoma.[7] Possible explanation of such variations may relate to histological type of human-derived tumors, many of which are strongly resistant to chemotherapy.[16] Designing the study, we have focused on two possible ways of therapeutic activity of the derivative. One of them is kinase-inhibitory property previously discussed by Fallah-Tafti et al.[8] Suppressing of ALK growth in tumor tissue of animals treated with AX-554 indicates the ability of the substance to activate the processes of apoptosis of tumor cells, and thus, inhibit their proliferation and tumor progression.[5] Apoptogenic action of 2-amino-4H-chromenes along with their direct cytotoxicity is considered now as leading way of their anticancer activity.[15] In an article, recently published by Kulshrestha et al. microtubule inhibitory effect of chromene derivative SP-6-27 with pro-apoptotic and cytotoxic property was shown for ovarian cancer cells.[17] AX-554 intragastric administration also caused the decrease in the concentration of the structural oncogene TUBB3 in lung xenograft tumor node. Therefore, the derivative likely inhibits cancer cell division process.[18] The absence of quantitative changes in pharmacological resistance marker c-MET/HGF in all experimental groups in our opinion is because the xenograft tissue did not undergo chemotherapeutic influence.[16,19] In summary, aminochromone derivative AX-554 (2-aminium-7-[diethylamino]-4-[4-metoxybenzo[d][1,3]dioxol-5-yl]-4H-chromene carbonitrile N-acetylamino-ethanoate) when administered 50 mg/kg intragastrically daily during a week suppresses patient-derived lung adenocarcinoma growth, prevents brain metastasis, causes remission in 60% cases, and prolongs survival of mice, tumor carriers. The effect is due to anti-ALK-mediated activation of tumor cells apoptosis and suppression of neoplastic cell proliferation.
CONCLUSIONS
Unlike cyclophosphamide, aminochromone derivative AX-554 has a greater inhibitory effect (P = 0.01) on the primary tumor node developed at the site of human lung adenocarcinoma transplantation in nu/nu BALB/c female mice, as well as significant anti-metastatic activity
About 50 mg/kg/day AX-554 intragastric course increases tumor carriers’ life expectancy of more than 3.3 times when compared with the control (P = 0.001) and induces the malignant process remission in 60% of mice
The anticancer effect of the derivative is due to anti-ALK-mediated activation of tumor cells apoptosis and suppression of neoplastic cell proliferation.
Financial support and sponsorship
The study has been financially supported by Ogarev National Research Mordovia State University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Russia State Prize holder Prof. Sofia Y. Skachilova under whose authority the studied formulation has been synthetized.
REFERENCES
- 1.Long JT, Cheang TY, Zhuo SY, Zeng RF, Dai QS, Li HP, et al. Anticancer drug-loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J Nanobiotechnology. 2014;12:37. doi: 10.1186/s12951-014-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tovar I, Expósito J, Jaén J, Alonso E, Martínez M, Guerrero R, et al. Pattern of use of radiotherapy for lung cancer: A descriptive study. BMC Cancer. 2014;14:697. doi: 10.1186/1471-2407-14-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parente Lamelas I, Abal Arca J, Fírvida Pérez JL. Directed therapies in lung cancer: New hope? Arch Bronconeumol. 2012;48:367–71. doi: 10.1016/j.arbres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Gold KA, Wistuba II, Kim ES. New strategies in squamous cell carcinoma of the lung: Identification of tumor drivers to personalize therapy. Clin Cancer Res. 2012;18:3002–7. doi: 10.1158/1078-0432.CCR-11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sève P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–75. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 6.Patil SA, Wang J, Li XS, Chen J, Jones TS, Hosni-Ahmed A, et al. New substituted 4H-chromenes as anticancer agents. Bioorg Med Chem Lett. 2012;22:4458–61. doi: 10.1016/j.bmcl.2012.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourdeau H, Leblond L, Hamelin B, Desputeau C, Dong K, Kianicka I, et al. Antivascular and antitumor evaluation of 2-amino-4- (3-bromo-4,5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol Cancer Ther. 2004;3:1375–84. [PubMed] [Google Scholar]
- 8.Fallah-Tafti A, Tiwari R, Shirazi AN, Akbarzadeh T, Mandal D, Shafiee A, et al. 4-aryl-4H-chromene-3-carbonitrile derivatives: Evaluation of src kinase inhibitory and anticancer activities. Med Chem. 2011;7:466–72. doi: 10.2174/157340611796799258. [DOI] [PubMed] [Google Scholar]
- 9.Bardashov IN, Alekseeva AU, Ershov OV, Grishanov DA. One-pot synthesis of 4-alkyl-2-amino-4H-chromene derivatives. Heterocycl Commun. 2015;21:175–7. [Google Scholar]
- 10.Blinov DS, Skachilova SY, Dudina MO, Samishina EA, Suslova IR, Blinova EV. Acute toxicity and anticancer activity of 4-aryl substituted 4H-chromene derivative on syngeneic Lewis model of lung carcinoma. Exp Clin Pharm. 2018;82:12–17. [Google Scholar]
- 11.Haubitz M. Acute and Long-term Toxicity of Cyclophosphamide. Tx Med. 2007;19:26–3. [Google Scholar]
- 12.Chijiwa T, Kawai K, Noguchi A, Sato H, Hayashi A, Cho H, et al. Establishment of patient-derived cancer xenografts in immunodeficient NOG mice. Int J Oncol. 2015;47:61–70. doi: 10.3892/ijo.2015.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247–50. doi: 10.1038/nprot.2007.25. [DOI] [PubMed] [Google Scholar]
- 14.Glantz SA. Primers in Biostatistics. 7th ed. San Francisco: The McGraw-Hill Companies, Inc; 2012. [Google Scholar]
- 15.Patil SA, Patil R, Pfeffer LM, Miller DD. Chromenes: Potential new chemotherapeutic agents for cancer. Future Med Chem. 2013;5:1647–60. doi: 10.4155/fmc.13.126. [DOI] [PubMed] [Google Scholar]
- 16.Lwin Z, Riess JW, Gandara D. The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J Thorac Dis. 2013;5(Suppl 5):S556–64. doi: 10.3978/j.issn.2072-1439.2013.08.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulshrestha A, Katara GK, Ibrahim SA, Patil R, Patil SA, Beaman KD, et al. Microtubule inhibitor, SP-6-27 inhibits angiogenesis and induces apoptosis in ovarian cancer cells. Oncotarget. 2017;8:67017–28. doi: 10.18632/oncotarget.17549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinho L, Mendes F, Rodrigues MM. Molecular targets in lung cancer therapy: A current review. J Integr Oncol. 2015;4:1–12. [Google Scholar]
- 19.Galimi F, Torti D, Sassi F, Isella C, Corà D, Gastaldi S, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: Response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17:3146–56. doi: 10.1158/1078-0432.CCR-10-3377. [DOI] [PubMed] [Google Scholar]


