Abstract
Objective
Functional near‐infrared spectroscopy (fNIRS) is an emerging noninvasive technology used to study cerebral cortex activity. Being virtually silent and compatible with cochlear implants has helped establish fNIRS as an important tool when investigating auditory cortex as well as cortices involved with hearing and language processing in adults and during child development. With respect to this review article, more recently, fNIRS has also been used to investigate central auditory plasticity following hearing loss and tinnitus or phantom sound perception.
Methods
Here, we review the currently available literature reporting the use of fNIRS in human studies with cochlear implants and tinnitus to measure human central auditory cortical circuits. We also provide the reader with detailed reviews of the technology and traditional recording paradigms/methods used in these auditory‐based studies.
Results
The purpose of this review article is to summarize theoretical advancements in our understanding of the neurocognitive mechanisms underlying auditory processes and their plasticity through fNIRS research of human auditory performance with cochlear implantation and plasticity that may contribute to the central percepts of tinnitus.
Conclusion
fNIRS is an emerging noninvasive brain imaging technology that has wide reaching application that can be applied to human studies involving cochlear implants and tinnitus.
Level of Evidence
N/A
Keywords: Auditory cortex, cochlear implants, functional near‐infrared spectroscopy, tinnitus
INTRODUCTION
Functional near‐infrared spectroscopy (fNIRS) has emerged as a noninvasive imaging modality used to measure cortical hemodynamic activity in many human auditory and nonauditory studies.1, 2 By measuring changing optical properties of activated brain tissue using near‐infrared light, this technology can quantify changes in oxygenated and deoxygenated hemoglobin.1 This method of measuring changes in localized hemoglobin serves as a surrogate/correlate of neural activity. By relying on the intrinsic optical properties of blood, fNIRS provides a more direct metabolic marker relative to the widely used blood‐oxygen‐level dependent imaging (BOLD) effect in functional magnetic resonance imaging (fMRI), which relies only on measured deoxyhemoglobin.3 The BOLD effect in MRI measures short‐lived changes in magnetic susceptibility that are functions of the relative presence of deoxyhemaglobin. Typically, when a brain region is activated, increased metabolic demand leads to increases in cerebral blood flow/volume to deliver oxygenated blood to active neurons. Deoxygenated blood is produced leading to measurable increases in BOLD signal intensity; a surrogate measure of the neural activation. fNIRS provides the added advantage of separately measuring oxygenated hemoglobin (HbO), deoxygenated hemoglobin (HbR) and total hemoglobin (HbT), and their relative contributions to measures of activation.3
Major limitations of the technology lie in the restricted depth of penetration (3 cm) and spatial resolution (1 cm) using scalp, “cap” configurations that limit studies to outer cortex only. The ability of fNIRS to detect cortical hemodynamic responses at various depths depends on factors including optode source power, source‐detector distance, detector sensitivity, and skin/skull optical properties. Typical fNIRS depth sensitivity is limited to 1.5 to 3 cm as extra‐cerebral tissues like thick‐dark hair attenuates infrared (IR) light leading to compromised spatial resolution. Exact spatial resolution of cortical hemodynamic response is complicated by partial volume error that relies on localized absorption change relative to source/detector position and wavelength of light that is affected by optical properties of the tissue. As a result, changes in chromophore concentration alters wavelength efficacy which can influence the response amplitudes from brain region to region within and across subjects. Concurrent optical tomographic imaging may enhance spatial uniformity during experimental procedures to improve data analysis.4 Despite these limitations, fNIRS is an attractive neuroimaging modality for hearing research as it is virtually silent and does not confound the recording environment, and is noninvasive and safe for repeated measurements with children and adults. These added advantages of the technology have catalyzed major contributions to the science of auditory processes and hearing restoration.
Cochlear implantation (CI) is a commonly used surgically placed hearing device within the cochlea to stimulate the inner ear and restore hearing. This is a brain‐based treatment for hearing and speech restoration, yet knowledge regarding the neural plasticity that underlies the success of this approach is limited. A lack of compatibility with fMRI,5 artifacts created by the device during electroencephalography (EEG) and event‐related potential (ERP) imaging, and positron emission tomography (PET‐CT) that employs ionizing radiation and radiotracers that limit repeated measures6 have all placed a premium on finding alternative imaging methods. fNIRS is well‐suited to measure central auditory changes with CI7 as it is compatible with the electrical and magnetic components of the device.4, 7 Moreover, fNIRS has improved temporal resolution over fMRI due to a sampling rate of 10 Hz and above.8, 9
Research and clinical practice find that language acquisition outcomes vary to a much greater extent among children with CI, regardless of age of implantation, than for normally hearing children.10 Interestingly, even among postlingually deafened CI recipients who are otherwise comparable in their hearing restoration, those with better‐specified neural response to language show better language recovery.11 One of the key advantages of fNIRS in CI studies is that it can be used safely and repeatedly in young learners to map neurocognitive characteristics of brain changes underlying language acquisition in relation to children's learning experiences, hearing, CI performance, and learning abilities. The study of CI therefore offers a unique tool for illuminating theoretical perspectives on the sources of individual variability in language acquisition and processing, with the potential to inform both theories as well as clinical and educational practice for children with CI.12 In the review section below we summarize the current, yet limited, studies to date that have utilized fNIRS to investigate the brain bases of language restoration with CI.
Another key potential contribution of fNIRS is to the study of central auditory mechanisms in tinnitus. Tinnitus is the phantom perception of sound in the absence of an extraneous sound source.13 Tinnitus is highly prevalent with an estimated 10% to 15% of US adults being affected.14 Military personnel are particularly at risk and tinnitus is one of the highest service‐related disabilities, with nearly 750,000 veterans receiving associated compensation at a cost of over $2 billion in 2014.14 The underlying etiology of tinnitus is not well defined, yet is typically associated with peripheral ear disease that leads to aberrant neural activity within central auditory circuits.15 A poor understanding of the central etiology of tinnitus has subsequently restricted effective therapies.
Neuroimaging strategies to study tinnitus, like hearing loss and in some instances CI, have traditionally utilized fMRI, PET with and without computer tomography (PET‐CT), EEG, and magnetoencephalography (MEG). Meaningful data captured from these imaging modalities have been limited by the potential confounding effects of external noise (fMRI), use of high production‐cost radioisotopes (PET), and limited spatial resolution (EEG and MEG).7, 16 Factors limiting EEG/MEG spatial resolution have more profound effect on fNIRS. Specifically, source localization (of cortical activity) represents the greatest differences between EEG/MEG and fNIRS as it is difficult to localize the source of a response within the volume of the brain with EEG/MEG despite advancements in technology including beam‐forming (etc.). Alternatively, with fNIRS the detected response must be within approximately 3 cm of the brain surface, and thus provides advantages over the other techniques.
Despite limitations to each modality, reported results have identified changes in tinnitus brains that may reflect correlates of anomalous neural activity (increased spontaneous neural firing rates and synchrony), described in animal models.17, 18 Alternative imaging technologies with minimal confounding effects on tinnitus perception are needed to potentially translate these putative correlates within human central auditory brain centers and circuits. fNIRS is well‐suited for this role as it measures changing optical properties of brain to extrapolate and quantify hemodynamic responses through neurovascular coupling,1 and has been utilized to measure resting functional states and brain connectivity.19 Here we also summarize the current, yet limited, studies to date that have utilized fNIRS to investigate human brain changes in tinnitus. Lastly, we provide the reader with a review of common methods used with fNIRS and audiologic studies like CI and tinnitus.
APPLICATION OF fNIRS AND HEARING LOSS
Cochlear Implantation: Children and Adults
Human brain organization for language arises as the result of complex interaction between neurobiology and language experiences.20 Children with CIs often vary in the age of implantation as well as quality of language therapy, thereby offering a unique window of opportunity in understanding how early language experience influences language organization in the brain. fNIRS neuroimaging is well‐suited for the study of language and hearing in CI recipients of all ages, yet relatively few studies have taken advantage of the technology to study language and hearing with CI.21, 22 In part, this has been due to the limitations in signal localization and processing protocols for the fNIRS method, many of which are being addressed in this review on fNIRS neuroimaging.
Sevy et al.7 were the first to establish that fNIRS could reliably detect hemodynamic responses to speech in the auditory cortices (bilateral superior temporal gyrus [STG] and temporal lobe regions) of CI users. To establish a connection between differential HbO/HbR levels and spoken language input, participants listened to five 20‐second vignettes from a children's story, interspersed with blocks of 25 to 55 seconds of silence. Participants came from four populations: normal‐hearing (NH) adults (n = 11) and children under 19 (n = 12), and two groups of CI‐using children: those who had used their implant for more than 4 months (n = 40), and those tested on the day their implant was first activated (n = 13). The fNIRS array used by Sevy et al.7 consisted of two detector probes on each hemisphere, on either side of an emitter located at the T3/T4 references points. Significant responses were seen in the auditory cortices while listening to speech compared to silence: in 100% and 82% of the NH adults and children, respectively, and 76% of the CI children with implant experience and 78% of CI children on the day of implant activation. There was wide variation in the laterality of the speech‐evoked responses in all groups, but bilateral response was the most common across all groups. In summary, not only was the Sevy et al.7 pioneering work successful in demonstrating the feasibility of fNIRS neuroimaging with CI, it offered the remarkable finding that hemodynamic response can be detected in the auditory cortex, noninvasively, in first‐time CI users during their initial experiences with the device. Since this seminal work, three additional studies (thus far only with adults) have used Sevy et al.'s7 promising result to begin research on neurological organization and potential re‐organization, in the face of CI‐filtered language exposure.
Olds et al.23 investigated the neural correlates of speech processing among 35 postlingual deafened CI adults (ages 23–86), again with a wide range of implant experience (1 day to 12 years), and sought to correlate speech‐specific cortical responses with speech perception skills. In this study, CI users and NH adult controls listened to four types of auditory stimuli in 20‐second blocks: normal read speech, excerpted from a story; vocoded (or “channelized”) speech, in which white noise at a series of frequency bandwidths was modulated with the average amplitude envelope across those frequencies in a real speech sample; scrambled speech, in which those amplitude envelopes were redistributed randomly across the frequency bandwidths; and environmental sounds as a nonspeech control. For NH adults, vocoded speech is distinctly recognizable although degraded—in fact, it approximates CI‐filtered speech for NH listeners—while scrambled speech is completely unintelligible. Both groups also performed behavioral tasks to assess their phoneme perception in consonant‐vowel‐consonant (CVC) syllables and their open sentence recognition. The fNIRS array included 8 emitters and 12 detectors on each hemisphere, centered around the T7/T8 coordinates, and thus aimed to capture activity in the lateral temporal lobe and STG regions (for converging fMRI support for these localizations see Pollonini et al.24). On the behavioral tasks, CI listeners showed a wide range of abilities—20% to 94% accuracy on syllables and 28% to 97% accuracy on sentences—and these accuracy scores were used to define two groups of good and poor speech perceivers within the CI cohort. Comparisons with the brain imaging data showed that good speech perceivers had similar cortical activation to the NH adults, namely strong responses for normal and vocoded speech, and much reduced responses to both scrambled speech and non‐speech controls—whereas poor speech perceivers showed considerable activation for all four types of stimuli, speech and nonspeech alike. The key finding here is that while CI participants' cortical activation correlated with their language proficiency, it did not correlate with their general auditory abilities. However, this study did not disclose how the general auditory abilities were measured (behavioral tasks presented were speech identification tasks). The findings highlight the promising nature of fNIRS research with CI: this approach can be used to understand the neurocognitive processes that contribute to successful language abilities and their restoration. These findings are also counter‐intuitive when taken with almost the inverse effect seen in good speech perceivers and NH adults. This discrepancy may highlight potential data acquisition variability within and between subjects using fNIRS. Fortunately, several reports have examined the test‐retest reliability of fNIRS measurements in adults during basic visual stimulation,25 verbal fluency tasks,26, 27 and in speech‐evoked, temporal‐lobe fNIRS responses in normal‐hearing adults.28 These studies have documented excellent reproducibility of fNIRS measurements to temporal‐lobe responses to auditory speech (with or without visual speech cues) and indicate that fNIRS is well‐suited to assess individual differences in responses to address plasticity within and between human test subjects.
Bisconti et al.12 provided an initial attempt to understand how degraded speech input to an otherwise‐typical brain, in the case of postlingual deafened adults with CI, might cause changes in neural wiring for aspects of language processing. This study reported fNIRS data from ten adult CI users with a wide range of ages and implant experience (ages 21–74; 1–24 years post‐implant) and 10 NH adult controls. The study's arrays of emitters and detectors were arranged bilaterally across the inferior and middle frontal regions, the superior and middle temporal regions, and the parietal cortex, all between the T3/T4 and F7/F8 coordinates. While wearing the fNIRS system, participants judged whether word pairs rhymed (eg, wall ∼ ball vs. fork ∼ spoon) to tap one aspect of their phonological awareness also listened passively to passages from the CELF‐4 standardized test followed by comprehension questions. Three types of trial blocks were used, each 20 to 25 seconds long: blocks with the target rhyme or passage tasks, blocks with silence, and control blocks akin to the rhyme task in which participants judged whether two pure tones matched. On the behavioral tasks, the CI users were significantly less accurate but still highly successful (between 89–100% accuracy across all groups) as compared to NH controls, and the overall imaging results demonstrated no significant differences between the NH and CI listeners. The authors concluded that postlingual deafened adults whose auditory language processing developed typically as children, and for whom CI was effective for language restoration, can reactivate the typical auditory cortical regions after implantation, and need not show neurological reorganization or other compensatory processing.
Chen et al.29 addressed the issue of neural plasticity due to hearing loss and how CI treatment interacts with this plasticity. They asked 20 postlingual deafened CI users and 20 NH controls to complete a visual and an auditory task. During the visual task participants saw reversing displays of circular checkerboard patterns that created the perception of movement. During the phonological task the participants listened to normally spoken words and acoustically reversed words. During the visual condition, CI participants showed significantly greater activation in the right auditory cortex than NH controls, suggesting that implantation does not completely reverse the effects of hearing loss during which visual functions can begin to take over the auditory cortex. Similarly, during the auditory task, the CI participants showed stronger activation in visual cortex than the NH adults, suggesting that there is also neural reorganization of visual cortex to support language following hearing loss and its restoration.
Dewey and Hartley30 reported increased activation to visual stimulation in right auditory cortex in deafness as compared to controls using fNIRS that in CI users had lower visual cortex activation indicating that that CI users process visual stimuli more efficiently than NH controls.31 Finally, brain‐behavior correlations revealed that individuals with lesser auditory cortex activation and greater visual cortex activation during language tasks had the best language outcomes. Taken together the findings suggest that the variability in language restoration outcomes in CI participants might depend on the degree of cross‐modal plasticity in the auditory and visual cortices to support the restoration of language function. van de Rijt and colleagues32 showed increased activation to auditory, visual, and audiovisual stimulation in temporal cortex of NH subjects and postlingual deafened CI users using fNIRS. These data exhibited the potential and reliability28 of fNIRS for studying neural mechanisms of audiovisual integration, both in NH and following CI.
In summary, fNIRS research with CI has effectively demonstrated both the feasibility of fNIRS neuroimaging with this population as well as the breadth of theoretical questions that can be asked using this research approach (Table 1). One exciting frontier of fNIRS research might focus on the nature of sensitive periods in language acquisition. For instance, the above‐mentioned research suggests that even in those who lose hearing later in life, sensorimotor and/or auditory capabilities might begin to “take over” the auditory‐temporal cortex. Is it then possible that children with older age of implantation find it more difficult to learn language33 because at a more advanced age newly available language input must now face greater neural competition within the temporal regions that have already become committed to other physiological or cognitive processes. To better answer these and other important questions about language and hearing with CI, we highlight below research directions to improve the fNIRS methodology for the study of language and hearing deficits and rehabilitation.
Table 1.
Summary Table of Manuscripts Reviewed
| Manuscript | Topic | Study population | Control | Main Finding |
|---|---|---|---|---|
| Sevy et al. (7) | CI | CI (>4 months) children (n = 40); CI (day of implantation) children (n = 13) | NH adults (n = 11), NH children (n = 12) | First to demonstrate hemodynamic response to speech in CI users; demonstrated hemodynamic response to speech on day of implantation |
| Olds et al. (23) | CI | Postlingually deafened CI adults (n = 35); implant experience: 1 day to 12 years | NH adults (n = 35) | fNIRS cortical activation correlates with speech proficiency; good speech perceivers in CI group had similar cortical responses to NH adults: strong responses to normal and vocoided speech, but reduced response to scrambled speech and environmental sound. Poor speech perceivers had strong responses to all 4 conditions. |
| Bisconti et al. (12) | CI | Postlingually deaf CI adults (n = 10); implant experience:1–24 years | NH adults (n = 10) | Postlingually deaf adults whose auditory language processing developed typically as children, and for whom CI was effective for language restoration, can reactivate the typical auditory cortical regions |
| Chen et al. (2015) | CI | Postlingually deaf CI adults (n = 20); implant experience: 6 months to 16 years | NH adults (n = 20) | Variability in language restoration outcomes in the CI subjects might depend on the degree of cross‐modal plasticity in the auditory and visual cortices to support the restoration of language function |
| Schecklmann et al. (36) | Tinnitus | Chronic tinnitus adults, received rTMS (n = 12) | Chronic tinnitus adults, sham rTMS (n = 11) | Proof‐of‐concept of noninvasive brain stimulation and neuroimaging with fNIRS; block‐design and event‐design resulted in different patterns of activation in auditory and temporoparietal cortices |
| Issa et al. (37) | Tinnitus | Chronic tinnitus adults (n = 10) | NH adults (n = 8) | Control participants demonstrated deactivation in both auditory and nonauditory regions during inter‐stimulus silent periods, while tinnitus participants demonstrated maintenance of activation |
| San Juan et al. (40) | Tinnitus | Chronic tinnitus adults (n = 10) | NH adults (n = 8) | Following sound stimulation, resting state functional connectivity of auditory cortex and non‐auditory cortices increased in tinnitus participants but decreased in controls. |
CI = cochlear implant; NH = normal‐hearing.
APPLICATION OF fNIRS AND TINNITUS
Despite its high prevalence, touted neural correlates of tinnitus (phantom perception of sound in the absence of a sound stimulus) found in animal models, such as increased spontaneous neural firing, enhanced neural synchrony in auditory cortex, and tonotopic map reorganization,34 have not been translated effectively in humans. Functional brain imaging studies of human tinnitus demonstrated that it is related to central auditory pathway neural changes associated with non‐auditory brain areas. This suggests that nonauditory neural networks play a role in tinnitus pathogenesis, including: 1) fronto‐parietal area for awareness/attention; 2) stress/emotion neural networks, like the anterior cingulate cortex, insula, and amygdala; and 3) hippocampus and para‐hippocampal regions involved in memory/cognition for symptom perception, anxiety and associated distress.35 Other neuroimaging modalities have provided significant contributions to the field, but drawbacks of those technologies—such as loud recording environments—limit their use. fNIRS could help bypass these limitations since it provides a noninvasive and silent recording environment.
Schecklman et al.36 were the first to describe the feasibility of studying tinnitus with fNIRS technology. They divided participants with tinnitus into a verum group to receive repetitive trans‐magnetic stimulation (rTMS) treatment and a sham group. The authors used binaural stimulation with noise using both a block (see methods section below for explanation of this paradigm) and an event‐related paradigm. Recordings were performed two weeks before treatment to establish a baseline for all participants, and on the day of the last treatment for tinnitus participants. At baseline, tinnitus participants exhibited higher activation in the right auditory cortex using the block design and frontal cortex showed decreased activation using the event‐related design. The trait‐related increased activity in the right auditory cortex was considered to represent at least one aspect of the tinnitus percept. Additionally, the sham group had higher oxygenation than the verum group in a channel in the left hemisphere at baseline during the block design, but this reversed following treatment. In the event‐related design, the opposite was observed: the sham group had lower oxygenation than the verum group in the left hemisphere at baseline, and no differences following the treatment. The authors attributed the differences in results produced by the two types of designs to the difference in the length of the auditory stimuli presented. The authors also stated that these findings suggest that oxygenation of the left auditory cortex may reflect state‐like or baseline effects, whereby baseline level (block design: sham > verum; event‐related design: verum > sham) and eventual intervention led to a decrease in the group with increased baseline activity and vice versa (block design: verum > sham; event‐related design: sham > verum). They admit that interpretation of these findings is challenging due baseline differences between the two groups and the inverse changes during treatment depend on the stimulation procedure (verum versus sham) or on the difference in baseline measurements. These data do not likely reflect lack of reproducibility in fNIRS as a technology as several reports have examined the test‐retest reliability of fNIRS measurements in adults during verbal fluency tasks,26, 27 and in speech‐evoked, temporal‐lobe fNIRS responses in NH adults.28
Overall, these data suggested that fNIRS could be used to detect brain changes between normal controls and those with subjective tinnitus. While unable to objectify what exactly the plasticity is that may underlie tinnitus perception, this proof of concept study provided a foundation for the only two other fNIRs studies that have investigated human tinnitus to date.
Issa et al.37 investigated hemodynamic responses within the region of interest (ROI; auditory cortex) and non‐ROI (adjacent nonauditory cortex) of NH participants with and without bilateral subjective tinnitus. They performed a block design paradigm of alternating sound (pure tones at 750 Hz and 8000 Hz and broadband noise) and silence (inter‐stimulus rest; ISR). Control participants demonstrated deactivation in both ROI and non‐ROI during ISR periods, while tinnitus participants demonstrated maintenance of activation. This spontaneous activation in the ROI in the absence of stimulation was thought to represent a human parallel of increased spontaneous and tone‐evoked neural firing rates in auditory cortex found in animal models.38 The increased activity seen in non‐ROI in tinnitus is congruent with previous findings of maladaptive changes in areas outside of auditory cortex in tinnitus brains.16, 39 Furthermore, tinnitus participants exhibited deactivation in the ROI during blocks of exposure to broadband noise, contrasting the activation seen in controls under the same conditions. These results are likely indicative of forward masking, or a form of temporal inhibition in which a loud sound suppresses the response to subsequent sounds and residual inhibition of external sound suppressing phantom perception. This diverges from studies of animal models that have shown increased tone‐evoked activation38 highlighting the importance of performing studies using human participants.
In recently published data, our group analyzed differences in resting state functional connectivity (RSFC) between control and tinnitus participants prior to and following auditory stimulation.40 RSFC measures the spatiotemporal relationship between two brain regions and is thought to represent contextual influences that affect local processing and perception.41, 42 The signal arises from low frequency (<0.1 Hz) spontaneous fluctuations in electrical activity, and thereby, hemodynamic activity as measured by fNIRS. To capture the signal, participants need to be in a fully resting state without stimulation. As such, the silent nature of the fNIRS equipment is of great benefit. RSFC has been proposed to represent a record of brain regions that have been modulated together in the past, and may predict which brain regions are likely to work together when processing information in the future, and/or represent networks that affect local processing or serve to coordinate neural activity.43 The authors found that following sound stimulation, RSFC of auditory cortex and nonauditory cortices increased in tinnitus participants but decreased in controls. These results point to the importance of plasticity of neural circuits in the pathogenesis of tinnitus and implicate cross‐modal plasticity as a significant contributor to the pathophysiology.
Taken together, the above three published studies (Table 1) are the only ones currently available that have used fNIRS technology to investigate the effects of tinnitus on central auditory cortices in humans. These studies have shown the efficacy of fNIRS as an effective tool in the study of tinnitus to measure not only stimulated activity through changes in hemodynamic responses, but, also to measure RSFC in both auditory and nonauditory cortices.
VIABILITY OF fNIRS
Functional NIRS has been verified to detect significant hemodynamic responses (concentration level changes in oxy‐ and deoxy‐hemoglobin), that can be conceptually compared to the BOLD signal detected by fMRI.44, 45 Specific to the auditory processing domain, Sevy et al.7 compared the cortical responses evoked by speech stimuli (digital recordings of an animated female voice reading children's stories) collected with both fNIRS and fMRI, at the same time, from three normal‐hearing participants.7 Both fNIRS and fMRI captured similar significant responses from the bilateral superior temporal gyrus. Other studies have found a similar tight correlation between the fNIRS and fMRI signals in motor tasks46, 47, 48, 49, 50 and language tasks.51
METHODS
Because fNIRS measures hemodynamic responses, correlates of neural activity rather than the original neurochemical response, studies must be carefully designed to capture changes in underlying neuronal activity. Hemodynamic response includes several physiological artifacts, such as blood pressure fluctuation (0.1 Hz), respiration (0.2∼0.3 Hz), and heart‐beat (1.2∼1.3 Hz). Therefore, the designed task frequency needs to avoid these frequency bands.
The pitfalls faced during fNIRS imaging are somewhat like fMRI, and therefore, the experimental approach to maximally capture brain activity is often similar. Since fNIRS is silent, it allows for continuous measurements throughout the experiment, circumventing the need for sparse or silent designs often used by auditory fMRI studies. These designs omit data collection during stimulus presentation due to the noise generated by data acquisition sequences.52 As with fMRI, fNIRS investigators also often implement two types of experimental designs: the block design and the event‐related design. Block designs are well‐suited to accumulate the task power for a potentially limited dataset (Fig. 1) and thus localize functional areas and investigate steady state processes (eg, A, and B types of hearing tasks). This makes fNIRS (vs. ERP) especially advantageous for the study of young infants or children who have a short attention span. Event‐related design consists of several types of tasks (conditions) with varied inter‐stimulus intervals (ISI) ranging from a few to 20 seconds. Such designs attempt to measure transient changes in brain activity. Unlike block designs, stimuli are presented in a random order rather than an alternating pattern offering a higher flexibility in experimental conditions. One potential advantage of fNIRS and event‐related design is that the high sampling frequency permits for shorter ISI distances than otherwise possible with fMRI. fNIRS has improved temporal resolution over fMRI due to a sampling rate of 10 Hz and above, thus one can identify more accurate onset and better filtering of physiological interference of brain activation of interest. This makes fNIRS more analytically and ecologically advantageous for such experiments as acoustic or phonological odd‐ball designs where target stimuli are only about 200 ms short and the efficacy of the experimental design benefits from having a 1000 ms or even shorter ISI.53 The optimal characteristics of rapid event‐related designs for fNIRS imaging have not been fully explored and present a promising frontier for improving experimental designs.
Figure 1.
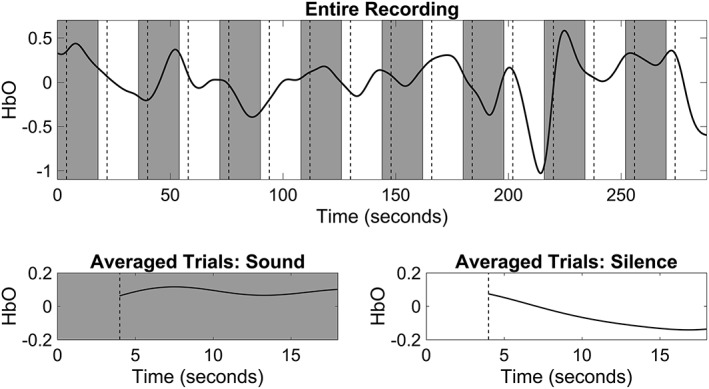
Example of block design and signal averaging. The top panel contains an example of block design paradigm composed of alternating sound stimuli (gray boxes) and silence (white boxes), with each stimulus lasting 18 seconds. The resulting hemodynamic response of the right auditory cortex is superimposed on the block design schematic. The vertical dashed lines mark 4 seconds after the start of each block, the time required for the hemodynamic response to return to baseline. The bottom panel shows the mean hemodynamic response during the sound blocks (left plot) and silent blocks (right plot) with first 4 seconds of each block excluded from analysis. Note the increased mean activation during the blocks of sound stimulation.
NEUROANATOMICAL LOCALIZATION
The regions of interest (ROI) selected for hearing research are usually primary and associative auditory cortices (Brodmann areas 41 and 42), middle/superior temporal gyrus (Brodmann areas 21 and 22), dorsolateral/anterior prefrontal cortex (Brodmann areas 8, 9, and 10), and temporo‐parietal area (Brodmann area 38). Because all these regions are on the side of the brain, the cap design is usually band‐like (Fig. 2). The importance of neuroanatomic mapping is that fNIRS is unable to provide precise localization of the specific brain regions that generate the hemodynamic response. To spatially assess fNIRS data, one must find the association between the scalp locations where fNIRS measurements are performed and the underlying anatomical information. Researchers have proposed three registration methods for fNIRS probe localization.54, 55, 56 The first and most popular method is to use the 10 to 20 reference system, which is standard for EEG scalp electrode positioning.57 The 10 to 20 system assumes there is a consistent association between scalp locations and their underlying anatomical structures. Such association has been verified on cadavers57 and by using multiple structural imaging techniques, including X‐ray‐radiation,58 computed tomography,59 and MRI.60 These associated reference points and predesigned fNIRS probes yield an estimation of the underlying structural brain region that generates the detected hemodynamic responses.55 However, data analysis and interpretation by this localization method can be imprecise by neglecting the individual‐ and group‐level registration results. The within‐ and between‐subject errors are defined as deviations from multiple measurements on the same individual and the individual participant's brain image subsequently undergoes spatial transformation onto a brain template; usually a brain image averaged across a group with similar demographics (eg, age).
Figure 2.
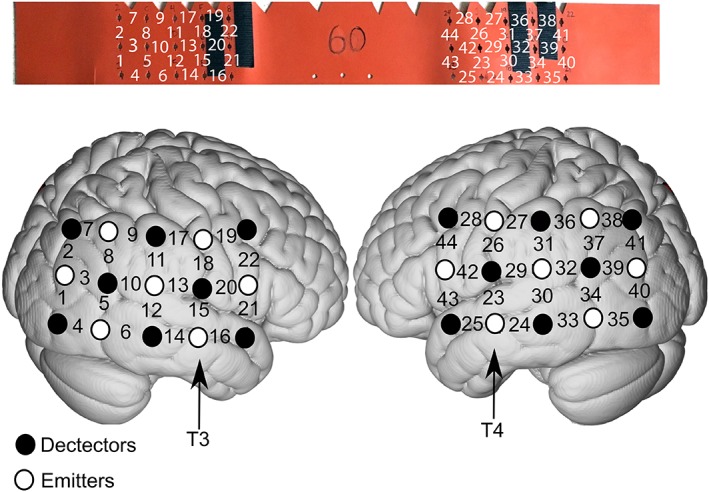
Example of cap configuration for auditory research based on the 10‐20 EEG system. T3 and T4 are used as anatomic references when placing the cap. For this design, channels 13 and 15 and channels 23 and 29 record from primary auditory cortex and surrounding belt regions of the right and left hemisphere, respectively. The top panel shows the cap/band used when a subject's head circumference measures 60 centimeters. The bottom panel is a schematic of the cortical regions associated with each optode and associated brain region.
If an investigator has access to an MRI scanner, the second method is to co‐register a participants fNIRS data to their structural MRI. This registration method allows fNIRS data obtained in a real‐world space to be merged onto the structural MRI obtained in another real‐world space. Given the higher spatial resolution of MRI scanning compared to fNIRS, as well as the consideration of within‐ and between‐participant variability, the registration results are much more reliable than the 10 to 20 system‐based registration method. However, access to an MRI scanner is not guaranteed in typical fNIRS experiments due to the additional cost and effort, which reduce the economical merits and convenience of fNIRS.55 In addition, in special cases such as with CI, MRI scanning is not possible due to the ferromagnetic incompatibility.
An alternative and convenient third method is to use a three‐dimensional (3D) digitizer. fNIRS probe or channel positions are recorded by a 3D digitizer (typically magnetic) together with the positions of at least three scalp landmarks (typically nasion and two pre‐auricular points). The positions of recorded fNIRS probes can then be projected onto an age‐specific standard brain template through a spatial transformation based on the scalp landmarks. Although this method does not adopt individualized anatomical information, it considers the within‐ and between‐subject variance that should improve fNIRS registration.61
DATA PROCESSING
Task‐Evoked Hemodynamic Responses
To analyze block‐design paradigms, block average (Fig. 1) is used for data analysis accompanied by t‐test or analysis of variance (ANOVA) as statistical examination. The statistical analysis of task‐evoked hemodynamic response detected by fNIRS is still not standardized, however.
General linear model approach offers several analytical advantages to the analyses of fNIRS data. It avoids subjective selection of periods of peak activity, takes the full time‐course information and takes advantage of all the available data, and provides efficient evaluation of data collected in experiments with short ISI. This method assumes that data can be represented as a linear combination of several sources (regressor); each regressor represents one modeled task‐evoked response or artifact related response. Instead of direct analysis of the hemodynamic response curve, investigators can summarize the difference between modeled and detected hemodynamic responses in a set of parameters. Such parametrized method facilitates multi‐level statistical analysis.62
RESTING‐STATE FUNCTIONAL CONNECTIVITY
Resting‐state functional connectivity (RSFC) approach investigates the spatiotemporal relation of various brain regions (Fig. 3). The signal arises from low frequency (<0.1 Hz) spontaneous fluctuations in electrical activity, and thereby, hemodynamic activity as measured by fNIRS. For the study of hearing loss, this approach helps reveal the mechanisms underlying audio production, transmission, integration and processing, and is especially helpful for studying patients with difficulty participating in tasks. In recent years, fNIRS has been intensively used to study RSFC of multidisciplinary areas including hearing research. The prevalent method for studying RSFC is seed‐based correlation analysis. In hearing research, auditory cortex is usually selected as a seed region, while the correlations between all other brain regions and the seed region are calculated (Fig. 3b). To assess group‐level functional connectivity, t‐tests are performed after correlation coefficients have undergone Fisher transformations.63
Figure 3.
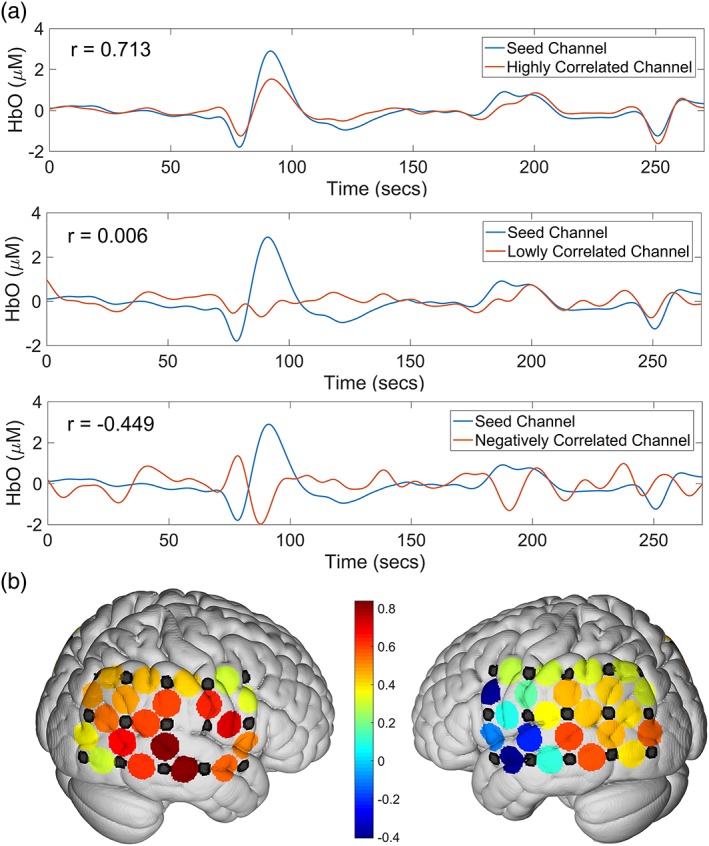
(a) Demonstration of resting state connectivity differences among various channels. From top to bottom, a channel with high connectivity to the seed region, a channel with negative connectivity to the seed region, and one with low connectivity to the seed region. (b) Heat map of connectivity to the right auditory cortex (channels left blank in the figure). Warmer colors indicate high connectivity to the seed region and cooler colors indicate negative connectivity to the seed region.
CONCLUSIONS
fNIRS technology continues to evolve and the nature of the approach provides distinct advantages when studying human hearing loss and rehabilitation with CI as well as the subjective nature of tinnitus. Despite the current limitations that are largely isolated to limited depth of penetration and spatial orientation, fNIRS has the distinct advantages of virtually silent recordings that are noninvasive and compatible with CI in both adults and children. Going forward, the wide application of fNIRS as a modality to study central human auditory circuits will continue to advance our understanding of normal and aberrant circuits that exist following hearing loss and tinnitus.
Editor's Note: This Manuscript was accepted for publication 04 June 2018.
Conflict of Interest: None
Financial Disclosure: None
BIBLIOGRAPHY
- 1. Ferrari M, Quaresima V. A brief review on the history of human functional near‐infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012;63:921–935. [DOI] [PubMed] [Google Scholar]
- 2. Ayaz H, Onaral B, Izzetoglu K, Shewokis P, McKendrick R, Parasuraman R. Continuous monitoring of brain dynamics with functional near infrared spectroscopy as a tool for neuroergonomic research: empirical examples and a technological development. Front Hum Neurosci 2013;7:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huppert T, Hoge R, Diamond S, Franceschini M, Boas D. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 2006;29:368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quaresima V, Bisconti S, Ferrari M. A brief review on the use of functional near‐infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang 2012;121:79–89. [DOI] [PubMed] [Google Scholar]
- 5. Bandettini P. Twenty years of functional MRI: the science and the stories. Neuroimage 2012;62:575–588. [DOI] [PubMed] [Google Scholar]
- 6. Talavage T, Gonzalez‐Castillo J, Scott S. Auditory neuroimaging with fMRI and PET. Hear Res 2014;307:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sevy A, Bortfeld H, Huppert T, Beauchamp M, Tonini R, Oghalai J. Neuroimaging with near‐infrared spectroscopy demonstrates speech‐evoked activity in the auditory cortex of deaf children following cochlear implantation. Hear Res 2010;270:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutini S, Moro S, Bisconti S. Functional near infrared optical imaging in cognitive neuroscience: an introductory review. J Infrared Spectrosc 2012;20:75–92. [Google Scholar]
- 9. Kovelman I, Mascho K, Millott L, Mastic A, Moiseff B, Shalinsky M. At the rhythm of language: brain bases of language‐related frequency perception in children. NeuroImage 2012;60:673–682. [DOI] [PubMed] [Google Scholar]
- 10. Tobey E, Thal D, Niparko J, Eisenberg L, Quittner A, Wang N. Influence of implantation age on school‐age language performance in pediatric cochlear implant users. Int J Audiol 2013;52:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fitzpatrick E, Olds J. Practitioners' perspectives on the functioning of school‐age children with cochlear implants. Cochlear Implants Int 2015;16:9–23. [DOI] [PubMed] [Google Scholar]
- 12. Bisconti S, Shulkin M, Hu X, Basura G, Kileny P, Kovelman I. Functional near‐infrared spectroscopy brain imaging investigation of phonological awareness and passage comprehension abilities in adult recipients of cochlear implants. J Speech Lang Hear Res 2016;59:239–253. [DOI] [PubMed] [Google Scholar]
- 13. Baizer J, Manohar S, Paolone N, Weinstock N, Salvi R. Understanding tinnitus: the dorsal cochlear nucleus, organization and plasticity. Brain Res 2012;1485:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theodoroff S, Lewis M, Folmer R, Henry J, Carlson K. Hearing impairment and tinnitus: prevalence, risk factors, and outcomes in US service members and veterans deployed to the Iraq and Afghanistan wars. Epidemiol Rev 2015;37:71–85. [DOI] [PubMed] [Google Scholar]
- 15. Savage J, Waddell A. Tinnitus. BMJ Clin Evid 2012;2012:pii0506. [PMC free article] [PubMed] [Google Scholar]
- 16. Vanneste S, De Ridder S. The auditory and non‐auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front Syst Neurosci 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lanting C, De Kleine E, Van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res 2009;255:1–13. [DOI] [PubMed] [Google Scholar]
- 18. Adjamian P. The application of electro‐and magneto‐encephalography in tinnitus research–methods and interpretations. Front Neurol 2014;5:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu C‐M, Zhang Y‐J, Biswal B, Zang Y‐F, Peng D‐L, Zhu C‐Z. Use of fNIRS to assess resting state functional connectivity. J Neurosci Methods 2010;186:242–249. [DOI] [PubMed] [Google Scholar]
- 20. Ip K, Hsu L, Arredondo M, Tardif T, Kovelman I. Brain bases of morphological processing in Chinese‐English bilingual children. Dev Sci 2017;20:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson C, Lazard D, Hartley D. Plasticity in bilateral superior temporal cortex: Effects of deafness and cochlear implantation on auditory and visual speech processing. Hear Res 2017;343:138–149. [DOI] [PubMed] [Google Scholar]
- 22. Saliba J, Bortfeld H, Levitin D, Oghalai J. Functional near‐infrared spectroscopy for neuroimaging in cochlear implant recipients. Hear Res 2016;338:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olds C, Pollonini L, Abaya H, et al. Cortical activation patterns correlate with speech understanding after cochlear implantation. Ear Hear 2016;37:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pollonini L, Olds C, Abaya H, Bortfeld H, Beauchamp M, Oghalai J. Auditory cortex activation to natural speech and simulated cochlear implant speech measured with functional near‐infrared spectroscopy. Hear Res 2014;309:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plichta M, Herrmann M, Baehne C, et al. Event‐related functional near‐infrared spectroscopy (fNIRS): are the measurements reliable? NeuroImage 2006;31:116–124. [DOI] [PubMed] [Google Scholar]
- 26. Kakimoto Y, Nishimura Y, Hara N, Okada, M , Tanii H, Okazaki Y. Intra‐subject reproducibility of prefrontal cortex activities during a verbal fluency task over two repeated sessions using multi‐channel near‐infrared spectroscopy. Psychiatry Clin Neurosci 2009;63:491–499. [DOI] [PubMed] [Google Scholar]
- 27. Schecklmann M, Ehlis A, Plichta M, Fallgatter A. Functional nearinfrared spectroscopy: A long‐term reliable tool for measuring brain activity during verbal fluency. NeuroImage 2008;43:147–155. [DOI] [PubMed] [Google Scholar]
- 28. Wiggins I, Anderson C, Kitterick P, Hartley D. Speech‐evoked activation in adult temporal cortex measured using functional near‐infrared spectroscopy (fNIRS): Are measurements reliable? Hear Res 2016;339:142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Sandmann P, Thorne J, Bleichner M, Debener S. Cross‐modal functional reorganization of visual and auditory cortex in adult cochlear implant users identified with fNIRS. Neural Plast 2016. 10.1155/2016/4382656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewey R, Hartley, D . Cortical cross‐modal plasticity following deafness measured using functional near‐infrared spectroscopy. Hear Res 2015;325:55–63. [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Stropahl M, Schonwiesner M, Debener S. Enhanced visual adaptation in cochlear implant users revealed by concurrent EEg‐fNIRS. Neuroimage 2017;146:600–608. [DOI] [PubMed] [Google Scholar]
- 32. van de Rijt L, van Opstal A, Mylanus E, et al. Temporal cortex activation to audiovisual speech in normal‐hearing and cochlear implant users measured with functional near‐infrared spectroscopy. Front Hum Neurosci 2016;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomblin J, Peng S, Spencer L, Lu N. Long‐term trajectories of the development of speech sound production in pediatric cochlear implant recipients. J Speech Lang Hear Res 2008;51:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baguley D, McFerran D, Hall D. Tinnitus. Lancet 2013;382:1600–1607. [DOI] [PubMed] [Google Scholar]
- 35. Simonetti P, Oiticica J. Tinnitus mechanisms and structural changes in the brain: the contribution of neuroimaging research. Int Arch Otorhinolaryngol 2015;19:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schecklmann M, Giani A, Tupak S, et al. Functional near‐infrared spectroscopy to probe state‐and trait‐like conditions in chronic tinnitus: a proof‐of‐principle study. Neural Plast 2014;2014:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Issa M, Bisconti S, Kovelman I, Kileny P, Basura G. Human auditory and adjacent nonauditory cerebral cortices are hypermetabolic in tinnitus as measured by functional near‐infrared spectroscopy (fNIRS). Neural Plast 2016;2016:7453149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Norena A, Eggermont J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 2003;183:137–153. [DOI] [PubMed] [Google Scholar]
- 39. Vanneste S, Van de Heyning P, De Ridder D. Contralateral parahippocampal gamma‐band activity determines noise‐like tinnitus laterality: A region of interest analysis. Neuroscience 2011;199:481–490. [DOI] [PubMed] [Google Scholar]
- 40. San Juan J, Hu X, Issa M, et al. Tinnitus alters resting state functional connectivity (RSFC) in human auditory and non‐auditory brain regions as measured by functional near‐infrared spectroscopy (fNIRS). PLoS One 2017;12:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Engel A, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci 2001;2:704–716. [DOI] [PubMed] [Google Scholar]
- 42. Fox M, Snyder A, Zacks J, Raichle M. Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 2006;9:23–25. [DOI] [PubMed] [Google Scholar]
- 43. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 44. Steinbrink J, Villringer A, Kempf F, Haux D, Boden S, Obrig H. Illuminating the BOLD signal: Combined fMRI‐fNIRS studies. Magn Reson Imaging 2006;24:495–505. [DOI] [PubMed] [Google Scholar]
- 45. Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage 2011;54:2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehagnoul‐Schipper DJ, van Colier WNJM, van Erning LJTO, et al. Simultaneous measurements of cerebral oxygenation changes during brain activation by near‐infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects. Human Brain Mapping 2002;16:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okamoto M, Dan H, Shimizu K, et al. Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. NeuroImage 2004;21:1275–1288. [DOI] [PubMed] [Google Scholar]
- 48. Hoge RD, Franceschini MA, Covolan RJM, Huppert T, Mandeville JB, Boas DA. Simultaneous recording of task‐induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin‐labeling MRI. Neuroimage 2005;25:701–707. [DOI] [PubMed] [Google Scholar]
- 49. Toronov V, Webb A, Choi JH, et al. Investigation of human brain hemodynamics by simultaneous near‐infrared spectroscopy and functional magnetic resonance imaging. Med Phys 2001;28:521–527. [DOI] [PubMed] [Google Scholar]
- 50. Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage. 2006b;29:368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kennan RP, Kim D, Maki A, Koizumi H, Constable RT. Non‐invasive assessment of language lateralization by Transcranial near infrared optical topography and functional MRI. Hum Brain Mapp 2002;16:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perrachione T, Gosh S, Ostrovskaya I, Gabrieli J, Kovelman I. Phonological working memory or words and nonwords in cerebral cortex. J Speech Lang Hear Res 2017;60:1959–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niu H, Li X, Chen Y, Ma C, Zhang J, Zhang Z. Reduced frontal activation during a working memory task in mild cognitive impairment: a non‐invasive near‐infrared spectroscopy study. CNS Neurosci Ther 2013;19:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lloyd‐Fox S, Richards J, Blasi A, Murphy D, Elwell C, Johnson M. Coregistering functional near‐infrared spectroscopy with underlying cortical areas in infants. Neurophotonics 2014;1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsuzuki D, Dan I. Spatial registration for functional near‐infrared spectroscopy: From channel position on the scalp to cortical location in individual and group analyses. Neuroimage 2014;85:92–103. [DOI] [PubMed] [Google Scholar]
- 56. Wijeakumar S, Spencer J, Bohache K, Boas D, Magnotta V. Validating a new methodology for optical probe design and image registration in fNIRS studies. Neuroimage 2015;106:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blume W, Buza R, Okazaki H. Anatomic correlates of the ten‐twenty electrode placement system in infants. Electroencephalogr Clin Neurophysiol 1974;36:303–307. [DOI] [PubMed] [Google Scholar]
- 58. Morris H, Lüders H, Lesser R, Dinner D, Klem G. The value of closely spaced scalp electrodes in the localization of epileptiform foci: A study of 26 patients with complex partial seizures. Electroencephalogr Clin Neurophysiol 1986;63:107–111. [DOI] [PubMed] [Google Scholar]
- 59. Homan R, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol 1987;66:376–382, 1987. [DOI] [PubMed] [Google Scholar]
- 60. Gevins A, Illes J. Neurocognitive Networks of the Human Braina. Ann N Y Acad Sci 1991;620:22–44. [DOI] [PubMed] [Google Scholar]
- 61. Okamoto M, Dan H, Sakamoto K, et al. Three‐dimensional probabilistic anatomical cranio‐cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2004;21:99–111. [DOI] [PubMed] [Google Scholar]
- 62. Tak S, Ye J. Statistical analysis of fNIRS data: a comprehensive review. Neuroimage 2014;85:72–91. [DOI] [PubMed] [Google Scholar]
- 63. Niu H, He Y. Resting‐state functional brain connectivity: Lessons from functional near‐infrared spectroscopy. Neuroscientist 2014;20:173–188. [DOI] [PubMed] [Google Scholar]


