Abstract
Here we propose to use bacterial cells as factories that generate EVs harboring both the RNP complex (or any other CRISPR‐mediated tool) and a specific ligand molecule. The ligand would allow specific binding of EVs to cells with the matching receptors, adding a level of specificity to the delivery, hence stimulating precise genome editing.
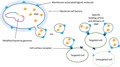
The production and release of extracellular vesicles (EVs) are a common property of cells spanning all domains of life (Deatherage and Cookson, 2012). These EVs enable the transport of various compounds, such as nucleic acids, proteins and viral particles, and are therefore potentially involved in cell communication, competition and survival (Kim et al., 2015; van Niel et al., 2018). From an application point of view, EVs could play an important role in health and disease, for instance in the development of novel strategies for vaccination and phage therapy (Liu et al., 2018). With respect to the latter, it has been shown for B. subtilis that phage‐resistant strains became susceptible for phages as a result of fusion with EVs carrying phage receptors (Tzipilevich et al., 2017).
Extracellular vesicles carry cargos both on or embedded in the membrane, as well as inside the lumen. Extracellular vesicles offer the enclosed cargos protection against (non)enzymatic degradation and are thus essential for exchange of RNA, proteins and other molecules that are prone to degradation (Mashburn‐Warren and Whiteley, 2006; Tsatsaronis et al., 2018). Moreover, EVs have the potential to facilitate the delivery of molecules that are generally excluded from entering target cells due to size, charge or hydrophobicity. Here, we argue that the production of EVs may also be harnessed to produce cell‐specific delivery systems for genome editing tools, such as the CRISPR toolbox including Cas9, Cas12a and base editors (Gaudelli et al., 2017; Knott and Doudna, 2018). One of the most important research directions in applying genome editing tools, especially in the context of medical applications to correct genetic disorders or to combat pathogens, is the delivery of the CRISPR toolbox to the right position in the human body or to the pathogens (Doudna and Charpentier, 2014; Sander and Joung, 2014). It is hereby preferred to deliver the nuclease enzyme (e.g. Cas9) in complex with the gRNA, the so‐called CRISPR‐Cas9 RNA–protein complex (or ribonucleoprotein complex; RNP), which after uptake allows precise genome editing. Currently, efforts are made to deliver RNP complexes via virus‐like particles, receptor‐mediated endocytosis or osmocytosis (D'Astolfo et al., 2015; Liu et al., 2015; Yin et al., 2016; Chaverra‐Rodriguez et al., 2018).
We propose to extend the delivery toolbox for genome editing approaches by bacterial EVs. The idea is to use bacterial cells as factories that generate EVs harbouring both the RNP complex (or any other CRISPR‐mediated tool) and a specific ligand molecule. The ligand molecules could be cell membrane‐anchored proteins. The presence of such ligand molecules on the surface of the EV would allow specific binding to cells with the matching receptors followed by fusion of EVs and cells and delivery of cargo. This adds a level of specificity to the delivery, hence stimulating precise genome editing. In practice, genes encoding a nuclease (e.g. Cas9) and gRNA will be introduced in the appropriate EV‐producing bacteria. These genes and gRNA should be constructed downstream of strong promoters to allow high production of the RNP complex. In the same bacteria, the DNA sequences of the ligand and a native bacterial membrane protein can be fused together to allow display of the ligand on the cell membrane. When EVs are generated from the cell membrane, they contain the ligand on the surface and most likely enclose the RNP complexes in the lumen, as the complexes are expressed in great abundance in the cytosol.
It should be noted that the above proposed model is most applicable for Gram‐positive producer bacteria, as they, in contrast to Gram‐negative bacteria that have an additional outer membrane, only have a cytoplasmic membrane acting as the origin of EV generation, and the membrane is in direct contact with cytosolic components to achieve engulfment of cargos (Toyofuku et al., 2018). Moreover, toxicity caused by lipopolysaccharides (LPS), which hampers the application of Gram‐negative bacteria (Acevedo et al., 2014), is also not of concern for EVs generated from Gram‐positive bacteria. In Gram‐negative bacteria, the outer membrane is the well‐known origin for EV generation, but such EVs generated cannot easily pack cytosolic components (Bonnington and Kuehn, 2014). Recently, evidence has been provided that inner‐membrane fragments can also be part of EVs, resulting in the release of merged inner–outer membrane vesicles (Pérez‐Cruz et al., 2015). Using this type of EVs for delivering genome editing complex is challenging, as the chance for all the required components to end up in the same EV could be considerably low.
In spite of the advantages that Gram‐positive bacteria can offer for the proposed application, it should be noted that Gram‐positive bacteria naturally produce EVs in lower quantity than the Gram‐negative. Nevertheless, knowledge on the biogenesis of EVs has been accumulated and pointed out ways to increase EV release. For example, explosive cell lysis is a source of EVs (Turnbull et al., 2016); cell wall degrading enzymes also play a role in EV release by weakening or penetrating the major barrier for EVs to escape (Toyofuku et al., 2017). By employing these enzymes, or inducing explosive cell lysis, EVs can be harvested in large amounts also from Gram‐positive bacteria.
When the proper bacterial cell factories are chosen, a crucial point to achieve the proposed cell‐specific delivery system is a justified design of the ligand molecule. This molecule should be chosen/designed in such a way that it binds specifically to the target cells without triggering unwanted signal cascades or immune responses. When the ligand molecule is fused to the bacterial membrane protein, proper folding of the ligand should be guaranteed for efficient targeting effect. With knowledge from multiple research fields, such as bioinformatics, biochemistry, microbiology and pharmacology, proper design of such a delivery system can be achieved.
We anticipate that the proposed strategy could pave the way for the production of cell‐specific delivery systems by exploiting bacteria as cell factories of both the genome editing tools and EVs. In our vision, this strategy can lead to the production of relatively low‐cost but high‐efficiency, high‐specificity delivery systems of genome editing tools for biotechnological and medical applications ranging from steering metabolite production to fighting pathogenic bacteria or correcting human genetic disorders.
Microbial Biotechnology (2019) 12(1), 71–73
References
- Acevedo, R. , Fernández, S. , Zayas, C. , Acosta, A. , Sarmiento, M.E. , Ferro, V.A. , et al (2014) Bacterial outer membrane vesicles and vaccine applications. Front Immunol 5: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnington, K.E. , and Kuehn, M.J. (2014) Protein selection and export via outer membrane vesicles. Biochim Biophys Acta Mol Cell Res 1843: 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaverra‐Rodriguez, D. , Macias, V.M. , Hughes, G.L. , Pujhari, S. , Suzuki, Y. , Peterson, D.R. , et al (2018) Targeted delivery of CRISPR‐Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat Commun 9: 3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Astolfo, D.S. , Pagliero, R.J. , Pras, A. , Karthaus, W.R. , Clevers, H. , Prasad, V. , et al (2015) Efficient intracellular delivery of native proteins. Cell 161: 674–690. [DOI] [PubMed] [Google Scholar]
- Deatherage, B.L. , and Cookson, B.T. (2012) Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 80: 1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna, J.A. , and Charpentier, E. (2014) The new frontier of genome engineering with CRISPR‐Cas9. Science 346: 1258096. [DOI] [PubMed] [Google Scholar]
- Gaudelli, N.M. , Komor, A.C. , Rees, H.A. , Packer, M.S. , Badran, A.H. , Bryson, D.I. , and Liu, D.R. (2017) Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Lee, J. , Park, J. , and Gho, Y.S. (2015) Gram‐negative and Gram‐positive bacterial extracellular vesicles. Semin Cell Dev Biol 40: 97–104. [DOI] [PubMed] [Google Scholar]
- Knott, G.J. , and Doudna, J.A. (2018) CRISPR‐Cas guides the future of genetic engineering. Science 361: 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Gaj, T. , Yang, Y. , Wang, N. , Shui, S. , Kim, S. , et al (2015) Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nat Protoc 10: 1842–1859. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Defourny, K.A.Y. , Smid, E.J. , and Abee, T. (2018) Gram‐positive bacterial extracellular vesicles and their impact on health and disease. Front Microbiol 9: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn‐Warren, L.M. , and Whiteley, M. (2006) Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol 61: 839–846. [DOI] [PubMed] [Google Scholar]
- van Niel, G. , D'Angelo, G. , and Raposo, G. (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228. [DOI] [PubMed] [Google Scholar]
- Pérez‐Cruz, C. , Delgado, L. , López‐Iglesias, C. , and Mercade, E. (2015) Outer‐inner membrane vesicles naturally secreted by gram‐negative pathogenic bacteria. PLoS ONE 10: e0116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, J.D. , and Joung, J.K. (2014) CRISPR‐Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku, M. , Cárcamo‐Oyarce, G. , Yamamoto, T. , Eisenstein, F. , Hsiao, C.C. , Kurosawa, M. , et al (2017) Prophage‐triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis . Nat Commun 8: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku, M. , Nomura, N. and Eberl, L. (2018) Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. [Epub ahead of print]. 10.1038/s41579-018-0112-2 [DOI] [PubMed] [Google Scholar]
- Tsatsaronis, J.A. , Franch‐Arroyo, S. , Resch, U. , and Charpentier, E. (2018) Extracellular vesicle RNA: a universal mediator of microbial communication? Trends Microbiol 26: 401–410. [DOI] [PubMed] [Google Scholar]
- Turnbull, L. , Toyofuku, M. , Hynen, A.L. , Kurosawa, M. , Pessi, G. , Petty, N.K. , et al (2016) Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7: 11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipilevich, E. , Habusha, M. , and Ben‐Yehuda, S. (2017) Acquisition of phage sensitivity by bacteria through exchange of phage receptors. Cell 168: 186–199. e12. [DOI] [PubMed] [Google Scholar]
- Yin, H. , Song, C.Q. , Dorkin, J.R. , Zhu, L.J. , Li, Y. , Wu, Q. , et al (2016) Therapeutic genome editing by combined viral and non‐viral delivery of CRISPR system components in vivo . Nat Biotechnol 34: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]


