Abstract
Objective
To understand the prevalence and transmission of carbapenem-resistant Klebsiella pneumoniae (CRKP) in ICU patients in Zhejiang Province, China, and determined the genetic and phenotypic characteristics of these CRKP strains.
Materials and Methods
A total of 202 ICU patients from eight tertiary hospitals were recruited and 55 non-duplicate CRKP strains were collected during July and August in 2017. These strains were subjected to determination of MICs, carriage of carbapenemase genes and tet(A) variants, PFGE, MLST and virulence potential using G. mellonella larvae infection model.
Results
A total of 55 CRKP strains were recovered from 42 patients, representing a carriage rate of 20.8%. CRKP strains were recovered from both the intestinal and respiratory tract of 13 patients. Importantly, strains isolated from sputum and fecal samples often displayed identical PFGE profiles, suggesting that CRKP may also colonize the respiratory tract. The most dominant ST type of these CRKP strains was ST11, accounting for 78% (43/55) of the test strains. The majority of CRKP strains were resistant to multiple antibiotics, with the exception of tigecycline and ceftazidime/avibactam. Interestingly, 32 strains were found to harbor the tet(A) variant, which is known to confer reduced tigecycline susceptibility. Assessment of the virulence potential of these CRKP strains by string test showed that results were negative for 53 of the 55 test strains. However, further assessment of virulence potential using a G. mellonella larvae infection model showed that CRKP isolated from sputum consistently exhibited a higher virulence level than strains recovered from fecal samples.
Conclusion
CRKP is highly prevalent in ICU patients in Zhejiang Province with strains isolated from respiratory exhibiting higher virulence potential than those from GI tract. These data provide essential insight into development of new infection control measures to halt the transmission of CRKP in clinical settings.
Keywords: carbapenem-resistant Klebsiella pneumoniae, surveillance, sputum, feces, ICU, resistance, virulence
Introduction
Carbapenems are considered antimicrobial agents of the last resort, especially in cases where extended-spectrum β-lactamase–producing organisms were involved. In 1996, Klebsiella pneumoniae carbapenemases (KPCs) were first identified in the USA,1 further limiting the therapeutic options that remained (colistin, tigecycline, or aminoglycosides) and causing infections that may result in significant mortality.2 To date, blaKPC-2-bearing K. pneumoniae strains have emerged worldwide and become a major public health threat.3
In China, the first KPC-positive, carbapenem-resistant K. pneumoniae isolate was recovered from a 75-year-old intensive care unit (ICU) patient in Zhejiang Province in 2004.4 Thereafter, KPC-2 has become the most common carbapenemase detectable in China, especially in Zhejiang Province.5 One dominant genotype of KPC-2-producing K. pneumoniae (KPC-2-KP) strains in China is ST11, which is closely related to ST258.6 KPC-2-KP strains readily spread via physical contact as well as contaminated food and water and have the propensity to acquire genetic materials, mostly in the form of plasmids and transposons, through horizontal gene transfer.7–9 Recently, several reports indicated that intestinal carriage of KPC-2-KP plays an important role in transmission of this organism.10,11 K. pneumoniae is a ubiquitous colonizer of the human intestinal tract,12 which may serve as a reservoir from which KPC-2-KP strains are disseminated in health care settings. To date, intestinal colonization with KPC-2-KP is known to be increasingly common among hospitalized patients. In addition, several reports have shown that the use of ventilator in hospitalized patients, in particular in ICU, plays an important role in transmission of CRKP in hospitals. In this work, we performed a cross-sectional surveillance of CRKP isolated from fecal and sputum samples of patients in ICUs and characterized their resistance and virulence potential. Findings of this study shall provide insight into the current prevalence rate and features of transmission of CRKP in hospitals in China, as well as development of intervention strategies.
Materials and methods
Research design
We first assessed the prevalence of CRKP in patients in ICUs of eight hospitals located in different regions of Zhejiang Province, China, during July and August 2017. Sputum and fecal samples were obtained from each patient in the ICU of each hospital and subjected to isolation of K. pneumoniae. The K. pneumoniae strains were isolated according to standard clinical procedures; species identity of the strains was confirmed by MALDI-TOF MS (Bruker Microflex LT; Bruker Daltonik GmbH, Bremen, Germany). Besides, this study was reviewed and approved by the Second Affiliated Hospital of Zhejiang University Ethics Committee with a waiver of informed consent. The reason for the support of waiver of patient consent for this study is because that this study only focuses on characterization of the bacterial strains and no information on the patients was used.
Antimicrobial susceptibility tests and phenotypic and genotypic characterization
Antimicrobial susceptibilities were performed by the micro-broth dilution method on all carbapenem-resistant K. pneumoniae (CRKP) isolates according to Clinical and Laboratory Standards Institute (CLSI) guidelines.13 The minimum inhibitory concentrations (MICs) of antimicrobial agents imipenem, meropenem, ertapenem, cefmetazole, ceftazidime, cefotaxime, cefepime, piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime/avibactam, tigecycline, ciprofloxacin, amikacin, and aztreonam against CRKP were interpreted according to CLSI breakpoints except for polymyxin B and tigecycline, which were interpreted according to the European Committee on Antimicrobial Susceptibility Testing criteria (available at http://www.eucast.org/clinical_breakpoints/).
PCR assays were used to assess the carriage rate of carbapenemase-encoding genes (blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48-like),14,15 β-lactamase genes (blaCTX−M, blaTEM, and blaSHV),16 and plasmid-mediated quinolone resistance determinants (qnrA, qnrB, qnrS, qnrC, qnrD, aac(6′)-Ib-cr, oqxA, oqxB, and qepA)17–19 in these CRKP strains. PCR was also performed to screen for carriage of the tigecycline resistance determinant tet(A) according to procedures described in a previous report,20 using the following primers, 5′-CGGCAGGCAGAGCAAGTAG-3′ (forward); 5′-ACGTGAAACCCAACAGACCC-3′ (reverse). The PCR products were then analyzed by agarose gel electrophoresis and sequencing. Pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) were performed on all CRKP isolates to investigate the scale of clonal dissemination of the test strains as previously described.21
Virulence assay
Expression of the hypermucoviscosity phenotype in the CRKP strains was determined by the string test as previously described.22 For virulence testing, we employed the Galleria mellonella model to further investigate toxicity. Eight randomly selected insects weighing about 300 mg each (purchased from Tianjin Huiyude Biotech Company, Tianjin, China) were used for the assessment of the virulence level of each isolate. Fresh K. pneumoniae strains were adjusted with PBS to concentrations of 1×106 CFU/mL. The subsequent infection assay was conducted as described previously,23 followed by recording of survival rate every 12 hours for 2 days. All experiments were performed in duplicate and repeated in the case of discrepancy. Unpaired t-test was performed to analyze the differences between two groups using Prism GraphPad (GraphPad Software, Inc., La Jolla, CA, USA).
Results
A total of 202 ICU patients in eight participating hospitals were recruited for this study. One sputum sample and one fecal sample were collected from each of these 202 patients, yielding a total of 404 samples for further analysis (Table 1). A total of 132 K. pneumoniae strains were isolated from clinical specimens of these patients, among which 51 were isolated from sputum and 81 from fecal samples. Among these 132 K. pneumoniae strains, 55 (41.7%) were found to be resistant to carbapenems and designated as CRKP (30 from fecal and 25 from sputum samples). These 55 CRKP strains were isolated from 42 of the 202 (20.8%) patients tested. The isolation rate of CRKP in each hospital was significantly different, with the highest rate being in YWC Hospital (YWCH) and the lowest in Huzhou People’s Hospital and Hangzhou First Hospital. Among these participating hospitals, there were three hospitals with isolation rate approaching or exceeding 50%. The rate of recovery of CRKP in the eight hospitals and carriage of specific carbapenemase genes are shown in Table 1 and Figure 1.
Table 1.
Information regarding ICU patients and CRKP strains recruited for this study
| Hospitals | No. of patients | No. of patients with CRKP | Positive rate (%) | No. of CRKP strains | Positive rate (%) | No. of fecal CRKP | Positive rate (%) | No. sputum CRKP | Positive rate (%) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ZJPPH | 41 | 14 | 34.1 | 16 | 57.1 | 9 | 52.9 | 7 | 63.6 |
| CXPH | 8 | 2 | 25 | 3 | 42.9 | 2 | 40 | 1 | 50 |
| HZPH | 16 | 2 | 12.5 | 2 | 22.2 | 2 | 33.3 | 0 | 0 |
| HZSH | 41 | 8 | 19.5 | 12 | 50 | 5 | 33.3 | 7 | 77.8 |
| SAHZJU | 30 | 4 | 13.3 | 6 | 33.3 | 2 | 25 | 4 | 40 |
| WYCH | 14 | 5 | 35.7 | 7 | 100 | 4 | 100 | 3 | 100 |
| HZFH | 20 | 2 | 10 | 2 | 22.2 | 1 | 16.7 | 1 | 33.3 |
| TZFH | 32 | 5 | 15.6 | 7 | 23.3 | 5 | 25 | 2 | 20 |
| Total | 202 | 42 | 20.8 | 55 | 41.7 | 30 | 37.0 | 25 | 49.0 |
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CXPH, Cixi People’s Hospital; HZFH, Hangzhou First Hospital; HZPH, Huzhou People’s Hospital; HZSH, Hangzhou Second Hospital; ICU, intensive care unit; SAHZJU; Second Affiliated Hospital of Zhejiang University; TZFH, Taizhou First Hospital; YWCH, YWC Hospital; ZJPPH, Zhejiang Provincial People’s Hospital.
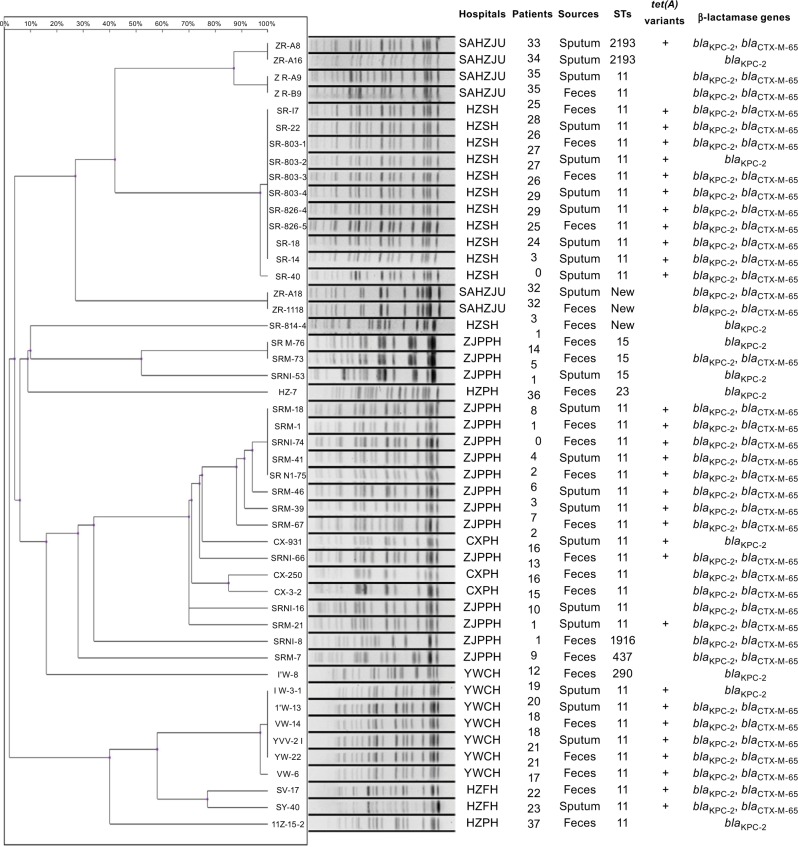
Figure 1.
Characteristics of CRKP strains isolated from patients in ICUs of various hospitals in Zhejiang Province, China.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CXPH, Cixi People’s Hospital; HZFH, Hangzhou First Hospital; HZPH, Huzhou People’s Hospital; HZSH, Hangzhou Second Hospital; ICU, intensive care unit; SAHZJU; Second Affiliated Hospital of Zhejiang University; TZFH, Taizhou First Hospital; YWCH, YWC Hospital; ZJPPH, Zhejiang Provincial People’s Hospital.
Clonal relationship of CRKP strains
To investigate if CRKP strains were clonally transmitted within a hospital or even among different hospitals, PFGE (it was considered to be homologous when the similarity was not less than 80%) and MLST analyses were performed, with results showing that clonal transmission was often observed in the same hospital, but not between different hospitals (Figure 1). In some hospitals, such as YWCH and HZSH, CRKP was found to belong to a single clone. ST type analysis led to the identification of seven ST types among the 55 CRKP isolates. The most dominant ST type was ST11 (43/55), followed by ST15 (3/55) and ST2193 (2/55). One strain each of ST437, ST1916, ST290, and ST23 was also found. On the other hand, three strains were found to belong to new ST types, with two isolates belonging to the same clone. Seven CRKP strains isolated in TZFH belonged to ST11 type.
Among the 42 patients who carried CRKP strains, 13 were found to simultaneously harbor CRKP in their intestine and respiratory tract. Strains isolated from different body sites or specimen types of a patient often showed identical PFGE profiles, suggesting that CRKP is readily transmitted between different body sites. Although the PFGE patterns of some strains recovered from sputum and fecal samples were not identical, they nevertheless belonged to the same ST type. However, there were also exceptions, for example, strains CX 250 and CX 931, which were, respectively, isolated from the respiratory and intestinal tract of the same patient, were found to be genetically unrelated.
Antibiotic susceptibility and potential mechanisms of resistance to important antibiotics in CRKP strains
All CRKP strains were found to exhibit resistance to the majority of antibiotics tested. Over 90% of the strains were resistant to all β-lactams, piperacillin/tazobactam, cefoperazone/sulbactam, and ciprofloxacin. Nevertheless, all except two strains remained susceptible to tigecycline and ceftazidime/avibactam (Table 2). Difference in drug susceptibility profiles was not observed among strains isolated from sputum and fecal samples. An interesting observation is that tigecycline susceptibility was reduced in some strains, with MIC being ≥0.5 µg/mL. It was previously reported that a novel tet(A) variant located in a conjugative plasmid could mediate reduced tigecycline susceptibility in CRKP. Among the 55 CRKP isolates tested, we found that 32 strains carried the tet(A) variant, including two which harbored a new tet(A) variant, accounting for 58.2% of the test isolates. Our data showed that the amino acid sequence of the new tet(A) variant exhibited 90% homology to the conventional tet(A) variant. As shown in Figure 2, the MIC values of strains harboring tet(A) variant were mostly 1 and 2 µg/mL (27/32; 84.4%), whereas the MIC values of strains without tet(A) variant were all ≤0.25 µg/mL (15/23; 65.2%). Interestingly, all CRKP isolates harboring the tet(A) variant were found to harbor qnrS1 gene.
Table 2.
Susceptibility of carbapenem-resistant K. pneumoniae strains isolated from respiratory and intestinal tract of ICU patients to commonly used antibiotics
| Antibiotic | Respiratory tract+intestinal tract (n=55) | Respiratory tract (n=25) | Intestinal tract (n=30) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Range | MIC50 | MIC90 | S% | I% | R% | MIC50 | MIC90 | S% | I% | R% | MIC50 | MIC90 | S% | I% | R% | |
|
| ||||||||||||||||
| IMP | 4–128 | 64 | 64 | 0 | 0 | 100 | 64 | 64 | 0 | 0 | 100 | 32 | 64 | 0 | 0 | 100 |
| MEM | 8–>128 | 128 | >128 | 0 | 0 | 100 | 128 | >128 | 0 | 0 | 100 | 64 | >128 | 0 | 0 | 100 |
| ETP | 16–>128 | >128 | >128 | 0 | 0 | 100 | >128 | >128 | 0 | 0 | 100 | 64 | >128 | 0 | 0 | 100 |
| CMZ | 4–>128 | 128 | >128 | 21.8 | 7.3 | 70.9 | 128 | >128 | 8 | 4 | 88 | 64 | >128 | 33.33 | 10.00 | 56.67 |
| CAZ | 8–>128 | 128 | >128 | 0 | 5.5 | 94.6 | >128 | >128 | 0 | 4 | 96 | 64 | >128 | 0 | 6.67 | 93.33 |
| CTX | 64–>128 | >128 | >128 | 0 | 0 | 100 | >128 | >128 | 0 | 0 | 100 | >128 | >128 | 0 | 0 | 100 |
| TZP | 256/4–>256/4 | >256/4 | >256/4 | 0 | 0 | 100 | >256/4 | >256/4 | 0 | 0 | 100 | >256/4 | >256/4 | 0 | 0 | 100 |
| SCF | 64/32–>256/128 | >256/128 | >256/128 | 0 | 0 | 100 | >256/128 | >256/128 | 0 | 0 | 100 | >256/128 | >256/128 | 0 | 0 | 100 |
| CAV | ≤0.5/4–>64/4 | 1/4 | 4/4 | 96.4 | 0 | 3.6 | 2/4 | 4/4 | 100 | 0 | 0 | 1/4 | 4/4 | 93.33 | 0 | 6.67 |
| FEP | 32–>64 | >64 | >64 | 0 | 0 | 100 | >64 | >64 | 0 | 0 | 100 | >64 | >64 | 0 | 0 | 100 |
| PB | ≤0.5–2 | ≤0.5 | 2 | 70.9 | 29.1 | 0 | 1 | 2 | 64.00 | 36.00 | 0 | ≤0.5 | 2 | 76.67 | 23.33 | 0 |
| TGC | ≤0.25–2 | 1 | 2 | 100 | 0 | 0 | 1 | 2 | 100 | 0 | 0 | 0.5 | 2 | 100 | 0 | 0 |
| CIP | ≤1–>32 | >32 | >32 | 10.9 | 0 | 89.1 | >32 | >32 | 4.00 | 0 | 96 | >32 | >32 | 16.67 | 0 | 83.33 |
| AK | ≤4–>128 | >128 | >128 | 25.5 | 0 | 74.6 | >128 | >128 | 12.00 | 0 | 88.00 | >128 | >128 | 36.67 | 0 | 63.33 |
| ATM | ≤4–>128 | >128 | >128 | 1.8 | 0 | 98.2 | >128 | >128 | 0 | 0 | 100 | >128 | >128 | 3.33 | 0 | 96.67 |
Abbreviations: AK, amikacin; ATM, aztreonam; CAV, ceftazidime/avibactam; FEP: cefepime; PB: polymyxin B; CIP, ciprofloxacin; CMZ, cefmetazole; CAZ: ceftazidime; CTX: cefotaxime; TZP: piperacillin/tazobactam; SCF: cefoperazones/sulbactam; ETP, ertapenem; ICU, intensive care unit; IMP, imipenem; MIC, minimum inhibitory concentration; MEM, meropenem; TGC, tigecycline.
Figure 2.
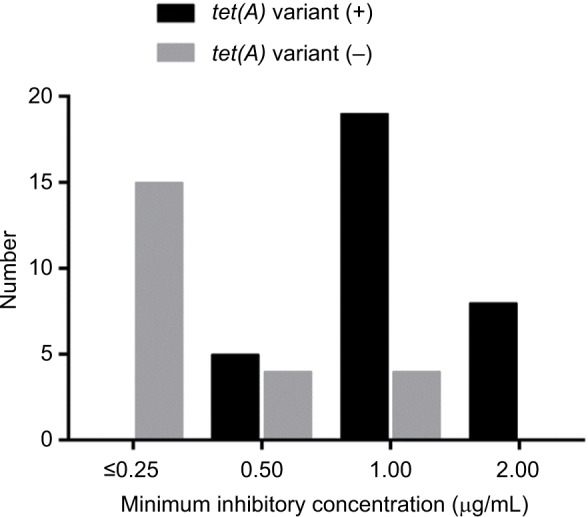
Tigecycline susceptibility of CRKP strains with or without the tet(A) variant.
Abbreviation: CRKP, carbapenem-resistant Klebsiella pneumoniae.
Virulence-associated features
All CRKP strains were subjected to string test to determine their virulence level, with results showing that 53 of the 55 strains were negative for the test. These strains were subjected to further assessment of virulence potential using a G. mellonella larvae infection model, using an inoculum of 1×106 CFU/mL. Importantly, the virulence level of CRKP isolated from respiratory tract was found to be consistently higher than that of strains recovered from the intestinal tract, which was basically consistent with the distribution of virulence genes (Figure 1). As shown in Figure 3, it was clear that the death rate caused by strains isolated from sputum samples had exceeded 10% by 12 hours, almost twice the mortality caused by stool specimens. Likewise, a similar trend in death rate was observed at the subsequent 24 and 36 hours. Of course, not all strains from different hospitals had shown consistent virulence. In one hospital (HZSH), however, intestinal strains were found to exhibit a higher level of virulence.
Figure 3.
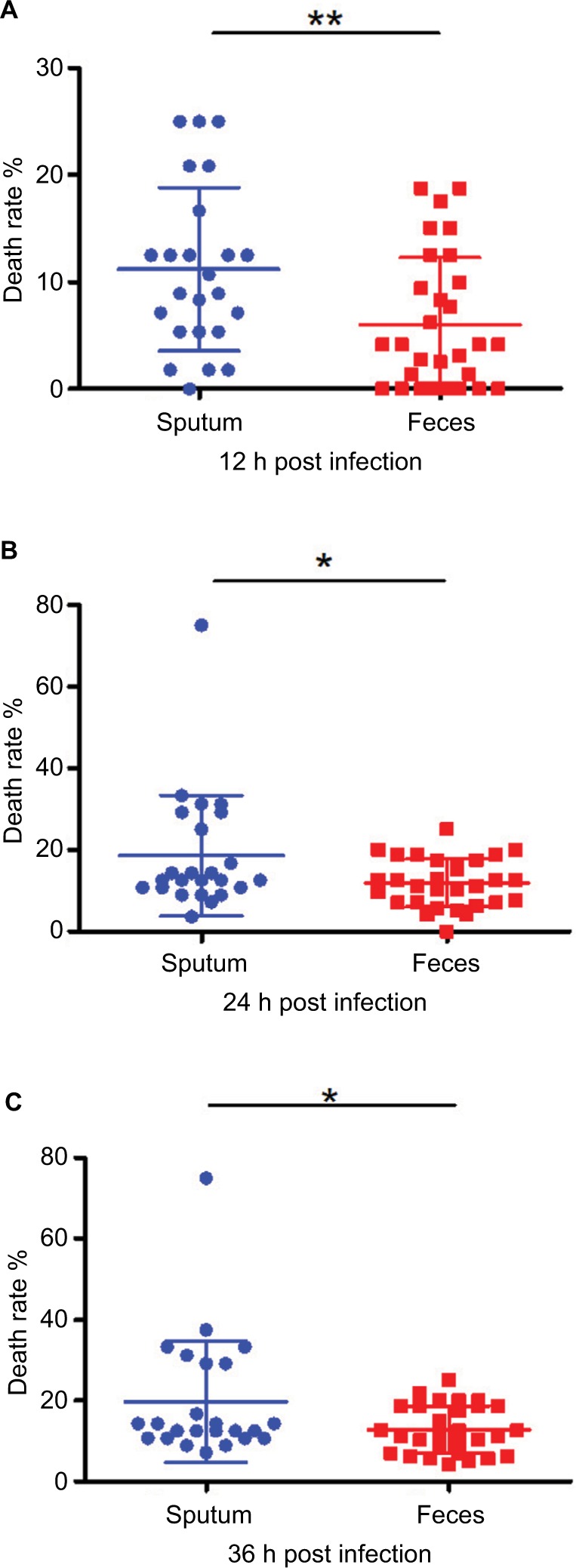
Virulence potential of CRKP strains as determined by means of a Galleria mellonella infection model.
Notes: G. mellonella was infected with 1×104 CFU of CRKP. The death rate of the worms infected by CRKP strains recovered from sputum and feces was recorded at 12 hours (A), 24 hours (B) and 36 hours (C). *P<0.05; **P<0.01.
Abbreviation: CRKP, carbapenem-resistant Klebsiella pneumoniae.
Discussion
This study provides important information regarding the current status of prevalence and transmission kinetics of CRKP in hospital ICUs in Zhejiang Province, China. First, our data showed that the prevalence of CRKP in ICU patients varied wildly between different hospitals, probably reflecting variation in effectiveness of infection control measures. Second, the isolation rate of CRKP was found to be higher in sputum than fecal samples, which is alarming as fecal carriage of CRKP is supposed to be much more common than other sites of human body. The high carriage of CRKP in sputum of ICU patients might be due to the use of ventilator, which has been reported to be a major risk factor of CRKP infections in ICU. Among these CRKP strains, car riage of the blaKPC-2 gene seems to be the major contributor of the carbapenem resistance phenotype. The finding that ST 11 was the most dominant type is consistent with previous studies in China. Importantly, clonal spread in a hospital was commonly observed, presumably due to ineffective infection control measures. Fortunately, widespread dissemination of specific strains between different hospitals has not occurred at this stage.
Among the CRKP strains tested, resistance to colistin has already reached 23%, a very high rate when compared to previous reports. In fact, colistin has not been officially used in China until the beginning of 2018. Instead, the drug has been sporadically used for the treatment of patients who purchased the drug from overseas. Since the use of colistin in clinical settings in China is uncommon, the high prevalence of colistin resistance in clinical CRKP strains is alarming, and suggests that clinical CRKP strains readily develop into colistin resistance. Upon the approval of use of colistin in hospitals in China, the rate of colistin resistance in CRKP is expected to increase further. In contrast, tigecycline has been widely used to treat infections caused by CRKP, often in combination with carbapenems. Yet resistance to tigecycline remained low in CRKP, with all strains displaying susceptibility to the drug. Interestingly, we found that a significant shift of MIC of tigecycline to the reduced susceptibility range in the test strains. Tigecycline resistance was reported to be due to the overexpression of efflux pumps such as AcrAB-TolC and OqxAB.24,25 A recent report showed that that a tet(A) variant gene could mediate tigecycline resistance in CRKP.26 In the present study, the MIC50 and MIC90 of tigecycline in these strains were found to be 1 and 2 µg/mL, respectively. Further analysis showed that carriage of the tet(A) variant in these strains contributed to an increase in MIC of tigecycline. Consistently, Akiyama et al found that the tet(A) variant could mediate a shift of tigecycline MIC from 0.19 to 3 µg/mL.27 In our study, we observed that a few strains carrying the tet(A) variant exhibited MIC as low as 0.5 µg/mL but the majority of strains exhibited MIC of 1–2 µg/mL. We therefore hypothesize that the tet(A) variant might be under strict regulation in these CRKP strains, so that a small number of the variant genes do not efficiently encode resistance to tigecycline. Further studies are needed to tract the transmission of tet(A) variants among CRKP strains and test how such gene is regulated in different host backgrounds.
Another important observation of this study is that we showed that the rate of isolation of CRKP from sputum was even higher than that of the gastrointestinal tract. Previous studies focused more on the surveillance of CRKP in the GI tract of patients, and the respiratory tract is not a common site from which CRKP is isolated. Yet our data show that carriage of CRKP in the airways of specific patients may be a reason why CRKP is commonly transmitted within an ICU. Whether the transmission process involves ventilators, which are commonly used in ICU, needs to be determined. These findings warrant the inclusion of sputum samples in screening of CRKP in ICU. More alarmingly, our data consistently showed that CRKP strains isolated from sputum exhibited significant higher virulence potential than those recovered from feces, suggesting that strains from sputum may cause more serious infections. The molecular basis of difference in virulence of CRKP strains recovered from the respiratory and GI tract needs to be investigated. Furthermore, these findings also appear to suggest that strains of differential virulence potential also exhibit difference in the ability in colonizing different body sites. The genetic basis of such difference also needs to be determined.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81772250) and Collaborative Research Fund of Hong Kong Research Grant Council (C5026-16G).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008;300(24):2911–2913. doi: 10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei ZQ, du XX, Yu YS, et al. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51(2):763–765. doi: 10.1128/AAC.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jj L, Sheng ZK, Deng M, et al. Epidemic of Klebsiella pneumoniae ST11 clone coproducing KPC-2 and 16S rRNA methylase RmtB in a Chinese University Hospital. Bmc Infect Dis. 2012;12(373) doi: 10.1186/1471-2334-12-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Y, Wei Z, Ji S, et al. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66(2):307–312. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20(9):821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 8.Montezzi LF, Campana EH, Corrêa LL, et al. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int J Antimicrob Agents. 2015;45(2):174–177. doi: 10.1016/j.ijantimicag.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Woodford N, Wareham DW, Guerra B, Teale C. Carbapenemase-producing Enterobacteriaceae and non-Enterobacteriaceae from animals and the environment: an emerging public health risk of our own making? J Antimicrob Chemother. 2014;69(2):287–291. doi: 10.1093/jac/dkt392. [DOI] [PubMed] [Google Scholar]
- 10.Lübbert C, Lippmann N, Busch T, et al. Long-term carriage of Klebsiella pneumoniae carbapenemase-2-producing K pneumoniae after a large single-center outbreak in Germany. Am J Infect Control. 2014;42(4):376–380. doi: 10.1016/j.ajic.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhao ZC, Xu XH, Liu MB, et al. Fecal carriage of carbapenem-resistant Enterobacteriaceae in a Chinese university hospital. Am J Infect Control. 2014;42(5):e61–e64. doi: 10.1016/j.ajic.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Schjørring S, Struve C, Krogfelt KA. Transfer of antimicrobial resistance plasmids from Klebsiella pneumoniae to Escherichia coli in the mouse intestine. J Antimicrob Chemother. 2008;62(5):1086–1093. doi: 10.1093/jac/dkn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI . Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement. CLSI document M100-S27. Wayne PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 14.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Ji S, Chen Y, et al. Resistance of strains producing extended-spectrum beta-lactamases and genotype distribution in China. J Infect. 2007;54(1):53–57. doi: 10.1016/j.jinf.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Dias D, Manageiro V, Francisco AP, et al. Assessing the molecular basis of transferable quinolone resistance in Escherichia coli and Salmonella spp. from food-producing animals and food products. Vet Microbiol. 2013;167(3-4):523–531. doi: 10.1016/j.vetmic.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006;50(8):2872–2874. doi: 10.1128/AAC.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones-Dias D, Manageiro V, Graça R, et al. QnrS1- and Aac(6’)-Ib-cr-producing Escherichia coli among Isolates from animals of different sources: susceptibility and genomic characterization. Front Microbiol. 2016;7:671. doi: 10.3389/fmicb.2016.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao H, Qin S, Chen S, et al. Emergence of carbapenem-resistant hyper-virulent Klebsiella pneumoniae. Lancet Infect Dis. 2018;18(1):25. doi: 10.1016/S1473-3099(17)30628-X. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Chen G, Wu X, et al. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the bla NDM-1 element and clonal spread of progenitor resistant strains. Front Microbiol. 2015;6:595. doi: 10.3389/fmicb.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 23.Mclaughlin MM, Advincula MR, Malczynski M, et al. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonellamodel and a pilot study to translate to patient outcomes. BMC Infect Dis. 2014;14(1):1. doi: 10.1186/1471-2334-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng ZK, Hu F, Wang W, et al. Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother. 2014;58(11):6982–6985. doi: 10.1128/AAC.03808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Majumdar S, Veleba M, Finn S, Fanning S, Schneiders T. Elucidating the regulon of multidrug resistance regulator RarA in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(4):1603–1609. doi: 10.1128/AAC.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao H, Qin S, Chen S, Shen J, du XD. Emergence of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. 2018;1825(1):25. doi: 10.1016/S1473-3099(17)30628-X. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama T, Presedo J, Khan AA. The tetA gene decreases tigecycline sensitivity of Salmonella enterica isolates. Int J Antimicrob Agents. 2013;42(2):133–140. doi: 10.1016/j.ijantimicag.2013.04.017. [DOI] [PubMed] [Google Scholar]