Abstract
Background
Penile warts are the most common sexually transmitted disease in males. Clinicians should be familiar with the proper evaluation and management of this common condition.
Objective
To provide an update on the current understanding, evaluation, and management of penile warts.
Methods
A PubMed search was completed in Clinical Queries using the key terms ‘penile warts’ and ‘genital warts’. The search strategy included meta-analyses, randomized controlled trials, clinical trials, observational studies, and reviews.
Results
Penile warts are caused by human papillomavirus (HPV), notably HPV-6 and HPV-11. Penile warts typically present as asymptomatic papules or plaques. Lesions may be filiform, exophytic, papillomatous, verrucous, hyperkeratotic, cerebriform, fungating, or cauliflower-like. Approximately one-third of penile warts regress without treatment and the average duration prior to resolution is approximately 9 months. Active treatment is preferable to watchful observation to speed up clearance of the lesions and to assuage fears of transmission and autoinoculation. Patient-administered therapies include podofilox (0.5%) solution or gel, imiquimod 3.75 or 5% cream, and sinecatechins (polypheron E) 15% ointment. Clinician-administered therapies include podophyllin, cryotherapy, bichloroacetic or trichloroacetic acid, oral cimetidine, surgical excision, electrocautery, and carbon dioxide laser therapy. Patients who do not respond to first-line treatments may respond to other therapies or a combination of treatment modalities. Second-line therapies include topical/intralesional/intravenous cidofovir, topical 5-fluorouracil, and topical ingenol mebutate.
Conclusion
No single treatment has been shown to be consistently superior to other treatment modalities. The choice of the treatment method should depend on the physician’s comfort level with the various treatment options, the patient’s preference and tolerability of treatment, and the number and severity of lesions. The comparative efficacy, ease of administration, adverse effects, cost, and availability of the treatment modality should also be taken into consideration.
Keywords: bichloroacetic/trichloroacetic acid, cimetidine, cryotherapy, electrocautery, human papillomavirus, imiquimod, laser, podofilox, podophyllin, sinecatechins, surgery
Introduction
Penile warts are the most common sexually transmitted disease in males and are caused by human papillomavirus (HPV).1–3 Penile warts typically present as soft flesh-colored to brown papules or plaques on the penile shaft and glans penis.4
To provide an update on the current understanding, evaluation, and management of penile warts, a narrative review was conducted based on a PubMed search using the key terms ‘penile warts’ and ‘genital warts’. The search strategy included meta-analyses, randomized controlled trials, clinical trials, observation studies, and reviews.
Epidemiology
Worldwide, HPV infection is the most common sexually transmitted disease.4 In the United States, 50–75% of sexually active individuals have been infected with genital HPV at some point during their lifetime.4,5 Infection with genital HPV does not mean that an individual will develop genital warts.5 It is estimated that 0.5–5% of sexually active young male adults have genital warts upon physical examination.6–9 The peak age is between 25 and 29 years.7,10 Generally, approximately 75% of males who have sexual contact with a partner who has genital warts develop penile warts within 8 months.8
Etiopathogenesis
HPV is a nonenveloped, capsid, double-stranded deoxyribonucleic acid (DNA) virus belonging to the Papillomavirus genus of the Papillomaviridae family and infects only humans.11 The virus has an eight kilobase circular genome that encodes eight genes, including the genes for the two encapsulating structural proteins, namely L1 and L2.11 The virus-like particle containing L1 is used in the manufacturing of HPV vaccines.11 L1 and L2 mediate HPV infectivity.11
More than 200 HPV types have been isolated thus far, of which more than 100 HPV genotypes have been sequenced.4,11,12 More than 40 HPV types can be transmitted sexually.4,11,12 HPV can be divided into high-risk or low-risk types based on their risk for cancer. Approximately 90% of genital warts are caused by the low-risk types HPV-6 and/or HPV-11.2–4,13 HPV-1, HPV-2, HPV-3, HPV-4, HPV-16, HPV-18, HPV-40, HPV-42, HPV-43, HPV-44, HPV-54, HPV-70, HPV-72, and HPV-81 account for the rest.14 It is possible to be infected with different HPV types at the same time.8 In adults, genital HPV infection is predominately transmitted by sexual intercourse and, less commonly, by oral sex, skin-to-skin transmission, and fomites.8 In children, HPV infection may result from sexual abuse, vertical transmission, autoinoculation, hetero-inoculation (nonsexual contact with a caregiver), and transmission via fomites.1,15 The HPV virus invades cells in the basal layer of the epidermis through micro-abrasions in the skin or mucosa.13 The incubation period for genital infection varies from 3 weeks to 8 months, with an average of 2–4 months.12,16,17 The disease is more common in individuals with the following factors: immunodeficiency, unprotected sexual intercourse, multiple sexual partners, sexual partner with multiple sexual partners, prior sexually transmitted disease, sexual intercourse at an early age, shorter time interval between meeting with a new partner and engaging in sexual activity, non-circumcision, and smoking.3,18–21 Moisture, maceration, trauma, and epithelial defects in the penile area are other predisposing factors.3
Histopathology
Histological findings include papillomatosis, focal parakeratosis, marked acanthosis, multiple vacuolated koilocytes, vascular distension, and coarse keratohyalin granules.4,17
Clinical manifestations
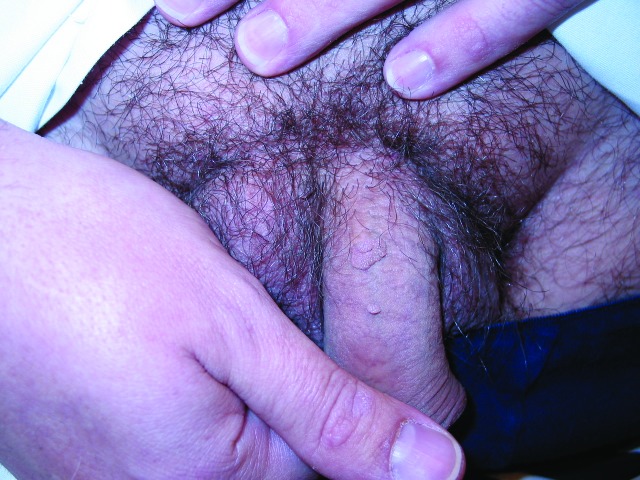
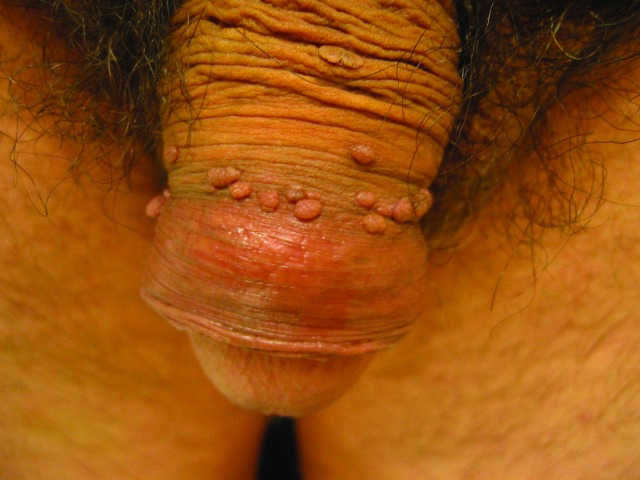
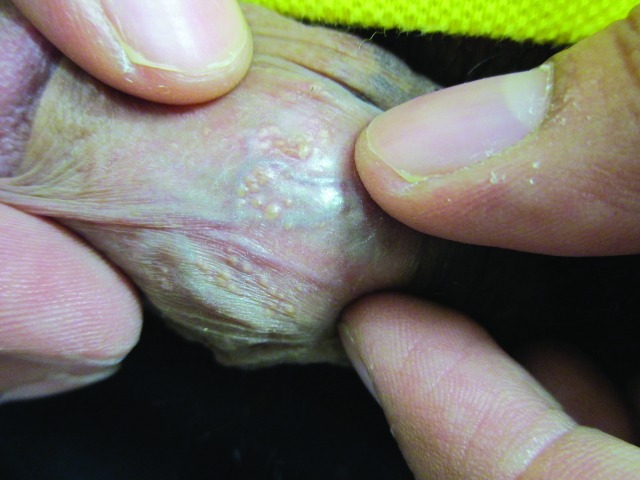
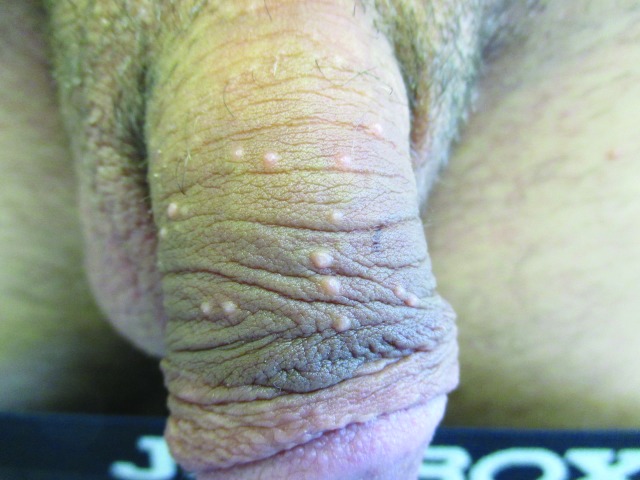
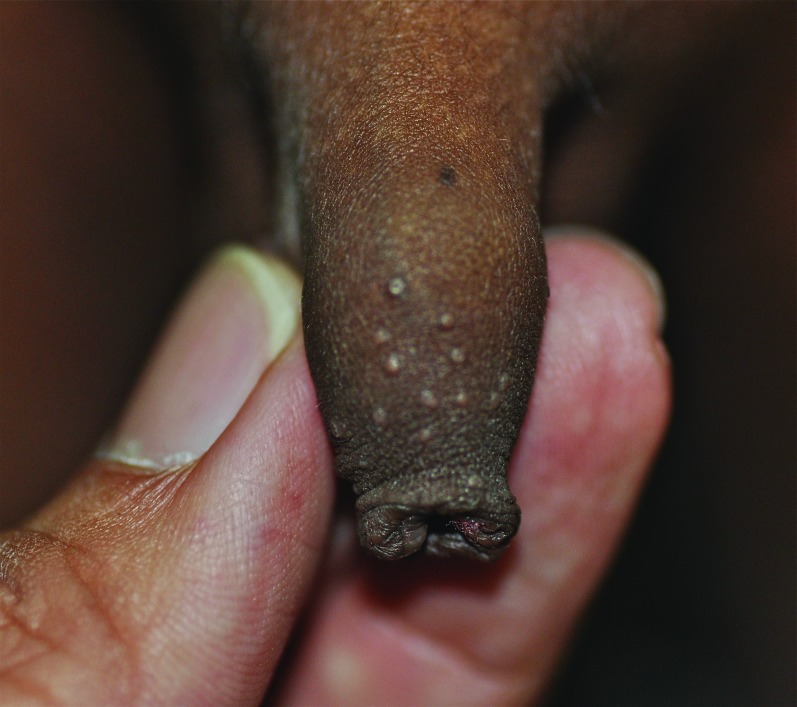
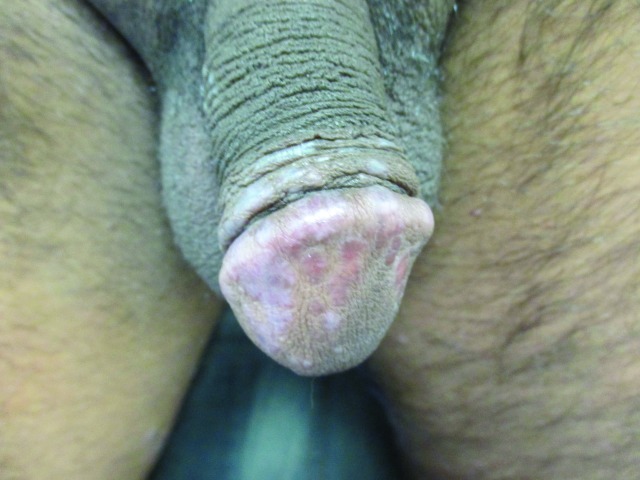
Penile warts are usually asymptomatic and rarely pruritic or painful.3,8,13 Penile warts are usually located on the frenulum, glans penis, inner surface of the prepuce, and coronal sulcus.3,8,13 Penile warts typically present as small, discrete, soft, smooth, pearly, dome-shaped papules at onset.3,7,14 Lesions can occur separately (Figure 1) or in clusters (Figure 2).8 They can be pedunculated or sessile. With time, the papules may coalesce into plaques.14 Lesions may be filiform, exophytic, papillomatous, verrucous, hyperkeratotic, cerebriform, fungating, or cauliflower-like (Figures 3–5).3,7,22 The color is variable and may be flesh-colored, pink, erythematous, brown, violaceous, or hyperpigmented.4,23
Figure 1.
Penile warts manifesting as small, discrete, soft, smooth, flesh-colored, dome-shaped papules.
Figure 2.
Penile warts manifesting as multiple, small, discrete, soft, smooth, flesh-colored, dome-shaped papules in clusters.
Figure 3.
Penile warts manifesting as multiple verrucous papules and plaques.
Figure 4.
Penile warts that are cauliflower-like.
Figure 5.
Penile warts manifesting as papillomas on the penile shaft.
Diagnosis
The diagnosis is mainly clinical, based on the history and physical findings. Dermoscopy and in vivo reflectance confocal microscopy help to increase the diagnostic accuracy.24 The morphological features may vary from finger-like, knob-like, to mosaic-like pattern.24–26 Among the vascular features, glomerular, hairpin, and dotted vessels are commonly seen.26 Papillomatosis is a constant feature.4 Some authors suggest the use of acetowhite test to aid the diagnosis of penile warts. While the sensitivity of acetowhite test is high for hyperplastic penile warts, the sensitivity is low for other types of penile warts and subclinically infected areas. Skin biopsy is seldom warranted but should be considered for atypical features (e.g., atypical pigmentation, induration, fixation to underlying structures, hard consistency, ulceration, or bleeding), when the diagnosis is in doubt or for warts recalcitrant to various treatment modalities. Although some authors suggest HPV DNA test to show the type of HPV that may affect the risk of malignant transformation, HPV typing is generally not recommended for routine diagnosis and not easily obtained.13
Differential diagnosis
Differential diagnosis includes pearly penile papules, Fordyce spots, acrochordons, condylomata lata of syphilis, molluscum contagiosum, granuloma annulare, lichen nitidus, lichen planus, seborrheic keratosis, epidermal nevus, lymphangioma circumscriptum, lymphogranuloma venereum, scabies, syringomas, traumatic neuromas, schwannomas, bowenoid papulosis, and squamous cell carcinoma.7
Pearly penile papules typically present as asymptomatic, small, smooth, soft, yellowish, pearly white, or flesh-colored, conical- or dome-shaped papules 1–4 mm in diameter (Figure 6).27 The lesions are usually uniform in size and shape and are symmetrically distributed.27 Typically, the papules occur in single, double, or multiple rows circumferentially around the corona and sulcus of the glans penis. The papules tend to be more prominent on the dorsum of the corona and less prominent towards the frenulum.27
Figure 6.
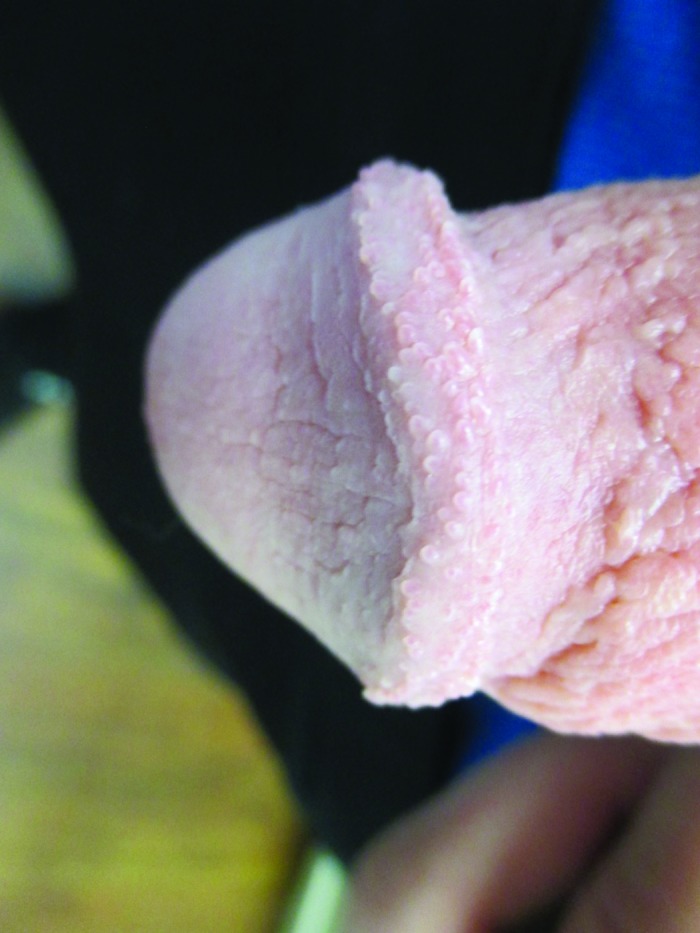
Pearly penile papules presenting as flesh-colored papules circumferentially distributed on the corona of the glans penis.
Fordyce spots are enlarged sebaceous glands. On the glans and shaft of the penis, Fordyce spots appear as asymptomatic, isolated or grouped, discrete, creamy yellow, smooth papules 1–2 mm in diameter (Figure 7).28–30 On the penile shaft, these papules are more obvious during penile erection or when the foreskin is stretched.28,30 A thick chalky or cheesy material can sometimes be expressed by squeezing the lesion.28
Figure 7.
Fordyce spots presenting as discrete, creamy yellow, smooth papules.
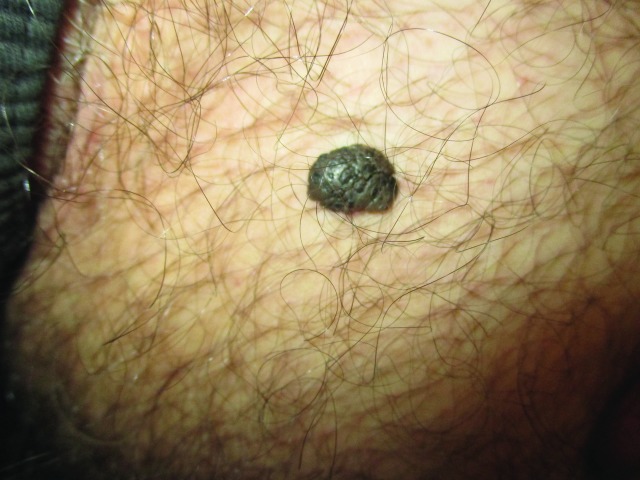
Acrochordons, also known as skin tags, are soft, flesh-colored to dark brown, sessile or pedunculated skin growths with a smooth contour (Figure 8).31,32 Occasionally, the lesions may be hyperkeratotic or have a warty appearance.33 Most acrochordons are 2–5 mm in diameter, although often larger in the groin.32 They can appear in almost any part of the body but are more frequently seen on the neck and intertriginous areas.31,32 When they occur on the penile area, they can mimic penile warts.
Figure 8.
Acrochordons presenting as soft, flesh-colored skin growths with a smooth contour.
Condylomata lata or condyloma latum are cutaneous lesions of secondary syphilis caused by the spirochete, Treponema pallidum.34,35 Clinically, condylomata lata manifest as moist, gray-white, velvety, flat-topped or cauliflower-like, broad papules or plaques.36 They tend to develop in warm, moist sites of the genitals and perineum.36 A nonpruritic, diffuse, symmetrical, maculopapular rash on the trunk, palms, and soles is characteristic of secondary syphilis.34 Systemic manifestations include headache, fatigue, pharyngitis, myalgia, and arthralgia.34 Alopecia, erythematous, or leukoplakic lesions on the oral mucosa and generalized lymphadenopathy may be present.36
Typically, molluscum contagiosum presents as discrete, smooth, firm, dome-shaped, yellow, pearly white or skin-colored, waxy papules with characteristic central umbilication (Figures 9 and 10).37–39 In the pediatric age group, lesions are most commonly seen on the trunk and intertriginous areas, whereas in adults, lesions are more commonly seen on the lower abdomen, upper thighs, and genital area (Figure 9).39 The size of lesions ranges from 1 to 5 mm in diameter and the number of lesions is usually less than 20.38,39
Figure 9.
Molluscum contagiosum presents as discrete, smooth, dome-shaped, pearly white papules on the penile shaft.
Figure 10.
Examination of a molluscum contagiosum lesion under a dermatoscope shows a discrete dome-shaped papule with central umbilication.
Granuloma annulare is a benign, self-limited inflammatory disease of the dermis and subcutaneous tissue. The condition is characterized by asymptomatic, firm, brown violaceous, erythematous, or flesh-colored papules, typically arranged in an annular, circinate configuration.40 As the condition progresses, central involution may be noted.40 The ring of papules often becomes coalescent to form an annular plaque. Granuloma annulare typically involves the extensor surfaces of the distal extremities but may also involve the shaft and glans of the penis.41,42
Lichen nitidus is a chronic inflammatory dermatosis characterized by numerous, asymptomatic, discrete, flat-topped, flesh-colored, shiny, pinhead- to pinpoint-sized papules (Figure 11).43,44 The abdomen, chest, extremities, and penis are sites of predilection.43–45 The lesions are usually arranged in groups.43
Figure 11.
Lichen nitidus presenting as multiple discrete, flat-topped, fleshed-colored papules on the penile shaft.
Cutaneous lichen planus is a chronic inflammatory dermatosis characterized by six Ps: pruritic, purple (violaceous), planar (flat-topped), polygonal, papules/plaques that affect the skin (Figure 12).46 Sites of predilection include the flexor aspects of the wrists, dorsa of hands, trunk, shins, ankles, and glans penis.47 Approximately 25% of the lesions occur on the genitalia.47
Figure 12.
Lichen planus presenting as purple, planar, polygonal papules on the glans penis.
Seborrheic keratosis typically presents as an asymptomatic, well-demarcated, round or oval, brown plaque with a ‘stuck on’ warty appearance (Figure 13).48 Often, lesions may appear shiny and oily and, hence, the misnomer ‘seborrheic’ (greasy) keratosis.48 Sites of predilection include the face, chest, back, and extremities. Occurrence on the penis is rare but has been reported.49 HPV is found in approximately 70% of cases of seborrheic keratosis in the genital area versus 5% in nongenital areas.49
Figure 13.
Seborrheic keratosis presenting as a well-demarcated, oval, brown plaque with a ‘stuck on’ warty appearance.
An epidermal nevus is a hamartoma arising from embryonic ectoderm that differentiates into keratinocytes, apocrine glands, eccrine glands, hair follicles, and sebaceous glands. The classic lesion is a solitary, asymptomatic, well-circumscribed plaque that follows Blaschko lines. The onset is usually within the first year of life. The color varies from flesh to yellow to brown. The lesion may thicken and become more verrucous with time.
Lymphangioma circumscriptum is a benign saccular dilatation of the cutaneous and subcutaneous lymphatics.50 The condition is characterized by clusters of vesicles resembling frog spawn (Figure 14). The color depends on the content: whitish, yellow, or light tan coloration is due to color of the lymph fluid while reddish or blue color is due to the presence of erythrocytes in the lymph fluid as a result of hemorrhage.51 The vesicles may undergo verrucous changes and have a warty appearance.50 Sites of predilection include the extremities and, less commonly, the genitalia.52
Figure 14.
Lymphangioma circumscriptum presenting as clusters of small firm dark-red blisters with warty surface on the distal penis, resembling frog spawn.
Lymphogranuloma venereum is a sexually transmitted disease caused by Chlamydia trachomatis.34 The condition is characterized by a transient painless genital papule and, less commonly, an erosion, ulcer, or pustule followed by inguinal and/or femoral lymphadenopathy with a characteristic ‘groove sign’ known as buboes.34,53
Human scabies, caused by the parasite mite Sarcoptes scabiei var hominis, is characterized by burrows, erythematous papular eruption, and intense pruritus (often worse at night).54 The papules, caused by a hypersensitivity reaction to the mite, are erythematous and usually 1–2 mm in diameter (Figure 15).54
Figure 15.
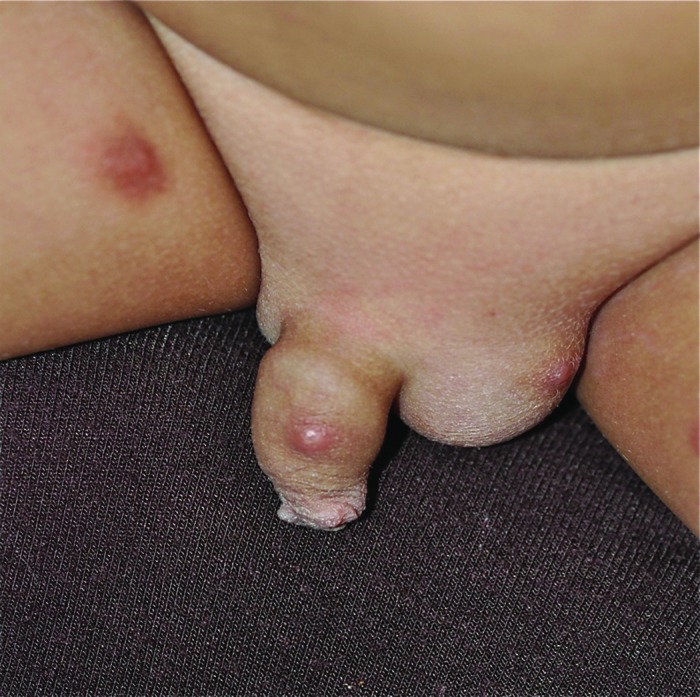
Human scabies presenting as erythematous papules, which are intensely pruritic.
Typically, syringomas present as asymptomatic, small, soft to firm, flesh- to tan-colored papules usually 1–3 mm in diameter.55 The lesions are usually found in the periorbital areas and on the upper cheeks.55 However, syringomas may appear on the penis and buttocks. When located on the penis, syringomas may be mistaken for penile warts.
Traumatic neuromas are nerve sheath tumors in which the ratio of axons to Schwann cell fascicles is approximately equal.56,57 The condition results from regenerative proliferation of traumatized or injured nerve fibers.56,57 Penile traumatic neuromas may result from trauma to the penis, such as circumcision or penile dorsal neurotomy to treat premature ejaculation.56–59 Clinically, penile traumatic neuromas present with skin-colored or erythematous papules or nodules on the glans or penile shaft.56–59
Schwannomas are neoplasms originated from Schwann cells.60 A penile schwannoma typically presents as a solitary, asymptomatic, slow-growing nodule on the dorsal aspect of the penile shaft.60,61
Bowenoid papulosis is a premalignant focal intraepidermal dysplasia that typically manifests as multiple red to brown papules or plaques in the anogenital area, such as the penis.62 The condition corresponds to in situ squamous cell carcinoma. Progression to invasive squamous cell carcinoma occurs in 2–3% of cases.62
Typically, squamous cell carcinoma of the penis presents as a nodule, ulcer, or erythematous lesion.63 The lesion may appear warty, leukoplakic, or sclerotic.63,64 The most common site is the glans penis, followed by the prepuce and shaft.63
Complications
Penile warts can be a cause of significant anxiety or distress to the patient and his sexual partner because of their disfiguring appearance and transmissibility, stigmatization, concerns about future fertility and risk of cancer, and their association with other sexually transmitted diseases.65,66 It is estimated that 20–34% of affected patients have concomitant sexually transmitted diseases.17 Affected patients often experience guilt, feelings of shame, low self-esteem, and angst.67 Individuals with penile warts have higher rates of sexual dysfunction, depression, and anxiety when compared with the normal population.65,68 The condition can have negative psychosocial effects on the patient and may adversely affect quality of life.66,69–72 Large exophytic lesions may bleed, cause urethral obstruction, and interfere with sexual intercourse.7,14,73 Malignant transformation is rare except in immunocompromised individuals.74,75 Individuals with penile warts are at increased risk for anogenital, head, and neck cancers as a result of coinfection with high-risk HPV genotypes.4,76
Prognosis
If left untreated, penile warts may resolve spontaneously, stay the same, or increase in size and number.3 Approximately one-third of penile warts regress without treatment and the average duration prior to resolution is approximately 9 months.4,77 Proper treatment has a clearance rate of 35–100% in 3–16 weeks.65 In spite of resolution of visible warts, HPV infection may persist and may result in recurrence of penile warts.4 The recurrence rate ranges from 25 to 67% within 6 months of treatment.7,78 Patients with subclinical infection, recurrent infection from sexual contact, and immunodeficiency have higher recurrence rate.79
Management
Active treatment of penile warts is preferable to watchful observation to speed up clearance of the lesions, assuage fears of transmission and autoinoculation, allay emotional stress, improve cosmetic appearance, reduce social stigma associated with penile lesions, and alleviate symptoms (e.g., pruritus, pain, or bleeding).3,7,77 Penile warts that persist for more than 2 years are less likely to resolve spontaneously and active treatment should be seriously considered.14 Sexual partners should be offered informational counseling. Testing for other sexually transmitted diseases is advisable.
Active treatments may be mechanical, chemical, immunomodulatory, and antiviral.80 Head-to-head comparisons of available modalities are scarce. Effectiveness varies among treatment modalities. Thus far, no single treatment has been shown to be consistently superior to other treatment modalities. The choice of the treatment method should depend on the physician’s comfort level with the various treatment options, the patient’s preference and tolerability of treatment, and the number and severity of lesions. The comparative efficacy, ease of administration, adverse effects, cost, and availability of the treatment modality should also be taken into consideration. In general, patient-administered therapies are less effective compared with clinician-administered ones.6
Patient-administered therapies
Patient-administered therapies include podofilox (0.5%) solution or gel, imiquimod 3.75 or 5% cream, and sinecatechins (polypheron E) 15% ointment.13,80,81 Podofilox (0.5%) solution or gel is derived from podophyllotoxin, a plant resin that works by disrupting microtubules during cell cycle replication, arresting mitosis, and causing tissue necrosis, and has been shown efficacious in the treatment of penile warts.79,82 Podofilox (0.5%) solution or gel is applied topically twice a day for 3 consecutive days per week and can be applied at home.7 The weekly cycle can be repeated up to four times.7 Clearance rates range from 45 to 77%.7 Adverse events include local discomfort, pruritus, irritation, burning, pain, xerosis, erythema, erosion, ulceration, and postinflammatory pigmentary changes at the site of application.7,14 To minimize local adverse events, podophyllotoxin should be washed off 1–4 hours after application.79 Sexual intercourse should be avoided when podophyllotoxin is still on the skin.79
Imiquimod is available as 3.75 and 5% cream. Imiquimod enhances the host’s innate and cellular immune response and combats HPV infection by binding to the dermal dendritic cells and macrophages through toll-like receptor-7.9 This results in the production and release of proinflammatory cytokines, such as interferon alpha (IFN-α), tumor necrosis factor alpha (TNF-α), and interleukin (IL)-1, IL-6, IL-8, and IL-12.7,79 The secreted cytokines stimulate local immune effects that are cytotoxic against HPV. Imiquimod also acts on cell-mediated immunity by activating Langerhans cells, thereby increasing the presentation of antigens for the T cells. Studies have shown that topical application of 5% imiquimod cream is beneficial in the treatment of penile warts.83 The 5% imiquimod is applied three times per week till total clearance of the lesion or for a maximum of 16 weeks, while the 3.75% imiquimod is applied nightly for up to 8 weeks.9,79 Clearance rates are similar to those of podofilox.7 Adverse events with topical imiquimod include local irritation, pruritus, burning, erythema, vesicles, erosions, ulceration, and hypopigmentation at the application site and, less frequently, flu-like symptoms.14,79 To minimize local adverse events, the treatment area should be washed with soap and water 6–10 hours after application.
Sinecatechins ointment is a water extract of leaves of green tea (Camellia sinensis) and available as 10 and 15% formulations. It is believed that the medication works by decreasing viral replication through upregulation of apoptosis-associated genes and modulation of genes involved in the proinflammatory response to HPV infection.7,79 Sinecatechins ointment is a safe and effective treatment of penile warts in immunocompetent males aged 18 years and older.13,84 It is recommended that sinecatechins ointment be applied three times a day for up to 16 weeks.6,7 It is not necessary to wash the ointment off prior to the next application.6 Two randomized, double-blind, phase III trials (n=1005) showed rates of complete clearance and recurrence after 12 weeks of genital warts were significantly superior to the control group (p<0.001 and p<0.001, respectively).6 Adverse effects include pruritus, burning, discomfort, irritation, pain, erythema, edema, induration, vesicular rash, erosion, and ulceration at the application site, which occur in approximately 20% of patients.6,7,13 Staining of underwear is another nuisance aspect. A single-blind, randomized controlled trial (n=42) showed that cryotherapy plus sinecatechins 15% ointment was more effective in the treatment of external genital warts compared to cryotherapy alone (−5.0 lesions versus −2.1 lesions, respectively, p=0.07).85
Clinician-administered therapies
Clinician-administered therapies include podophyllin, cryotherapy with liquid nitrogen, bichloroacetic or trichloroacetic acid, oral cimetidine, surgical excision, electrocautery, and carbon dioxide laser therapy.80,81 Podophyllin 25% liquid, derived from podophyllotoxin, works by arresting mitosis and causing tissue necrosis.7 The medication is applied directly to the penile wart once a week for up to 6 weeks, with a maximum of 0.5 ml per treatment.7 Podophyllin 25% liquid should be washed off 1–4 hours post-treatment and should not be applied to any raw area.7 The clearance rate is approximately 62%.7 Because of the reported toxicity including death associated with its use, podofilox, its purified form, is preferred, which has a much better safety profile.7
Liquid nitrogen, a treatment of choice for penile warts, can be applied via a spray gun or a cotton-tip applicator directly to and 2 mm surrounding the lesion.79 Liquid nitrogen works by causing tissue damage and cell death through rapid freezing with formation of ice crystals.79,86 The minimum temperature required for destruction of the warts is −50°C, although some authors believe that a temperature of −20°C is also effective.86 The success rate is about 75%.79 Adverse effects include pain during treatment, erythema, peeling, vesicle, erosion, ulceration, and dyspigmentation at the application site.82 A recent phase II parallel-randomized study on 16 Iranian men with genital warts showed that cryotherapy using Wartner compound that contains a mixture of 75% of dimethyl ether and 25% of propane is also effective. Further studies need to be done to confirm or refute this finding.86 Suffice to say, cryotherapy using Wartner compound is less effective than cryotherapy using liquid nitrogen.86
Bichloroacetic and trichloroacetic acid can be used for the treatment of small penile warts, as their ability to penetrate skin is limited.79 Either medication works by protein coagulation with resultant cell destruction and eradication of the penile wart.7 A burning sensation may be experienced at the application site. Recurrences following the application of bichloroacetic or trichloroacetic acid are common. The medication can be applied up to three times weekly.7 Clearance rates range from 64 to 88%.7
Oral cimetidine, a H2-receptor antagonist, presumably works by enhancing cell-mediated immunity against HPV.87 The recommended dose is 25–40 mg/kg/day. The medication is safe, painless, and well tolerated.88
Electrocautery, pulsed dye laser therapy, carbon dioxide laser therapy, or surgical excision works by mechanical destruction of the penile wart, and they may be required if the lesion is extensive, bulky, or recalcitrant to conservative treatment.14,79,89,90 These modalities of treatment have the highest success rate but also have the potential of leaving scars on the skin. Topical anesthesia, such as with Nanorap (a hydrogel with 2.5% lidocaine and 2.5% prilocaine with 50% of active products in nanocapsules) applied over the lesions without occlusion 20 minutes before the procedure or a eutectic mixture of local anesthetics (EMLA) applied over the lesions with occlusion for an hour before the procedure, should be considered to reduce the discomfort/pain, which can be disturbing for some individuals.44 General anesthesia may be necessary for surgical removal of large and extensive lesions.
Alternate therapies
Patients who do not respond to first-line treatments may respond to other therapies or a combination of treatment modalities.75,79 Second-line therapies include topical/intralesional/intravenous cidofovir, topical 5-fluorouracil, and topical ingenol mebutate.
Antiviral therapy with topical, intralesional, or intravenous cidofovir may be considered for immunocompromised patients with severe, refractory lesions.14 Cidofovir is an acyclic nucleoside phosphonate that competitively inhibits viral DNA polymerase, thereby preventing viral replication.79,91 Adverse effects of topical or intralesional cidofovir include irritation, erosion, post-inflammatory pigmentary changes, and superficial scars at the site of application.88 The main adverse effect of intravenous cidofovir is nephrotoxicity, which can be prevented by saline hydration and probenecid.91
Topical 5-fluorouracil has been shown to be effective in the treatment of penile warts.92 5-fluorouracil is an antimetabolite fluoropyrimidine analog of the nucleoside pyrimidine. The medication works by blocking the action of thymidylate synthase and thereby stopping the production of DNA. A Cochrane database systematic review of six randomized, placebo-controlled trials involving 988 patients with genital warts showed that topical 5-fluorouracil was more effective than no treatment or placebo (relative risk: 0.39; 95% confidence interval: 0.23–0.67).92 The authors cautioned that further studies are necessary due to lack of high-quality randomized, placebo-controlled trials.92 Adverse events of topical 5-fluorouracil include pain, burning, inflammation, and ulceration at the application site.79
Preliminary studies showed that topical application of ingenol mebutate gel is efficacious in the treatment of genital warts.93–95 Ingenol mebutate works by induction of cell death in proliferating keratinocytes and by stimulation of an inflammatory response characterized by infiltration of neutrophils.95 Local skin irritation may occur after 24–48 hours.94,95 The recurrence rate is unknown.
Prevention
Genital warts can be prevented, to a certain extent, by delaying sexual initiation and limiting the number of sexual partners.3 Latex condoms, when used consistently and properly, reduce HPV transmission. Sexual partners with anogenital warts should be treated.
HPV vaccines prior to the onset of sexual activity are effective in the primary prevention of HPV infection.5 This is because the vaccines do not provide protection against diseases from vaccine HPV types that the individual has acquired through prior sexual activity.96 The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, the American Academy of Family Practice, and the International Papillomavirus Society recommend routine vaccination with HPV vaccine for girls and boys.81,97–101 The target age for vaccination is 11–12 years for girls and boys.100–102 The vaccine can be administered as early as 9 years of age.97 Three doses of HPV vaccine should be given at 0, 1–2 (typically 2), and 6 months.97 Catch-up vaccination is indicated for males through age 21 years and females through age 26 years if not vaccinated in the target age.99–102 Vaccination is also recommended for men who are homosexual or immunocompromised through age 26 years if not vaccinated before.100,101 HPV vaccination reduces the chance of HPV infection and subsequent penile warts as well as penile cancers. Vaccinating both males and females is more beneficial in reducing penile warts than by vaccinating only males, as males may acquire HPV infection from their female sexual partners. The prevalence of anogenital warts decreased significantly from 2008 to 2014, thanks to the administration of the HPV vaccine.103 The annual percentage change among males aged 20–24 years was −6% (p=0.005).103 The decreased prevalence likely results from herd protection from vaccination among females. A 2017 systematic review and meta-analysis of six studies (n=27,078) showed that HPV quadrivalent vaccine is efficacious in preventing anogenital warts in both males and females.104 The 9-valent vaccine (Gardasil-9) which has replaced the 4-valent vaccine (Gardasil) targets HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 and contains those virus-like particles.96,101,105 As such, the 9-valent vaccine is effective in the prevention of penile warts.105 The bivalent vaccine (Cervarix) targets only HPV types 16 and 18 and is not effective in the prevention of penile warts.97 Safety data for the HPV vaccines are reassuring.102
Conclusion
Penile warts are sexually transmitted disease caused by HPV. The condition can have negative psychosocial effects on the patient and may adversely affect quality of life. Although approximately one-third of penile warts regress without treatment, active treatment is preferred to speed up clearance of the lesions, assuage fears of transmission and autoinoculation, allay emotional stress, improve cosmetic appearance, reduce social stigma associated with penile lesions, and alleviate symptoms. Active treatments may be mechanical, chemical, immunomodulatory, and antiviral, and often a combination of these. Thus far, no single treatment has been shown to be consistently superior to other treatment modalities. The choice of the treatment method should depend on the physician’s comfort level with the various treatment options, the patient’s preference and tolerability of treatment, and the number and severity of lesions. The comparative efficacy, ease of use, adverse effects, cost, and availability of the treatment modality should also be taken into consideration. HPV vaccines prior to the onset of sexual activity are effective in the primary prevention of HPV infection. The target age for vaccination is 11–12 years for both girls and boys.
Acknowledgments
The photo images were taken by the authors with verbal consent of the respective patient. The patients are unidentifiable and the photo images have not been previously published.
Footnotes
Contributions: Professor Alexander KC Leung is the principal author. Dr Benjamin Barankin, Dr Kin Fon Leong, and Professor Kam Lun Hon are the coauthors who contributed and helped with the drafting of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that there is no conflict of interest in preparing this article. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/11/dic.212563-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2018 Leung AKC, Barankin B, Leong KF, Hon KL. https://doi.org/10.7573/dic.212563. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/penile-warts-an-update-on-their-evaluation-and-management
Provenance: invited; externally peer reviewed.
Peer review comments to author: 16 November 2018
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Costa-Silva M, Fernandes I, Rodrigues AG, Lisboa C. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92(5):675–681. doi: 10.1590/abd1806-4841.201756411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung AK, Kellner JD, Davies D. Genital infection with human papillomavirus in adolescents. Adv Ther. 2005;22(3):187–197. doi: 10.1007/bf02849928. [DOI] [PubMed] [Google Scholar]
- 3.Leung AK, Barankin B. Genital warts. Consultant360.com. [Accessed September 1, 2018]. http://www.consultant360.com/articles/genital-warts.
- 4.Scheinfeld N. Condylomata acuminata (anogenital warts) in adults: epidemiology, pathogenesis, clinical features, and diagnosis. In: Post TW, editor. UpToDate. Waltham, MA: [Accessed September 1, 2018]. [Google Scholar]
- 5.Richards S. An overview of genital warts. Nurs Stand. 2014;28(24):46–50. doi: 10.7748/ns2014.02.28.24.46.e8344. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Daigle D. Sinecatechins 10% ointment: a green tea extract for the treatment of external genital warts. Skin Therapy Lett. 2015;20(1):6–8. [PubMed] [Google Scholar]
- 7.Karnes JB, Usatine RP. Management of external genital warts. Am Fam Physician. 2014;90(5):312–318. [PubMed] [Google Scholar]
- 8.Leslie SW, Shenot PJ. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2018 Jan–2018 Mar 15. Wart, Genital. [Google Scholar]
- 9.Yuan J, Ni G, Wang T, et al. Genital warts treatment: beyond imiquimod. Hum Vaccin Immunother. 2018;14(7):1815–1819. doi: 10.1080/21645515.2018.1445947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palefsky JM. Human papillomavirus infections: epidemiology and disease associations. In: Post TW, editor. UpToDate. Waltham, MA: [Accessed September 5, 2018]. [Google Scholar]
- 12.Steben M, Garland SM. Genital warts. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1063–1073. doi: 10.1016/j.bpobgyn.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg G, Taylor M, Berman B, Kaufmann M, Abramovits W, Zeichner J. Sinecatechins ointment, 15% for the treatment of external genital and perianal warts: proceedings of an expert panel roundtable meeting. J Clin Aesthet Dermatol. 2016;9(3 Suppl 1):S2–S15. [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin LT. Condylomata acuminata (anogenital warts) in children. In: Post TW, editor. UpToDate. Waltham, MA: [Accessed August 25, 2018]. [Google Scholar]
- 15.Stefanaki C, Barakas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31(4):422–424. doi: 10.1097/inf.0b013e318245a589. [DOI] [PubMed] [Google Scholar]
- 16.Dunne EF, Burstein GR, Stone KM. Anogenital human papillomavirus infection in males. Adolesc Med. 2003;14:613–632. doi: 10.1016/S1041349903500482. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal VN, Koranne RV, Srivastava SB. Genital warts: current status. Int J Dermatol. 1989;28(2):75–85. doi: 10.1111/j.1365-4362.1989.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 18.Albero G, Castellsagué X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis. 2012;39(2):104–113. doi: 10.1097/olq.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 19.Firnhaber C, Sello M, Maskew M, et al. Human papillomavirus types in HIV seropositive men with penile warts in Johannesburg, South Africa. Int J STD AIDS. 2011;22(2):107–109. doi: 10.1258/ijsa.2010.010306. [DOI] [PubMed] [Google Scholar]
- 20.Kaderli R, Schnüriger B, Brügger LE. The impact of smoking on HPV infection and the development of anogenital warts. Int J Colorectal Dis. 2014;29(8):899–908. doi: 10.1007/s00384-014-1922-y. [DOI] [PubMed] [Google Scholar]
- 21.Zetola N, Klausner JD. Male circumcision reduces human papillomavirus incidence and prevalence: clarifying the evidence. Sex Transm Dis. 2012;39(2):114–115. doi: 10.1097/olq.0b013e318242b4f3. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu A, Hattori M, Kaira K, Ishikawa O. Keratotic condyloma acuminatum. J Dermatol. 2016;43(6):716–717. doi: 10.1111/1346-8138.13266. [DOI] [PubMed] [Google Scholar]
- 23.Lopaschuk CC. New approach to managing genital warts. Can Fam Physician. 2013;59(7):731–736. [PMC free article] [PubMed] [Google Scholar]
- 24.Veasey JV, Framil VM, Nadal SR, et al. Genital warts: comparing clinical findings to dermatoscopic aspects, in vivo reflectance confocal features and histopathologic exam. An Bras Dermatol. 2014;89(1):137–140. doi: 10.1590/abd1806-4841.20141917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos MA, Sousa A, Lage G, et al. Blue-gray plaque of the penis. JAAD Case Rep. 2018;4(6):531–533. doi: 10.1016/j.jdcr.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong H, Shu D, Campbell TM, Frühauf J, Soyer HP, Hofmann-Wellenhof R. Dermatoscopy of genital warts. J Am Acad Dermatol. 2011;64(5):859–864. doi: 10.1016/j.jaad.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Leung AK, Barankin B. Pearly penile papules. J Pediatr. 2014;165(2):409. doi: 10.1016/j.jpeds.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Leung AK, Barankin B. Fordyce spots. Consultant. 2016;56:377–378. https://www.consultant360.com/articles/fordyce-spots. [Google Scholar]
- 29.Radhakrishnan S, Agarwal DC. Fordyce spots masquerading as penile warts. Med J Armed Forces India. 2016;72(4):384–385. doi: 10.1016/j.mjafi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rane V, Read T. Penile appearance, lumps and bumps. Aust Fam Physician. 2013;42(5):270–274. [PubMed] [Google Scholar]
- 31.Bahce ZS, Akbulut S, Sogutcu N, Oztas T. Giant acrochordon arising from the thigh. J Coll Physicians Surg Pak. 2015;25(11):839–840. [PubMed] [Google Scholar]
- 32.Ma H, Xia Y, Yin S, Lai W. Giant skin tag on the labium majorum. Int J Womens Dermatol. 2015;1(4):175–176. doi: 10.1016/j.ijwd.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipoff JB, Chatterjee K. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; 2018 Jan–2018 Jul 5. Acrochordon. [Google Scholar]
- 34.Bartels A, Crandall M, Spring L. Recalcitrant genital papules. J Fam Pract. 2017;66(7):457–460. [PubMed] [Google Scholar]
- 35.Leung AKC, Leong KF, Lam JM. A case of congenital syphilis presenting with unusual skin eruptions. Case Rep Pediatr. 2018 Mar 25;2018 doi: 10.1155/2018/1761454. 1761454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015 Mar 17;350:h1259. doi: 10.1136/bmj.h1259. [DOI] [PubMed] [Google Scholar]
- 37.Leung AK, Kong AY. Discrete papules on the thigh of a child. Molluscum contagiosum. Am Fam Physician. 2010;81(4):511. [PubMed] [Google Scholar]
- 38.Leung AK. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15(2):136–137. doi: 10.1016/s1473-3099(14)71061-8. [DOI] [PubMed] [Google Scholar]
- 39.Leung AKC, Barankin B, Hon KLE. Molluscum contagiosum: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(1):22–31. doi: 10.2174/1872213x11666170518114456. [DOI] [PubMed] [Google Scholar]
- 40.Leung AK, Barankin B. An annular lesion on the elbow. Am Fam Physician. 2016;93(5):397–398. [PubMed] [Google Scholar]
- 41.Fathi K, Harangi F, Kravjak A, Pinter A. Subcutaneous granuloma annulare of the penis associated with a urethral anomaly: case report and review of the literature. Pediatr Dermatol. 2014;31(4):e100–e103. doi: 10.1111/pde.12322. [DOI] [PubMed] [Google Scholar]
- 42.Mercieca L, Carabot P, Boffa MJ. Penile granuloma annulare. Skinmed. 2016;14(3):233–236. [PubMed] [Google Scholar]
- 43.Chu J, Lam JM. Lichen nitidus. CMAJ. 2014;186(18):E688. doi: 10.1503/cmaj.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung AK, Ng J. Generalized lichen nitidus in identical twins. Case Rep Dermatol Med. 2012;2012 doi: 10.1155/2012/982084. 982084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee WJ, Park OJ, Won CH, et al. Penile lichen nitidus successfully treated with topical pimecrolimus 1% cream. J Dermatol. 2013;40(6):499–500. doi: 10.1111/1346-8138.12089. [DOI] [PubMed] [Google Scholar]
- 46.Leung AK, Barankin B. Lichen planus. Consultant. 2014;54:137–138. https://www.consultant360.com/articles/lichen-planus. [Google Scholar]
- 47.Badri T, Kenani N, Benmously R, Debbiche A, Mokhtar I, Fenniche S. Isolated genital annular lichen planus. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20(1):31–33. [PubMed] [Google Scholar]
- 48.Leung AK, Leung AA. Seborrheic keratosis. Consultant. 2012;52:573–575. [Google Scholar]
- 49.Part M, Svecová D, Brezová D, Breza J. Giant seborrheic keratoses on penis. J Sex Med. 2014;11(12):3119–3122. doi: 10.1111/jsm.12676. [DOI] [PubMed] [Google Scholar]
- 50.Adikari S, Philippidou M, Samuel M. A rare case of acquired lymphangioma circumscriptum of the penis. Int J STD AIDS. 2017;28(2):205–207. doi: 10.1177/0956462416657238. [DOI] [PubMed] [Google Scholar]
- 51.Zaballos P, Del Pozo LJ, Argenziano G, et al. Dermoscopy of lymphangioma circumscriptum: a morphological study of 45 cases. Australas J Dermatol. 2018;59(3):e189–e193. doi: 10.1111/ajd.12668. [DOI] [PubMed] [Google Scholar]
- 52.Ayhan E. Lymphangioma circumscriptum: good clinical response to isotretinoin therapy. Pediatr Dermatol. 2016;33(3):e208–209. doi: 10.1111/pde.12839. [DOI] [PubMed] [Google Scholar]
- 53.Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61( Suppl 8):S865–873. doi: 10.1093/cid/civ756. [DOI] [PubMed] [Google Scholar]
- 54.Leung AK, Barankin B, Hon KL. Scabies. Consult Pediatr. 2016;15:613–616. https://www.consultant360.com/articles/scabies-extremely-pruritic-rash-nighttime-intensity. [Google Scholar]
- 55.Dhossche JM, Brodell RT, Al Hmada Y, Ward KH. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32(1):145–146. doi: 10.1111/pde.12403. [DOI] [PubMed] [Google Scholar]
- 56.Cardoso TA, dos Santos KR, Franzotti AM, Avelar JC, Tebcherani AJ, Pegas JR. Traumatic neuroma of the penis after circumcision – case report. An Bras Dermatol. 2015;90(3):397–399. doi: 10.1590/abd1806-4841.20153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoverson KR, Sasaki GT, Wohltmann WE. Traumatic neuroma of the penis. Dermatol Online J. 2014;20(1):21252. [PubMed] [Google Scholar]
- 58.Park HJ, Kim TN, Baek SR, Lee KM, Choi KU, Park NC. Penile traumatic neuroma: a late complication of penile dorsal neurotomy to treat premature ejaculation. Sex Med. 2016;4(3):e221–224. doi: 10.1016/j.esxm.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salcedo E, Soldano AC, Chen L, et al. Traumatic neuromas of the penis: a clinical, histopathological and immunohistochemical study of 17 cases. J Cutan Pathol. 2009;36(2):229–233. doi: 10.1111/j.1600-0560.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 60.Han H, Lei HE, Tian L, Zhang XD. Isolated penis schwannoma: a rare case report. Urol Case Rep. 2018;21:12–13. doi: 10.1016/j.eucr.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen AH, Smith ML, Maranda EL, Punnen S. Clinical features and treatment of penile schwannoma: a systematic review. Clin Genitourin Cancer. 2016;14(3):198–202. doi: 10.1016/j.clgc.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Marcucci C, Sabban EC, Friedman P, Peralta R, Calb I, Cabo H. Dermoscopic findings in bowenoid papulosis: report of two cases. Dermatol Pract Concept. 2014;4(4):61–63. doi: 10.5826/dpc.0404a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deem S, Keane T, Bhavsar R, El-Zawahary A, Savage S. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108(9):1378–1392. doi: 10.1111/j.1464-410x.2011.10647.x. [DOI] [PubMed] [Google Scholar]
- 64.Thapa S, Ghosh A, Shrestha S, Ghartimagar D, Narasimhan R, Talwar OP. Warty carcinoma penis: an uncommon variant. Case Rep Pathol. 2017;2017 doi: 10.1155/2017/2937592. 2937592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lacey CY, Woodhall SC, Wikstrom A, et al. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2013;27(3):e263–e270. doi: 10.1111/j.1468-3083.2012.04493.x. [DOI] [PubMed] [Google Scholar]
- 66.Steben M, LaBelle D. Genital warts: Canadians’ perception, health-related behaviors, and treatment preferences. J Low Genit Tract Dis. 2012;16(4):409–415. doi: 10.1097/lgt.0b013e3182466ee3. [DOI] [PubMed] [Google Scholar]
- 67.Jeynes C, Chung MC, Challenor R. ‘Shame on you’ – the psychosocial impact of genital warts. Int J STD AIDS. 2009;20(8):557–560. doi: 10.1258/ijsa.2008.008412. [DOI] [PubMed] [Google Scholar]
- 68.Kucukunal A, Altunay IK, Mercan S. Sexual dysfunction in men suffering from genital warts. J Sex Med. 2013;10:1585–1591. doi: 10.1111/jsm.12132. [DOI] [PubMed] [Google Scholar]
- 69.Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010 Mar 7;10:113. doi: 10.1186/1471-2458-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paradisi A, Capizzi R, Ricci F, et al. Quality of life in patients with anogenital warts. Eur J Dermatol. 2013;23(6):837–842. doi: 10.1684/ejd.2013.2171. [DOI] [PubMed] [Google Scholar]
- 71.Tan LS, Chio MT, Sen P, et al. Assessment of psychosocial impact of genital warts among patients in Singapore. Sex Health. 2014;11(4):313–318. doi: 10.1071/sh13189. [DOI] [PubMed] [Google Scholar]
- 72.Vriend HJ, Nieuwkerk PT, van der Sande MA. Impact of genital warts on emotional and sexual well-being differs by gender. Int J STD AIDS. 2014;25(13):949–955. doi: 10.1177/0956462414526706. [DOI] [PubMed] [Google Scholar]
- 73.Cervigni M, Scotto V, Panei M, et al. Acute urethral obstruction due to condylomata acuminata. Obstet Gynecol. 1991;78:970–972. [PubMed] [Google Scholar]
- 74.Gormley RH, Kovarik CL. Human papillomavirus-related genital disease in the immunocompromised host: part I. J Am Acad Dermatol. 2012;66(6):867.e1–14. doi: 10.1016/j.jaad.2010.12.050. quiz 881–2. [DOI] [PubMed] [Google Scholar]
- 75.Gormley RH, Kovarik CL. Human papillomavirus-related genital disease in the immunocompromised host: part II. J Am Acad Dermatol. 2012;66(6):883.e1–17. doi: 10.1016/j.jaad.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 76.Blomberg M, Friis S, Munk C, et al. Genital warts and risk of cancers: a Danish study of nearly 50000 patients with genital warts. J Infect Dis. 2012;205(10):1544–1553. doi: 10.1093/infdis/jis228. [DOI] [PubMed] [Google Scholar]
- 77.Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1988;39(6):951–955. doi: 10.1016/s0190-9622(98)70268-3. [DOI] [PubMed] [Google Scholar]
- 78.Jablonska S. Traditional therapies for the treatment of condylomata acuminata (genital warts) Australas J Dermatol. 1998;39(Suppl 1):S2–S4. [PubMed] [Google Scholar]
- 79.Scheinfeld N. Condylomata acuminata (anogenital warts): management of external condylomata acuminata in men. In: Post TW, editor. UpToDate. Waltham, MA: [Accessed September 5, 2018]. [Google Scholar]
- 80.Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5(6):25–36. [PMC free article] [PubMed] [Google Scholar]
- 81.Workowski KA, Bolan GA Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–37. [PMC free article] [PubMed] [Google Scholar]
- 82.Recanati MA, Kramer KJ, Maggio JJ, Chao CR. Cantharidin is superior to trichloroacetic acid for the treatment of non-mucosal genital warts: a pilot randomized controlled trial. Clin Exp Obstet Gynecol. 2018;45(3):383–386. doi: 10.12891/ceog4112.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen T, Nelson A, Ault K. Imiquimod cream 2.5% and 3.75% applied once daily to treat external genital warts in men. Cutis. 2015;96(4):277–282. [PubMed] [Google Scholar]
- 84.Stockfleth E, Meyer T. The use of sinecatechins (polyphenon E) ointment for treatment of external genital warts. Expert Opin Biol Ther. 2012;12(6):783–793. doi: 10.1517/14712598.2012.676036. [DOI] [PubMed] [Google Scholar]
- 85.On SC, Linkner RV, Haddican M, et al. A single-blinded randomized controlled study to assess the efficacy of twice daily application of sinecatechins 15% ointment when used sequentially with cryotherapy in the treatment of external genital warts. J Drugs Dermatol. 2014;13(11):1400–1405. [PubMed] [Google Scholar]
- 86.Firooz A, Hosseini H, Izadi Firouzabadi L, Nassiri Kashani M, Nasrollahi SA. The efficacy and safety of other cryotherapy compounds for the treatment of genital warts: a randomized controlled trial. J Dermatolog Treat. 2018;27:1–3. doi: 10.1080/09546634.2018.1480745. [DOI] [PubMed] [Google Scholar]
- 87.Franco I. Oral cimetidine for the management of genital and perigenital warts in children. J Urol. 2000;164(3 Pt 2):1074–1075. doi: 10.1097/00005392-200009020-00038. [DOI] [PubMed] [Google Scholar]
- 88.Leung AKC, Barankin B, Hon KLE. Molluscum contagiosum: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(1):22–31. doi: 10.2174/1872213x11666170518114456. [DOI] [PubMed] [Google Scholar]
- 89.Azizjalali M, Ghaffarpour G, Mousavifard B. CO(2) Laser therapy versus cryotherapy in treatment of genital warts; a Randomized Controlled Trial (RCT) Iran J Microbiol. 2012;4(4):187–190. [PMC free article] [PubMed] [Google Scholar]
- 90.Veitch D, Kravvas G, Al-Niaimi F. Pulsed dye laser therapy in the treatment of warts: a review of the literature. Dermatol Surg. 2017;43(4):485–493. doi: 10.1097/dss.0000000000001023. [DOI] [PubMed] [Google Scholar]
- 91.Blaizot R, Dutkiewicz AS, Guillet S, Pham-Ledard A, Beylot-Barry M. Intravenous cidofovir for diffuse genital warts in the setting of multifactorial immunosuppression. J Eur Acad Dermatol Venereol. 2017;31(3):e162–e163. doi: 10.1111/jdv.13880. [DOI] [PubMed] [Google Scholar]
- 92.Batista CS, Atallah AN, Saconato H, da Silva EM. 5-FU for genital warts in non-immunocompromised individuals. Cochrane Database Syst Rev. 2010 Apr;14(4):CD006562. doi: 10.1002/14651858.cd006562.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braun SA, Gerber PA. Ingenol mebutate for the management of genital warts in sensitive anatomic locations. J Am Acad Dermatol. 2017;77(1):e9–e10. doi: 10.1016/j.jaad.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 94.Dika E, Vaccari S, Fanti PA, Patrizi A. Ingenol mebutate: an unconventional treatment proposal. Dermatol Ther. 2016;29(1):72. doi: 10.1111/dth.12265. [DOI] [PubMed] [Google Scholar]
- 95.Schopf RE. Ingenol mebutate gel is effective against anogenital warts – a case series in 17 patients. J Eur Acad Dermatol Venereol. 2016;30(6):1041–1043. doi: 10.1111/jdv.13097. [DOI] [PubMed] [Google Scholar]
- 96.Foerster V, Khangura S, Severn M. HPV vaccination in men: a review of clinical effectiveness, cost-effectiveness, and guidelines [Internet] Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2017. Mar 24, [PubMed] [Google Scholar]
- 97.Cox JT. Human papillomavirus vaccinations. In: Post TW, editor. UpToDate. Waltham, MA: [Accessed September 15, 2018]. [Google Scholar]
- 98.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 99.Committee opinion no 641: human papillomavirus vaccination. Obstet Gynecol. 2015;126(3):e38–e43. doi: 10.1097/aog.0000000000001052. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 100.Park IU, Introcaso C, Dunne EF. Human papillomavirus and genital warts: a review of the evidence for the 2015 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2015;61( Suppl 8):S849–855. doi: 10.1093/cid/civ813. [DOI] [PubMed] [Google Scholar]
- 101.Petrosky E, Bocchini JA, Jr, Hariri S, et al. plus Centers for Disease Control and Prevention (CDC) Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 102.Committee Opinion No. 704 Summary: human papillomavirus vaccination. Obstet Gynecol. 2017;129(6):1155–1156. doi: 10.1097/aog.0000000000002111. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 103.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018;108(1):112–119. doi: 10.2105/ajph.2017.304119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tejada RA, Vargas KG, Benites-Zapata V, Mezones-Holguín E, Bolaños-Díaz R, Hernandez AV. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: systematic review and meta-analysis. Salud Publica Mex. 2017;59(1):84–94. doi: 10.21149/7824. [DOI] [PubMed] [Google Scholar]
- 105.Pitisuttithum P, Velicer C, Luxembourg A. 9-Valent HPV vaccine for cancers, pre-cancers and genital warts related to HPV. Expert Rev Vaccines. 2015;14(11):1405–1419. doi: 10.1586/14760584.2015.1089174. [DOI] [PubMed] [Google Scholar]