Abstract
Objectives
The concept of disease control incorporates independent disease characteristics that are longitudinally reflective of disease status and which can be used to make treatment decisions. Chronic rhinosinusitis (CRS) is a chronic condition for which the determination of disease control by both the patient and the treating physician is important. Our objectives were to determine CRS disease characteristics that are associated with patient‐reported and physician‐rated CRS disease control.
Study Type
Cross‐sectional.
Methods
A total of 209 participants were prospectively recruited. Participants were asked to rate their global level of CRS control as “not at all,” “a little,” “somewhat,” “very,” and “completely.” All participants completed a 22‐item Sinonasal Outcome Test (SNOT‐22) and also reported the number of sinus infections, CRS‐related antibiotic courses taken, CRS‐related oral corticosteroid courses taken, and missed days of work or school due to CRS, all in the last 3 months. Clinical and demographic characteristics were also collected from each participant. A Lund‐Kennedy endoscopy score was calculated for each participant from nasal endoscopy. Two rhinologists were then given each participant's SNOT‐22 score (as well as SNOT‐22 nasal, sleep, otologic/facial pain, and emotional subdomain scores), endoscopy score, and the number of sinus infections, CRS‐related antibiotics, CRS‐related oral corticosteroid courses and missed days of work or school due to CRS in the preceding 3 months as reported by the patient. The two rhinologists were blinded to all other participant characteristics and each rhinologist independently rated every participant's global control level as “not at all,” “a little,” “somewhat,” “very,” and “completely.” Associations were sought between CRS disease characteristics (SNOT‐22 score, endoscopy score, sinus infections, CRS‐related antibiotic usage, CRS‐related oral corticosteroid usage, and lost productivity due to CRS) and patient‐reported CRS control as well as mean physician‐rated CRS control.
Results
Patient‐reported global CRS control was associated only with SNOT‐22 (adjusted relative risk [RR] = 0.99, 95% CI: 0.98–0.99, P < .001) but no other CRS disease characteristic. Patient‐reported CRS control was specifically associated only with nasal symptoms and not extra‐nasal symptoms of CRS. Physician‐rated CRS control was associated with SNOT‐22 score (adjusted RR [for each 1‐unit increase of SNOT‐22] = 0.99, 95% CI: 0.98–0.99, P < .001), number of acute bacterial CRS exacerbations—reflected by number of antibiotic courses taken (or sinus infections)—in the last 3 months (adjusted RR = 0.89, 95% CI: 0.82–0.98, P = .014) and the number of CRS‐related oral corticosteroid courses taken in the last 3 months (adjusted RR = 0.87, 95% CI: 0.78–0.97, P = .012). Nasal, sleep, and otologic/facial pain symptoms were all associated with physician‐rated CRS control. Having used at least one course of antibiotics or oral corticosteroids in the last 3 months was the optimal threshold for detecting poorly controlled CRS.
Conclusions
Patients and physicians use different criteria to determine the level of CRS control. While both rely on the burden of CRS symptomatology, patients consider primarily nasal symptoms while physicians include nasal and extra‐nasal symptoms of CRS in determining CRS control. Physicians also independently consider CRS‐related antibiotic use, as a reflection of acute bacterial CRS exacerbations, and CRS‐related oral corticosteroid use in the determination of global CRS control.
Level of Evidence
2c.
Keywords: Chronic rhinosinusitis, control, SNOT‐22, antibiotics, oral corticosteroids, endoscopy, productivity loss
INTRODUCTION
Chronic rhinosinusitis (CRS) is an inflammatory disease of the paranasal sinuses that affects up to 5% of the US population.1 With heterogeneous etiologies,2, 3, 4, 5, 6 CRS impacts affected individuals by causing a significant decrease in quality of life (QOL), which is comparable to that caused by other severe chronic diseases such as asthma and diabetes.7 The decreased QOL experienced by patients with CRS may be due to chronic symptomatology, acute CRS exacerbations as well exacerbations of comorbid pulmonary disease.8, 9, 10, 11, 12, 13 These clinical manifestations of CRS and the resultant QOL decrease lead patients to seek treatment totaling billions of dollars in direct healthcare costs every year.14 The clinical manifestations of CRS also impact affected individuals' abilities to pursue normal day‐to‐day activities, such as the performance of workplace tasks.15 This lost productivity attributable to CRS also accounts for billions of dollars in indirect costs. In total, the direct and indirect costs of CRS exceed 20 billion dollars every year in the United States alone.16 Therefore, CRS is not only a significant burden on afflicted individuals but also on society as a whole.
The goal of treatment of CRS focuses on reducing the manifestations of the disease. However, there are many distinct disease manifestations of CRS that may differentially influence a patient's or physician's assessment of the disease. The most significant aspects of CRS from the patient's and/or treating physician's perspectives may include chronic symptomatology (which includes both nasal and extra‐nasal symptoms), objective CRS‐related findings on physical examination (for example, the size of nasal polyps, the severity of mucosal inflammation or quality of sinonasal drainage), the frequency of acute CRS exacerbations, the need for systemic medication usage (including both antibiotics and corticosteroids) and lost productivity due to the disease.8, 9, 15, 17, 18 The magnitude of each of these manifestations of CRS may therefore influence decisions about treatment. The treatment of CRS is multifaceted and must be individualized, with respect to medication choice and dosage, to the needs of each patient. In the medical management of CRS, the highest level of evidence supports the use of intranasal saline irrigation and topical intranasal corticosteroids, the frequency and dosage of which may be titrated to meet patient‐specific needs.17 There is no definitive role for long‐term systemic antibiotics and systemic corticosteroids in maintenance medical management of CRS, although these medications are often used in certain circumstances such as acute CRS exacerbations or sinus infections.19, 20 Endoscopic sinus surgery may also be offered to those patients who are failing appropriate medical management.17, 18 Escalation, de‐escalation or continuation of a maintenance CRS treatment regimen is motivated not only by the patient‐perceived impact of CRS disease manifestations but also by the physician‐determined impact of the disease on the patient's overall health. For example, a patient may focus on the severity of CRS symptoms as the cause of decreased QOL, while a physician may focus on frequent use of systemic corticosteroids for CRS as a risk factor for serious medication side effects. In both cases, escalation of therapy may be warranted but in each case to address different aspects of the disease. One challenge in developing uniform treatment plans based on CRS disease status is that it is still unclear how each of the many disease characteristics may independently contribute to patients' or physicians' perceptions of CRS status and, therefore, upon which disease characteristics treatment decisions should be made.
The notion of disease “control” has been developed for a number of incurable conditions that must be chronically managed based on real‐time disease status.21, 22 Disease control is a metric of disease status that incorporates important but independent longitudinal measures of disease severity and their impact on patients. Classification of a disease as well‐ or poorly controlled is indicative of how well disease parameters are maintained within certain acceptable limits. Disease control therefore informs the treatment of chronic disease: escalate therapy for patients with poor control but maintain or possibly de‐escalate therapy for patients with well‐controlled disease. As an example in clinical practice, control is an important metric for standardized treatment decisions for asthmatics.23 Asthma control incorporates independent disease characteristics such as the subjective severity of symptoms (eg, patient complaints about wheezing), interference of the disease with normal day‐to‐day activities, as well as objective measures of the disease, including spirometry, and the utilization of systemic medications (eg, corticosteroids). Based on how a patient's asthma status measures according to these various criteria—how well‐ or poorly controlled the asthma is—a standardized and consensus approach to escalation, de‐escalation or maintenance of asthma therapy has been developed.23
CRS is considered to be an incurable inflammatory condition, like asthma, that must be chronically managed with treatment decisions made based on complex interaction of the real‐time needs of the patient and status of the disease.17 At present, subjective physician‐dependent perception of CRS is a primary driver of treatment of this disease. However, epidemiologic data suggests most physician visits for CRS are to primary care physicians or internists, rather than subspecialists with specific expertise in CRS.24 A measure of CRS control that incorporates multiple independent disease manifestations could be utilized to improve the quality of patient care by serving as the basis for a standardized CRS treatment algorithm that may be used independent of physicians' experience or expertise with CRS. At present, however, there is no consensus agreement for what disease characteristics should be incorporated into the notion of CRS disease control. Several prior studies have investigated CRS symptom control25, 26 and comprehensively attempted to develop scales for determining overall CRS disease control.27, 28 These studies, while providing useful insight into the development of the concept of control for CRS, have been limited in scope and methodology. In this study, we sought to establish groundwork for the concept of CRS control by taking a systematic approach to studying the determinants of how a large cohort of patients self‐rated their global CRS control as well as how physicians rated these patients' CRS control level. We focused on baseline CRS symptomatology, the frequency of sinus infections, CRS‐related antibiotic usage, CRS‐related oral corticosteroid usage, endoscopic findings and lost productivity due to CRS as potential determinants of global CRS control because these disease manifestations have been identified as the most significant with respect to QOL, objective disease burden and societal cost burden.17, 18 We hypothesized that these disease manifestations would be differentially associated with CRS control as determined by patients and by physicians. In order to study this hypothesis, we first established the independence of these disease manifestations as distinct entities that could be used in measuring CRS control and then determined the degree to which each manifestation was associated with patients' and physicians' ratings of CRS control.
METHODS
Study Participants
This study was approved by the candidate's institutional review board, which oversaw all research described in this study. Adult patients of age 18 years or older with consensus guideline criteria for CRS29 were recruited prospectively and provided informed consent for inclusion in this study. Exclusion criteria included comorbid diagnoses of vasculitis, cystic fibrosis, sarcoidosis, and immunodeficiency. Patients with an active acute CRS exacerbation were excluded so that all study participants' CRS characteristics would be reflective of their general, baseline symptomatology. In order to remove the confounding effect of recent endoscopic sinus surgery, patients who had endoscopic sinus surgery within the last 6 months were also excluded.
Study Design and Data Collection
This is a cross‐sectional study to determine how and in what manner the SNOT‐22 reflects patient‐reported CRS symptom control. All data were collected at enrollment. The age, gender, race, and smoking history of all participants was requested and recorded. Any participant who reported current or former tobacco use was considered a smoker for this study.30 All participants completed a SNOT‐22 survey.31 SNOT‐22 subdomain scores were calculated as previously described.32 The SNOT‐22 nasal subdomain score was calculated by summing SNOT‐22 items 1–6, 21, and 22. The SNOT‐22 otologic/facial pain subdomain score was calculated by summing SNOT‐22 items 7–10. The SNOT‐22 sleep subdomain score was calculated by summing SNOT‐22 items 11–18. The SNOT‐22 emotional subdomain score was calculated by summing SNOT‐22 items 19 and 20. All participants were asked to recall the number of courses of oral antibiotics and oral corticosteroids they had taken in the last 3 months for their CRS. Recall of past systemic antibiotic or corticosteroid use without specifying the exact medication name or dosing has been previously identified and used as a meaningful method of assessing patients' systemic medication usage for both asthma and CRS.23, 33, 34, 35 Patients were also asked to recall the number of sinus infections they had experienced in the last 3 months.9 To evaluate for CRS‐related productivity loss, all participants were asked how many days of work or school they missed in the last 3 months due to CRS, as previously described and validated.36, 37, 38 At enrollment, participants were assessed by the treating rhinologist for a history of prior sinus surgery, aeroallergen hypersensitivity based on formal allergy testing, and asthma based on clinical history23 and prior diagnosis. The treating rhinologist also performed a nasal endoscopy to evaluate for the presence or absence of nasal polyps, and from which the Lund‐Kennedy endoscopy score was calculated.39 The Lund‐Kennedy score reflects the severity of polyps, edema and discharge, as well as scarring and crusting in the post‐ESS patient, that is seen on nasal endoscopy.39
Rating of Chronic Rhinosinusitis Control
Using a previously described scale, all participants were asked to rate the overall level of control of their CRS as “not at all,” “a little,” “somewhat,” “very,” or “completely.”40 These levels of CRS control were also translated to numeric values (1–5) for subsequent analyses (Fig. 1). Two rhinologists, one of whom was the candidate, also independently rated the overall level of control for each participant's CRS, using the same scale as the one the participants used (Fig. 1). These rhinologists, henceforth referred to as Physician #1 and Physician #2, were blinded to the participants' identities when rating CRS control. The only participant‐related information that was revealed to the physicians was SNOT‐22 score, individual SNOT‐22 subdomain scores, the number of patient‐reported sinus infections in the last 3 months, the number of patient‐reported antibiotic courses for CRS in the last 3 months, the number of patient‐reported oral corticosteroids for CRS in the last 3 months, and the number of missed days of work or school due to CRS in the last 3 months.
Figure 1.

Grading scheme for overall CRS control used by patients and physicians.
Statistical Analysis
All analyses were performed with the statistical software package R (www.r-project.org).41 A total of 209 participants were recruited during the study period (July 1, 2016–June 30, 2017). This recruitment provided greater than 95% power to detect associations of medium effect size (r = 0.15) between CRS control level (as the dependent variable) and CRS disease characteristics (as the independent variable), while controlling for 14 other covariates—the largest multivariable model used in this study—at a significance level of 0.05.
In order to determine agreement between CRS control level as graded by Physician #1 and Physician #2, we employed several measures of inter‐rater agreement. We determined the overall match between physician‐rated CRS control level by calculating the joint probability of agreement. Because CRS control level is an ordinal variable, we used Pearson correlation to determine qualitative agreement as well as a two‐way intraclass correlation to determine absolute agreement.
To better understand multicollinearity within our CRS characteristic data (SNOT‐22 score, endoscopy score, number of sinus infections in the past 3 months, number of CRS‐related antibiotic courses in the last 3 months, number of CRS‐related oral corticosteroid courses in the last 3 months, and number of days of work or school missed due to CRS in the last 3 months), we used Pearson correlation to determine relationships between the different CRS disease characteristic and also performed a principal component analysis (PCA). Determination of variable loading onto each principal component was determined with a varimax rotation on the original PCA using the principal function of the psych package.
In order to calculate the relationship between the level of CRS control and CRS disease characteristic, the levels of symptom control were assigned an integer score from 1 to 5 and analyzed as 5‐level ordinal data (Fig. 1). In the case of physician‐rated CRS control, the mean value of Physician #1's and Physician #2's CRS control rating—which could obviously take values of 1.5, 2.5, 3.5, or 4.5—was used and therefore analyzed as 9‐level ordinal data ranging from 1 to 5 by increments of 0.5. Associations between the level of CRS control (as dependent variable) and CRS disease characteristics (including SNOT‐22 score or SNOT‐22 subdomain scores, number of sinus infections in the preceding 3 months, CRS‐related antibiotic use over the preceding 3 months, CRS‐related oral corticosteroid use in the preceding 3 months, and the number of missed days of work or school due to CRS in the preceding 3 months) as the independent variables were then evaluated using univariate and multivariable negative binomial regression.
In order to identify as well as characterize the sensitivity and specificity of participants' CRS characteristics for identifying patient‐reported or physician‐determined poor CRS control (which we define as control levels of “not at all,” “a little,” or “somewhat”), we analyzed receiver operating characteristic (ROC) curves with the pROC package.42 The area under the ROC curve (AUC) was calculated with the trapezoid rule using the auc function and the 95% confidence interval of the AUC was calculated by performing 2000 bootstraps of the data with the ci function. P‐value for significance of the ROC curve was determined by Wilcoxon rank‐sum test.
RESULTS
Characteristics of Study Participants
We recruited a total of 209 participants whose demographic and clinical characteristics are summarized in Table 1. Participants' CRS‐specific characteristics included a mean SNOT‐22 score of 34.1 (standard deviation [SD]: 21.2). The distributions of participants' SNOT‐22 scores and SNOT‐22 subdomain scores (which reflect severity of nasal, sleep‐related, otologic/facial pain, and emotional symptoms, respectively) are shown in Figure 2. Participants had a mean Lund‐Kennedy endoscopy score of 3.1 (SD: 2.6) and reported missing a mean of 2.0 days (SD: 7.4 days) of work or school in the last 3 months due to their CRS. Of all participants, 78.0% had at least some endoscopic findings (endoscopy score greater than 0) and 30.1% reported missing at least one day of work or school due to their CRS in the last 3 months. The distributions of participants' endoscopy scores and lost productivity due to CRS are shown in Figure 3. As reported by participants, in the last 3 months the mean number of sinus infections was 0.6 (SD: 0.8), the mean number of CRS‐related antibiotic courses taken was 0.5 (SD: 0.9) and the mean number of CRS‐related oral corticosteroid courses taken was 0.3 (SD: 0.8). Of all participants, 47.4% reported having at least one sinus infection, 34.0% reported using at least one antibiotic course for their CRS, and 20.6% reported using at least one oral corticosteroid course for their CRS in the last 3 months. The distributions of participants' number of sinus infections as well as CRS‐related antibiotic and oral corticosteroid usage in the 3 months are shown in Figure 4. The distribution of how the participants self‐reported their level of global CRS control is shown in Figure 5.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Study participants (N = 209) | |
|---|---|
| Demographics | |
| Age, mean in years, (SD) | 54.0 (16.6) |
| Gender | |
| Male | 50.7% |
| Female | 49.3% |
| Race | |
| White | 52.1% |
| Black of African American | 0.5% |
| Other | 4.3% |
| Declined to respond | 43.1% |
| Smoking | 34.9% |
| Comorbidities | |
| Asthma | 27.8% |
| Aeroallergen hypersensitivity | 42.1% |
| Aspirin sensitivity | 5.3% |
| CRS characteristics | |
| Nasal polyps | 46.9% |
| Previous sinus surgery | 45.9% |
| Intranasal steroid use | 47.4% |
| Endoscopy score, mean (SD) | 3.1 (2.6) |
| SNOT‐22 score, mean (SD) | 34.1 (21.2) |
| Missed days of work or school due to CRS in the last 3 months, mean (SD) | 2.0 (7.4) |
| Sinus infections in the last 3 months, mean (SD) | 0.6 (0.8) |
| CRS‐related antibiotic courses in the last 3 months, mean (SD) | 0.5 (0.9) |
| CRS‐related oral corticosteroid courses in the last 3 months, mean (SD) | 0.3 (0.8) |
Figure 2.
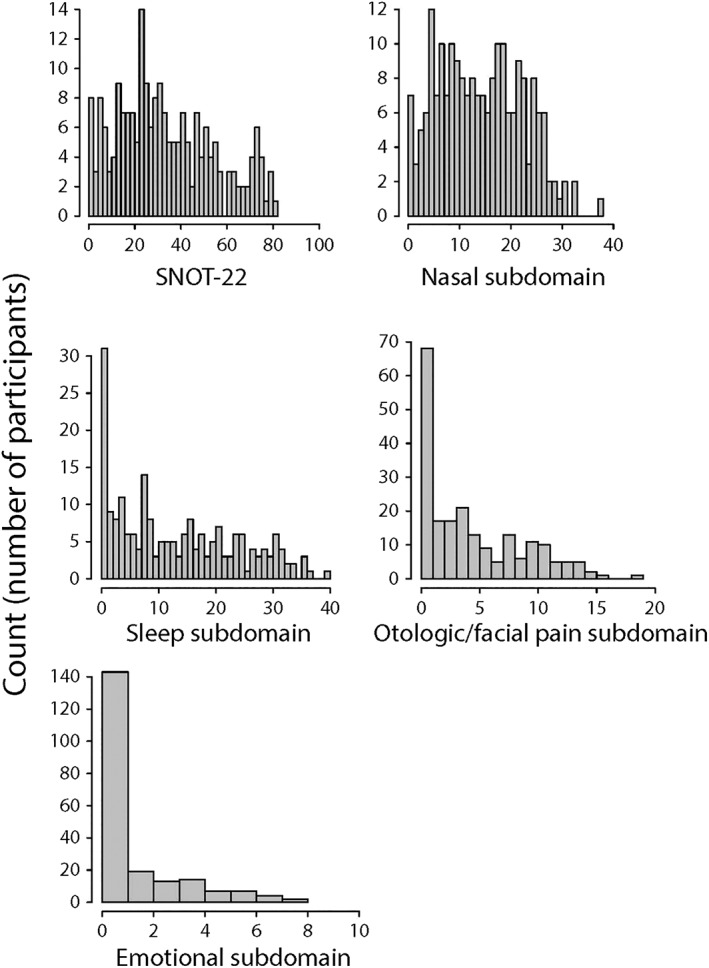
Histogram plots of participants' SNOT‐22 scores, as well as nasal, sleep, otologic/facial pain and emotional SNOT‐22 subdomain scores.
Figure 3.
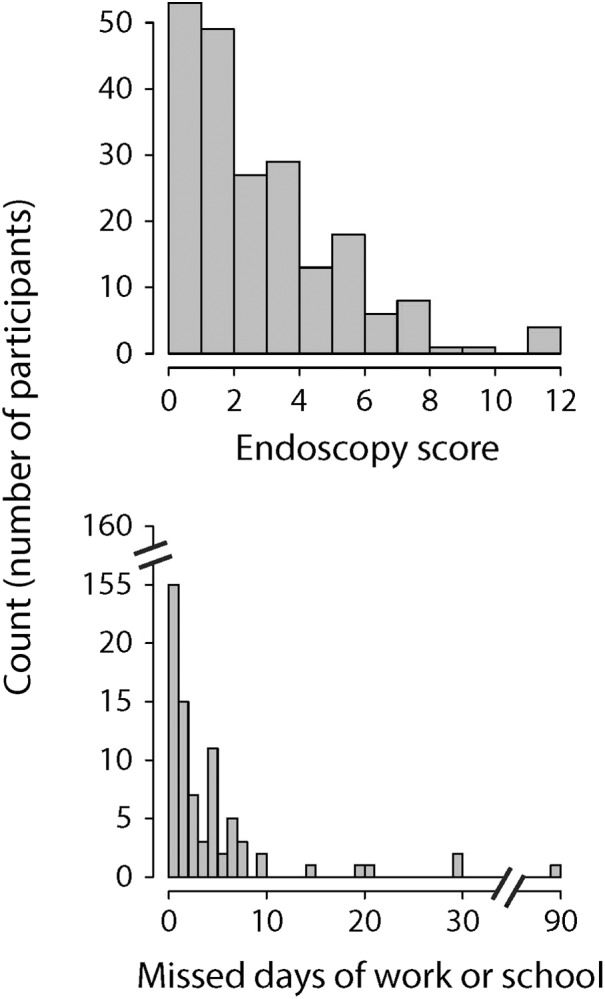
Histogram plots of participants' Lund‐Kennedy endoscopy scores and number of missed days of work or school due to CRS in the last 3 months.
Figure 4.
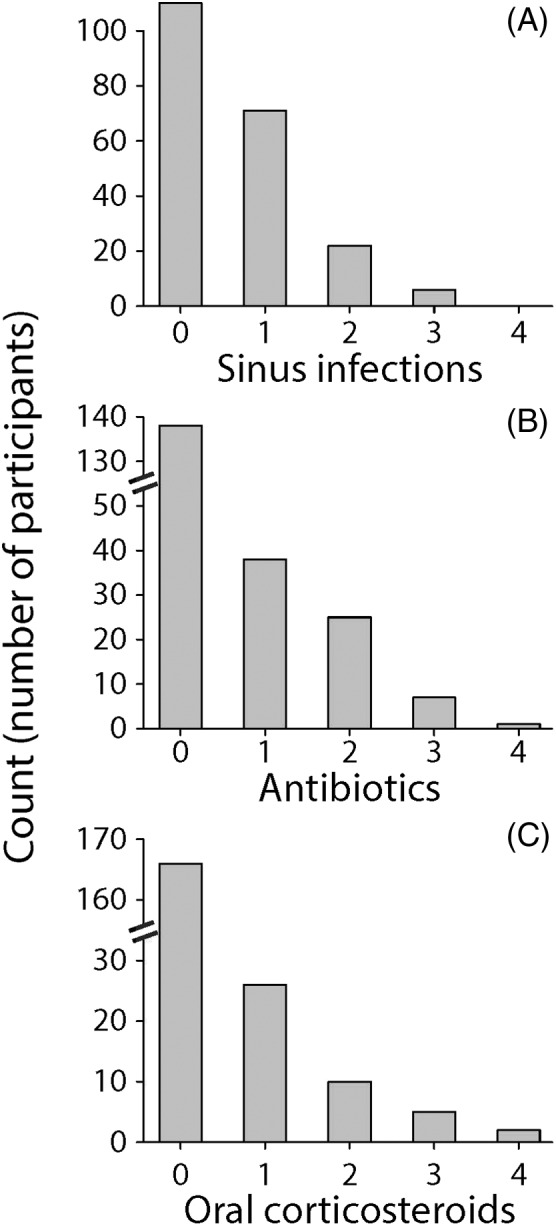
Histogram plots of participants' number of (A) sinus infections, (B) CRS‐related antibiotic courses, and (C) CRS‐related oral corticosteroid courses, all in the last 3 months.
Figure 5.
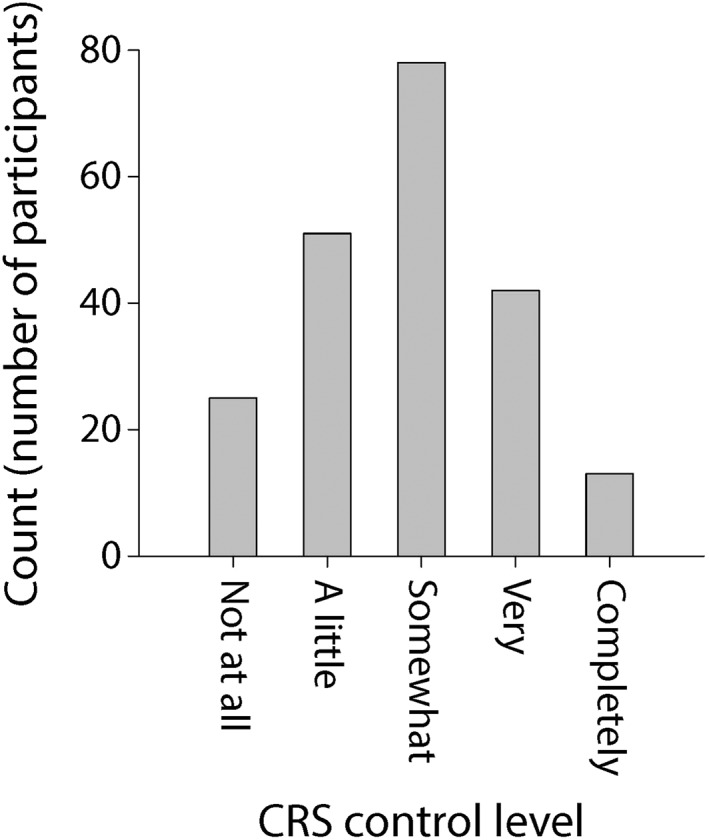
Histogram plot of participants' self‐reported level of CRS control.
Multicollinearity of CRS Disease Characteristics
We next sought to determine how participants' CRS disease characteristics might associate with their self‐reported global CRS control level. Previous studies suggest that the CRS disease characteristics we are studying—SNOT‐22 score, nasal endoscopy score, sinus infection frequency, frequency of systemic antibiotic/oral corticosteroid usage and productivity loss—may be correlated.9, 35, 43 Strong correlation between variables in a multivariable model—termed multicollinearity—may artificially increase standard errors and therefore reduce the ability to detect statistical significance of individual predictor variables amongst the correlated variables.44 We therefore sought correlations between all of the CRS disease characteristics that we have studied here (Fig. 6). Upon examining the correlation matrix shown in Figure 6, which illustrates a heat map for the coefficients of correlation between CRS disease characteristics, five distinct variable clusters are apparent reflecting: CRS symptomatology (SNOT‐22 score), acute bacterial CRS exacerbations (patient‐reported sinus infections and CRS‐related antibiotic use), CRS‐related oral corticosteroid use, endoscopy score and lost productivity.
Figure 6.
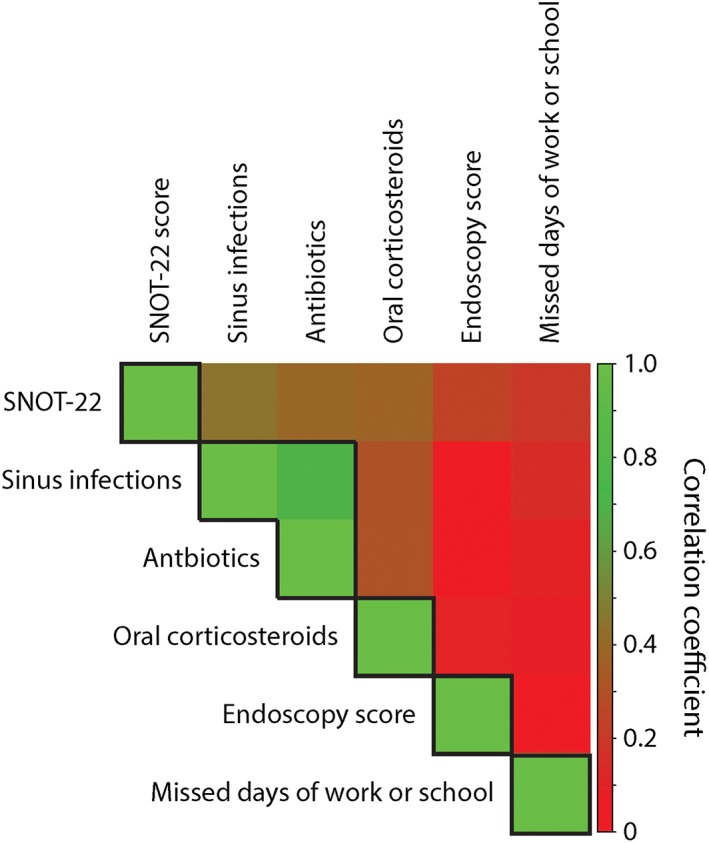
Correlation matrix of CRS disease characteristic data, reflecting SNOT‐22 score, endoscopy score, number of sinus infections in the last 3 months, the number of CRS‐related antibiotic courses in the last 3 months, the number of CRS‐related oral corticosteroids in the last 3 months, and number of days of missed work or school due to CRS in the last 3 months. Correlation coefficients are color‐coded on a scale (shown on the right) from 0.0, representing no correlation, as red to 1.0, representing perfect correlation, as green. Boxes are drawn around variables reflecting putatively distinct CRS disease characteristics.
PCA, an analytical technique, can also be used to detect collinear variables. When PCA is performed on a set of variables, the most collinear variables will be found to cluster together on common principal components so that the primary variables that comprise each principal component are deemed to be variables that likely reflect the same underlying process.44 In order to confirm collinearity within our data, which we suspected based on the correlation matrix shown in Figure 6, we performed PCA on our participants' CRS disease characteristic data. Because we specifically hypothesized that the CRS disease characteristics, which we studied, reflected five independent variable clusters based on our findings in Figure 6, we examined the first five principal components of the PCA. The contributions (loadings) of each CRS disease characteristic onto the first five principal components, which account for 95% of the total variability in the data, most dominantly reflected CRS symptomatology (SNOT‐22 and associated subdomain scores), acute bacterial CRS exacerbations (patient‐reported sinus infections and CRS‐related antibiotic use in the last 3 months), CRS‐related oral corticosteroid use in the last 3 months, endoscopy score and lost productivity due to CRS (Table 2). These results confirmed the suspected pattern of variable collinearity suggested by Figure 6. These results also suggest that the number of patient‐reported sinus infections and the number of patient‐reported CRS‐related antibiotics are highly correlated and inclusion of both in the same multivariable model could interfere with detection of either as a statistically significant predictor for global CRS control. Henceforth, we utilize patient‐reported CRS‐related antibiotic usage in multivariable analyses but for each multivariable analysis we also checked that our results would not qualitatively change by using the number of sinus infections instead.
Table 2.
Loadings of CRS Disease Characteristics on PC
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| SNOT‐22 | 0.27 | 0.14 | 0.10 | 0.17 | 0.93 |
| Endoscopy score | −0.02 | 0.99 | −0.02 | 0.04 | 0.11 |
| Sinus infections in the last 3 months | 0.88 | 0.02 | 0.07 | 0.10 | 0.21 |
| CRS‐related antibiotics in the last 3 months | 0.91 | −0.04 | 0.03 | 0.14 | 0.11 |
| CRS‐related oral corticosteroids in the last 3 months | 0.18 | 0.04 | 0.03 | 0.97 | 0.16 |
| Missed days of work or school in the last 3 months due to CRS | 0.06 | ‐0.02 | 0.99 | 0.03 | 0.09 |
Loadings that are shown in bold and italics are those that most dominantly influence the correspond principal component.
Factors Associated With Patient‐Reported Global CRS Control
We next determined associations between patient‐reported global CRS control and CRS characteristics of SNOT‐22 score, the number of sinus infections in the last 3 months, the number of CRS‐related antibiotic courses in the last 3 months, the number of CRS‐related oral corticosteroid courses in the last 3 months, and the number of missed days of work or school due to CRS in the last 3 months (Table 3). We did not consider nasal endoscopy score as a factor that would be associated with patient‐reported CRS control in a meaningful way since we would not expect patients to know their nasal endoscopy score. In other words, we would not expect patients to be fully cognizant of their nasal endoscopy findings, or to consider those findings in their own assessment of CRS disease control. On univariate analysis we found that patient‐reported CRS control was significantly associated with SNOT‐22 score, the number of sinus infections in the last 3 months, and the number of CRS‐related antibiotic courses taken in the last 3 months (Table 3). However, in a multivariable regression model that used SNOT‐22 score, the number of CRS‐related antibiotic courses, the number of CRS‐related oral corticosteroid courses, and the number of missed days of work or school due to CRS as independent variables, only SNOT‐22 (RR = 0.99, 95% CI: 0.98–0.99, P < .001) score was significantly associated with patient‐reported CRS control level (Table 3). This result indicates, for example, that a 26‐point SNOT‐22 increase would be associated with a 25% reduction in patient‐reported CRS control. We additionally repeated the multivariable analysis controlling for characteristics that might confound how participants may rate CRS control level including characteristics of age, gender, smoking history, comorbid asthma, aeroallergen hypersensitivity, polyps, use of intranasal steroids, and history of prior sinus surgery. Even after controlling for these covariates, we still found that only SNOT‐22 score was associated with patient‐reported CRS control. Utilizing the number of sinus infections instead of the number of CRS‐related antibiotic courses used in the last 3 months did not qualitatively change these results either. These results indicate that patients most prominently utilize severity of their CRS symptoms in assessing their global CRS control.
Table 3.
Association Between Patient‐Reported CRS Control Level and CRS Disease Characteristics
| Univariate analysis | Multivariable analysisa | |||
|---|---|---|---|---|
| RRb (95% CI) | P value | RRb (95% CI) | P value | |
| SNOT‐22 score | 0.99 (0.98–0.99) | < 0.001 | 0.99 (0.98–0.99) | <.001 |
| Number of sinus infection in last 3 months | 0.81 (0.73–0.91) | < 0.001 | — | — |
| Number of CRS‐related antibiotics in last 3 months | 0.86 (0.77–0.95) | 0.003 | 0.94 (0.84–1.05) | .294 |
| Number of CRS‐related oral corticosteroids in last 3 months | 0.91 (0.81–1.02) | 0.116 | 1.04 (0.92–1.18) | .493 |
| Number of days of missed work or school due to CRS in last 3 months | 0.99 (0.98–1.01) | 0.240 | 1.00 (0.99–1.01) | .986 |
Including SNOT‐22 score, number of CRS‐related antibiotics, number of CRS‐related oral corticosteroids and missed days of work or school due to CRS as independent variables.
Relative risk
Symptoms of CRS Differentially Associate With Patient‐Reported CRS Control Level
Since we found SNOT‐22 score to most dominantly associate with patient‐reported CRS control, we next sought to further define the CRS symptoms that most associated with patients' reported level of CRS control. We therefore sought associations between patient‐reported CRS control and SNOT‐22 subdomain scores (Table 4). We found that all subdomain scores were associated with patient‐reported CRS control on univariate analysis (Table 4). This result was confirmed and further characterized by finding that the severity of each symptom represented on the SNOT‐22 was associated with patient‐reported CRS control (Table A1 in Appendix). However, in a multivariable regression model that accounted for all subdomain scores simultaneously, only the nasal subdomain score (RR = 0.98, 95% CI: 0.97–0.99, P = .006) remained significantly associated with patient‐reported CRS control. This result indicates, for example, that a 15‐point increase in the nasal subdomain score of the SNOT‐22 is associated with a 25% reduction in patient‐reported CRS control. This association between patient‐reported CRS control and the nasal subdomain score remained statistically significant (while all other SNOT‐22 subdomains were not significantly associated) even after controlling for other CRS disease characteristics (CRS‐related antibiotic usage or sinus infections, CRS‐related oral corticosteroid usage, and CRS‐related lost productivity) as well as patient characteristics of age, gender, smoking history, comorbid asthma, aeroallergen hypersensitivity, polyps, use of intranasal steroids, and history of prior sinus surgery, any of which might potentially influence how patients would perceive their CRS disease status. These results indicate that the severities of nasal symptoms, such as obstruction or drainage, are most prominently associated with patients' perception of their global CRS control.
Table 4.
Association Between Patient‐Reported CRS Control Level and SNOT‐22 Subdomain Scores
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| RRa (95% CI) | P value | RR1 (95% CI) | P value | |
| Nasal subdomain | 0.97 (0.96–0.98) | <.001 | 0.98 (0.97–0.99) | .006 |
| Sleep subdomain | 0.98 (0.97–0.99) | <.001 | 0.99 (0.98–1.01) | .224 |
| Otologic/facial pain subdomain | 0.96 (0.94–0.98) | <.001 | 0.99 (0.98–1.01) | .570 |
| Emotional subdomain | 0.92 (0.88–0.97) | .001 | 0.99 (0.93–1.05) | .738 |
Relative risk
Inter‐Physician Reliability of Rating CRS Control
We next sought to determine how participants' CRS disease characteristics might influence physician‐rated CRS control. However, we first sought to explore the degree of consistency between the two physicians (Physician #1 and Physician #2) who independently rated participants' CRS control level (Fig. 7). We found that the joint probability of agreement—the percentage of study participants who were rated to have the same exact level of CRS control by the physician graders—was 41.6%. However, we found that 95.7% of participants received CRS control ratings by the two physician graders that were exactly the same or differed by one level. We also found that the CRS control ratings by Physician #1 and Physician #2 were strongly correlated (r = 0.85, 95% CI: 0.81–0.88, P < .001). Likewise, we found excellent agreement with Intraclass Correlation (Intraclass Correlation Coefficient = 0.75, 95% CI: 0.57–0.84, P < .001). These results indicate that the rating of CRS control was a consistent metric between the two physicians who independently rated participants' global CRS control level.
Figure 7.
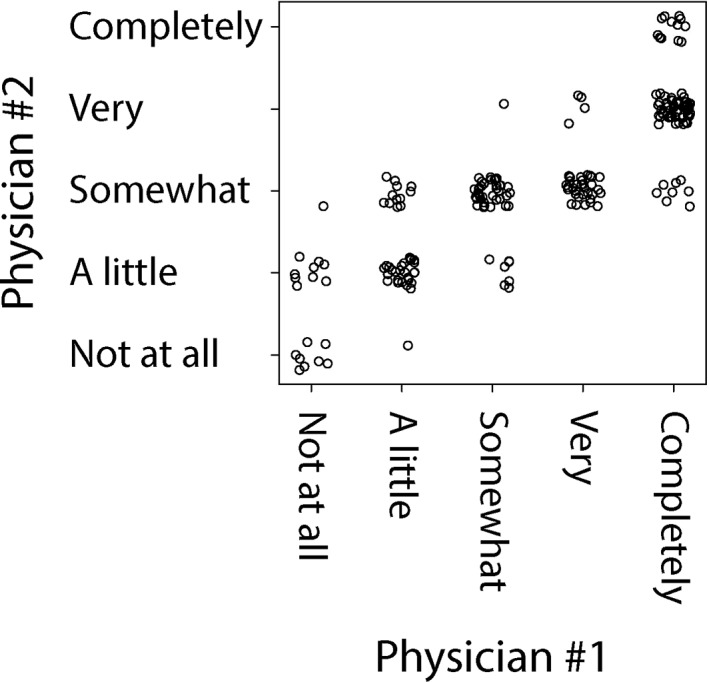
Scatter plot of participants' CRS control level as graded by Physician #1 vs. Physician #2.
Factors Associated With Physician‐Rated Global CRS Control
Physician‐rated CRS control was only moderately correlated with patient‐reported CRS control (r = 0.58, 95% CI: 0.48–0.66, P < .001), indicating that there are likely different factors that may influence physician‐rated versus patient‐reported global CRS control. We next sought to characterize physician‐rated CRS control by determining associations between physician‐rated CRS control and CRS characteristics of SNOT‐22 score, endoscopy score, the number of CRS‐related antibiotic courses in the last 3 months, the number of CRS‐related oral corticosteroid courses in the last 3 months, and the number of missed days of work or school due to CRS in the last 3 months (Table 5). We found that on univariate analysis all of these CRS disease characteristics were associated with physician‐rated CRS control (Table 5). However, in a multivariable regression model that used all of these CRS disease characteristics as independent variables, only SNOT‐22 score (RR = 0.99, 95% CI: 0.98–0.99, P < .001), the number of CRS‐related antibiotics (RR = 0.89, 95% CI: 0.82–0.98, P = .014) and the number of oral corticosteroid courses (RR = 0.87, 95% CI: 0.78–0.97, P = .012) taken in the last 3 months were significantly associated with physician‐rated CRS control level. These results reflect, for example, that a 25% reduction in physician‐reported CRS control is associated with a 20‐point increase in SNOT‐22, two courses of CRS‐related antibiotics or two courses of CRS‐related oral corticosteroids in the last 3 months. Missed days of work or school due to CRS reported by patients trended towards association (RR = 0.99, 95% CI: 0.98–1.00, P = .065) while endoscopy score was not at all associated with physician‐rated CRS control (RR = 1.00, 0.98–1.02, P = .938). We did not include any other patient characteristics in our multivariable model since the physician graders did not have access to any other clinical or demographic patient data when rating global CRS control. Additionally, these results did not qualitatively change by using the number of sinus infections instead of the number of CRS‐related antibiotics courses in the last 3 months in the multivariable model. These results indicate that the physician graders in this study considered CRS symptom severity, acute bacterial CRS exacerbation frequency (reflected by patient‐reported sinus infections or CRS‐related antibiotics usage) and CRS‐related oral corticosteroids utilization in assessing participants' global CRS control.
Table 5.
Association Between Physician‐Rated CRS Control Level and CRS Disease Characteristics
| Univariate analysis | Multivariable analysisa | |||
|---|---|---|---|---|
| RRb (95% CI) | P value | RRb (95% CI) | P value | |
| SNOT‐22 score | 0.98 (0.97–0.99) | <.001 | 0.99 (0.98–0.99) | <.001 |
| Endoscopy score | 0.97 (0.95–0.99) | .008 | 1.00 (0.98–1.02) | .938 |
| Number of sinus infection in last 3 months | 0.71 (0.66–0.78) | <.001 | — | — |
| Number of CRS‐related antibiotics in last 3 months | 0.74 (0.69–0.81) | <.001 | 0.89 (0.82–0.98) | .014 |
| Number of CRS‐related oral corticosteroids in last 3 months | 0.70 (0.63–0.78) | <.001 | 0.87 (0.78–0.97) | .012 |
| Number of days of missed work or school due to CRS in last 3 months | 0.96 (0.94–0.98) | <.001 | 0.99 (0.98–1.00) | .065 |
Including SNOT‐22 score, number of CRS‐related antibiotics, number of CRS‐related oral corticosteroids and missed days of work or school due to CRS as independent variables.
Relative risk
Relationship of CRS Symptoms With Physician‐Rated CRS Control Level
Just as we found for patient‐reported CRS control, we also found SNOT‐22 score to be strongly associated with physician‐rated CRS control level. Therefore, we next sought to determine if the severity of particular categories of CRS symptomatology were differentially associated with physician‐rated CRS control level. Both physician graders in this study were provided with the participants' SNOT‐22 nasal, sleep, otologic/facial pain and emotional subdomain scores to consider in their rating of overall CRS control level. We therefore sought associations between SNOT‐22 subdomains scores and physician‐rated CRS control level (Table 6). On univariate analysis, we found that all SNOT‐22 subdomain scores were significantly associated with physician‐rated CRS control (Table 6). Because the physician graders did not view participants' SNOT‐22 surveys while grading participants' global CRS control, we did not calculate associations between individual SNOT‐22 items scores and physician‐rated CRS control. Using a multivariable regression model that accounted for all subdomain scores simultaneously, the nasal (RR = 0.98, 95% CI: 0.97–0.99, P < .001), sleep (RR = 0.98, 95% CI: 0.97–0.99, P = .001) and otologic/facial pain (RR = 0.98, 95% CI: 0.96–0.99, P = .012) subdomain scores remained significantly associated with physician‐reported CRS control. These results indicate that the contribution of CRS symptoms to physician‐rated CRS control includes not only the nasal symptoms classically associated with CRS but also the extra‐nasal symptoms of CRS associated with patients' functional status (sleep) and discomfort (ear pressure or facial pain).
Table 6.
Association Between Physician‐Rated CRS Control Level and SNOT‐22 Subdomain Scores
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| RRa (95% CI) | P value | RR1 (95% CI) | P value | |
| Nasal subdomain | 0.96 (0.95–0.97) | <.001 | 0.98 (0.97–0.99) | <.001 |
| Sleep subdomain | 0.97 (0.96–0.98) | <.001 | 0.98 (0.97–0.99) | .001 |
| Otologic/facial pain subdomain | 0.93 (0.92–0.94) | <.001 | 0.98 (0.96–0.99) | .012 |
| Emotional subdomain | 0.86 (0.83–0.89) | <.001 | 0.97 (0.92–1.01) | .151 |
Relative risk
Quantitative CRS Disease Characteristics Accurately Detect Poorly Controlled CRS
We next sought to determine if CRS disease characteristics could be used to detect poorly controlled CRS, which we define as control levels of “not at all,” “a little,” or “somewhat” as previously described, using ROC analysis.45 We first evaluated whether threshold values for any of the CRS disease characteristics that we studied here could be used to detect participants who rated their CRS as poorly controlled. We found that all characteristics (excluding endoscopy score, which we felt was not meaningful for patient‐reported CRS control) could be used to accurately detect patient‐reported poor CRS control (Table 7). The ROC curves of all CRS disease characteristics in detecting patient‐determined poor CRS control are shown in Figure 8. We likewise found that all of the same CRS disease characteristics could be used to accurately detect physician‐rated poor CRS control (which we defined as a mean physician control score of ≤ 3.5) (Table 8). The ROC curves of all CRS disease characteristics in detecting physician‐determined poor CRS control are shown in Figure 9. By comparing the results in Table 7 and Table 8, it is apparent that utilizing at least one course of CRS‐related antibiotic or oral corticosteroid in the last 3 months, reporting at least one sinus infection or missing at least one day of work or school due to CRS in the last 3 months, all served as optimal thresholds for detecting patient‐reported or physician‐rated poor CRS control. Additionally, a similar level of CRS symptom severity (as reflected by SNOT‐22) was associated with poor disease control as rated by both patients and physicians. We likewise found similar thresholds of CRS disease characteristics for detecting patients whose CRS disease control was rated as poor by both the patient and physician (Table A2 and Figure A1 in the Appendix).
Table 7.
Accuracy of CRS Disease Characteristics to Detect Patient‐Reported Poorly Controlled CRS
| P value | AUC (95% CI) | Optimal cut‐off valuea | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| SNOT‐22 score | <.001 | 0.87 (0.81–0.92) | >20 | 85.7% | 72.7% |
| No. of sinus infectionsb | <.001 | 0.76 (0.70–0.81) | >0 | 60.4% | 89.1% |
| No. of CRS‐related antibioticsb | <.001 | 0.67 (0.62–0.73) | >0 | 42.9% | 90.9% |
| No. of CRS‐related oral corticosteroidsb | <.001 | 0.61 (0.57–0.66) | >0 | 26.6% | 96.4% |
| No. of missed days or work or school due to CRS2 | <.001 | 0.68 (0.64–0.73) | >0 | 39.6% | 96.4% |
Maximizes the sum of sensitivity and specificity
In the last 3 months
Figure 8.
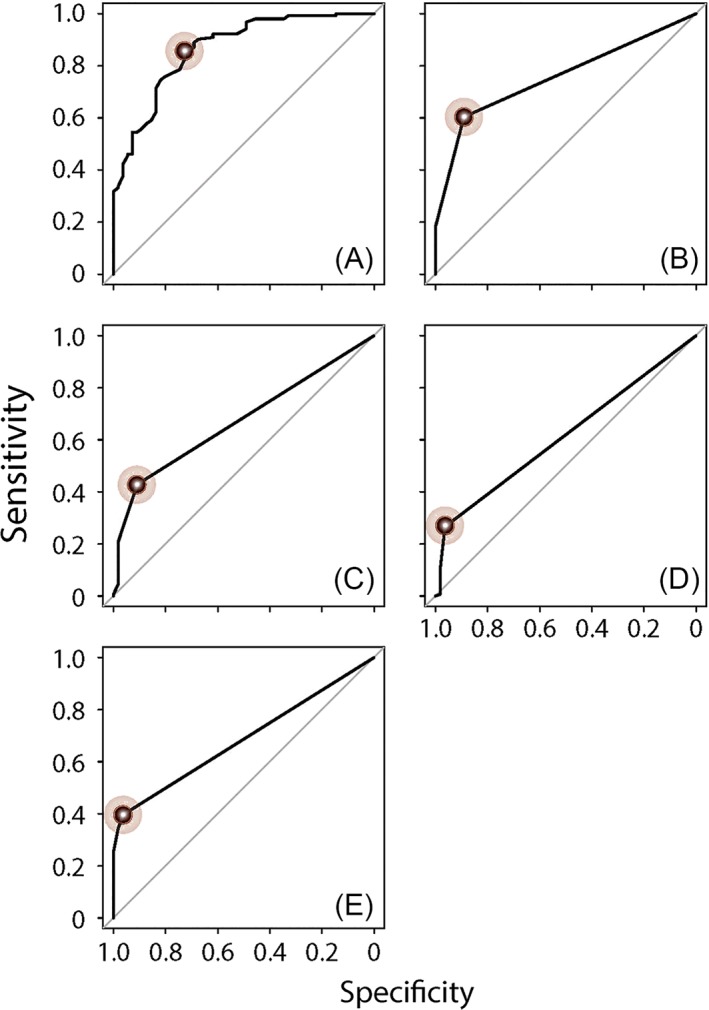
ROC curves to detect patient‐reported poorly controlled CRS using independent variables of (A) SNOT‐22 score, (B) number of sinus infections in last 3 months, (C) CRS‐related antibiotics courses in last 3 months, (D) CRS‐related oral corticosteroids courses in the last 3 months, and (E) number of missed days of work or school due to CRS in last 3 months. The points reflecting the optimal cut‐off for each statistically significant independent variable, which are summarized in Table 7 and maximized the sum of sensitivity and specificity, are marked in each panel.
Table 8.
Accuracy of CRS Disease Characteristics to Detect Physician‐Reported Poorly Controlled CRS
| P value | AUC (95%CI) | Optimal cut‐off valuea | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| SNOT‐22 score | <.001 | 0.97 (0.94–0.99) | >25 | 88.9% | 93.2% |
| Endoscopy score | .025 | 0.59 (0.51–0.67) | >0 | 83.7% | 32.4% |
| No. of sinus infectionsb | <.001 | 0.75 (0.70–0.81) | >0 | 64.4% | 83.8% |
| No. of CRS‐related antibioticsb | <.001 | 0.71 (0.66–0.76) | >0 | 48.1% | 91.9% |
| No. of CRS‐related oral corticosteroidsb | <.001 | 0.63 (0.59–0.67) | >0 | 29.6% | 95.9% |
| No. of missed days or work or school due to CRSb | <.001 | 0.72 (0.67–0.76) | >0 | 45.2% | 97.3% |
Maximizes the sum of sensitivity and specificity
In the last 3 months
Figure 9.
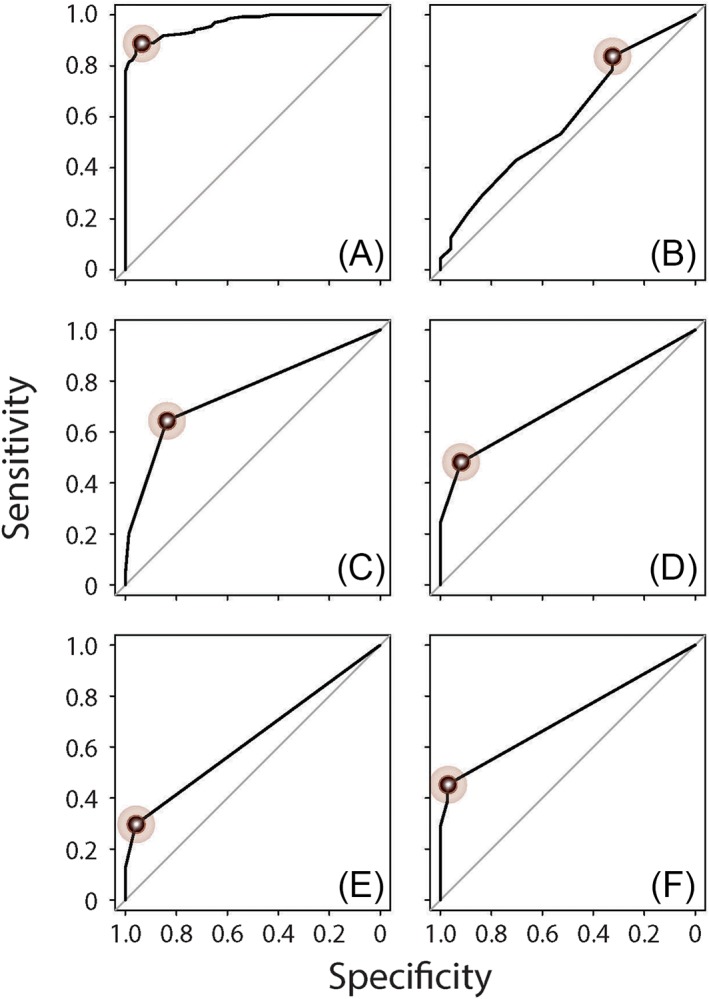
ROC curves to detect physician‐determined poorly controlled CRS using independent variables of (A) SNOT‐22 score, (B) endoscopy score, (C) number of sinus infections in last 3 months, (D) CRS‐related antibiotics courses in last 3 months, (E) CRS‐related oral corticosteroids courses in the last 3 months, and (F) number of missed days of work or school due to CRS in last 3 months. The points reflecting the optimal cut‐off for each statistically significant independent variable, which are summarized in Table 8 and maximized the sum of sensitivity and specificity, are marked in each panel.
DISCUSSION
CRS is a highly prevalent disease that imparts a significant QOL detriment on afflicted individuals as well as a significant cost burden on society as a whole. A standardized approach to treatment of CRS could lead to significant improvements in patient outcomes and reduction in the overall impact of this disease by providing all patients a minimum level of high quality care, independent of care provider experience with or expertise in CRS. For chronic incurable diseases, the concept of control is often used and translated into a comprehensive quantitative metric of disease status upon which treatment decisions can be made in real‐time. The concept of disease control incorporates important but independent longitudinal measures of disease severity and their impacts on patients. The clinical utility of defining disease control has been illustrated in the care of asthmatics where a standardized definition of asthma control now serves as the foundation upon which long term therapy is titrated in a standardized manner.21, 23 One way in which CRS differs from asthma is that the subjective perception of CRS by patients is a primary driver of treatment in addition to physicians' evaluation of patients' disease. Although clinical decisions regarding titration of therapy for CRS are made every day based on patients' and physicians' implicit assessment of CRS control level, currently there is no formal definition of CRS control. In this study, we used a top‐down approach to understanding CRS control from the perspectives of patients and physicians. We asked a large cohort of CRS patients to rate their own level of global CRS control. Two rhinologists also independently rated these patients' global CRS control level with knowledge of only six CRS disease characteristics for each patient: SNOT‐22 score, nasal endoscopy score, and in the last 3 months: the number of sinus infections, CRS‐related antibiotic courses used, CRS‐related oral corticosteroid courses used and missed days of work or school due to CRS. We found that the only disease characteristic that independently associated with patients' rating of their global CRS control was the SNOT‐22 score (most prominently, the nasal subdomain score of the SNOT‐22). In comparison, we found that physician‐rated CRS control was associated with the SNOT‐22 score (including nasal and extra‐nasal symptom subdomains) as well as the number of CRS‐related antibiotics (likely a reflection of acute bacterial CRS exacerbations) and the number of CRS‐related oral corticosteroid courses taken in the last 3 months.
Previous work has sought to develop a definition of CRS control. The 2012 European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) proposed a staging system for disease control in CRS as a metric that could be used longitudinally to assess improvements in patients' CRS symptoms and the health of their sinonasal mucosa (eg, reduction of edema, inflammation or mucopurulent drainage), upon which decisions could be made for titration of treatment.18 The EPOS staging system for CRS control was based on symptoms (nasal obstruction, rhinorrhea, facial pain, hyposmia/anosmia, and sleep disturbance), nasal endoscopy and the need for any systemic antibiotics or corticosteroids in the last 3 months. Based on how much each of these disease characteristics posed a problem to the patient, the EPOS staging system recommended a rating of “controlled,” “partly controlled,” or “uncontrolled.” The major limitation of this staging system, which was acknowledged by the 2012 EPOS, was that this staging system was not data driven but, instead, was designed based solely on expert opinion. This staging system was developed based on criteria that experts in the field thought they would use to assess a patient's CRS control without determining whether this standard was being employed in actual practice. In addition, this staging system did not consider patient perception of CRS control.
A subsequent study by Snidvongs et al. sought to evaluate the predicative efficacy of the EPOS staging system for CRS control.27 The authors analyzed data from 106 patients who had undergone endoscopic sinus surgery (ESS) and who were followed longitudinally at 6‐ and 12‐month time points after ESS. Physician‐rated CRS disease control was assessed by one of the authors who used the same “controlled,” “partly controlled,” or “uncontrolled” scale as the EPOS staging system but used a predefined set of criteria that was similar but distinct from the EPOS staging system. Patients were asked to rate their degree of improvement or deterioration of nasal function after ESS using a 13‐point Likert scale ranging from ‐6 (significant deterioration) to + 6 (significant improvement), which the authors referred to as the global anchor score. The authors then categorized patients' level of CRS control based on the global anchor score. CRS disease characteristic data, including CRS‐related systemic medication usage, SNOT‐22 scores, and endoscopy score, were collected from each participant. In this study, only nasal obstruction symptoms associated with patient‐reported CRS control while only endoscopic findings associated with the physician‐rated CRS control level. Based on these findings, the authors developed a simplified staging system that solely incorporated nasal obstruction symptomatology and endoscopic findings. The authors also recommended using systemic medication usage in their staging system since these medications can artificially improve symptomatology and endoscopy score, and must therefore be accounted for in any measure of control. The authors found that this simple scale, based on nasal symptomatology, endoscopy score, and systemic medication usage, outperformed the EPOS staging system in associating with patient‐ and physician‐rated CRS control for their cohort. This study by Snidvongs et al. was the first to take a systematic approach to understanding CRS disease control. However, this study also suffers from several limitations. As this study was conducted in postsurgical patients, patient‐reported control is based on a global anchor score that was assessed by asking patients how their nasal function improved or deteriorated and thus the authors' measure of patient‐reported CRS control was more reflective of improvement in nasal function after surgery rather than a global assessment of patients' CRS. Moreover, because the authors' metric for patient‐reported CRS control was derived from a question that specifically asked patients about nasal symptoms, a significant bias may have been introduced that could explain why the authors' metric for patient‐reported CRS control was only associated with the severity of nasal obstruction, but no other symptom represented on the SNOT‐22. The authors also considered the frequency of systemic medication by assessing for at least one course of either an antibiotic or oral corticosteroid in the last 3 months with no evidence‐based justification for this threshold of antibiotic or oral corticosteroid use. Finally, the physician‐rated CRS control determination was performed by a single physician, which itself could lead to measurement (experimenter) bias. Additionally, the predefined criteria for how the physician grader would rate CRS control was dominated by endoscopic findings. For example, complete CRS control was determined by “normal mucosa on endoscopy … regardless of symptomatology.” This inherent bias towards endoscopic findings in the a priori criteria for physician‐rated CRS control level may explain why only endoscopy score, and no other CRS disease characteristic, was associated with physician‐rated CRS control level.
A more recent study by Banglawala et al. sought to develop greater understanding of global CRS control.28 Banglawala et al. used a comprehensive approach to identifying disease characteristics that would associate with patient‐ and physician‐rated CRS control. Through literature review, a moderated focus group interview with 20 patients, and a multidisciplinary working group of 11 experts in the field of rhinosinusitis, allergy, pediatrics, and primary care who were invited to participate, a preliminary set of 12 CRS disease characteristics were chosen as reflective of global CRS control, including CRS‐related symptomatology (nasal obstruction, nasal discharge, facial pain, hyposmia/anosmia, sneezing, cough, headache), lost productivity, impact on daily activities, use of any systemic antibiotics or corticosteroids, escalation of maintenance medical therapy and patient‐reported CRS symptom control. Moreover the authors chose to assess these criteria over a two‐week period. A total of 50 patients were enrolled, and they were asked to rate their level of CRS disease control as “not at all,” “a little,” “somewhat,” “very well,” or “completely.” For each patient, the treating physician rated the patient's global CRS disease control on the same three‐level scale used by EPOS and Snidvongs et al. (“controlled,” “partly controlled,” or “uncontrolled”), based on history, nasal endoscopy, and computed tomography (if available). By seeking association between patient‐reported and physician‐rated global CRS disease control and scores for each of their 12 preliminary CRS disease characteristics, Banglawala et al. were able to reduce their preliminary 12 control‐related CRS characteristics to a final set of four CRS disease characteristics relating to nasal obstruction, nasal discharge, lost productivity and systemic medication use for CRS in the last 2 weeks. The authors found that the severity of nasal obstruction and need for systemic medications due to CRS was associated with physician‐rated CRS disease control while the severity of nasal discharge and lost productivity due to CRS was associated with patient‐reported CRS disease control. This work by Banglawala et al.,28 which has been further assessed by Kohli et al.,46 is a notable first step toward the development of a global CRS assessment tool. However, this work was limited in several ways. Although the authors designed their survey based on the input of experts across different medical specialties, all patients' CRS control was assessed by a single physician (the treating physician). Thus although multiple otolaryngologists were involved in this study, each patient's CRS control was assessed by one physician. Noise and variability that is introduced into the data by rating CRS control levels of subsets (of the already small number) of participants by different physicians, each of whom may have used different physician‐specific criteria, may have reduced the power to detect other disease characteristics associated with physician‐rated CRS disease control. Additionally, physician‐rated CRS disease control was not assessed in a standardized manner. Although it is a strength of this study that physician‐rated CRS control was not based on predefined criteria, which could bias associations between disease characteristics and physician‐rated CRS control, it is unclear if the patient histories contained uniform information for the physician graders to evaluate. Therefore the physician graders in this study may have had different information from which to assess patients' global CRS control level, which could have introduced additional noise or variability in the data. This study also included only 50 patients, which was a sample size chosen by the authors to meet previous recommendations for development of patient‐reported outcome measures. However, because the authors used multivariable regression to detect CRS disease characteristics associated with patient‐reported or physician‐rated CRS disease control, it is possible that this study was underpowered to detect all of the CRS disease characteristics that could be associated with CRS control. The authors also used a time frame of two weeks over which to assess CRS control. The utilization of a uniform time frame over which to assess CRS control is a strength of the study, however, it is possible that this time frame is too short to assess several disease manifestations such as the need for systemic antibiotics or corticosteroids or lost productivity. In our cohort, for example, 20% to 34% reported missing work or school, or using a systemic antibiotic or corticosteroid due to CRS in the last 3 months—a time scale that is six times longer than that assessed by Banglawala et al. Although it is impossible to know how many patients responded affirmatively to any of these metrics over the preceding two‐week period, it is likely considerably smaller than 20% to 34%, which could lead to difficulty in detecting association between these metrics and patient‐reported or physician‐rated CRS control. Finally, like Snidvongs et al., Banglawala et al. did not differentiate between antibiotic usage and oral corticosteroid usage, nor did they consider the absolute number of courses of these medications taken by the patients.
In our study, we performed a systematic study of CRS disease characteristics that are most associated with patient‐reported and physician‐rated global CRS control. Our study design included asking patients to rate their own CRS control and then asking two rhinologists, blinded to every patient attribute except the six disease characteristics that we studied, to independently rate the patients' global CRS control. Although similar to past studies by determining associations between global CRS control level and CRS disease characteristics,27, 28 our study design included some improvements over previous studies. For example, we used the same scale for grading CRS disease control by patients and physicians so that any associations with patient‐reported and physician‐rated control would be comparable. We also enrolled a large number of study participants,27 which provided sufficient power to detect associations between CRS control and CRS disease characteristics while controlling for multiple confounding variables. Two rhinologists independently graded every study participant's global CRS control using the same standardized patient data, which we believed would reduce the impact of idiosyncratic biases of either of the physician graders. After compiling our list of CRS disease characteristics that could potentially influence perception of CRS control, we performed a PCA to determine which of those characteristics were distinct and independent CRS disease characteristics so that unnecessary noise and variability, which could reduce our power to detect statistically significant associations, would not be introduced into our multivariable models. Finally, our study design allowed consideration for the number of antibiotic or oral corticosteroid courses that patients took in the determination of physician‐rated CRS control. Moreover, rather than using a predetermined criteria for systemic medication usage, we used ROC analysis to determine optimal thresholds of CRS‐related systemic medication usage for poor‐ versus well‐controlled CRS.
Our results showed that the dominant (and only) factor associated with patient‐reported global CRS control was symptom severity. By further dissecting this association, we found that only the severity of nasal symptoms was associated with patient‐reported global CRS control, while none of the extra‐nasal symptoms of CRS were associated. In comparison, physician‐rated global CRS control was also associated with symptom severity but this association was with the severities of nasal symptoms as well as symptoms related to poor sleep and otologic or facial pain symptoms. Moreover, physician‐rated CRS control was associated with antibiotics usage and oral corticosteroids usage, independent of each other. Based on our PCA analysis, physician‐rated CRS control associated with antibiotics is likely related to consideration for the frequency of acute bacterial CRS exacerbations while the association with oral corticosteroids may be related to nonbacterial exacerbations or progression of disease. In both cases, we found that having used one or more courses of CRS‐related antibiotics or CRS‐related oral corticosteroids was most optimal for detecting poorly controlled versus well‐controlled CRS.
Our results, derived through the systematic top‐down study of patient‐reported and physician‐rated CRS control, are largely supportive of previous recommendations and findings.18, 27, 28 For example, like Snidvongs et al. and Banglawala et al., we found that nasal symptoms are most strongly associated with patient‐reported global CRS control.27, 28 Similar to the EPOS proposed staging system, we also found that severity of sleep and facial pain symptoms are used in the determination of physician‐rated CRS control. Additionally, similar to recommendations by EPOS and Snidvongs et al., we find that the use of at least one course of systemic antibiotics or oral corticosteroids in the past 3 months was most associated with poorly controlled versus well‐controlled CRS. However, our medication usage results differ from previous studies not only through our data‐driven derivation for usage thresholds, but also in our finding that antibiotic usage and oral corticosteroids usage are independent predictors of physician‐rated CRS control and should therefore be considered separately. In contrast to other studies, we also do not find any association between lost productivity due to CRS or endoscopy scores with patient‐reported or physician‐rated CRS control (although we did not seek association between endoscopy score and patient‐reported CRS control). That we find no association between CRS control and lost productivity or endoscopy score is not surprising given that previous work has found lost productivity due to CRS to be primarily associated with emotional symptoms,43, 47 which were not associated with CRS control in our study, and endoscopy score has traditionally been found to be weakly associated with patients' sinonasal QOL,48 which we find to be the primary determinant of global CRS control.
Although we studied six well‐established CRS disease characteristics that may influence perception of global CRS control and are consistent with previous studies of CRS control, interpretation of our study is limited to these characteristics as there may be other CRS disease characteristics that influence the associations we have determined here. Additionally, while we feel that the rating of all participants' global CRS control by two physicians is a strength, it may be viewed as a limitation that participants' CRS control was judged based on only six data points rather than an overall clinical picture, which may be apparent to a treating physician in the clinic setting. For example, CRS control in an asthmatic or polyp patient may be judged differently than in a nonasthmatic or non‐polyp CRS patient.49 It is possible that each of the associations we have found, in particular those associations with physician‐rated CRS disease control, may pertain primarily to one CRS variant or another (eg, those patients with polyps or those without polyps) and future work may certainly seek to determine whether disease control is perceived differently for different CRS variants. However, evaluation of CRS control in such a standardized way may be optimally suited to future development of treatment algorithms for escalation, de‐escalation or maintenance of therapy, which should be developed based on standardized criteria and not a treating physician's intuition or “gut feeling.” Moreover, although we use the same 3‐month time scale for CRS‐related systemic medication usage, sinus infections and CRS‐related lost productivity, we did not specifically query patients about problems related to their CRS symptoms over the same 3‐month time scale. However, because we only included patients who were at their baseline CRS state (not in the midst of an acute exacerbation) it is likely that participants' CRS symptomatology as reflected by the SNOT‐22 at the time of enrollment was representative of symptoms over the preceding 3 months. Nevertheless, further characterization of CRS control should ideally include assessment of CRS disease characteristics over a uniform time period. Additionally, our measure of lost productivity only included missed days of work or school but did not include any measure of “presenteeism” (going to work or school but having less productivity there). Finally, although our study participants' CRS control level was graded by two physicians, it is possible that personal or idiosyncratic biases in the physicians' grading impacted our measures of physician‐rated CRS control. Future work should seek to study physician‐rated CRS control amongst many physicians who grade global CRS control of the same patient populations, while using the same data points in order to identify a comprehensive set of disease characteristics that can be used to define CRS control. At the same time, it will ultimately be important to consider and weigh the clinical relevance of CRS disease characteristics against physician biases for characteristics that determine control. An ideal measure of CRS control will incorporate CRS disease characteristics that are not only important to a broad range of expert practitioners but also shown to be of clinical relevance in CRS.
Supporting information
Supporting Information
Financial Support: None
Conflicts of Interest: None
2018 Trio Society Thesis
BIBLIOGRAPHY
- 1. Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the united states. Ann Otol Rhinol Laryngol 2011;120:423–427. [DOI] [PubMed] [Google Scholar]
- 2. Carey RM, Adappa ND, Palmer JN, Lee RJ, Cohen NA. Taste receptors: Regulators of sinonasal innate immunity. Laryngoscope Investig Otolaryngol 2016;1:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. London NR, Lane AP. Innate immunity and chronic rhinosinusitis: What we have learned from animal models. Laryngoscope Investig Otolaryngol 2016;1:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamilos DL. Chronic rhinosinusitis in patients with cystic fibrosis. J Allergy Clin Immunol Pract 2016;4:605–612. [DOI] [PubMed] [Google Scholar]
- 5. Stevens WW, Peters AT. Immunodeficiency in chronic sinusitis: Recognition and treatment. Am J Rhinol Allergy 2015;29:115–118. [DOI] [PubMed] [Google Scholar]
- 6. Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: One airway disease. Immunol Allergy Clin North Am 2004;24:19–43. [DOI] [PubMed] [Google Scholar]
- 7. DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 2016;30:134–139. [DOI] [PubMed] [Google Scholar]
- 8. Hoehle LP, Phillips KM, Bergmark RW, Caradonna DS, Gray ST, Sedaghat AR. Symptoms of chronic rhinosinusitis differentially impact general health‐related quality of life. Rhinology 2016;54:316–322. [DOI] [PubMed] [Google Scholar]
- 9. Phillips KM, Hoehle LP, Bergmark RW, Caradonna DS, Gray ST, Sedaghat AR. Acute exacerbations mediate quality of life impairment in chronic rhinosinusitis. J Allergy Clin Immunol Pract 2017;5:422–426. [DOI] [PubMed] [Google Scholar]
- 10. Phillips KM, Hoehle LP, Caradonna DS, Gray ST, Sedaghat AR. Association of severity of chronic rhinosinusitis with degree of comorbid asthma control. Ann Allergy Asthma Immunol 2016;117:651–654. [DOI] [PubMed] [Google Scholar]
- 11. Phillips KM, Hoehle LP, Bergmark RW, et al. Chronic rhinosinusitis severity is associated with need for asthma‐related systemic corticosteroids. Rhinology 2017;55:211–217. [DOI] [PubMed] [Google Scholar]
- 12. Speth MM, Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Changes in chronic rhinosinusitis symptoms differentially associate with improvement in general health‐related quality of life. Ann Allergy Asthma Immunol 2018;121(2):195–199. doi: S1081‐1206(18)30395‐8 [pii]. [DOI] [PubMed] [Google Scholar]
- 13. Banoub RG, Hoehle LP, Phillips KM, et al. Depressed mood modulates impact of chronic rhinosinusitis symptoms on quality of life. J Allergy Clin Immunol Pract 2018. doi: S2213‐2198(18)30311‐8 [pii]. [DOI] [PubMed] [Google Scholar]
- 14. Caulley L, Thavorn K, Rudmik L, Cameron C, Kilty SJ. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US medical expenditure panel survey. J Allergy Clin Immunol 2015;136:1517–1522. [DOI] [PubMed] [Google Scholar]
- 15. Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope 2014;124:2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rudmik L. Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep 2017;17:5. [DOI] [PubMed] [Google Scholar]
- 17. Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016;6(Suppl 1):S209. [DOI] [PubMed] [Google Scholar]
- 18. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 2012;23:298. [PubMed] [Google Scholar]
- 19. Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AG. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4:CD011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Head K, Chong LY, Piromchai P, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev 2016;4:CD011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: A roadmap to asthma control. Eur Respir J 2015;46:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Del Prato S, Felton AM, Munro N, et al. Improving glucose management: Ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract 2005;59:1345–1355. [DOI] [PubMed] [Google Scholar]
- 23. EPR‐3 . NAEPP Expert Panel Report 3: Guidelines for the Diagnosis and Treatment of Asthma. Bethesda: US Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 24. Smith WM, Davidson TM, Murphy C. Regional variations in chronic rhinosinusitis, 2003–2006. Otolaryngol Head Neck Surg 2009;141:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray ST, Hoehle LP, Phillips KM, Caradonna DS, Sedaghat AR. Patient‐reported control of chronic rhinosinusitis symptoms is positively associated with general health‐related quality of life. Clin Otolaryngol 2017;42:1161–1166. [DOI] [PubMed] [Google Scholar]
- 26. Campbell AP, Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Symptom control in chronic rhinosinusitis is an independent predictor of productivity loss. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135(4):237–241. doi: S1879‐7296(18)30063‐2 [pii]. [DOI] [PubMed] [Google Scholar]
- 27. Snidvongs K, Heller GZ, Sacks R, Harvey RJ. Validity of european position paper on rhinosinusitis disease control assessment and modifications in chronic rhinosinusitis. Otolaryngol Head Neck Surg 2014;150:479–486. [DOI] [PubMed] [Google Scholar]
- 28. Banglawala SM, Schlosser RJ, Morella K, et al. Qualitative development of the sinus control test: A survey evaluating sinus symptom control. Int Forum Allergy Rhinol 2016;6:491–499. [DOI] [PubMed] [Google Scholar]
- 29. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol Head Neck Surg 2015;152(2 Suppl):S39. [DOI] [PubMed] [Google Scholar]
- 30. Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. A contemporary analysis of clinical and demographic factors of chronic rhinosinusitis patients and their association with disease severity. Ir J Med Sci 2018;187:215–221. [DOI] [PubMed] [Google Scholar]
- 31. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item sinonasal outcome test. Clin Otolaryngol 2009;34:447–454. [DOI] [PubMed] [Google Scholar]
- 32. Sedaghat AR, Gray ST, Caradonna SD, Caradonna DS. Clustering of chronic rhinosinusitis symptomatology reveals novel associations with objective clinical and demographic characteristics. Am J Rhinol Allergy 2015;29:100–105. [DOI] [PubMed] [Google Scholar]
- 33. Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the composite asthma severity index—an outcome measure for use in children and adolescents. J Allergy Clin Immunol 2012;129:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gliklich RE, Metson R. Techniques for outcomes research in chronic sinusitis. Laryngoscope 2015;125:2238–2241. [DOI] [PubMed] [Google Scholar]
- 35. Yamasaki A, Hoehle LP, Phillips KM, et al. Association between systemic antibiotics and corticosteroid use for chronic rhinosinusitis and quality of life. Laryngoscope 2018;128:37–42. [DOI] [PubMed] [Google Scholar]
- 36. Campbell AP, Hoehle LP, Phillips KM, Caradonna DS, Gray ST, Sedaghat AR. Smoking: An independent risk factor for lost productivity in chronic rhinosinusitis. Laryngoscope 2017;127:1742–1745. [DOI] [PubMed] [Google Scholar]
- 37. Revicki DA, Irwin D, Reblando J, Simon GE. The accuracy of self‐reported disability days. Med Care 1994;32:401–404. [DOI] [PubMed] [Google Scholar]
- 38. Zhang W, Bansback N, Anis AH. Measuring and valuing productivity loss due to poor health: A critical review. Soc Sci Med 2011;72:185–192. [DOI] [PubMed] [Google Scholar]
- 39. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg 1997;117(3 Pt 2):35. [DOI] [PubMed] [Google Scholar]
- 40. Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the rhinitis control assessment test in patients with rhinitis. J Allergy Clin Immunol 2013;131:379–386. [DOI] [PubMed] [Google Scholar]
- 41. R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. Available at: http://www.R-project.org/. Accessed July 17, 2017. [Google Scholar]
- 42. Robin X, Turck N, Hainard A, et al. pROC: An open‐source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell AP, Phillips KM, Hoehle LP, et al. Depression symptoms and lost productivity in chronic rhinosinusitis. Ann Allergy Asthma Immunol 2017;118:286–289. [DOI] [PubMed] [Google Scholar]
- 44. Belsley DA. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Hoboken, NJ: Wiley; 1980. [Google Scholar]
- 45. Gray ST, Phillips KM, Hoehle LP, Caradonna DS, Sedaghat AR. The 22‐item sinonasal outcome test accurately reflects patient‐reported control of chronic rhinosinusitis symptomatology. Int Forum Allergy Rhinol 2017;7:945–951. [DOI] [PubMed] [Google Scholar]
- 46. Kohli P, Soler ZM, Storck KA, Shahangian A, Banglawala SM, Schlosser RJ. Responsiveness and reliability of the sinus control test in chronic rhinosinusitis. Rhinology 2017;55:39–44. [DOI] [PubMed] [Google Scholar]
- 47. Chowdhury NI, Mace JC, Smith TL, Rudmik L. What drives productivity loss in chronic rhinosinusitis? A SNOT‐22 subdomain analysis. Laryngoscope 2018;128:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in‐office computed tomography in post‐surgical chronic rhinosinusitis patients. Laryngoscope 2011;121:674–678. [DOI] [PubMed] [Google Scholar]
- 49. Dennis SK, Lam K, Luong A. A review of classification schemes for chronic rhinosinusitis with nasal polyposis endotypes. Laryngoscope Investig Otolaryngol 2016;1:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


