Summary
Hydroxymethylnitrofurazone (NFOH) is a nitrofurazone prodrug effective in vivo during acute infections, and it has less hepatotoxicity effect than the standard drug benznidazole (BZN) which has been used during short‐ and long‐term treatment. In the present study, we induced the indeterminate form of Chagas disease in mice with a Y strain of Trypanosoma cruzi and analysed the histopathological data about the effects of NFOH and BZN on different tissues, including the heart, skeletal muscle, liver, kidney, colon, spleen and brain. After infection, BALB/c mice were treated with NFOH (150 mg/kg) and BZN (60 mg/kg) for 60 days and then submitted to immunosuppression using dexamethasone (5 mg/kg) for 14 days. Two trained analysts, as part of a blind evaluation, examined the results using serial sections of 3 mm diameter in two different moments. The results showed reactivation of the disease only in the infected nontreated group (POS). After treatment, amastigote nests were found in the heart, colon, liver and skeletal muscle in the POS group and in the heart and liver of the BZN group. Interestingly, amastigote nests were not found in the NFOH and NEG groups. The histopathological analysis showed fewer tissue lesions and parasite infiltrates in the NFOH group when compared with the BZN and POS groups. We have not observed any increase in the levels of hepatocellular injury biomarkers (AST/ALT) in the NFOH group. These in vivo studies show the potential for NFOH as an effective and safe compound useful as an anti‐T. cruzi agent.
Keywords: benznidazole, Chagas disease, chronic phase, hydroxymethylnitrofurazone, indeterminate form, Trypanosoma cruzi
1. INTRODUCTION
American trypanosomiasis or Chagas disease is a tropical disease caused by the Trypanosoma cruzi (T. cruzi) that affects approximately 5‐6 million people in the world.1 The globalized transport system has led to the disease being seen in developed countries such as the United States, Japan, Germany, Spain, the Netherlands, France, Portugal, Sweden, Switzerland, the United Kingdom, Italy, Belgium, and Australia. Despite this, only two drugs remain available for treatment during the acute phase of Chagas disease: nifurtimox and benznidazole (BZN).2, 3 For both adults and children, these drugs have demonstrated limited action in the indeterminate form.4, 5, 6, 7, 8, 9, 10, 11, 12
Chung et al13 obtained the hydroxymethylnitrofurazone (NFOH), as a synthetic intermediate aiming to obtain nitrofurazone (NF)‐primaquine prodrugs. The NFOH exhibited trypanocidal activity against trypomastigotes and amastigote forms of T. cruzi using infected LLC‐MK2 cells.13 The NFOH was four times less mutagenic than NF through the AMES test,14 and it did not show in vivo genotoxicity.15 In addition, the pharmacokinetic study has shown an increased bioavailability that is around 20 times more in rats and 10% in rabbits.16, 17
The improved activity of NFOH in comparison with NF can be explained by the nucleophilic attack of Cys25 residue present in the enzyme cruzain of T. cruzi to the carbonyl group present in the semicarbazone subunit of NFOH. At the same concentration, NF inhibited 30% of cruzain, while for NFOH, a value of 60% was recorded.18, 19, 20
Two studies described the in vivo efficacy of NFOH in the acute phase of Chagas disease. Ekins et al,21 using infected transgenic T. cruzi Brazil (Luc strains) with firefly luciferase, showed 78.5% of efficacy after four days of treatment with NFOH (50 mg/kg).21 In another study, Davies et al,22 which used the Tulahuen strain of T. cruzi, demonstrated negative PCR after six months of the last dose of NFOH treatment (150 mg/kg, 60 days). In addition, NFOH caused low animal mortality after experiments (NFOH, 16%; BZN, 33%; NF, 75%; positive control, 66%).22
Melo23 reported an increase in the rat lethal dose (LD50) for NFOH (2000 mg/kg) in comparison with NF (556.3 mg/kg).23 A cytotoxicity assay using HepG2 cells revealed that BZN was able to reduce 30% of the cells, while NFOH did not cause cell death. Moreover, in vivo analysis for short‐term (ST, 21 days) and long‐term (LT, 60 days) treatment demonstrated that NFOH (150 mg/kg/d) did not show hepatotoxicity, while BZN (150 mg/kg/d) showed extensive liver inflammation and fibrosis.24
In summary, the previous studies showed the in vitro and in vivo efficacy of NFOH against trypomastigotes forms of T. cruzi in the early acute phase of Chagas disease with decreased toxicity compared to BZN. The current available drugs are effective in the acute phase of the disease and act against amastigotes of some strains of T. cruzi in late acute, indeterminate, and chronic phases of Chagas disease. But they do not exhibit activity against the Y strain of T. cruzi during the later phases.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 So, the purpose of this work was to evaluate the effect of NFOH in several tissues (heart, colon, liver, kidney, spleen, brain and skeletal muscle) during the indeterminate form of the disease, induced by the Y strain of T. cruzi in BALB/c male mice.
2. MATERIAL AND METHODS
2.1. Ethical approval
The study and all the procedures were approved by the Research Ethics Committee of Animal Experimentation of the School of Pharmaceutical Sciences of Araraquara, São Paulo State, Brazil—UNESP (39/2014). Male mice were used, BALB/c isogenic, with weights between 18 and 21 g. All animals were kept under controlled temperatures (23 ± 1°C), automatic lighting (12/12 hours cycle), humidity (55% ± 5%), ration and water ad libitum.
2.2. Parasites and animal infection
The induction of the Chagas disease was performed with an inoculum of 102 trypomastigotes forms (blood transfusions),28, 31, 36 Y strain of T. cruzi, by intraperitoneal injection. This inoculum was obtained, standardized and maintained by the Parasitology Laboratory of the School of Pharmaceutical Sciences, São Paulo State University—UNESP, Araraquara/SP—Brazil.
2.3. Experimental design and treatment
Figure 1 shows the experimental design. After three days of inoculation, 5 μL of fresh blood samples from the tails of the mice was collected weekly until no parasites were observed in the blood, which was confirmed by three negative parasitaemia on alternate days, showing subpatent parasitaemia and a simulation of the indeterminate form of chronic Chagas disease. Using a parasitaemia curve, in order to establish the infection pattern, 5 μL of blood obtained from the Balb/c mice's tails was examined microscopically (100 fields), and the number of parasites was established according to Brener's technique (1962).
Figure 1.
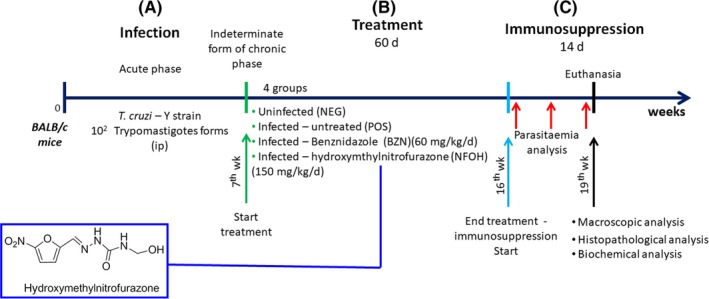
Experimental protocol. A, Infection and induction of chronic phase with a Y strain of T. cruzi; B, treatment period with NFOH and BZN (indeterminate form of chronic phase); C, immunosuppression [Colour figure can be viewed at wileyonlinelibrary.com]
Four groups were separated into positive (POS, infected), negative (NEG, not infected) control, treatment with BZN (infected, 60 mg/kg/d) and treatment with NFOH (infected, 150 mg/kg/d)22 (N = 42; each infected groups, n = 12; no infected group, n = 6). The drug was administrated by gavage in a suspension of arabic gum (4%)37, 38, 39 once a day for 60 consecutive days.22 The experiment was performed with all groups at one time.
2.4. Recrudescence induced
Twenty‐four hours after treatment, the immunosuppression was induced by dexamethasone (5 mg/kg/d) through i.p. route for 14 consecutive days. The parasitaemia analyses were observed in three distinct periods, analysed at the first, seventh and fourteenth day.40
2.5. Anatomopathological evaluation—Relative weight
The weight of animals was measured weekly to identify gain or loss of weight during the experimental protocol, as well as any change at any moment of research. The weight of animals was measured weekly to identify any change at any moment of research. After euthanizing, the organs, heart, liver, kidney, spleen and brain, were weighed to establish the relative weight ([organ/animal weight] × 100), and the results were expressed as percentage.41, 42, 43, 44, 45 The colon and skeletal muscle were excluded from this analysis due to the importance of weight of bone and faeces (bias).
2.6. Macroscopic analysis
After euthanizing, the organs were immersed in tamponed formaldehyde (10%). All organs and tissues were codified to blind all groups on the subsequently analysis. The macroscopic analyses were carried out to identify any alterations on surface of organs and tissues. Specifically, relevant to the liver, the gallbladder was measured and classified into normal (+, <1 mm), enhanced (++, 1‐3 mm) and mega‐gallbladder (+++, >3 mm).
2.7. Histopathological analysis
The organs were analysed as follows: heart (all cardiac tissue—portions transversal); brain (the internal half of the both hemispheres—portions transversal); large intestine (colon segment—fragment collected just after the ileocecal valve—portions transversal); kidney (both ventral half—portions transversal); spleen (all distal part—portions transversal); liver (biggest lobule—portions transversal); gallbladder (entire organ—macroscopic analysis); skeletal muscle (quadriceps muscles—portions transversal). All organs/tissues were sectioned each 3 mm for analysis of whole organ/tissue (Figure 2).
Figure 2.
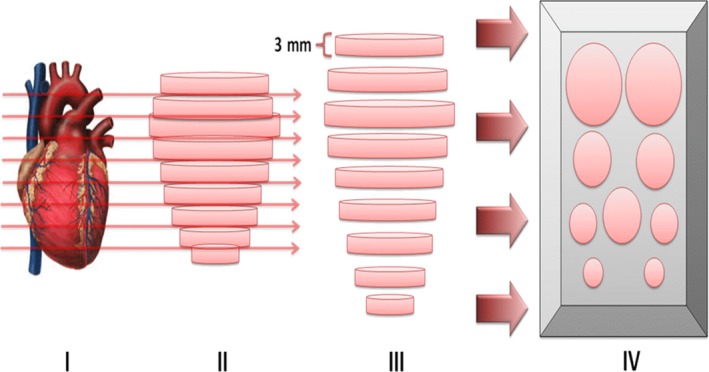
Representation of serial histopathological sections (3 mm). (I) Example of an anatomical organ; (II) geometric representation of the organ/tissue; (III) serial sections of 3 mm throughout the organ/tissue; and (IV) representation of organs/tissue in paraffin for histopathological sections. In this study, the following organs/tissues were serially analysed: heart, colon, spleen, liver, kidney, brain and skeletal muscle [Colour figure can be viewed at wileyonlinelibrary.com]
All codified organs and tissues were placed in paraffin (in a 4‐μmol/L section), and haematoxylin and eosin staining (H and E) was performed. The histopathological analyses were performed by optical microscopy (Primo Star; Carl Zeiss Mycroscopy® GmbH, Gottingen, Germany) at 10×, 20×, 40× and 100× magnifications. A pathologist carried out the analysis, and 15% of samples were used to calculate the intra‐examined index (Kappa index, 0.83). Photographs were taken at 400 × with an Olympus X‐785 digital camera coupled to the microscope. The histopathology analysis was based in the following pathological alterations:
In the heart, the following aspects were identified: cellular hypertrophy, inflammatory infiltrate (Table 1), dystrophic calcification and amastigote nests.
Table 1.
Numerical criteria for the classification of inflammatory parameters according to the type of cell found during histopathological analysis
| Infiltrates parameters | Intensity | Type | |||||
|---|---|---|---|---|---|---|---|
| Absent (0%) | Mild (1%‐33%) | Moderate (33%‐66%) | Intense (>66%) | Acute | Mixed | Chronic | |
| Inflammatory Infiltrates | −1 | 0 | 1 | 2 | −1 to 0 | 0.1‐1 | 1.1‐2 |
In the colon, epithelial mucosa, gut‐associated lymphoid tissue (GALT), dystrophic calcification and amastigote nests were analysed. The ascending, transverse, descending and sigmoid colon was all examined.
In the spleen, the presence of capsule, white/red pulp, marginal zone and follicles in the organ (Table 2); dystrophic calcification; and amastigote nests were analysed.
Table 2.
Numerical criteria for the classification of splenic parameters according to the type found during histopathological analysis
| Lymphoid parameters (spleen) | Decreased | Increased |
|---|---|---|
| White pulp | 1‐2 | 2.1‐3 |
| Red pulp | 1‐2 | 2.1‐3 |
| −1 to 0 | 0.1‐1 | |
| Lymphoid follicles | Disorganized | Organized |
The liver analyses involved the general structure, hepatocyte morphology, necrosis, lipidosis (Table 3), inflammatory infiltrate (Table 1), dystrophic calcification and presence of amastigote nests.
Table 3.
Numerical criteria for the classification of hepatic parameters according to the histopathological measures
| Hepatic parameters (Liver) | Intensity | |||
|---|---|---|---|---|
| Absent (0%) | Mild (1%‐33%) | Moderate (33%‐66%) | Intense (>66%) | |
| Lipidosis | −1 | 0 | 1 | 2 |
| Necrosis | −1 | 0 | 1 | 2 |
In the kidneys, the presence of Bowman's capsule space, the proximal and distal ducts, circulatory alteration (haemorrhage), dystrophic calcification and amastigote nests were analysed.
In the brain, Purkinje cells, circulatory alteration (haemorrhage), dystrophic calcification and amastigote nests were analysed.
The skeletal muscle analyses involved the general structure of the muscle cells, inflammatory infiltrate (Table 1), dystrophic calcification and amastigote nests.
2.8. Cellular damage markers
The activities of alanine (ALT) and aspartate (AST) aminotransferases at the end of the treatment followed by immunosuppression were determined in BALB/c mice by using commercially available assay kits (Labtest®, Lagoa Santa, MG, Brazil). The data are expressed as average ± SD.
2.9. Statistical analysis
For statistical analysis, the GraphPad Prism Software® (La Jolla, CA, USA) statistical program 6.0 was used by applying analysis of variance (P < 0.05, P < 0.001) by Tukey multiple comparisons and by ANOVA parametric test and performed using analysis of the Kruskal‐wallis nonparametric test (P < 0.05).
3. RESULTS
3.1. NFOH does not cause mortality during the treatment
The acute phase showed 25% of mortality. By the end of the immunosuppressive phase, one animal from each treated group died (NFOH and BZN), due to alpha male attacks.46, 47, 48, 49, 50 No animal died during the treatment phase.
3.2. NFOH is able to prevent parasitaemia recrudescence
The parasitaemia was accomplished, as shown in Figure 3. After immunosuppression, the highlighted area details the reactivation of parasitaemia. Parasitaemia reactivation was found in the nontreated group (POS), which demonstrated the efficacy of immunosuppression to induce the parasitaemia during the indeterminate form of chronic Chagas disease in mice. Both NFOH and BZN groups did not exhibit parasitaemic reactivation (Figure 3).
Figure 3.
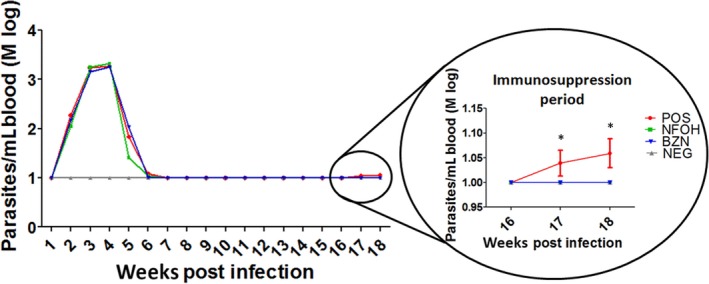
Trypanosoma cruzi (Y strain) parasitaemia curves from infected isogenic BALB/c mice (POS and NFOH and BZN treatments), expressed as logarithmic mean (Mlog). *Statistically different of NFOH, BZN and NEG groups (P < 0.0001) [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. NFOH is able to prevent the formation of splenomegaly, mega‐kidney and mega‐gallbladder (without mega‐stone) compared to POS
Organ relative weight was another parameter evaluated in order to verify the health of the animals. The mean of relative weights of organs, including the heart, brain, liver, kidney and spleen, is shown in Figure 4. The POS group had increased weight of the brain, liver, kidney and spleen compared with the NEG group. The NFOH group exhibited low weights of organs, such as the kidney and spleen, when compared to the POS and BZN groups. The BZN group showed an increased weight of the brain, kidney and spleen compared with the NEG group. In addition, this group exhibited a decreased liver weight, compared with the NFOH group, and a reduced spleen weight when compared to the POS group (Tables S1‐S8).
Figure 4.
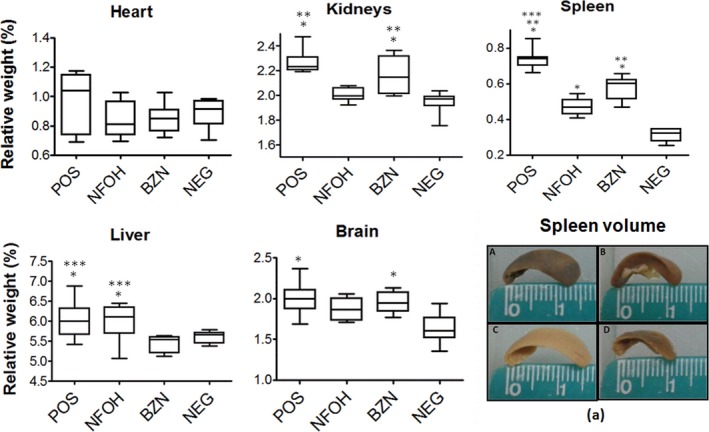
Relative weight of the organs/tissue (spleen, kidneys, heart, brain and liver). (A) POS, (B) NFOH, (C) BZN and (D) NEG. *Statistically significant in the NEG; **Statistically significant in the NFOH; ***Statistically significant in the BZN (P < 0.05); (a) spleen photograph (Tables S1‐S8) [Colour figure can be viewed at wileyonlinelibrary.com]
Macroscopic analyses have shown a statistical difference in the gallbladder size. The NEG group exhibited smaller gallbladders compared with the POS and BZN groups. The POS group showed enhanced gallbladders in 22.2% and mega‐gallbladder in 44.4% of animals. For the BZN group, enhanced gallbladders and mega‐gallbladder were observed in 12.5% of animals. In the NFOH group, no alterations of this organ were observed, being 100% normal gallbladder; therefore, it is statistically different from the group POS (Table 4).
Table 4.
Macroscopic analysis of the gallbladder (size) expressed in percentage (%) of animals per group
| Groups | Gallbladder size (macroscopic analysis) | ||
|---|---|---|---|
| Normal (+, <1 mm) | Enhanced (++, 1‐3 mm) | Mega‐gallbladdera (+++, >3 mm) | |
| POS | 33.3% | 22.2% | 44.4% |
| NFOH | 100% | 0% | 0% |
| BZN | 75% | 12.5% | 12.5% |
| NEG | 100% | 0% | 0% |
Presence of mega‐stones in gallbladder.
3.4. NFOH reduced intensity of tissue parasitism and inflammation compared to POS
The histopathological analyses were performed, and the results are shown in Figures 5 and 6. All infected animals (POS, BZN and NFOH) showed cardiac hypertrophy and mild and chronic inflammatory infiltrates that were statistically different from the NEG group. There was no statistically significant difference between the groups infected in relation to these cardiac parameters. Dystrophic calcification was found in the cardiac tissue of 44.4% of animals in the POS group, 25% in the NFOH group and 12.5% in the BZN group, but it was absent in the animals of the NEG group. In addition, the presence of amastigote nests was found in 55.5% of the animals in the POS group and 50% in the BZN group, but none were recorded in the NFOH group (Figures 5 and 7).
Figure 5.
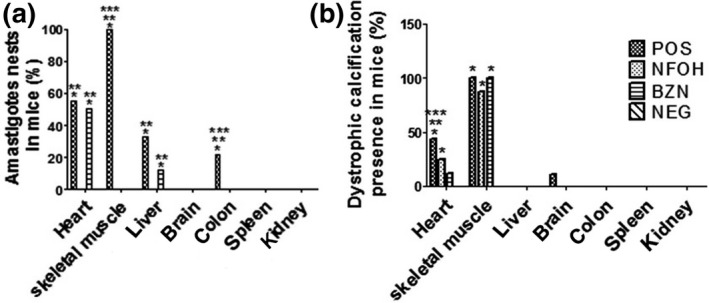
Histopathological analysis of the organs (H&E). A, Percentage of animals/organs with amastigote nests; B, percentage of animals/organs with dystrophic calcification. *Statistically different of NEG; **Statistically different of NFOH; ***Statistically different of BZN (P < 0.05)
Figure 6.
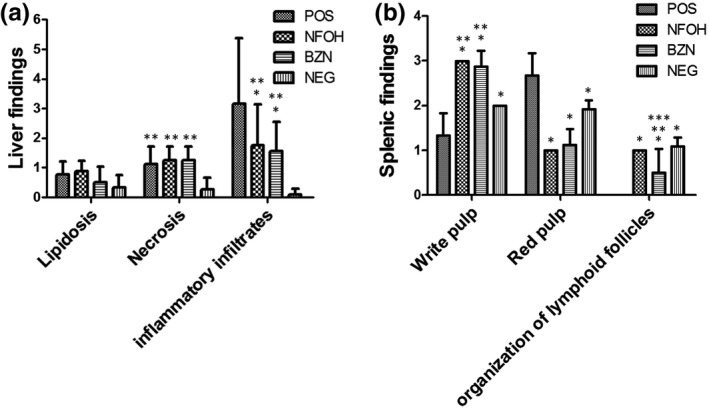
Histopathological analysis of the organs (H&E). A, Percentage of animals with disorganization of lymphoid tissues; B, quantification of inflammatory infiltrates in hepatic tissue (according to Tables 1, 2, 3). *Statistically different of POS (P < 0.05); **Statistically significant in the NEG; ***Statistically significant in the NFOH (P < 0.05)
Figure 7.
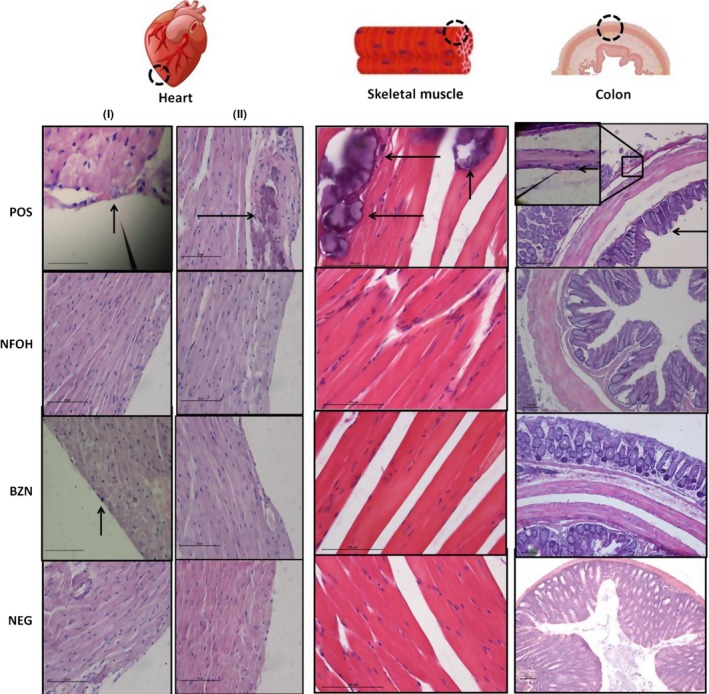
Photograph of the heart (I‐II), skeletal muscle (H&E, 40 X) and colon tissues (H&E, 10X). Heart: I—POS and BZN presence of amastigote nest (small arrow), NFOH and NEG: healthy cardiac muscle cells; II—dystrophic calcification (large arrow) presence in the POS. The BZN, NFOH and NEG: healthy cardiac muscle cells. Skeletal muscle: POS presence of amastigote nest (small arrow) and dystrophic calcification region (large arrow), NFOH, BZN and NEG: normal tissue. Colon: POS presence of amastigote nest (small arrow) and hypertrophy of the muscle layer (large arrow), NFOH, BZN and NEG normal colon [Colour figure can be viewed at wileyonlinelibrary.com]
In the colon, amastigotes were found only in the POS group. The presence of amastigote nests was found around the myenteric plexus, and focal inflammatory infiltrates were recorded in the POS group in 22.2% of the animals. Amastigotes and inflammatory infiltrates were absent in the NFOH, BZN and NEG groups (Figures 5 and 7).
The analysis of skeletal muscle identified amastigote nests and dystrophic calcification in all animals of the POS group. The treatment with NFOH and BZN groups showed 87.5% and 100% of animals with dystrophic calcification respectively. However, amastigote nests were not found in these groups (Figures 5 and 7).
For the liver, all groups showed normal hepatocytes and normal sinusoid spaces. Focal necrotic regions were identified, mainly in the POS and BZN groups, but there was no statistical difference between the infected groups. The presence of acute, chronic and mixed inflammatory infiltrates was observed in the POS group, and this was statistically increased, compared with the BZN and NFOH groups. The presence of amastigote nests was verified in 33.3% of animals in the POS group and 12.5% in the BZN group. There were no amastigote nests found in the NFOH group (Figures 6 and 8).
Figure 8.
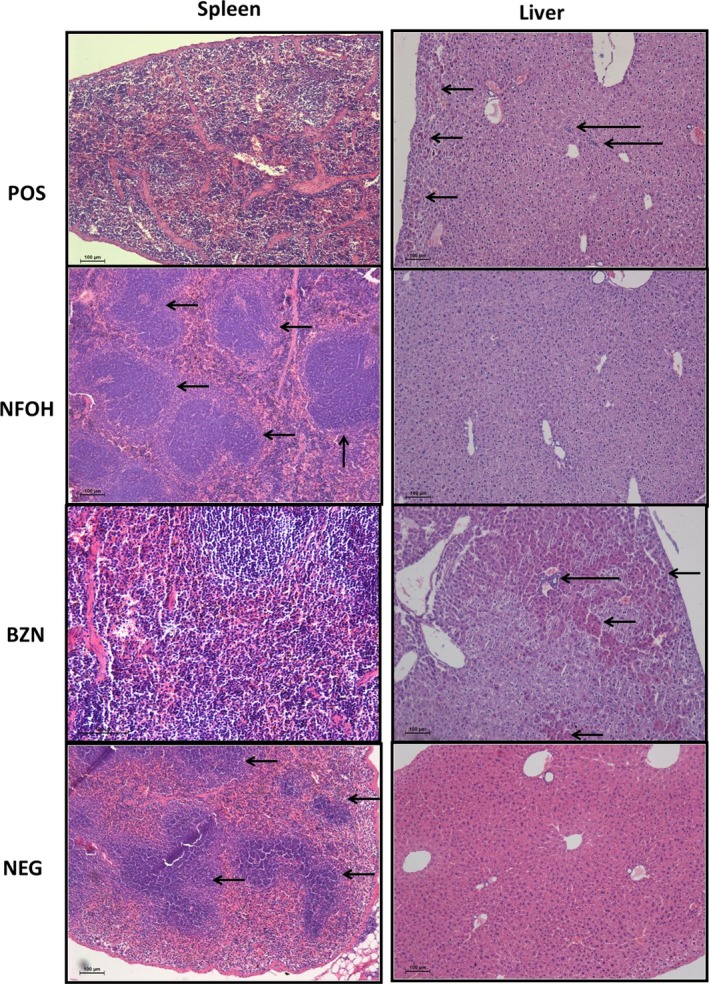
Photograph of the spleen and liver tissues (H&E, 10×). Spleen: POS and BZN lymphoid follicles unorganized, NFOH and NEG lymphoid follicles organized (small arrow); liver: POS and BZN presence of inflammatory infiltrates (larger arrow) and necrosis areas (small arrow), NFOH and NEG healthy hepatocytes [Colour figure can be viewed at wileyonlinelibrary.com]
A disorganized lymphoid follicle in the POS group was found in all animals during splenic tissue analysis. The BZN group showed that 50% of the animals had disorganized lymphoid tissue. In addition, organized lymphoid follicles were found for both the NEG and NFOH groups (Figures 6 and 8). Kidney analysis showed normal proximal, distal ducts and glomerular structure, and a focal region was identified containing inflammatory infiltrates (acute and mild) in all infected groups. The brain analysis identified one animal in the POS group exhibiting dystrophic calcification. The BZN and NFOH groups did not show dystrophic calcification. Atrophy of the granular layer was observed in all infected groups. However, the data analysis did not show statistical differences among the groups.
3.5. NFOH during long period of treatment does not cause increased serum alanine to aspartate aminotransferase (ALT/AST) levels
The BALB/c mice received an oral dose of NFOH (150 mg/kg) and BZN (60 mg/kg) during 60 days of treatment to determine the liver toxicity of antiparasite drugs. All groups showed normal AST levels. NFOH group, as well as BZN group, does not cause increase in AST levels when compared to POS respectively (Figure 9).
Figure 9.
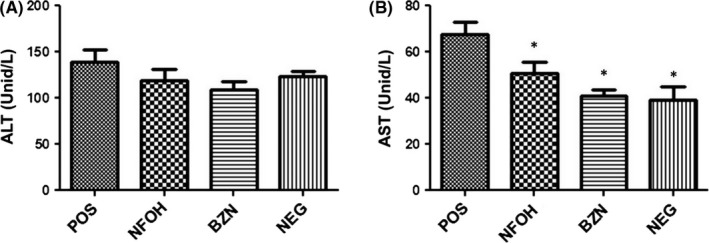
Toxicity evaluation in BALB/c mice after treatment an oral dose of 150 mg/kg/d of NFOH, 60 mg/kg/d of BZN. Serum levels of aspartate aminotransferase (AST) (A) and alanine aminotransferase (ALT) (B). The data are expressed as average ± SD. *Statistically significant difference with the infected animals (POS; P < 0.05)
4. DISCUSSION
The current drugs in the global market for the treatment of Chagas diseases have been used for more than 40 years. Their common problem is inefficacy during indeterminate and chronic phases of the disease, which culminates with the appearance of the latent form of the parasite.51, 52, 53, 54, 55 Silva and Nussenzweig56 isolated the Y strain (T. cruzi) from an acute human case reported in the city of Marília, São Paulo State, Brazil. Because of its high virulence (type II) and resistance to the current drugs, most of the animals died in the experiments during the acute phase.56, 57, 58, 59, 60, 61
According to the literature, the pre‐manifestation period occurs before the acute phase, and it lasts until the 7th day postinfection (dpi). The parasitaemia peaks after inoculation of the Y strain (T. cruzi) and occurs in 21‐28 dpi. The acute phase is considered to be under 30 dpi, indeterminate form (latent period) between 30 and 90 dpi and chronic phase at more than 120 dpi.62, 63, 64, 65
The immunosuppression can be reactivated and induced by drugs for animals and humans with a T. cruzi infection, after transplantation or even in association with autoimmune diseases, such as AIDS.10, 66, 67, 68 According to Pereira et al,40 dexamethasone can be used to induce reactivation of disease in animals, and Andrade et al69 reported 6%‐25% of deaths after immunosuppression of animals.
In this study, we inoculated 102 cells of trypomastigotes forms. We noticed that 25% of infected animals died during the acute phase, as expected. We used a dose of 60 mg/kg/d for the BZN group and 150 mg/kg/d for the NFOH group, following a previous report from Davies et al,22 to guarantee the maximal number of surviving animals up until the end of the procedure, after the immunosuppression stage.22, 24 In animal models, the BZN concentration in the chronic phase of Chagas disease, is directly related to side effects; this is favourable to use lower concentration in order to increase the period of treatment.70
The treatment started after 7 weeks of infection during the period of subacute parasitaemia. The period of drug treatment was performed according to previous procedures described in the literature.22, 24, 35 For the reactivation of the parasitaemia, 5 mg/kg of dexamethasone was administered for 14 days.40 The results showed that duration of 60 days of treatment using both drugs (BZN and NFOH) could inhibit the parasite growth even after immunosuppression.
Histopathological analysis of hearts from patients with chronic Chagas cardiomyopathy reveals a diffuse infiltrating inflammatory, hypertrophy, fibrosis and myocarditis with scarce tissue parasitism.71, 72, 73 In the previous literature, the immunosuppression during the chronic phase of Chagas disease (Y strain) caused development of myocardial lesions in mice.69 In our study, 100% of the infected animals were found to have cardiac hypertrophy and inflammatory infiltrates that were statistically different from the NEG group. Amastigote nests were found in 55.5% of cardiac tissue for the POS group and 50% for the BZN group. Interestingly, amastigote nests were not observed in NFOH and NEG groups.
The dystrophic calcification is a physiological condition that appears after necrosis, and occurs in either the acute or the chronic phase of infection.74, 75, 76, 77 However, heart sizes were not significantly different across all groups. Herein, we have observed the appearance of this dystrophic calcification in cardiac muscle tissues in 25% of animals treated with NFOH and 12.5% of animals from the BZN group, while for the POS group, it was identified in 44.4% of animals. These data suggest that all infected animals had heart necrosis that was most likely promoted by the amastigote infection and that the treatment with the NFOH was more effective than with the BZN (even though this group had less dystrophic calcification, it was found in 50% of animals with amastigote nests).
In histological analysis of skeletal tissues, amastigote nests and dystrophic calcifications were found in 100% of the POS animals. In the NFOH and BZN groups, we observed the appearance of this dystrophic calcification in 87.5% and 100% respectively. No amastigote nests were found after treatments.
In the liver, inflammatory infiltrate was observed with the presence of amastigote nests, focal regions of inflammatory infiltrate and necrosis in 33.3% of animals of the POS group and 12.5% of the BZN group. However, through observation, we saw that the area of necrosis was greater in the POS group than in the BZN group. The treatment with NFOH showed similar tissue profiles as the negative group. These results are in accordance with the results of Davies et al,24 who studied the in vivo hepatotoxicity using the NFOH and BZN at identical concentrations (150 mg/kg/d) during short‐term (ST: 21 days) and long‐term treatments (LT: 60 days). These authors have reported that the NFOH effect on the liver causes lower quantities of inflammatory infiltrates than in the BZN group in both periods (ST and LT). The same is observed for liver fibrotic remodelling during ST.24
Moreover, the NFOH treatment (150 mg/kg/d) did not cause liver injury in T. cruzi‐infected BALB/c mice, as demonstrated by levels of the ALT/AST assay when compared to reported references values.24 On the other hand, the NFOH group showed lower levels of AST compared to POS. It is interesting to note that biomarkers of hepatic levels (ALT/AST) are proportional to the increased liver damage (histopathological analysis) and can be attributed to T. cruzi tropism in this organ.
In the colonic tissue, amastigote nests were found in 22.2% of animals, only in the POS group. Ganglioneuritis in organs such as the colon, oesophagus and stomach has been reported,78 but it was not observed in this work. However, a significant increase in relative weight was observed in the spleen, liver and kidney. In fact, the infection enlarges secondary organs such as the spleen, liver, kidneys, gallbladder, pancreas, stomach, duodenum and jejunum,44, 79, 80 by destruction of the enteric nervous system (ESN) neurons which are present throughout the gastrointestinal tract. Once the ESN is dependent on the parasympathetic functions of the autonomic nervous system (ASN), the destruction of ESN alters the secretion regulations, motility, blood flow and immunological control of gastrointestinal organs.79, 81, 82 The parasympathetic denervation of the digestive tract and the consequent morphological and functional alterations have been extensively described.83, 84, 85, 86 The involvement of the glands and secondary organs of the digestive tract in Chagas disease has not been studied much.81, 82, 87 When ESN is destroyed, the bile secretion is blocked, increasing the gallbladder size.79, 81, 82 In our experiments, macroscopic analysis of the gallbladder revealed that no treated animal belonging to the NFOH group had an increase to this specific organ. On the other hand, an increase was observed in 66.6% of the POS and 25% of the BZN animals, suggesting the possible action of NFOH in intracellular parasites of T. cruzi and its ability to prevent the destruction of the myenteric plexus of this organ.
The congestive splenomegaly is a haemodynamic change caused from congestive heart failure in Chagas disease and is characterized by hyperplasic cells and disorganization of the spleen lymphoid follicles. The alteration of the lymphoid follicles also indicates an ongoing inflammatory process.45, 88, 89 Our results showed splenomegaly with a disorganization of lymphoid follicles in all the POS animals and 50% of the BZN animals. For the NFOH group, a reversible effect, a normal morphologic condition, was observed, suggesting a haemodynamic heart condition recovery and decrease in the inflammation process in 100% of the animals.
The BZN showed limited effectiveness in the disease. Several drawbacks, including its high toxicity, inability to eliminate amastigote forms present in tissues and absence of a protective effect in the tissues caused by the chronic stage of the disease, are still serious limitations that justify the discovery of new drugs.4, 7, 8, 9, 10, 12, 35, 90, 91 Even after more than 40 years since its discovery, there is not an efficient and safe treatment for chronic phase lesions. In some countries such as Brazil, BZN is the only drug available.
In this work, we describe a new alternative drug candidate for the indeterminate form of Chagas disease which is capable of preventing the reactivation of parasitaemia after immunosuppression, reducing lesions, and decreasing parasite infiltrates on organs/tissues. Moreover, NFOH has presented easy synthetic preparation, low cost and high yield.
5. CONCLUSION
We have succeeded in inducing the experimental indeterminate form of chronic Chagas disease, with reactivation of parasitaemia only in the POS group after immunosuppression. The histopathological studies demonstrate that animals without treatment (POS) showed the most severe parasite infiltrates in organs such as the heart, skeletal muscle, colon and liver tissue. The BZN treatment showed the presence of heart and liver amastigote infiltrates. The NFOH treatment removed amastigotes from all tissues studied; being intact fixed organism, these parasites were carefully analysed, as well as seen directly in situ. Furthermore, the gallbladder of animals treated with NFOH exhibited similar profiles to noninfected animals. Concerning inflammatory infiltrates of the liver, we identified the following order: POS > BZN ≅ NFOH > NEG. In this model, NFOH was not able to increase the levels of liver biomarkers. These results suggest that the NFOH is a promising drug candidate and alternative to treat Chagas disease.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally to the manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Proc. 2014/14980‐0, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Proc. 312404/2013‐1, Programa de Apoio ao Desenvolvimento Científico da Faculdade de Ciências Farmacêuticas da UNESP (PADC/FCF‐UNESP) and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for research fellowships and financial assistance. Also, the authors are grateful for the technical assistance of Isabel Martinez for the T. cruzi inoculation and to Rafael Consolin Chelucci and Diego Eidy Chiba who kindly helped in the euthanasia of the animals.
Scarim CB, de Andrade CR, da Rosa JA, dos Santos JL, Chin CM. Hydroxymethylnitrofurazone treatment in indeterminate form of chronic Chagas disease: Reduced intensity of tissue parasitism and inflammation—A histopathological study. Int J Exp Path. 2018;99:236–248. 10.1111/iep.12289
Contributor Information
Cauê B. Scarim, Email: cauebenitos@gmail.com.
Chung M. Chin, Email: chung@fcfar.unesp.br
REFERENCES
- 1. WHO . Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Geneva: World Health Organization; 2015, 90, 33‐44. [PubMed] [Google Scholar]
- 2. Maya JD, Orellana M, Ferreira J, Kemmerling U, López‐Muñoz R, Morello A. Chagas disease: present status of pathogenic mechanisms and chemotherapy. Biol Res. 2010;43:323‐331. [PubMed] [Google Scholar]
- 3. WHO . Control of Chagas disease: second report of the WHO expert committee World Health Organization (2000: Brasilia, Brazil). Geneva: World Health Organization; 2002, 905, 109. [PubMed] [Google Scholar]
- 4. Andrade MC, Oliveira MDF, Nagao‐Dias AT, et al. Clinical and serological evolution in chronic Chagas disease patients in a 4‐year pharmacotherapy follow‐up: a preliminary study. Rev Soc Bras Med Trop. 2013;46:776‐778. [DOI] [PubMed] [Google Scholar]
- 5. Da Silva GMS, Mediano MFF, Do Brasil PEAA, et al. A clinical adverse drug reaction prediction model for patients with chagas disease treated with benznidazole. Antimicrob Agents Chemother. 2014;58(11):6371‐6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Andrade ALSS, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407‐1413. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes CD, Tiecher FM, Balbinot MM, et al. Efficacy of benznidazol treatment for asymptomatic chagasic patients from state of Rio Grande do Sul evaluated during a three years follow‐up. Mem Inst Oswaldo Cruz. 2009;104:27‐32. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez ML, Riarte AR, Marson ME, et al. Pharmacokinetic and pharmacodynamic responses in adult patients with Chagas disease treated with a new formulation of benznidazole. Mem Inst Oswaldo Cruz. 2016;111:218‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasslocher‐Moreno AM, do Brasil PEAA, de Sousa AS, Xavier SS, Chambela MC, da Silva GMS. Safety of benznidazole use in the treatment of chronic Chagas’ disease. J Antimicrob Chemother. 2012;67:1261‐1266. [DOI] [PubMed] [Google Scholar]
- 10. Morillo CA, Marin‐Neto JA, Avezum A, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295‐1306. [DOI] [PubMed] [Google Scholar]
- 11. Sosa‐Estani S, Segura E, Ruiz A, Velazquez E, Porcel B, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas’ disease. Am J Trop Med Hyg. 1998;59:526‐529. [DOI] [PubMed] [Google Scholar]
- 12. Soy D, Aldasoro E, Guerrero L, et al. Population pharmacokinetics of benznidazole in adult patients with Chagas disease. Antimicrob Agents Chemother. 2015;59(6):3342‐3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung MC, Carvalho Güido RV, Favarato Martinelli T, et al. Synthesis and in vitro evaluation of potential antichagasic hydroxymethylnitrofurazone (NFOH‐121): a new nitrofurazone prodrug. Bioorg Med Chem. 2003;11:4779‐4783. [DOI] [PubMed] [Google Scholar]
- 14. Guido RVC, Ferreira EI, Nassute JC, Varanda EA, Chung MC. Diminuição da atividade mutagênica do pró‐fármaco NFOH‐121 em relação ao nitrofural (nitrofurazona). Rev Ciênc Farm. 2001;22(2):319‐333. [Google Scholar]
- 15. Bosquesi PL. Avaliação Da Presença Do Grupo Nitro Na Atividade Antichagásica E Mutagênica Do Candidato a Fármaco Hidroximetilnitrofural (NFOH). Universidade Estadual Paulista Júlio de Mesquita Filho – UNESP; 2009.
- 16. Nogueira Filho MAF, Carvalho EC, De Campos ML, et al. Pharmacokinetics of Hydroxymethylnitrofurazone and Its Parent Drug Nitrofurazone in Rabbits. Drug Metab Lett. 2014;7(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 17. Serafim EOP, De Albuquerque E, Silva AT, et al. Pharmacokinetics of hydroxymethylnitrofurazone, a promising new prodrug for chagas’ disease treatment. Antimicrob Agents Chemother. 2013;57:6106‐6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trossini GHG, Chung MC, Menezes CMS, Ferreira EI. Molecular modeling suggests cruzain specificity for peptide primaquine prodrugs. Lett Drug Des Discovery. 2010a;7:528‐533. [Google Scholar]
- 19. Trossini GHG, Giarolla J, de Rezende L, et al. Synthesis optimization of hydroxymethylnitrofurazone, an antichagasic candidate, using 32 factorial design. Lett Org Chem. 2010b;7:191‐195. [Google Scholar]
- 20. Trossini GHG, Malvezzi A, T‐do Amaral A, et al. Cruzain inhibition by hydroxymethylnitrofurazone and nitrofurazone: investigation of a new target in Trypanosoma cruzi . J Enzyme Inhib Med Chem. 2010c;25:62‐67. [DOI] [PubMed] [Google Scholar]
- 21. Ekins S, de Siqueira‐Neto JL, McCall L‐I, et al. Machine learning models and pathway genome data base for Trypanosoma cruzi drug discovery. PLoS Negl Trop Dis. 2015;9:e0003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davies C, Cardozo RM, Negrette OS, Mora MC, Chung MC, Basombrío MA. Hydroxymethylnitrofurazone is active in a murine model of Chagas’ disease. Antimicrob Agents Chemother. 2010;54(9):3584‐3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melo MFF. Preparação e determinanação da atividade toxicológica do pró‐fármaco hidroximetilnitrofural, potencialmente antichagásico. Universidade Estadual Paulista Júlio de Mesquita Filho – UNESP; 2006. http://www2.fcfar.unesp.br/#!/pos-graduacao/ciencias-farmaceuticas/dissertacoes-teses/2006/
- 24. Davies C, Dey N, Negrette OS, Parada LA, Basombrio MA, Garg NJ. Hepatotoxicity in mice of a novel anti‐parasite drug candidate hydroxymethylnitrofurazone: a comparison with benznidazole. PLoS Negl Trop Dis. 2014;8(10):e3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camandaroba ELP, Reis EAG, Gonçalves MS, Reis MG, Andrade SG. Trypanosoma cruzi: susceptibility to chemotherapy with benznidazole of clones isolated from the highly resistant Colombian strain. Rev Soc Bras Med Trop. 2003;36(2):201‐209. [DOI] [PubMed] [Google Scholar]
- 26. Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum Exp Toxicol. 2006;25:471‐479. [DOI] [PubMed] [Google Scholar]
- 27. Cencig S, Coltel N, Tuyens C, Carlier Y. Evaluation of benznidazole treatment combined with nifurtimox, posaconazole or Am Bisome® in mice infected with Trypanosoma cruzi strains. Int J Antimicrob Agents. 2012;40:527‐532. [DOI] [PubMed] [Google Scholar]
- 28. Esperandim VR, Da Silva Ferreira D, Toldo MPA, Saraiva J, Augusto MB, De Albuquerque S. New method for quantification of Trypanosoma cruzi in animal's tissue in the chronic phase of experimental Chagas’ disease. Parasitol Res. 2010;106:1471‐1473. [DOI] [PubMed] [Google Scholar]
- 29. Francisco AF, Lewis MD, Jayawardhana S, Taylor MC, Chatelain E, Kelly JM. Limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob Agents Chemother. 2015;59(8):4653‐4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francisco AF, Jayawardhana S, Lewis MD, et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci Rep. 2016;6:35351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia S, Ramos CO, Senra JFV, et al. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother. 2005;49(4):1521‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis MD, Francisco AF, Taylor MC, Kelly JM. A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen. 2015;20(1):36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedrosa RC, Cançado JR, Decache W. Estudo longitudinal do eletrocardiograma na doença de Chagas desde a fase aguda. Rev Soc Bras Med Trop. 1993;26:163‐174. [DOI] [PubMed] [Google Scholar]
- 34. Toledo MJDO, Bahia MT, Carneiro CM, et al. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob Agents Chemother. 2003;47(1):223‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaidenberg A, Luong T, Lirussi D, et al. Treatment of experimental chronic chagas disease with trifluralin. Basic Clin Pharmacol Toxicol. 2006;98:351‐356. [DOI] [PubMed] [Google Scholar]
- 36. Pereira IR, Vilar‐Pereira G, Moreira OC, et al. Pentoxifylline reverses chronic experimental chagasic cardiomyopathy in association with repositioning of abnormal CD8+ T‐cell response. PLoS Negl Trop Dis. 2015;9:1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caldas IS, Guedes PMM, dos Santos FM, et al. Myocardial scars correlate with eletrocardiographic changes in chronic Trypanosoma cruzi infection for dogs treated with Benznidazole. Tropical Med Int Health. 2013;18:75‐84. [DOI] [PubMed] [Google Scholar]
- 38. dos Santos FM, Caldas S, Cáu SBA, et al. Trypanosoma cruzi: induction of benznidazole resistance in vivo and its modulation by in vitro culturing and mice infection. Exp Parasitol. 2008;120:385‐390. [DOI] [PubMed] [Google Scholar]
- 39. Ferraz ML, Gazzinelli RT, Alves RO, Urbina JA, Romanha AJ. The anti‐Trypanosoma cruzi activity of posaconazole in a murine model of acute Chagas’ disease is less dependent on gamma interferon than that of benznidazole. Antimicrob Agents Chemother. 2007;51:1359‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pereira A, De Araújo RS, Brito RR, et al. Camundongos imunossuprimidos farmacologicamente como modelo para estudo de infecções parasitárias oportunistas. Rev Bras Parasitol Vet. 2008;17(Suppl 1):292‐295. [PubMed] [Google Scholar]
- 41. Calabrese KS, Paradela ASRC, Do Valle TZ, et al. Study of acute chagasic mice under immunosuppressive therapy by cyclosporin A: modulation and confocal analysis of inflammatory reaction. Immunopharmacology. 2000;47:1‐11. [DOI] [PubMed] [Google Scholar]
- 42. De Oliveira GM, Medeiros MM, Batista WDS, Santana R, Araújo‐Jorge TC, De Souza AP. Applicability of the use of charcoal for the evaluation of intestinal motility in a murine model of Trypanosoma cruzi infection. Parasitol Res. 2008;102:747‐750. [DOI] [PubMed] [Google Scholar]
- 43. Francisco AF. Efeito do tratamento com Efeito do tratamento com benzonidazol sobre o curso da infecção pela cepa y do Trypanosoma cruzi em camundongos submetidos à alteração dos estoques de ferro pelo uso da desferrioxamina. Universidade Federal de Ouro Preto‐MG; 2007. http://www.repositorio.ufop.br/bitstream/123456789/2455/1/DISSERTACAO_EfeitoTratamentoBenzonidazol.PDF
- 44. Jelicks LA, Tanowitz HB. Advances in imaging of animal models of Chagas disease. Adv Parasitol. 2011;75:193‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pereira SA, Rodrigues DB, Castro EC, dos Reis MA, Teixeira VPA. Morphometric study of the spleen in chronic Chagas’ disease. Am J Trop Med Hyg. 2002;66(4):401‐403. [DOI] [PubMed] [Google Scholar]
- 46. Clancy AN, Coquelin A, Macrides F, Gorski RA, Noble EP. Sexual behavior and aggression in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2222‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poole TB, Morgan TR. Difference in aggressive behaviour between male mice (Mus musculus L.) in colonies of different sizes. Anim Behav. 1973;21:788‐795. [DOI] [PubMed] [Google Scholar]
- 48. Poole TB, Morgan TR. Aggressive behavior of male mice (Mus musculus) toward familiar and unfamiliar opponents. Anim Behav. 1975;23:470‐479. [DOI] [PubMed] [Google Scholar]
- 49. Poole TB, Morgan TR. Social and territorial behaviour of laboratory mice (Mus musculus L.). Anim Behav. 1976;24:476‐478. [Google Scholar]
- 50. Ulrich J. The social hierarchy in albino mice. J Comp Psychol. 1938;25:373‐413. [Google Scholar]
- 51. Coura JR. Chagas disease: what is known and what is needed–a background article. Mem Inst Oswaldo Cruz. 2007;102:113‐122. [DOI] [PubMed] [Google Scholar]
- 52. Coura JR, Borges‐Pereira J. Chagas disease: what is known and what should be improved: a systemic review. Rev Soc Bras Med Trop. 2012;45(3):286‐296. [DOI] [PubMed] [Google Scholar]
- 53. Coura JR, De Castro SL. A critical review on chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3‐24. [DOI] [PubMed] [Google Scholar]
- 54. Coura JR, Viñas PA. Chagas disease: a new worldwide challenge. Nature. 2010;465(7301):S6‐S7. [DOI] [PubMed] [Google Scholar]
- 55. Pereira PCM, Navarro EC. Challenges and perspectives of Chagas disease: a review. J Venom Anim Toxins Incl Trop Dis. 2013;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silva LHP, Nussenzweig V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Fol Clin Biol. 1953;20(3):191‐207. [Google Scholar]
- 57. Andrade SG. Caracterização de cepas do Trypanosoma cruzi isoladas no Recôncavo Baiano (Contribuição ao estudo da patologia geral da Doença de Chagas em nosso meio). Rev Pat Trop. 1974;3:65‐121. [Google Scholar]
- 58. Martínez‐Díaz R, Escario J, Nogal‐Ruiz J, Gómez‐Barrio A. Biological characterization of Trypanosoma cruzi strains. Mem Inst Oswaldo Cruz. 2001;96:53‐59. [DOI] [PubMed] [Google Scholar]
- 59. Martinez I. Caracterização de duas cepas de Trypanosoma cruzi chagas, 1909 (kinetoplastida, trypanosomatidae) isoladas de exemplares de triatoma rubrovaria (blanchard, 1843) (hemiptera, reduviidae). Faculty of Pharmaceutical Sciences ‐ Thesis (Doctorate); 2004. http://www2.fcfar.unesp.br/#!/pos-graduacao/biociencias-e-biotecnologias-aplicadas-a-farmacia/dissertacoes-teses/2004/
- 60. Pinto FH, Ribeiro RD, Belda Neto FM, Prado Júnior JC. Estudo comparativo do comportamento da infecção de camundongos, através da inoculação subcutânea e intraperitoneal, utilizando‐se duas cepas de Trypanosoma cruzi . Rev Saude Publica. 1986;20:133‐140. [DOI] [PubMed] [Google Scholar]
- 61. Ribeiro AR, De Oliveira RC, Junior WC, et al. Trypanosoma cruzi isolated from a triatomine found in one of the biggest metropolitan areas of Latin America. Rev Soc Bras Med Trop. 2016;49(2):183‐189. [DOI] [PubMed] [Google Scholar]
- 62. Bustamante JM, Rivarola HW, Fernández AR, et al. Indeterminate Chagas’ disease: Trypanosoma cruzi strain and re‐infection are factors involved in the progression of cardiopathy. Clin Sci (Lond). 2003;104:415‐420. [DOI] [PubMed] [Google Scholar]
- 63. Okumura M. Pathogenesis of chagasic myocarditis: an experimental study. Rev Hosp Clin Fac Med Sao Paulo. 1996;51(5):166‐174. [PubMed] [Google Scholar]
- 64. Taniwaki NN, Gonçalves VM, Romero JK, Da Silva CV, Da Silva S, Mortara RA. Trypanosoma cruzi strains in the Calomys callosus: parasitemia and reaction of intracellular forms with stage‐specific antibodies in the acute and chronic phase of infection and after immunosuppression. Parasitol Res. 2011;109:431‐440. [DOI] [PubMed] [Google Scholar]
- 65. Torres A, Dávila DF, Gottoberg CF, Donis JH, De Bellabarba GA, Ramoni‐Perazzi P. Heart rate responses to a muscarinic agonist in rats with experimentally induced acute and subacute chagasic myocarditis. Rev Inst Med Trop Sao Paulo. 2000;42(4):219‐224. [DOI] [PubMed] [Google Scholar]
- 66. Ferreira M, Ishioka S, Rocha A, et al. Acute fatal Trypanosoma cruzi meningoencephalitis in human immunodeficiency virus‐positive hemophiliac patient. Am J Trop Med Hyg. 1991;45:723‐727. [DOI] [PubMed] [Google Scholar]
- 67. Libow LF, Beltrani VP, Silvers DN, Grossman ME. Post‐cardiac transplant reactivation of Chagas’ disease diagnose by skin biopsy. Cutis. 1991;48:37‐40. [PubMed] [Google Scholar]
- 68. Stolf NA, Bocchi E, Auler JOC. Heart transplantation in patients with Chagas’ disease cardiomyopathy. J Heart Transplant. 1987;6:3017‐3312. [PubMed] [Google Scholar]
- 69. Andrade S, Carneiro FA, de Souza A, de Lima E, Andrade Z. Influence of treatment with immunosuppressive drugs in mice chronically infected with Trypanosoma cruzi . Int J Exp Pathol. 1997;78(6):391‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scarim CB, Ribeiro AR, da Rosa JA, Chung MC. Response to different benznidazole doses in animal models of chronic phase Chagas disease: a critical review. Rev Soc Bras Med Trop. 2018;51:133‐140. [DOI] [PubMed] [Google Scholar]
- 71. Ferreira LR, Ferreira FM, Nakaya HI, et al. Blood gene signatures of chagas disease cardiomyopathy with or without ventricular dysfunction. J Infect Dis. 2016;215:387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Higuchi M, De Morais C, Barreto A, et al. The role of active myocarditis in the development of heart failure in chronic Chagas’ disease: a study based on endomyocardial biopsies. Clin Cardiol. 1987;10:665‐670. [DOI] [PubMed] [Google Scholar]
- 73. Higuchi ML. Chagas disease. Importance of the parasite in the pathogenesis of the cardiac chronic disease. Arq Bras Cardiol. 1995;64:251‐254. [PubMed] [Google Scholar]
- 74. Fauro R, Lo Presti S, Bazan C, et al. Use of clomipramine as chemotherapy of the chronic phase of Chagas disease. Parasitology. 2013;140:917‐927. [DOI] [PubMed] [Google Scholar]
- 75. Maldonado IRSC, Ferreira ML, Camargos ERS, Chiari E, Machado CRS. Skeletal muscle regeneration and Trypanosoma cruzi‐induced myositis in rats. Histol Histopathol. 2004;19:85‐93. [DOI] [PubMed] [Google Scholar]
- 76. Pittella JEH, Meneguette C, Barbosa AJA. Histopathological and immunohistochemical study of the brain and heart in the chronic cardiac form of chagas’ disease. Arq Neuropsiquiatr. 1993;51(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 77. Vizcaíno‐Castillo A, Jiménez‐Marín A, Espinoza B. Exacerbated skeletal muscle inflammation and calcification in the acute phase of infection by Mexican Trypanosoma cruzi DTUI strain. Biomed Res Int. 2014;2014:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dickerson MF, Astorga NG, Astorga NR, Lewis AD. Chagas disease in 2 geriatric rhesus macaques (Macaca mulatta) housed in the pacific northwest. Comp Med. 2014;64(4):323‐328. [PMC free article] [PubMed] [Google Scholar]
- 79. Matsuda NM, Miller SM, Evora PRB. The chronic gastrointestinal manifestations of Chagas disease. Clinics (Sao Paulo). 2009;64(12):1219‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nogueira‐Paiva N, Fonseca K, Vieira P, et al. Myenteric plexus is differentially affected by infection with distinct Trypanosoma cruzi strains in Beagle dogs. Mem Inst Oswaldo Cruz. 2014;109:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Crema E, Beatriz L, Ribeiro P, et al. Gallbladder neuron count in cholelithiasis patients with and without Chagas disease Contagem de neurônios da vesícula biliar de pacientes chagásicos e não chagásicos portadores de colelitíase. Rev Soc Bras Med Trop. 2007;40(1):15‐17. [DOI] [PubMed] [Google Scholar]
- 82. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286‐294. [DOI] [PubMed] [Google Scholar]
- 83. Adad SJ, Resende AV, Jorge BH. Estudo sistematizado do plexo mioentérico nos diferentes terços do esôfago de chagásicos crônicos com e sem megaesôfago. Rev Soc Bras Med Trop. 1992;25(suppl III):101.1308935 [Google Scholar]
- 84. Köberle F. Patogenia da moléstia de Chagas. Rev Goiana Med. 1957;3:155‐180. [Google Scholar]
- 85. Köberle F. Patogenia do megaesôfago brasileiro e europeu. Rev Goiana Med. 1963;9:79‐116. [Google Scholar]
- 86. Köberle F. Chagas disease and Chagas syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63‐116. [DOI] [PubMed] [Google Scholar]
- 87. Oliveira LC, Nascimento RS, Rocha A, et al. Cholelithiasis in chronic Chagas disease patients. Arq Gastroenterol. 1997;34:222‐226. [PubMed] [Google Scholar]
- 88. Bermejo DA, Amezcua Vesely MC, Khan M, et al. Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B‐cell response which is a high source of non‐parasite‐specific antibodies. Immunology. 2011;132:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Lima VMF, Fattori KR, de Souza F, et al. Apoptosis in T lymphocytes from spleen tissue and peripheral blood of L. (L.) chagasi naturally infected dogs. Vet Parasitol. 2012;184:147‐153. [DOI] [PubMed] [Google Scholar]
- 90. Bustamante JM, Presti MSL, Rivarola HW, et al. Treatment with benznidazole or thioridazine in the chronic phase of experimental Chagas disease improves cardiopathy. Int J Antimicrob Agents. 2007;29:733‐737. [DOI] [PubMed] [Google Scholar]
- 91. Khare S, Liu X, Stinson M, et al. Antitrypanosomal treatment with benznidazole is superior to posaconazole regimens in mouse models of Chagas disease. Antimicrob Agents Chemother. 2015;59(10):6385‐6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


