Abstract
Mushrooms have been highly regarded as possessing enormous nutritive and medicinal values. In the present study, we evaluated the anti-oxidative and anti-atherosclerotic potential of shiitake mushroom (Lentinula edodes) using its solvent–solvent partitioned fractions that consisted of methanol:dichloromethane (M:DCM), hexane (HEX), dichloromethane (DCM), ethyl acetate (EA) and aqueous residue (AQ). The hexane fraction (1 mg/mL) mostly scavenged (67.38%, IC50 0.55 mg/mL) the 2,2-diphenyl-1-picryl hydrazyl (DPPH) free radical, contained the highest reducing capacity (60.16 mg gallic acid equivalents/g fraction), and most potently inhibited lipid peroxidation (67.07%), low density lipo-protein oxidation and the activity of 3-hydroxy 3-methyl glutaryl co-enzyme A reductase (HMGR). GC–MS analyses of the hexane fraction identified α-tocopherol (vitamin E), oleic acid, linoleic acid, ergosterol and butyric acid as the bio-functional components present in L. edodes. Our findings suggest that L. edodes possesses anti-atherosclerotic bio-functionality that can be applied as functional food-based therapeutics against cardiovascular diseases.
Keywords: Cardiovascular disease, Functional food, HMG Co-A reductase, Lipid peroxidation, Low density lipoprotein
1. Introduction
Food and food bio-components having anti-oxidative and anti-hypercholesterolemic bio-functionalities have been highly advocated as remedies for oxidative stress-induced pathophysiological complications (Orekhov et al., 2013). Atherosclerosis, a cardiovascular disease (CVD) hallmark, has been intricately linked with an oversupply of food-based cholesterol and excessive production of oxidised low density lipoproteins (ox-LDL). The International Atherosclerotic Society (IAS) formulated global recommendations for the management of hyperlipidemia, emphasising control of the level of LDL in order to reduce the risk of atherosclerosis (Grundy et al., 2013). As atherosclerosis is a long-term cause and effect process, anti-atherosclerosis therapy should parallel its course. In this endeavour, inhibitors of the key enzyme in cholesterol biosynthesis, 3-hydroxy 3-methyl glutaryl co-enzyme A reductase (HMGR), have been screened from micro-organisms followed by their synthetic development designated as “statins”. But the long-term use of statins and other cholesterol lowering drugs seem inept when considering tachyphylaxis, drug toxicity and cost management (Martin et al., 2012). In addition, not all people can tolerate statins and their application comes with some downside along with muscle aches upon discontinuation (Karanth et al., 2013). In order to mitigate oxidative stress towards LDL, boosting the anti-oxidative defence arsenal has been suggested (Cherubini et al., 2005). In this connection, food and food bio-component based approaches have received due attention (Sirtori et al., 2009). The European Food Safety Association (EFSA) recommended two types of functional foods: phytosterol-based and β-glucan-based, for their potency in reducing the risk of atherosclerosis en route to CVD (EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010). These bio-components work by lowering the absorption of cholesterol and/or curbing its biosynthesis. The anti-oxidative mode of action of these bio-functional food components can be divided into two broad spectra: electron transfer (ET) and hydrogen atom transfer (HAT) through which the anti-oxidant itself becomes a “less harmful oxidant for a while” followed by its return to the main structural form with the help of other “network anti-oxidants”, ultimately mitigating oxidative stress.
The macro fungi, mushrooms have been reported to possess immense nutritional and medicinal bio-components and spur excellence in maintaining global public health. Mushrooms abound with phytosterol derivatives, including ergosterol and fungisterol as well as β-D-glucan. The ergosterol concentration in mushroom has been reported to be as high as 9.61 mg/g dry weight (Kalač, 2009, Mattila et al., 2002). β-D-glucan and its derivatives present in both edible and medicinal mushrooms confer their cholesterol lowering effects through reduced absorption and/or increased faecal excretion (Cheung, 2010, Wasser, 2011). The hypocholesterolemic effect of mushrooms through their inhibitory role on HMG CoA-reductase has also been documented (Gil-Ramírez et al., 2013b, Rahman et al., 2014). In addition, mushrooms have been highly regarded for possessing anti-oxidative prowess (Cheung and Cheung, 2005, Abdullah et al., 2012).
Lentinus edodes, commonly known as the “shiitake mushroom”, ranks second in the global mushroom market based on consumer demand (Bisen et al., 2010). Its nutritional components include bio-active polysaccharides such as β-D-glucan, heteroglucan, xylomannan, lentinan and eritadenine; free sugars including arabinose, arabitol, mannose, mannitol, trehalose and glycerol; vitamins (B2, B12, D2) and dietary fibre (Hobbs, 2000). Numerous bio-components present in L. edodes aid in its pharmacological potency against hypertension, hyperlipidemia and cardiovascular complications, depressed immunity, hepatic disorders and cancer. In addition, its anti-oxidative, anti-fungal and anti-microbial aspects have been duly attributed to its bio-functional components (Bisen et al., 2010). Although much work has been done on various aspects of other mushrooms and also on shiitake, studies relating to its anti-atherosclerotic effect through direct anti-oxidative and inhibitory effects upon LDL oxidation and HMG Co-A reductase activity are scarce. Thus, the present work had been designed to evaluate the anti-atherosclerotic potential of L. edodes along with identifying the relevant bio-functional components.
2. Materials and methods
2.1. Preparation of gradually partitioned solvent fractions of L. edodes
Proper separation and purification of the functionally active bio-components is an important criterion in demonstrating their bio-functionality. Based on the polarity and density gradient differences among five solvents (methanol, dichloromethane, ethyl acetate, hexane and water), we gradually fractionated the mushroom bio-components following a method described elsewhere (Rahman et al., 2014). Briefly, L. edodes fruiting bodies were sliced, sun-dried and ground to powder. Two hundred grams of powder was used for extraction with 4 L of methanol:dichloromethane (2:1) in conical flasks at room temperature with occasional stirring and shaking for 3 days followed by filtration through Whatman No. 1 filter paper. The extraction was repeated twice and the total organic solution, collected from each step of extraction, was evaporated using a rotary evaporator (Büchi Rotavapor R-114, Switzerland) that yielded the crude (M:DCM) extract. The dried, crude extract was dissolved in 90% aqueous methanol and partitioned with hexane (3 × 100 mL). The upper hexane layer was separated using a separatory funnel and later rota-evaporated. The bottom aqueous methanolic layer left was rota-evaporated that yielded a semisolid fraction. Re-dissolving of the semisolid fraction in distilled water (100 mL) was followed by successive partitioning with dichloromethane (DCM, 3 × 100 mL). We collected the partitioned DCM fraction and rota-evaporated. Then, we re-partitioned the aqueous fraction with ethyl acetate (EA, 3 × 100 mLs) followed by collection of the resultant upper EA layer and rota-evaporation. Finally, we freeze-dried the lower aqueous part and obtained the aqueous fraction.
2.2. Evaluation of the anti-oxidant properties of L. edodes fractions
We evaluated the anti-oxidative effects of the L. edodes fractions compared with the positive control quercetin while carrying out the standard tests described below.
2.2.1. Scavenging effect on 2,2-diphenyl-1-picrylhydrazil (DPPH) radical
We followed a previously developed method in our lab for determining the DPPH free radical scavenging effects of the L. edodes fractions (Abdullah et al., 2012). In brief, 3.9 mL of 0.06 mM DPPH dissolved in methanol was mixed with 0.1 mL of each solvent fraction (1.0 mg/mL conc.). After shaking the mixture in darkness, we measured the absorbance at 515 nm. Using methanol as the blank, we calculated the percentage of DPPH free radical scavenging using the following equation:
where A0 is the absorbance of the 0.06 mM methanolic DPPH alone, and As is the absorbance of the reaction mixture. The IC50 value (concentration of the fraction necessary to produce half maximal inhibition/scavenging) of the most potent solvent fraction was calculated from the graph of the radical scavenging activity against fraction concentration.
2.2.2. Folin–Ciocalteu assay using the L. edodes fractions
Folin–Ciocalteu reagent (10%, 250 μL) was added to an equal volume of the solvent fraction and kept at darkness for 3 min while shaking. To this mixture, 500 μL of 10% sodium carbonate was then added, followed by incubation in the dark for 1 h, followed by measurement of the absorbance at 750 nm. The calibration curve of gallic acid (2–10 μg/mL) was used to express the performance of the Folin–Ciocalteu assay as milligrams of gallic acid equivalents (mg GAE) per gram of fraction (Berger et al., 2004).
2.2.3. Inhibitory effects of the L. edodes fractions upon lipid peroxidation
We applied our previously established method to determine the inhibitory effect of each of the fractions on lipid peroxidation of buffered egg yolk (Rahman et al., 2014). Briefly, Fowl egg yolk was emulsified with 0.1 M phosphate buffer (pH 7.4) to prepare a solution of 25 g/L. Lipid peroxidation was induced by ferrous sulphate (1 M, 100 μL). The L. edodes fractions (100 μL) were then added, shaken and incubated at room temperature for 1 h. Then, 15% (v/v) of trichloroacetic acid (TCA, 500 μl) and thiobarbituric acid (TBA, 1 mL), both freshly prepared, were added. Following incubation in a boiling water bath for 10 min, the reaction mixtures were cooled and centrifuged at 3500g for 10 min to precipitate the proteins. The absorbance of an aliquot of the supernatant (100 μL) was measured at 532 nm. Buffered egg yolk with Fe+2 alone was used as the control. Calculation of the percentage inhibition of lipid peroxidation was performed according to the following equation:
where A0 is the absorbance of the control and As is the absorbance of the reaction mixture containing the fraction. The IC50 value of the most potent solvent fraction was calculated from the graph of the inhibition of lipid peroxidation against fraction concentration.
2.2.4. FeSO4–induced LDL oxidation and L. edodes fraction-mediated inhibition
Following our previously established method, we determined the L. edodes fraction-mediated inhibition of human LDL (Sigma–Aldrich, St. Louis, USA) oxidation in vitro (Rahman et al., 2014). The observed aspects included:
2.2.4.1. Effect of the L. edodes fractions upon the lag time of conjugated diene (CD) formation
We induced Fe+2 (FeSO4, 50 μg/mL)-mediated oxidative stress towards human LDL (150 μg/mL) at room temperature and pH 7.4. Subsequently, we observed the kinetics of both Fe+2 – induced LDL oxidation and the L. edodes fraction-mediated (1 μg/mL) corresponding inhibition at 234 nm at 20 min intervals for a period of 180 min. FeSO4 in ultrapure water (pH 7.4) was used as the blank. Along the time course of oxidation, the L. edodes fraction-mediated protection phase denoted as the “lengthened lag time of CD formation” was identified and measured.
2.2.4.2. Effect of L. edodes fractions upon malondialdehyde (MDA) production
Oxidative modification of human LDL was performed using 10 mM ferrous sulphate followed by the addition of L. edodes fractions at a 1 mg/mL concentration. Trichloroacetic acid (TCA, 500 μL, 15% v/v) and thiobarbituric acid (TBA, 1 mL, 1% v/v) were added and incubated at 100 °C for 10 min. After cooling, an aliquot (300 μL) was taken to the ELISA reader and the absorbance read at 532 nm. For the blank, FeSO4 in water, pH 7.4, was used. We used a standard assay kit (Cayman Chemicals’ TBARS assay kit, item No. 1000955) and followed the manufacturer’s recommendations to calculate the production of MDA.
2.3. Determination of HMG Co-A reductase (HMGR) inhibitory effect
The in vitro HMG Co-A reductase (HMGR) inhibitory effect of the various fractions of L. edodes was tested using the HMGR assay kit (Sigma-Aldrich, catalogue No. CS1090) (Gholamhoseinian et al., 2010). The human catalytic domain of the HMGR enzyme (concentration 0.6 mg protein/mL) and pravastatin as a positive control were used according to the recommended conditions. In order to attain a final mixture of volume 200 μL and concentration 400 μM, 4 μL of NADPH and 12 μL of HMG-CoA substrate were added with 2 μL of the catalytic domain of human recombinant HMGR together with an appropriate amount of 100 mM potassium phosphate buffer (containing 120 mM KCl, 1 mM EDTA, and 5 mM dithiothreitol, pH 7.4). Aliquots (1 μL) of pravastatin and L. edodes fractions (1 mg/mL) were added and the rate of NADPH oxidation by HMGR was monitored every 20 s at 340 nm for a period of 10 min using a Bio Tek H1 synergy hybrid multi-mode plate reader equipped with Gen 5 data analysis software (Bio Tek, VT, USA).
2.4. Identification of bio-functional components by GC–MS
As the hexane fraction of L. edodes showed the best performance in terms of anti-oxidative and HMG Co-A reductase inhibitory activities, it was subjected to GC–MS analysis to identify the bio-functional and anti-atherosclerotic components. We utilised gas chromatography directly coupled to a mass spectrometer system (Agilent 7000 C triple quadruple GC/MS system, USA). Utilising a HP-5 ms silica capillary column (30 m × 250 μm, 0.25 μm film) the instrumental conditions maintained were: oven temperature 70 °C–300 °C (finally maintained for 29 min); inert helium gas as the carrier, flow rate 1 mL/min; injection volume 1.5 μL; injection technique split less; injector temperature 250 °C; ionisation energy 70 eV; mode electronic ionisation (EI); temperature of ion source 200 °C; range of masses scanned 50–550 m/z; and interface line temperature 300 °C. Mass spectra of the National Institute of Standards and Technology (NIST 08 and NIST08 s) library data were used as a reference to identify the peaks.
2.5. Statistical analyses
We conducted all the experiments in triplicate and the data are presented as mean ± SD. Using statistical package SPSS version 16 we performed one-way analysis of variance (ANOVA). The differences among means were further analysed by the least significance difference (LSD) test at a 95% confidence level (p ⩽ 0.05).
3. Results and discussion
3.1. DPPH-free radical scavenging activity
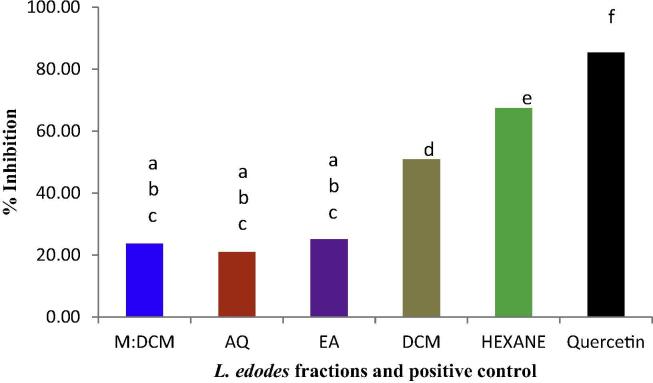
The DPPH-free radical scavenging performance of the L. edodes fractions was evaluated at a concentration of 1 mg/mL and the results are presented in Fig. 1. All the fractions were capable of scavenging free radicals but varied in their potency. The hexane fraction had the highest scavenging activity (67.38%, IC50 0.55 mg/mL), followed by that of DCM (50.85%, IC50 0.68 mg/mL). The other three fractions did not differ significantly in terms of DPPH free radical scavenging.
Figure 1.
DPPH free radical scavenging effect of the L. edodes fractions compared to the positive control, quercetin. Mean values (n = 3) having dissimilar superscripts are indicative of a significant statistical difference at the 95% level (p ⩽ 0.05) following a post hoc least significant difference (LSD) test.
The anti-oxidative test evaluating the scavenging effect of the L. edodes fractions upon the DPPH free radical is an ET based assay. All the fractions possessed free radical scavenging potential but the scavenging effect of the hexane fraction surpassed that of all the others (Fig. 1). It measured the cumulative antioxidant potential of the mushroom fractions whereby the scavenging antioxidants present in the respective fractions reduced the stable, purple radical DPPH˙ and converted it into the yellow coloured, non-radical form (DPPH-H) (Fig. 2).
Figure 2.
Basic mechanisms of DPPH free radical scavenging by the anti-oxidant molecule (AO-H).
3.2. Folin–Ciocalteu assay
The reducing capacity of the bio-components is a special feature of their anti-oxidative potency. In connection with this, we performed the Folin–Ciocalteu assay compared to quercetin. Reducing prowess was expressed in terms of milligrams of gallic acid equivalents per gram of fraction (mg GAE/g fraction). The results are presented in Fig. 2, indicating the hexane fraction possessed the highest reducing capacity (60.16 GAE/g fraction), followed very closely by those of DCM (58.86 mg GAE/g fraction) and EA (55.32 mg GAE/g fraction).
The Folin–Ciocalteu assay performed in the present experiment (Fig. 3) is another ET-based assay through which the total reducing capacities of the L. edodes bio-components were measured. The data presented in Fig. 2 are in line with a previous study undertaken by Abdullah et al. (2012). As compared to the natural reducing agent gallic acid, L. edodes showed promising results and thus we further studied its effects upon lipid peroxidation and LDL oxidation.
Figure 3.
Folin–Ciocalteu assay of the L. edodes fractions compared to the positive control, quercetin. Mean values (n = 3) having dissimilar superscripts are indicative of a significant statistical difference at the 95% level (p ⩽ 0.05) following a post hoc least significant difference (LSD) test.
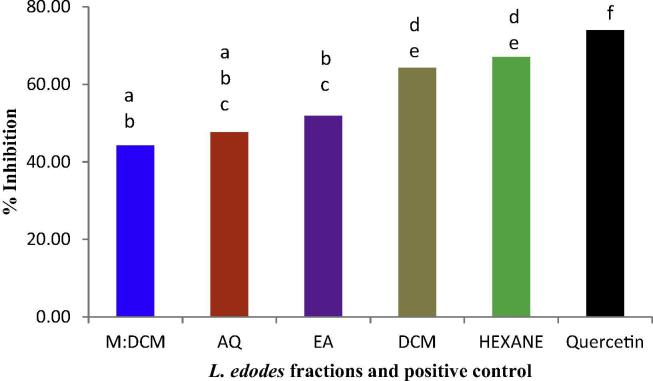
3.3. Lipid peroxidation inhibitory effect
The inhibitory effect of the L. edodes fractions upon lipid peroxidation is shown in Fig. 3. We induced oxidative modification of lipid structures with a transition metal, Fe+2, and assessed whether the L. edodes fractions could lower the inhibition or result in a reduced rate of oxidised lipid structural byproduct generation. We found that the hexane fraction had the most potent inhibitory effect on lipid peroxidation (67.07%), followed by DCM (64.23%) (Fig. 4). This finding was in line with the free radical scavenging and reducing capacities of the fractions and suggested these effects might be attributed to the lipophilic bio-components present in the hexane fraction of L. edodes.
Figure 4.
Lipid peroxidation inhibitory effect of L. edodes fractions compared to the positive control, quercetin. Mean values (n = 3) having dissimilar superscripts are indicative of a significant statistical difference at the 95% level (p ⩽ 0.05) following a post hoc least significant difference (LSD) test.
3.4. Inhibition of low density lipoprotein (LDL) oxidation by L. edodes fractions
The kinetics of LDL oxidation incorporates an initial lag period of slow oxidation followed by a log period of enhanced oxidation. During the lag period conjugated dienes (CD) are formed, and during the log period malondialdehydes (MDA) are generated (Yoshida and Kisugi, 2010). The anti-atherosclerotic performance of bio-components involves their mediating effect upon increasing the lag time and decreasing MDA production (Li and Mehta, 2005). The hexane fraction of L. edodes also performed the best in this regard.
3.4.1. Effect of L. edodes fractions upon the lag time of CD formation
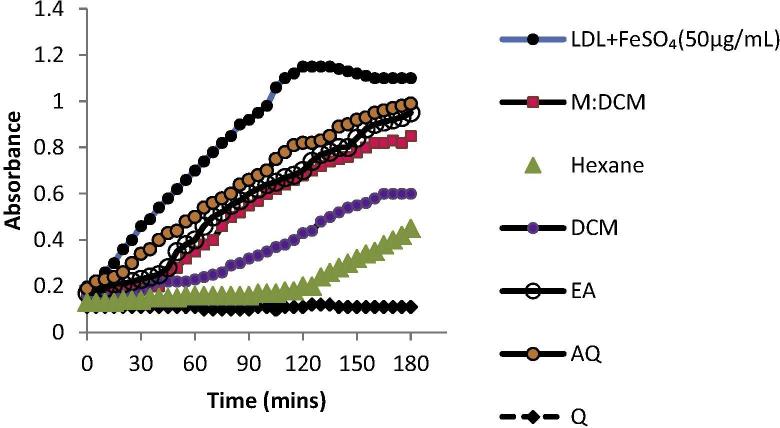
Oxidative stress exerted upon LDL molecules by Fe+2 affects the polyunsaturated fatty acids (PUFAs) present in the LDL molecule and produces conjugated dienes (CD). Fig. 5 represents the kinetics of CD production and the effect of L. edodes fractions upon this kinetic pattern. The hexane fraction lengthened the lag time (125 min) of CD formation the most, indicating it possessed the highest inhibitory activity against LDL oxidation.
Figure 5.
Kinetics of conjugated diene (CD) formation. L. edodes fractions mediated an increased lag time of CD formation at 234 nm, indicating their inhibitory effect on FeSO4-induced LDL oxidation. All the fractions and the positive control, quercetin, were used at a 1 μg/mL conc.
3.4.2. Effect of L. edodes fractions upon MDA formation
In the LDL oxidation cascade, CD formation is rapidly followed by the production of MDA through the breakage of the lipid hydro-peroxide double bonds. Thus, the level of MDA corresponds to the severity of LDL oxidation. We found that all L. edodes fraction-treated samples had a lower MDA production (data not shown for the sake of brevity). Among all the solvent fractions of L. edodes, the hexane fraction rendered the smallest amount of MDA (21.57 μM/mg LDL), indicating it had the highest content of bio-components capable of withstanding LDL oxidation.
3.5. Inhibitory effect of L. edodes fractions upon HMG Co-A reductase (HMGR) activity
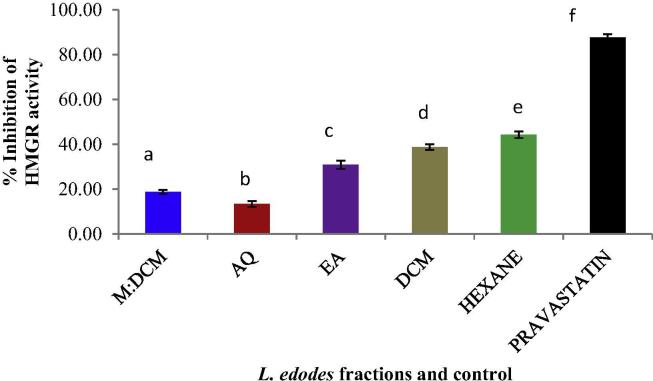
We assessed whether the L. edodes fractions could inhibit the activity of the cholesterol biosynthetic regulatory enzyme HMG Co-A reductase (HMGR) in vitro. All the fractions could inhibit the activity of the catalytic domain of human HMGR at 1 mg/mL concentration. As depicted in Fig. 6, here also, the hexane fraction-mediated inhibitory effect was the most potent (44.26%).
Figure 6.
Inhibitory effect of L. edodes fractions (1 mg/mL) on HMG Co-A reductase (HMGR) activity compared to the positive control, pravastatin (used directly according to the manufacturer’s recommendation). Mean values (n = 3) having dissimilar superscripts are indicative of a significant statistical difference at the 95% level (p ⩽ 0.05) following a post hoc least significant difference (LSD) test.
An increased level of total cholesterol is an independent risk factor for atherosclerosis. Interruption of cholesterol biosynthesis through direct inhibition of its regulatory enzyme HMG Co-A reductase has been a target for the last few decades. We also evaluated whether L. edodes could inhibit HMG Co-A reductase activity in vitro. Consistent with our other findings, the hexane fraction of L. edodes inhibited the activity of HMG Co-A reductase the most (44.26%) as compared to the synthetic statin drug pravastatin (87.64%). The anti-oxidative effect of the statin drugs has been regarded as conferring their anti-atherosclerotic effect (Puttananjaiah et al., 2011). L. edodes possesses the top-ranking position among 26 different edible and medicinal mushrooms in terms of exerting inhibitory effects towards HMG Co-A reductase activity (Gil-Ramirez et al., 2013a, Gil-Ramírez et al., 2013b). However, different parts of the mushroom body differ from one another in terms of potency, with the L. edodes cap possessing the best HMG Co-A reductase inhibitory activity.
3.6. Identification of the anti-atherosclerotic bio-functional components by GC–MS
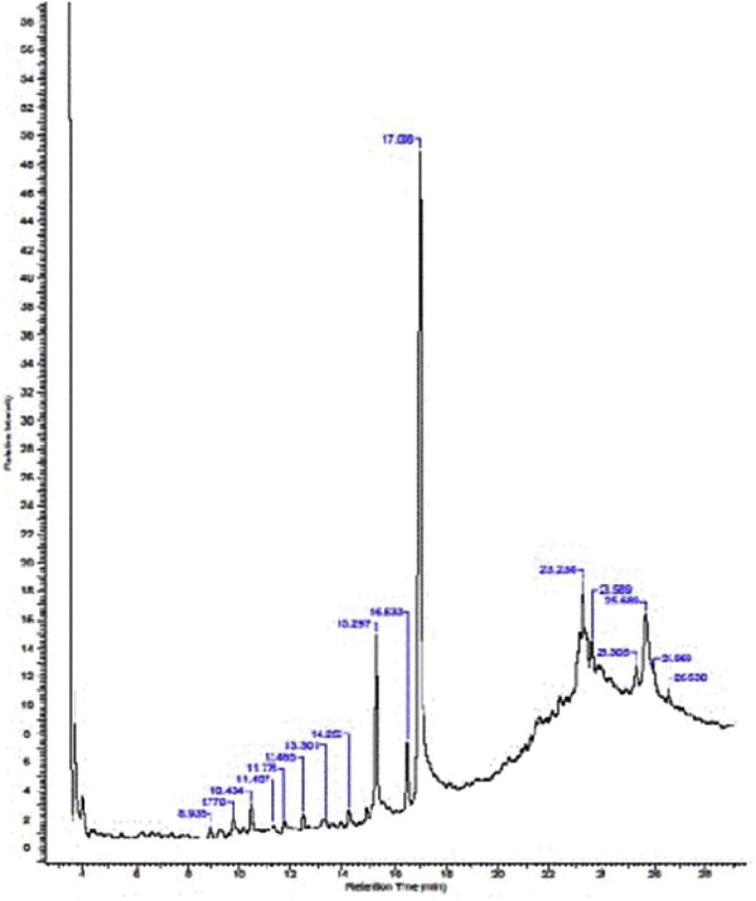
The bio-functional components in the hexane fraction of L. edodes that exhibited anti-atherosclerotic effects were identified through GC–MS analyses. The chromatograph presented in Fig. 7 demonstrates the peaks of the identified bio-components. Among them, the most abundant were α-tocopherol (vitamin E), oleic acid, linoleic acid, ergosterol and butyric acid (Table 1).
Figure 7.
GC–MS chromatograph of the hexane fraction of L. edodes.
Table 1.
Identification of the bio-active components of the hexane fraction of L. edodes by GC–MS.
| No | Bio-component | Structure | Molecular formula | Molecular weight (g) |
|---|---|---|---|---|
| 1 | α-Tocopherol | 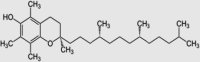 |
C29H50O2 | 430.71 |
| 2 | Oleic acid |  |
C18H34O2 | 282.46 |
| 3 | Linoleic acid |  |
C18H32O2 | 280.45 |
| 4 | Ergosterol | 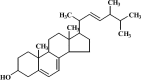 |
C28H44O | 396.65 |
| 5 | Butyric acid | 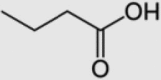 |
C4H8O2 | 88.11 |
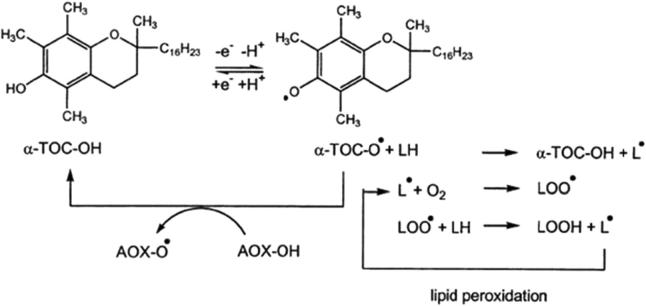
GC–MS analysis identified the presence of the chain breaking anti-oxidant α-tocopherol (vitamin E) in the hexane fraction. The inhibitory effect of L. edodes towards LDL oxidation observed in the present study might be attributed to the α-tocopherol content of this mushroom. Vitamin E scavenges free radicals by following hydrogen atom transfer (HAT) mechanism. It reacts with a free radical such as a lipid peroxyl molecule and interrupts further peroxidation by producing a hydroperoxide and a radical form of α-tocopherol. Both of these intermediates are less harmful as they are almost unreactive. The radical form of α-tocopherol can either react with another peroxyl molecule to turn into a functionally unreactive product or revert back to its original functional form of vitamin E (Fig. 8).
Figure 8.
Basic mechanisms demonstrating the lipid peroxidation inhibitory effect of α-tocopherol.
Despite some exceptions, studies involving animal models and clinical trials as well as human epidemiological data suggest the protective role of α-tocopherol against atherosclerosis (O’Byrne et al., 2002, Munteanu et al., 2004). Vitamin E was able to reduce atherosclerotic lesions up to 60% in experimentally induced atherosclerosis-prone mice (Peluzio et al., 2001, Schwenke et al., 2002). Its anti-atherosclerotic role in hypercholesterolemic rabbits has also been documented (Schwenke et al., 2002). At physiological concentrations (10–30 μM), α-tocopherol could lengthen the lag time of CD formation from 59.6 to 98.9 min (Baldi et al., 2003).
Ergosterol, another bio-component identified in the hexane fraction of L. edodes, has been suggested to act as a membrane antioxidant upon ingestion. Ohnuma et al. (2000) isolated and elucidated the structural features of ergosterol from L. edodes. The inhibitory effect of ergosterol peroxide isolated from the mushroom Armillariella mellea on lipid peroxidation was greater than those of α-tocopherol and thiourea (Wiseman et al., 1993). Supplementation of ergosterol and oleic acid in medium containing Saccharomyces cerevisiae has been reported to lower the production of reactive oxygen species, oxidative stress and the resultant damage at the cellular and biomolecular levels (Ohnuma et al., 2000). Ergosterol peroxide and ergosterol have been reported to suppress LPS-induced inflammatory responses through inhibiting NF-kB and C/EBPb transcriptional activity, and phosphorylation of MAPKs (Kim et al., 1999). Mushrooms’ antioxidant effects relating to DPPH free radical scavenging and Folin–Ciocalteu assay (reducing capacity test) has been attributed towards its content of different bio-components (Landolfo et al., 2010). However, anti-oxidative performance varies depending on mushroom species and also based on the extracting solvent utilised. In case of polar solvent-based mushroom bioseparation, phenolic and polyphenolic group of reducing substances have been regarded for providing with DPPH free-radical scavenging and Folin–Ciocalteu assay performance while triterpenoids have been linked with metal chelating activities (Landolfo et al., 2010, Kobori et al., 2007). For non-polar solvent such as hexane, ergosterol has been identified as the principal anti-oxidative mushroom bio-component (Kalogeropoulos et al., 2013). Thus, 5,6-dihydroxy ergosterol and structurally relevant components such as 7,22-ergostadienol, ergosta 7,22-dien-3-ol present in the hexane fraction might have been involved in chelating transition metal ion, Fe+2 and thus mediating reduced oxidative stress and consequent lowered LDL oxidation. In case of Grifola frondosa, the anti-oxidative pattern of ergosterol has been documented to be second only to fatty acids (Nieto and Chegwin, 2008).
Fatty acids are important functional bio-components of both edible and medicinal fungi. In the hexane fraction of L. edodes we identified the presence of oleic acid, linoleic acid and butyric acid. Oleic acid is a monounsaturated fatty acid that has been reported to provide anti-atherosclerotic effects in different diets, especially the Mediterranean diet (Shao et al., 2010). Its anti-atherosclerotic mode of action involves dampening of endothelial activity by reducing the expression of vascular cell adhesion molecule – 1 (VCAM-1) and the activity of NF–Kb (Zhang et al., 2002). As a result, the adherence of monocytes to endothelial walls is reduced and the associated pro-inflammatory and pro-atherogenic processes slowed down. Butyric acid, another short chain fatty acid found in the L. edodes fraction, has also been reported to confer an anti-atherosclerotic effect through modulating VCAM-1 and NF-κB activity (Massaro et al., 1999).
Linoleic acid (LA, cis-9, cis-12 octadecadienoic acid) has been among the most abundant fatty acids found in some other species of edible mushrooms (Kalogeropoulos et al., 2013). Linoleic acid and its constitutional and stereoisomers termed “conjugated linoleic acid (CLA)” have become an attractive subject of anti-atherosclerotic studies. Research involving the effects of linoleic acid upon animal models has demonstrated its considerable inhibitory effect on the establishment and progression of atherosclerosis. Dietary supplementation of conjugated linoleic acid (CLA) could lower blood levels of LDL and triacylaglyceride as well as slow down the progression of atherosclerotic lesion formation (Carluccio et al., 1999). The proposed mechanism involved CLA-induced enhanced expression of the nuclear transcription factor peroxisome proliferator activated receptor γ (PPARγ), especially in vascular smooth muscle cells. Although some in vitro studies indicate a pro-oxidative and pro-atherogenic role of linoleic acid, compelling in vivo evidence has yet to be established. In human subjects, linoleic acid rich diets have been credited with reducing the risk of coronary heart diseases (Zapolska-Downar et al., 2004).
Inhibitory effect towards the activity of HMG Co-A reductase might also be attributed to ergosterol present in the hexane fraction of L. edodes. Our proposition is backed by the findings of both in vitro, ex vivo and in vitro studies (Rudel, 1999, Kritchevsky et al., 2000, Gil-Ramirez et al., 2013a, Gil-Ramírez et al., 2013b). Ergosterol peroxide from the edible mushroom Sarcodon aspratus, had been found to down-regulate the expression of low-density lipoprotein receptor (LDLR) and HMG-CoA reductase (HMGCR) genes in RAW264.7 cells (Kim et al., 1999). Supplementation of ergosterol and oleic acid in the medium containing Saccharomyces cerevisiae had been reported to lower the production of reactive oxygen species, oxidative stress and the resultant damage towards cellular and biomolecular levels (Ohnuma et al., 2000). The HMG Co-A reductase inhibitory effect is mediated by the statins. Contrary to the established notion of the statins for hypocholesterolemic performance, the present study reports a different mechanistic approach of the mushroom bio-components. As the present study had not searched for the presence of statins in the L. edodes fractions, this remains as an important scope of our future studies.
4. Conclusion
L. edodes (shiitake mushroom) has been found possessing bio-active food components capable of conferring anti-oxidative defence and curtailing LDL oxidation as well as being potent in inhibiting the activity of the rate limiting enzyme in cholesterol biosynthesis, thus supporting its use as an anti-atherosclerotic agent. Food bio-components including α-tocopherol (vitamin E), oleic acid, linoleic acid, ergosterol and butyric acid present in L. edodes enabled it to act as an anti-atherosclerotic agent. The current findings can be further explored in in vivo models to substantiate the therapeutic use of L. edodes with a view to aiding atherosclerosis-affected people around the world.
Author contribution
Noorlidah Abdullah and Norhaniza Aminudin planned and supervised the research, edited the manuscript. Mohammad Azizur Rahman conducted the research, statistical analyses and prepared the manuscript.
Acknowledgements
The authors gratefully thank the University of Malaya, Malaysia for the UMRG Grant (RP014D-13AFR), IPPP Grant (PG109-2013B) and Mohammad Azizur Rahman is grateful for the fellowship supported by the Bright Sparks Unit, University of Malaya.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullah N., Ismail S.M., Aminudin N., Shuib A.S., Lau B.F. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. eCAM. 2012 doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi, S., Maurich, T., Lubrano, V., Turchi, G., 2003. Antioxidant properties of the chalcone plicatin B. In: Proceedings of the Eighth International Conference on Mechanisms of Anti-Mutagenesis and Anti-Carcinogenesis, Pisa, Italy.
- Berger A., Rein D., Kratky E., Monnard I., Hajjaj H., Meirim I., Piguet-Welsch C., Hauser J., Mace K., Niederberger P. Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs. Lipids Health Dis. 2004;3 doi: 10.1186/1476-511X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen P.S., Baghel R.K., Sanodiya B.S., Thakur G.S., Prasad G.B.K.S. Lentinus edodes: a macrofungus with pharmacological activities. Curr. Med. Chem. 2010;17:2419–2430. doi: 10.2174/092986710791698495. [DOI] [PubMed] [Google Scholar]
- Carluccio M.A., Massaro M., Bonfrate C., Siculella L., Maffia M., Nicolardi G., Caterina R.D. Oleic acid inhibits endothelial activation – a direct vascular antiatherogenic mechanism of a nutritional component in the Mediterranean diet. Arterioscler. Thromb. Vasc. Biol. 1999;19:220–228. doi: 10.1161/01.atv.19.2.220. [DOI] [PubMed] [Google Scholar]
- Cherubini A., Vigna G.B., Zuliani G., Ruggiero G.C., Senin U., Felin R.R. Role of antioxidants in atherosclerosis: epidemiological and clinical update. Curr. Pharm. Des. 2005;11:2017–2032. doi: 10.2174/1381612054065783. [DOI] [PubMed] [Google Scholar]
- Cheung P. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010;35:292–299. [Google Scholar]
- Cheung L., Cheung P.C. Mushroom extracts with antioxidant activity against lipid peroxidation. Food Chem. 2005;89:403–409. [Google Scholar]
- EFSA panel on dietetic products, nutrition and allergies, 2010. Scientific opinion on the substantiation of a health-claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (EC) no. 1924/2006. EFSA J. 8, 1885.
- Gholamhoseinian A., Sharifi-Far F., Shahouzehi B. Inhibitory activity of some plant methanol extracts on 3-Hydroxy-3-methylglutaryl coenzyme a reductase. Int. J. Pharmacol. 2010 [Google Scholar]
- Gil-Ramirez A., Clavijo C., Palanisamy M., Ruiz-Rodriguez A., Navarro-Rubio M., Perez M. Study on the 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitory properties of Agaricus bisporus and extraction of bioactive fractions using pressurised solvent technologies. J. Sci. Food Agric. 2013;93:2789–2796. doi: 10.1002/jsfa.6102. [DOI] [PubMed] [Google Scholar]
- Gil-Ramírez A., Clavijo C., Palanisamy M., Ruiz-Rodríguez A., Navarro-Rubio M., Pérez M., Soler-Rivas C. Screening of edible mushrooms and extraction by pressurized water (PWE) of 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitors. J. Funct. Foods. 2013;5:244–250. doi: 10.1002/jsfa.6102. [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Arai H., Barter P., Bersot T.P., Betteridge D.J., Carmena R., Genest J. An international atherosclerosis society position paper: global recommendations for the management of dyslipidemia. J. Clin. Lipidol. 2013;7:561–565. doi: 10.1016/j.jacl.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Hobbs C. Medicinal value of Lentinus edodes (Berk.) Sing. (Agaricomycetideae) – a literature review. Intl. J. Med. Mushr. 2000;2(90) [Google Scholar]
- Kalač P. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113:9–16. [Google Scholar]
- Kalogeropoulos N., Yanni A.E., Koutrotsios G., Aloupi M.M. Bioactive micro constituents and antioxidant properties of wild edible mushrooms from the island of Lesvos, Greece. Food Chem. Toxicol. 2013;55:378–385. doi: 10.1016/j.fct.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Karanth S., Tran V.M., Kuberan B., Schlegel A. Polyunsaturated fatty acyl-coenzyme As are inhibitors of cholesterol biosynthesis in zebrafish and mice. Dis. Mod. Mech. 2013;6:1365–1377. doi: 10.1242/dmm.013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Park S.S., Min T.J., Yu K.H. Antioxidant activity of ergosterol peroxide (5,8-epidioxy-5α, 8α-ergosta-6, 22E-dien-3β-ol) in Armillariella mellea. Bull. Kor. Chem. Soc. 1999;20:819–823. [Google Scholar]
- Kobori M., Yoshida M., Ohnishi-Kameyama M., Shnmoto H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007;150:209–219. doi: 10.1038/sj.bjp.0706972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S.A., Wright S., Tso P., Czarnecki S.K. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J. Am. Col. Nutr. 2000;19:472S–477S. doi: 10.1080/07315724.2000.10718950. [DOI] [PubMed] [Google Scholar]
- Landolfo S., Zara G., Zara S., Budroni M., Ciani M., Mannazzu M.I. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2010;141:229–235. doi: 10.1016/j.ijfoodmicro.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Li D., Mehta J.L. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc. Res. 2005;68:353–354. doi: 10.1016/j.cardiores.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Martin S.S., Bluementhal R.S., Miller M. LDL cholesterol: the lower the better. Med. Clin. N Am. 2012;96:13–26. doi: 10.1016/j.mcna.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Massaro M., Carluccio M., Caterina R.D. Vol. 44. Cardiologia; Rome, Italy: 1999. Direct vascular anti-atherogenic effects of oleic acid: a clue to the cardio-protective effects of the Mediterranean diet. pp. 507–513. [PubMed] [Google Scholar]
- Mattila P., Lampi A.M., Ronkainen R., Toivo J., Piironen V. Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem. 2002;76:293–298. [Google Scholar]
- Munteanu A., Zingg J.M., Azzi A. Anti-atherosclerotic effects of vitamin E – myth or reality? J. Cell. Mol. Med. 2004;8:59–76. doi: 10.1111/j.1582-4934.2004.tb00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto I.J., Chegwin A.C. Triterpenoids and fatty acids identified in the edible mushroom Pleurotus sajor-caju. J. Chil. Chem. Soc. 2008;53:515–1517. [Google Scholar]
- O’Byrne D.J., Devaraj S., Grundy S.M., Jialal I. Comparison of the antioxidant effects of concord grape juice flavonoids α-tocopherol on markers of oxidative stress in healthy adults. Am. J. Clin. Nutr. 2002;76:1367–1374. doi: 10.1093/ajcn/76.6.1367. [DOI] [PubMed] [Google Scholar]
- Ohnuma N., Amemiya K., Kakuda R., Yaoita Y., Machida K., Kikuchi M. Sterol constituents from two edible mushrooms, Lentinula edodes and Tricholoma matsutake. Chem. Pharm. Bull. 2000;48:749–751. doi: 10.1248/cpb.48.749. [DOI] [PubMed] [Google Scholar]
- Orekhov A.N., Sobenin I.A., Melnichenko A.A., Myasoedova V.A., Bobryshev Y. Use of natural products for direct anti-atherosclerotic therapy. In: Rezzani R., editor. Current Trends in Atherogenesis. INTECH; 2013. pp. 187–217. [Google Scholar]
- Peluzio M.C.G., Homem A.P.P.P., Cesar G.C., Azevedo G.S., Amorim R., Cara D.C., Alvarez-Leite J. Influences of alpha-tocopherol on cholesterol metabolism and fatty streak development in apolipoprotein E-deficient mice fed an atherogenic diet. Braz. J. Med. Biol. Res. 2001;34:1539–1545. doi: 10.1590/s0100-879x2001001200005. [DOI] [PubMed] [Google Scholar]
- Puttananjaiah M.K.H., Dhale M.A., Ganokar V., Keni S. Statins: 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 2011;163:215–222. doi: 10.1007/s12010-010-9031-z. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Abdullah N., Aminudin N. Inhibitory effect on in vitro LDL Oxidation and HMG Co-A reductase activity of the liquid-liquid partitioned fractions of Hericium erinaceus (Bull.) Persoon (lion’s mane mushroom) BioMed Res. 2014 doi: 10.1155/2014/828149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L.L. Atherosclerosis and conjugated linoleic acid. Br. J. Nutr. 1999;81:177–179. [PubMed] [Google Scholar]
- Schwenke D.C., Rudel L.L., Sorci-Thomas M.G., Thomas M.J. Α-tocopherol protects against diet induced atherosclerosis in New Zealand white rabbits. J. Lipid Res. 2002;43:1927–1938. doi: 10.1194/jlr.m200261-jlr200. [DOI] [PubMed] [Google Scholar]
- Shao S., Hernandez M., Kramer J.K., Rinker D.L., Tsao R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010;58:11616–11625. doi: 10.1021/jf102285b. [DOI] [PubMed] [Google Scholar]
- Sirtori C.R., Galli C., Anderson J.W., Sirtori E., Arnoldi A. Functional foods for dyslipidemia and cardiovascular risk prevention. Nutr. Res. Rev. 2009;22:244–261. doi: 10.1017/S0954422409990187. [DOI] [PubMed] [Google Scholar]
- Wasser S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011;89:1323–1333. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- Wiseman H., Cannon M., Arnstein H.R., Halliwell B. Enhancement by tamoxifen of the membrane antioxidant action of the yeast membrane sterol ergosterol: relevance to the anti yeast and anticancer action of tamoxifen. BBA-Mol. Basis Dis. 1993;1181:201–206. doi: 10.1016/0925-4439(93)90021-r. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kisugi R. Mechanisms of LDL oxidation. Clin. Chim. Acta. 2010;411:1875–1882. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Zapolska-Downar D., Siennicka A., Kaczmarczyk M., Kołodziej B., Naruszewicz M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: the role of NF-κB and PPARα. J. Nutr. Biochem. 2004;15:220–228. doi: 10.1016/j.jnutbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mills G.L., Nair M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002;50:7581–7585. doi: 10.1021/jf0257648. [DOI] [PubMed] [Google Scholar]