Abstract
Qatar has a dry, subtropical desert climate, with minimum annual rainfall and intensely hot and humid summers. Using indigenous grass, those adapted to local conditions have the potential to be used for fodder and can also be used for restoration or rehabilitation of degraded rangelands. Chloris virgata, Coelachyrum brevifolium and Cenchrus ciliaris bloom twice a year from April to May (summer) and September to October (winter) under the nursery condition. Therefore, it is important to understand, how seeds produced in different seasons affect the dormancy as well as germination of these species. Seeds of C. virgata, C. brevifolium and C. ciliaris, three desert grasses, were collected from the plants growing on Shahniya nursery in two different seasons, summer (May) and winter (October). The seeds collected in May (summer) were stored up to winter. However seeds collected in October (winter) were immediately used for experiment. We compared the germination potential of seeds that matured in different season at different alternating temperatures at 15/25, 20/30 and 25/35 °C. Lower temperatures correspond to the dark period, while higher temperatures reflect the light period. Seeds collected in summer season (old seeds) were heavier as compared to seeds collected in winter season (new seeds). Winter seeds of C. virgata seem to be dormant, while summer seeds, germinated well in all the tested temperature regimes. However, C. ciliaris seeds showed opposite trends.
Keywords: Native grasses, Old and new seeds, Storage seeds, Temperature, Light, Desert climate
1. Introduction
Grasses serve as a significant source of fodder in arid environments due to their palatable and nutritive value (Haase et al., 1995, Mansoor et al., 2002). The environmental conditions experienced by plants during seed development and maturation have been reported to affect seed germination and dormancy (Donohue, 2009, Gorecki et al., 2012). Maternal processes supply the seed with nutrients, hormones and proteins which influence the metabolism of seeds during its development and these processes are strongly regulated by various environmental factors such as temperature, photoperiod, water availability and nutrient supply (Fenner, 1991, Gutterman, 2000, Donohue, 2009). Temperature, rainfall and day length are the main factors that affect maternal plants during the growing season (Baskin and Baskin, 1998, Fenner, 1992). Temperature during seed development and maturation has been reported to affect seed germination (El-Keblawy and Al-Ansari, 2000, Jensen and Eriksen, 2001, Qaderi et al., 2003, Donohue et al., 2005). Moreover, seeds that develop with a low water supply have been reported to be less dormant (Meyer and Allen, 1999, Luzuriaga et al., 2006). Day length experienced by maternal plants also affects germination responses (El-Keblawy and Al-Rawai, 2006). Therefore, seeds from the same plant species but maturating at different seasons can exhibit differences in germination and dormancy responses (El-Keblawy et al., 2009). Moreover, environmental conditions can affect chemical composition and seed provisioning (e.g., mineral and phytohormone resources) throughout the growing season and therefore influence the germination (Baskin and Baskin, 1998, Galloway, 2002). Previous studies have reported that seeds which develop at a higher temperature or with shorter days are mostly less dormant (Fenner, 1991, Gutterman, 2000, Donohue, 2009).
All desert grasses studied here (Chloris virgata, Coelachyrum brevifolium and Cenchrus ciliaris) have a C4 photosynthetic pathway, which make them drought resistant and therefore they are able to grow in desert condition (Waramit, 2010). The use of native forage species will enhance the possibility of fodder production in a more sustainable manner with a low cost. These species have been successfully used as a fodder in Pakistan, India and Arab countries (Khan and Ansari, 2008, Patel et al., 2012, El-Keblawy, 2013). Besides used as a fodder, these species could also be used to enhance the rangeland productivity through restoration or rehabilitation of degraded desert rangelands and control the soil erosion (Peacock et al., 2003, Osman et al., 2008). C. virgata, C. brevifolium and C. ciliaris bloom twice a year from April to May (summer) and September to October (winter) under the nursery conditions. Therefore, it is important to understand, how seeds produced in different seasons affect the dormancy as well as germination of these grass species, which could enable us to make predictions about dormancy loss, select for reduced dormancy in the field and storage of seeds more successfully. We hypothesized that seed germination and dormancy can be affected by the parental environment during different seasons. Therefore, we articulate that the parental environment in which seeds develop (two different seasons) will determine differences in dormancy and germination statuses of these species. To test these hypotheses, we examined (i) do seeds differ in germination depending on when they are produced during the year? (ii) are there any light and temperature mediated mechanisms affecting germination under laboratory condition?
2. Materials and methods
2.1. Seed collection and seed storage
Mature seeds of all three species were collected from the plants growing at Shahniya nursery (Doha, Qatar). They were collected in May and October 2014 (referred to as old and new seeds, respectively). The seeds collected in May were cleaned and stored for four months at room temperature (20 ± 2 °C). However seeds collected in October were cleaned and immediately used for experiments. Three replicates of 50 seeds each were used to determine the seed fresh weight for both new and old seeds.
2.2. Seed germination assessment
To assess the effects of temperature, light and their interaction on seed matured during different seasons, germination tests were conducted in incubators set at different temperatures 15/25, 20/30 and 25/35 °C in 12 h dark/12 h light cycle. Lower temperatures correspond to the dark period, while higher temperatures the light period. The germination was conducted in 9-cm tight-fitting Petri-dishes containing one disk of Whatman No. 1 filter paper, moistened with 10 ml of distilled water. Four replicates of 25 seeds each were used for each treatment. For 22 days, the number of germinated seeds were counted and removed every alternate day. Seeds were considered to have germinated upon emergence of radicle. The experiment was stopped after 22 days because no new germination occurred for a consecutive five day period. These data were processed by GerminaQuant version 1.0 (Marques et al., 2015) to determine the germinability, mean germination time (t), speed index (SI), germination synchrony (Z), and uncertainty (U), as described by Miranda et al. (2014).
3. Results
The average mass of old seeds of C. vergata was lower (6.3 ± 0.5 mg) compared to new seeds (8.3 ± 1.1 mg). Similar trends were observed for C. brevifolium (12 ± 1 mg for old and 15 ± 1 mg for new seeds) and C. ciliaris (42.6 ± 4.9 mg for old and 45.6 ± 4.1 mg for new seeds).
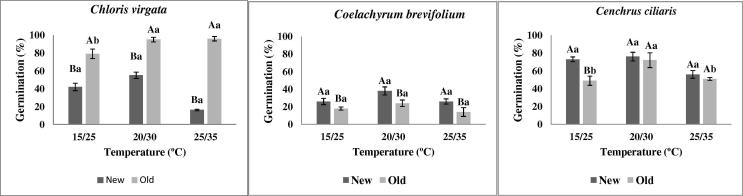
The seeds of C. vergata did not show any significant difference in germination percentage between all evaluated temperatures independent of age. Moreover, regardless of the temperature regime, the old C. vergata seeds showed a significantly higher germination rate when compared to new seeds. The major difference in germination rate in this species was shown between new (<20%) and old (100%) seeds at higher a temperature (25/35 °C). Similarly, C. brevifolium seeds also did not show any significant difference in germination percentage between different evaluated temperatures. However, there was a significant variation in germination percentage between new and old seeds in all incubated temperatures. The new seeds showed maximum germination at moderate temperature (38%) which was significantly higher than the old seeds (24%). Seeds of C. ciliaris did not show any significant variation in their germination response at moderate and high temperatures in both age seeds. However, new seeds incubated in 15/25 °C germinated 49% more than the old seeds at the same temperature (Fig. 1).
Figure 1.
Germinability (%) of C. virgata, C. brevifolium and C. ciliaris at different temperatures. The values represent the media (±SE) of four replicates of 25 seeds each. Different capital letters denote significant differences between means for each parameters within each age, and different small letters denote significant differences for each parameter between means within each temperature (p ⩽ 0.05, Newman–Keuls’ test).
Seeds of C. vergata required significantly a lower germination time at higher and moderate temperatures compared to lower temperature in both new and old seeds. While for new seeds the highest MGT was recorded at a lower temperature, there were no significant difference in MGT for new and old seeds at a higher temperature (Fig. 2). Similar trends were obtained for C. brevifolium, where the highest time taken by the seeds (both new and old seeds) incubated at a lower temperature; although the differences for new and old seeds at moderate and higher temperatures were non-significant. C. ciliaris seeds took relatively less time for germination compared to the other two species. Both new and old seeds take a longer time to germinate at a lower temperature. However, the lowest MGT was recorded for summer seeds that were incubated at a higher temperature (25/35 °C) (Fig. 2).
Figure 2.
Mean germination time (MGT) of C. virgata, C. brevifolium and C. ciliaris at different temperatures. For more details of the treatments and statistical analysis, see Fig. 1.
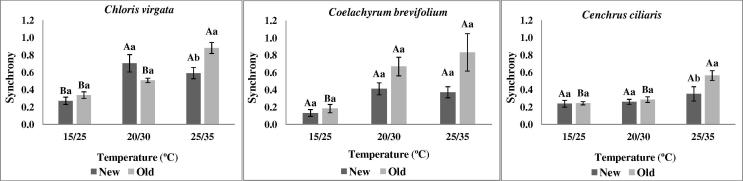
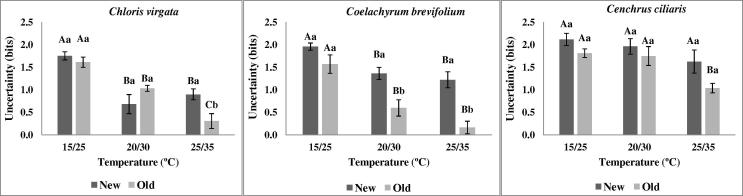
Overall, synchrony increased with the incubation temperature and few or no significant differences were verified between old and new seeds (Fig. 3). In other way, the uncertainty was inversely influenced by the temperature increase (Fig. 4). Additionally, only seeds incubated in higher temperatures showed significant differences between new and old seeds.
Figure 3.
Germination synchrony of C. virgata, C. brevifolium and C. ciliaris at different temperatures. For more details of the treatments and statistical analysis, see Fig. 1.
Figure 4.
Uncertainty of C. virgata, C. brevifolium and C. ciliaris at different temperatures. For more details of the treatments and statistical analysis, see Fig. 1.
4. Discussion
In this study we show that the seeds collected in summer season (old seeds) are heavier in all the studied species compared to seeds collected in winter season (new seeds). Seeds matured during different season have been reported to be responsible for variation in seed mass due to variation in environmental conditions (Wulff, 1986). Therefore in the present study, temporal variability in seed weight variation could be explained by the variation in climatic conditions during seed maturation. Similar results were obtained for Halopyrum mucronatum where summer seeds are heavier as compared to winter seeds (Noor and Khan, 1995). Seed weight significantly affects the germination characteristics. By the way, heavier (old) seeds germinated better and synchronously and show lower MGT and uncertainty. Seed germination, survival and seedling growth in numerous species and poor performance of lighter seeds has been related to their lower endosperm content (Harper, 1977). In the present study, this hypothesis can be applied to C. virgata, whereby in older seeds (summer) germinability was better than new and lighter seeds. However, in case of C. brevifolium and C. ciliaris lighter (new) seeds germinated better. We assumed that heavier seeds of these two species might be devoted for constituting soil seed bank whereas lighter seeds likely to be more widely dispersed and have a potential to rapidly colonize. Besides that, seeds produced during winter have an advantage because the chances of rainfall are high during this season (Böer, 1997). This fact might have ecological implication on seed germination particularly in a desert climate which favors germination in winter (November–February) and the chances of their seedling survival will be high during that time.
Winter seeds of C. virgata seem to be dormant, while summer seeds do not have any innate dormancy and they germinated well in all the tested temperature regimes. A confirmation of this is given by the lower MGT and greater synchrony of these seeds in all the evaluated temperatures. Winter seeds of C. ciliaris are non-dormant, C. brevifolium seeds seem to have dormancy for both new and old collections with a slightly higher dormancy for old seeds. These findings are similar to those of El-Keblawy (2013), who reported that C. brevifolium seeds have a high level of innate dormancy. This variation in dormancy pattern could be related to variation in temperature, light, photoperiod, water and nutrients that determine the degree of seed dormancy and seed performance during germination (Holdsworth et al., 2008, Donohue, 2009, Bewley et al., 2013). Therefore, it is reasonable to assume that variations between seeds collected during different seasons are not an expression of different ecotypes, since seeds of all these species were collected within a restricted area without any variation in local climate.
Germination percentage, MGT and germination requirement of these species differ significantly between different seasons of seed collection (new and old). New seeds (C. brevifolium and C. ciliaris) showed better germination. The seeds that matured in October (new seeds), have a good chance for seedling establishment because the temperature will be lower during that time and chances of rainfall will be higher. However, the old seeds that matured in May (summer), might enter the soil seed bank, since existing environmental conditions at the time of seed dispersal are not favorable for germination and seedling survival (El-Keblawy et al., 2013). The seeds of these species that matured in winter, faced more severe temperatures during seed formation and maturation (monthly average temperature between June to October 33.4 °C) as compared to seeds that matured in May (monthly average temperature between January to May 24.04 °C – Islam et al., 2009). Generally, seeds that develop and mature at warmer temperatures are less dormant at maturity than those that develop at cooler temperatures (Donohue et al., 2008, Kendall et al., 2011, Kendall and Penfield, 2012, Huang et al., 2014). Higher temperatures during seed development have been reported to increase seed germination in many species (Drew and Brocklehurst, 1990, Llorens et al., 2008). Kendall et al. (2011) reported that low temperatures during seed development increased abscisic acid (ABA) content and reduced gibberellic acid (GA) levels, which can act together to decrease the levels of mRNA for α-amylases and hydrolytic enzymes, strongly decreasing the future seed germination rates (Al-Helal, 1996, Nanjo et al., 2004).
This variation in germination could ensure that only a portion of the seed will germinate at one time even under the optimal condition and help their survival in desert condition where there is spatial and temporal unpredictability in rainfall (Gutterman, 1991). This indicates that these seeds will have a higher chance of survival over the season because there are high chances of rainfall during winter in a desert area (Böer, 1997). However, in summer there is no chance of rain and if the seed germinated during that time they won’t be able to survive due to extreme temperature and they might entered the soil seed bank. This is supported by the high synchrony on seed germination in seeds incubated at high temperatures and water availability. Entering the soil seed bank will help them to persist in harsh desert climatic condition and it will also have an impact on mitigating the risk of species extinction under such a condition (Wang and Liang, 1995).
Seeds of C. virgata that matured in summer season attained significantly higher germination percentage and germination speed (measure by 1/GMT) than those matured in winter season. This trend has been reported for Portulaca oleracea seeds in similar conditions (El-Keblawy and Al-Ansari, 2000). We assumed that seed which matured at a higher temperature might contribute to form the soil seed bank since at that time high temperature that coincide with dry season and chances of their germination are less due to unavailability of rain during summer. However, higher dormancy of winter seeds as in C. virgata could be attributed to lower water availability because these seeds faced more severe temperatures during their formation and maturation which might hasten the natural dehydration process of seed by changing integument structure and enhancing its permeability (Clua and Gimenez, 2003). Each species has a unique temperature requirement during germination. It has been shown that different grass species have different temperature requirement for seed germination (Mcwilliam et al., 1970, Palazzo and Brar, 1997). Results indicate that seeds of all the studied species have ability to germinate in wide range of temperature. This adaptation might have ecological amplitude under such harsh desert condition and allow these species to germinate and develop into seedlings between November and February (winter) when the chances of rainfall are high. However, the optimum temperature required for seed germination varies from species to species. In the present study, C. virgata seeds germinated better at higher temperatures. However, C. brevifolium and C. ciliaris seeds germinated better at moderate temperatures indicating that these species required relatively a high temperature for germination. Similar results were obtained for many native desert grass species such as C. ciliaris, C. setigerus, Lasiurus sindicus, Panicum turgidum (El-Keblawy et al., 2011). Further, C. virgata and C. ciliaris are C4, warm-season grass and, therefore they might require relatively warm temperature for germination as reported for other C4 grasses such as Miscanthus sinensis (Jones, 1985) and Bouteloua gracilis (Qi and Redmann, 1993).
Acknowledgements
This work was partially supported by a Grant from the Qatar National Research Fund, QNRF Grant # 5-260-1-053). Arvind Bhatt would like to acknowledge Dr. Yousef Al Horr, Dr. Esam Elsarrag (Gulf Organization for Research and Development) and Dr. Ali A. El-Keblawy (Dept. of Applied Biology, Faculty of Science and Sharjah Research Academy, University of Sharjah, Sharjah, UAE) for their unlimited support. We are also very grateful to Dr. Syd Ramdhani, University of KwaZulu-Natal (UKZN), South Africa, for his critical revision of a final version of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Helal A.A. Studies on germination of Rumex dentatus L. seeds. J. Arid. Environ. 1996;33:39–47. [Google Scholar]
- Baskin C.C., Baskin J.M. Academic Press; San Diego, CA: 1998. Seeds: Ecology, Biogeography, and Evolution of Seed Dormancy and Germination. [Google Scholar]
- Bewley J.D., Hilhorst H.W.M., Bradford K.J., Nonogaki H. Springer; New York: 2013. Seeds: Physiology of Development, Germination and Dormancy. [Google Scholar]
- Böer B. An introduction to the climate of the United Arab Emirates. J. Arid. Environ. 1997;35:3–16. [Google Scholar]
- Clua A.A., Gimenez D.O. Environmental factors during seed development of narrow leaved bird’s-foot-trefoil (Lotus tenuis) influences subsequent dormancy and germination. Grass Forage Sci. 2003;58:333–338. [Google Scholar]
- Donohue K. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical transactions of the royal society. Biol. Sci. 2009;364:1059–1074. doi: 10.1098/rstb.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K., Dorn L., Griffith C., Kim E., Aguilera A., Polisetty C.R., Schmitt J. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution. 2005;59:758–770. [PubMed] [Google Scholar]
- Donohue K., Heschel M.S., Butler C.M., Barua D., Sharrock R.A., Whitelam G.C., Chiang G.C.K. Diversification of phytochrome contributions to germination as a function of seed-maturation environment. New Phytol. 2008;177:367–379. doi: 10.1111/j.1469-8137.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- Drew R.L.K., Brocklehurst P.A. Effects of temperature of mother-plant environment on yield and germination of seeds of lettuce (Lactuca sativa) Ann. Bot. 1990;66:63–72. [Google Scholar]
- El-Keblawy A. Impacts of dormancy-regulating chemicals on innate and salinity-induced dormancy of four forage grasses native to Arabian deserts. Grass Forage Sci. 2013;68:288–296. [Google Scholar]
- El-Keblawy A., Al-Ansari F. Effect of site of origin, time of seed maturation and seed age on germination behavior of Portulaca oleracea L. from old and new world. Can. J. Bot. 2000;78:279–287. [Google Scholar]
- El-Keblawy A., Al-Rawai A. Effects of seed maturation time and dry storage on light and temperature requirements during germination in invasive prosopis juliflora. Flora. 2006;201:135–143. [Google Scholar]
- El-Keblawy A., Al-Sodany Y.M., Al-Hadad F.A. Effects of time of seed maturation on dormancy and germination requirements of Sporobolus spicatus (Vahl) Kunth, a native desert grass of the United Arab Emirates. Grassland Sci. 2009;55:11–17. [Google Scholar]
- El-Keblawy A., Al-Ansari F., Al-Shamsi N. Effects of temperature and light on salinity tolerance during germination in two desert glycophytic grasses, Lasiurus scindicus and Panicum turgidum. Grass Forage Sci. 2011;66(2):173–182. [Google Scholar]
- El-Keblawy A., Bhatt A., Gairola S. Perienth colour affect germination behavior in the wind pollinated Salsola rubescens in the Arabian Deserts. Can. J. Bot. 2013;92:69–75. [Google Scholar]
- Fenner M. The effects of parent environment on seed germinability. Seed Sci. Res. 1991;1:75–84. [Google Scholar]
- Fenner M. Environmental influences on seed size and composition. Hortic. Rev. (Am. Soc. Hortic. Sci.). 1992;13:183–213. [Google Scholar]
- Galloway L.F. The effect of maternal phenology on offspring characters in the herbaceous plant Campanula americana. J. Ecol. 2002;90:851–858. [Google Scholar]
- Gorecki M.J., Long R.L., Flematti G.R., Stevens J.C. Parental environment changes the dormancy state and karrikinolide response of Brassica tournefortii seeds. Ann. Bot. 2012 doi: 10.1093/aob/mcs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterman Y. Seeds: the ecology of regeneration in plant communities. In: Fenner M., editor. Maternal Effects on Seeds During Development. 2nd ed. CABI Publishing; Wallingford, England: 2000. pp. 59–84. [Google Scholar]
- Gutterman Y. Comparative germination study on seeds matured during winter or summer of some bi-seasonal flowering perennial desert plants from the Aizoaceae. J. Arid. Environ. 1991;21:283–291. [Google Scholar]
- Haase P., Pugnaire F.I., Incoll L.D. Seed production and dispersal in the semi-arid tussock grass Stipa tenacissima L. during masting. J. Arid. Environ. 1995;31:55–65. [Google Scholar]
- Harper J.L. Academic Press Inc; London: 1977. Population Biology of Plants. [Google Scholar]
- Holdsworth M.J., Bentsink L., Soppe W.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Huang Z., Footitt S., Finch-Savage W.E. The effect of temperature on reproduction in the summer and winter annual Arabidopsis thaliana ecotypes Bur and Cvi. Ann. Bot. 2014;113:921–929. doi: 10.1093/aob/mcu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.D., Kubo I., Ohadi M., Alili A.A. Measurement of solar energy radiation in Abu Dhabi, UAE. Appl. Energy. 2009;86:511–515. [Google Scholar]
- Jensen M., Eriksen E.N. Development of primary dormancy in seeds of Prunus avium during maturation. Seed Sci. Technol. 2001;29:307–320. [Google Scholar]
- Jones C.A. John Wiley & Sons; New York: 1985. C4 grasses and cereals: growth, development, and stress response. [Google Scholar]
- Kendall S.L., Hellwege A., Marriot P., Whalley C., Graham I.A., Penfield S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell. 2011;23:2568–2580. doi: 10.1105/tpc.111.087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall S.L., Penfield S. Maternal and zygotic temperature signalling in the control of seed dormancy and germination. Seed Sci. Res. 2012;22:S23–S29. [Google Scholar]
- Khan M.A., Ansari R. Biosaline Agriculture and High Salinity Tolerance. Birkhäuser Basel; 2008. Potential use of halophytes with emphasis on fodder production in coastal areas of Pakistan. pp. 157–162. [Google Scholar]
- Llorens L., Pons M., Gil L., Boira H. Seasonality of seed production and germination trends of Fumana ericoides (Cistaceae) in the west semiarid Mediterranean region. J. Arid. Environ. 2008;72:121–126. [Google Scholar]
- Luzuriaga A.L., Escudero A., Perez-Garcia F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae) Weed Res. 2006;46:163–174. [Google Scholar]
- Mansoor U., Hameed M., Wahid A., Rao A.R. Ecotypic variability for drought resistance in Cenchrus ciliaris L. germplasmfrom Cholistan desert in Pakistan. Int. J. Agr. Biol. 2002;4:392–397. [Google Scholar]
- F.R.F. Marques. GerminaQuant: a new tool for germination measurements. Seed Sci. Technol. 2015 (submitted to publication) [Google Scholar]
- Mcwilliam J.R., Clements J.R., Dowling P.M. Some factors influencing the germination and early seedling development of pasture plants. Aust. J. Agric. Res. 1970;21:19–32. [Google Scholar]
- Meyer S.E., Allen P.S. Ecological genetics of seed germination regulation in Bromus tectorum L. II. Reaction norms in response to a water stress gradient imposed during seed maturation. Oecologia. 1999;120:35–43. doi: 10.1007/s004420050830. [DOI] [PubMed] [Google Scholar]
- Miranda R.D.Q., Correia R.M., De Almeida-Cortez J.S., Pompelli M.F. Germination of Prosopis juliflora (Sw.) DC seeds at different osmotic potentials and temperatures. Plant Species Biol. 2014;29(3):E9–E20. [Google Scholar]
- Nanjo Y., Asatsuma S., Itoh K., Hori H., Mitsui T., Fujisawa Y. Posttranscriptional regulation of α-amylase II-4 expression by gibberellin in germinating rice seeds. Plant Physiol. Biochem. 2004;42:477–484. doi: 10.1016/j.plaphy.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Noor M., Khan M.A. Factors affecting germination of summer and winter seeds of Halopyrum mucronatum under salt stress. In: M.A. Khan, I.A. Ungar., editors. Biology of Salt Tolerant Plants. University of Karachi; Karachi, Pakistan: 1995. pp. 51–58. [Google Scholar]
- Osman A.E., Makawi M., Ahmed R. Potential of the indigenous desert grasses of the Arabian Peninsula for forage production in a water-scarce region. Grass Forage Sci. 2008;63:495–503. [Google Scholar]
- Palazzo A.J., Brar G.S. Screening of 12 Festuca cultivars for rapid root development. J. Turfgrass. Manag. 1997;2:15–25. [Google Scholar]
- Patel Y., Dabgar Y.B., Joshi P.N. Distribution and diversity of grass species in Banni Grassland, Kachchh District, Gujarat, India. Int. J. Sci. Res. Rev. 2012;1:43–56. [Google Scholar]
- Peacock J.M.F., Erguson M.E., Alhadrami G.A., Mccann I.R., Al-Hajoj A.S., Aleh A., Karnik R. Conservation through utilization: a case study of the indigenous forage grasses of the Arabian Peninsula. J. Arid. Environ. 2003;54:15–28. [Google Scholar]
- Qaderi M.M., Cavers P.B., Bernards M.A. Pre- and post-dispersal factors regulate germination patterns and structural characteristics of Scotch thistle (Onopordum acanthium) cypselas. New Phytol. 2003;159:263–278. doi: 10.1046/j.1469-8137.2003.00777.x. [DOI] [PubMed] [Google Scholar]
- Qi Q.M., Redman R.E. Seed germination and seedling survival of C3 and C4 grasses under water stress. J. Arid. Environ. 1993;24:277–285. [Google Scholar]
- Wang G., Liang X.G. The dynamics of seed bank on Shapotou artificially stabilized dunes. Act. Bot. Sin. 1995;37:231–237. [Google Scholar]
- Waramit N. Graduate Theses and Dissertations. Iowa State University; 2010. Native warm-season grasses: species, nitrogen fertilization, and harvest date effects on biomass yield and composition. [Google Scholar]
- Wulff R.D. Seed size variation in Desmodium paniculatum: II. Effects on seedling growth and physiological performance. J Ecol. 1986;1:99–114. [Google Scholar]