Summary
The present study investigated the therapeutic potential of omega‐6 fatty acids, according to their effects on antioxidant markers and matrix metalloproteinases (MMPs), in coronary heart disease‐induced rats. Rats were grouped into group I (sham control), group II (control), group III (0.5 g/kg bwt of omega‐6 fatty acids) and group IV (1 g/kg bwt of omega‐6 fatty acids). Reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GSH), catalase, glutathione peroxidase (Gpx) and acetylcholinesterase (AChE) enzyme activities were determined. ROS and MDA were substantially reduced, whereas SOD, catalase, Gpx and AChE were significantly increased, following supplementation with omega‐6 fatty acids. MMP‐2 mRNA expression was drastically increased by 95% in group II. Treatment significantly reduced MMP‐2 mRNA expression by 12.3% and 26.7% in groups III and IV respectively. MMP‐9 mRNA expression drastically increased, by 121%, in group II. Treatment significantly reduced MMP‐9 mRNA expression by 22.6% and 29.4% in groups III and IV respectively. MMP‐2 protein expression was drastically increased, by 81%, in group II. Treatment significantly reduced MMP‐2 protein expression by 9.4% and 26% in groups III and IV respectively. MMP‐9 protein expression was drastically increased, by 100%, in group II. Treatment significantly reduced MMP‐9 protein expression by 18.9% and 26.9% in groups III and IV respectively. In summary, the consumption of omega‐6 fatty acids significantly decreased MDA and ROS, while SOD, catalase, GHS, Gpx and AChE were increased. Furthermore, omega‐6 fatty acids significantly downregulated MMP‐2 and MMP‐9 expression in our coronary heart disease‐induced rat model.
Keywords: antioxidant, enzymes, matrix metalloproteinases, omega‐6 fatty acids, rats
1. INTRODUCTION
Reactive oxygen species (ROS) are products of oxidative stress.1 Several researchers have reported that dietary supplementation with fish oil containing omega‐6 fatty acids exerts a protective effect against coronary heart disease and cancer.2, 3 Popović et al4 reported that supplementation with fish oil containing omega‐6 fatty acids exerts a beneficial effect on total cholesterol, uric acid and triglycerides. Verschuren et al5 reported that a diet containing anti‐inflammatory agents is effective against atherosclerosis in mice. Essential fatty acids not synthesized in humans and animals are due to a lack of the required enzymes.6
Matrix metalloproteinases (MMPs), which are well‐known proteinases in animals and humans, are zinc‐containing enzymes that participate in extracellular matrix degradation. Several researchers have reported that MMPs play a crucial role in heart physiology, function and pathology. In addition, several researchers have reported the functional impact of essential fatty acids and fish oil on membrane fluidity, neural membrane oxidation and lipid peroxidation in neural tissues.7, 8 Research indicates a negative role for MMPs in vascular smooth muscle cell migration, cardiovascular remodelling and the formation of atherosclerotic plaque, which can lead to the development of coronary heart disease and heart failure.9 Furthermore elevated MMP‐2 and MMP‐9 levels may be associated with atherosclerotic plaques. Hence, investigation of MMPs in a coronary heart disease model would be helpful to understand the pathophysiology of heart disease and have already led to attempts to modulate MMP function in coronary heart disease for therapeutic interventions.
Omega‐6 fatty acids are essential and polyunsaturated fatty acids that provide several biological and therapeutic effects.10 A preliminary investigation was carried out at different concentrations of an omega‐6 fatty acid over the range 0.1‐1 g/kg bwt. An optimum and significant impact was observed from 0.5 to 1 g/kg bwt of omega‐6 fatty acid. Therefore, we selected 0.5‐1 g/kg bwt for this study. Thus, our study evaluated the protective effects of omega‐6 fatty acids on anti‐oxidants and MMPs in Sprague Dawley rats.
2. MATERIALS AND METHODS
2.1. Experimental rats
Sprague Dawley male rats were purchased from the animal house of Kunming General Hospital of Chengdu Military Area, Kunming Medical University, Kunming, Yunnan, China. Rats weighing 190‐200 g were used in the experiment, and rats were assigned to one of four homogeneous groups. A 12‐hours light and dark cycle was maintained throughout all experiments. Six rats were included in each homogenous group. All rats were provided with water and food ad libitum. All animals were kept under adaptive feeding for 7 days before experiments.
2.2. Ethical approval
This study was approved by the ethics committee of Kunming Medical University (Approval number: TXY12341).
2.3. Induction of coronary heart disease
Coronary heart disease was induced according to a previously described method, by knockout of the low‐density lipoprotein‐receptor gene and the hypercholesterolaemic apolipoprotein E gene.11 Rats were also supplemented with an atherosclerosis‐inducing diet (10% Butter, 2% cholesterol, 0.5% bile salt, 6% peanut oil and 81.5% base material). The base material composed of 48% corn, 20% wheat middlings, 15% soya bean cake, 12% rice bran and 5% fish meal. General clinical manifestations and serological indexes were detected. The results showed rats were quite susceptible to the atherosclerosis‐inducing diet. Induction of coronary heart disease was confirmed by determination of total cholesterol (261 ± 21.2 mg/dL), LDL cholesterol (185.5 ± 7.3 mg/dL), HDL cholesterol (45 ± 2.6 mg/dL), triglycerides (175.4 ± 10.7 mg/dL) and fibrinogen (471.7 ± 25.8 mg/dL).
2.4. Experimental groups and treatment
After establishing coronary heart disease, rats were grouped into group I (sham control), group II (control), group III (0.5 g/kg bwt of omega‐6 fatty acids) and group IV (1 g/kg bwt of omega‐6 fatty acids). Each group contained six rats. Control rats were given the same volume of normal saline. Omega‐6 fatty acids (P1547, Sigma‐Aldrich Shanghai China) were dissolved in normal saline. Omega‐6 fatty acids were adjusted to 0.5 and 1 g in 1 mL and given for 15 consecutive days via the oral route. All laboratory experiments were monitored and approved by the ethical committee of Kunming Medical University.
2.5. Preparation of heart tissue homogenate
At the end of 15 days, the rats were sacrificed by decapitation following anaesthesia. Blood was collected, and the heart was surgically removed and excised. Tissues were washed in normal saline and homogenized in Tris‐HCl buffer (0.1 mol/L, pH 7.4) and centrifuged at 11 200 g for 10 minutes. The supernatant was collected and centrifuged again at 100 000 × g for 60 minutes, and the resulting cytosolic fractions were used to determine biochemical markers.
2.6. Determination of antioxidant and oxidative markers
Reactive oxygen species in the heart tissue homogenate were determined using the dichlorofluorescein assay.12 Lipid peroxidation in the heart tissue homogenate was determined in the heart tissue homogenate according to malondialdehyde (MDA) content by measuring thiobarbituric acid reactive species. The final product was measured at 534 nm.13 Glutathione peroxidase (Gpx), superoxide dismutase (SOD), catalase and acetylcholinesterase (AChE) enzyme activities in the heart tissue homogenate were determined using spectrophotometry.13 Reduced glutathione (GSH) content in the heart tissue homogenate was determined by measuring the final product at 405 nm.14
2.7. RT‐PCR
Total RNA from rat heart tissue homogenate was extracted and converted into cDNA using oligo (dt) primers. Quantitative polymerase chain reaction (qPCR) was used to quantify MMP‐2 (forward: 5′‐GGCCCTGTCACTCCTGAGAT‐3′, reverse: 5′‐GGCATCCAGGTTATC GGGGA‐3′) and MMP‐9 (forward: 5′‐CGGAGCACGGAGACGGGTAT‐3′, reverse: 5′‐TGAAGGGGAAGACGCACAGC‐3′) mRNA expression. GAPDH (forward: 5′‐GGTCACCAGGGCTGCTTTT‐3′, reverse: 5′‐ATCTCGCTCCTGGAAGATGGT‐3′) was used as an internal control for the quantification of caspase‐3 expression. mRNA expression was calculated according to a previously described method.15
2.8. Western blot analysis
Protein levels in the heart tissue homogenate were estimated by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis. Proteins were then transferred to polyvinylidene membranes and incubated with a primary antibody against MMP‐2 (ab37150; Abcam, Cambridge, UK) or MMP‐9 (ab38898; Abcam) for 12 hours. Membranes were incubated with horseradish peroxidase (HRP)‐IgG (goat anti‐rabbit, A0545‐1ML; Sigma‐Aldrich, St. Louis, MO, USA) for 1 hour. MMP‐2 and MMP‐9 were determined by enhanced chemiluminescence.16 The bands were quantified by densitometry using Li‐Cor Odyssey software.
2.9. Immunohistochemistry
A microtome was used to cut rat heart tissue sections, which were stored at 4°C for further use. Hydrogen peroxide was used to abolish endogenous peroxidase activity, and sections were incubated with bovine serum albumin for 30 minutes to abolish non‐specific binding. Next, sections were treated with primary antibodies against MMP‐2 (ab37150; Abcam) or MMP‐9 (ab38898; Abcam) for 12 hours. Sections were treated with FITC Polyclonal Antibody, HRP (AB_930865, ThermoFischer Scientific) following repeated washes.17 Sections were then washed with phosphate‐buffered saline and mounted using the aqueous mounting medium. Images were obtained under a fluorescent microscope. The integrated pixel intensities were measured for the traced areas using image analysis software (Image‐Pro, Media Cybernetics, Silver Spring, MD). The intensity was normalized for potential alteration in nuclear size by dividing the integrated pixel intensity by the nuclear area. Relative signal intensities provided MMP‐2 and MMP‐9 protein concentrations.
2.10. Statistical analysis
Values are given as mean with standard error of the mean (SEM). Differences between the control and taurine groups were evaluated using the unpaired Student's t test. One‐way ANOVA (SPSS 17, IBM China/Hong Kong Limited, Hong Kong) was applied for statistical analysis of data, and post hoc Tukey's test was used for multiple comparisons. P‐value <0.05 was considered statistically significant.
3. RESULTS
Our study investigated the therapeutic potential of omega‐6 fatty acids, according to their effects on antioxidant markers and MMPs, in a coronary heart disease‐induced rat model. ROS were measured and are expressed in relative fluorescence units: the intracellular ROS level was 37.66 RFU in group I, which increased by 461.65% in group II. Supplementation with omega‐6 fatty acids significantly reduced the intracellular ROS levels by 30.63% and 67.46% in groups III and IV respectively (P < 0.05; Figure 1). MDA content, measured as the end product of lipid peroxidation, was 20.27 nmol/g in normal control rats (group I) and drastically increased by 193.8% in group II. Compared to the untreated control (group II), omega‐6 fatty acid treatment significantly reduced MDA content 24.14% and 52.23% in groups III and IV respectively (Figure 2, P < 0.05).
Figure 1.
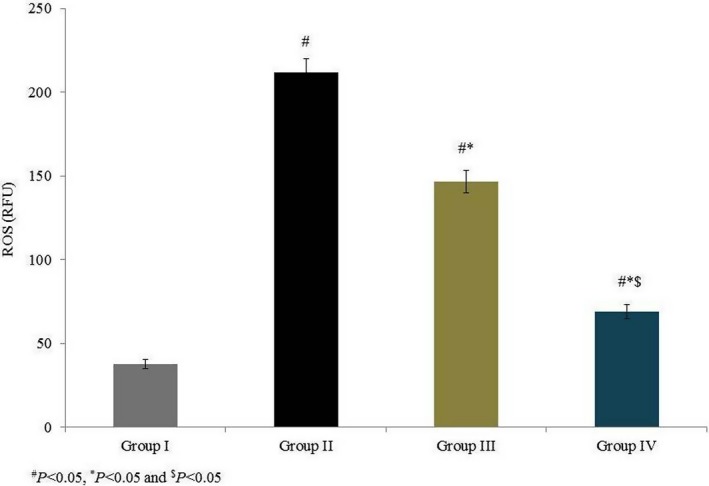
Effects of omega‐6 fatty acids on intracellular reactive oxygen species (ROS) in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
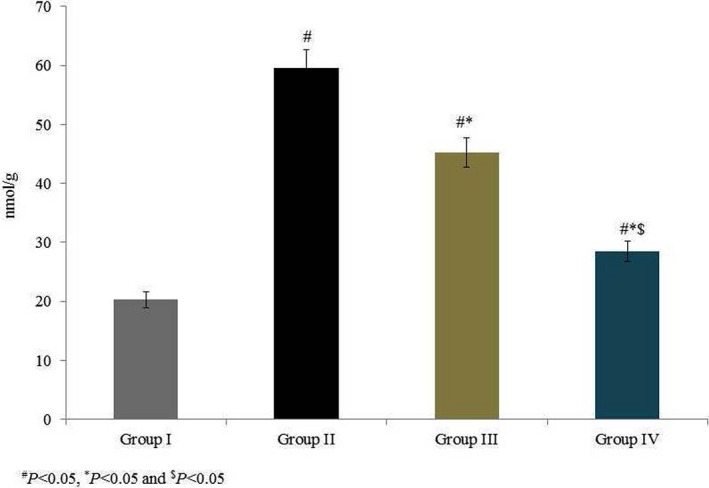
Effects of omega‐6 fatty acids on lipid peroxidation in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Superoxide dismutase activity was 6.7 U/mg in group I and was substantially reduced, by 41.8%, in group II. Rats supplemented with omega‐6 fatty acids showed a significant increase in SOD activity (by 23% and 48.2% in groups III and IV, respectively, P < 0.05; Figure 3). Catalase activity was 11.5 U/g in group I and was substantially reduced, by 53%, in group II. Rats supplemented with omega‐6 fatty acids showed a significant increase in catalase activity (by 35.2% and 87% in groups III and IV, respectively, P < 0.05; Figure 3). GPx activity was 0.93 (mg/protein) in group I and was reduced by 55.9% in group II. GPx activity was increased by 43.9% and 104.5% in groups III and IV respectively (P < 0.05; Figure 4). AChE activity was 9.1 (μmol/min/mg of protein) in group I and was reduced by 52.7% in group II. Rats supplemented with omega‐6 fatty acids showed a significant increase in AChE activity (by 41.9% and 95.3% in groups III and IV, respectively, P < 0.05; Figure 4). GSH content was 79.67 mg/g in group I and was drastically reduced, by 56.7%, in group II. Treatment significantly increased the GSH content by 49.5% and 89.6% in groups III and IV respectively (P < 0.05 Figure 5).
Figure 3.
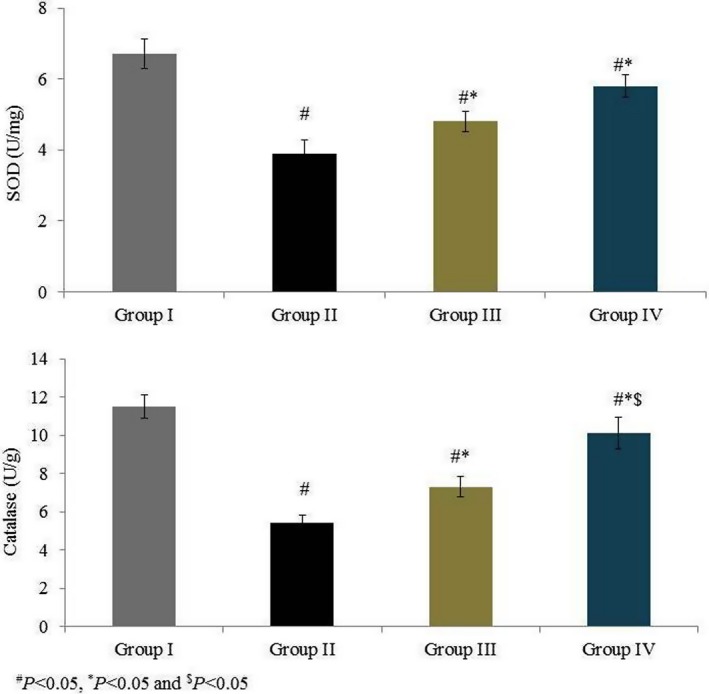
Effects of omega‐6 fatty acids on superoxide dismutase (SOD) and catalase activities in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
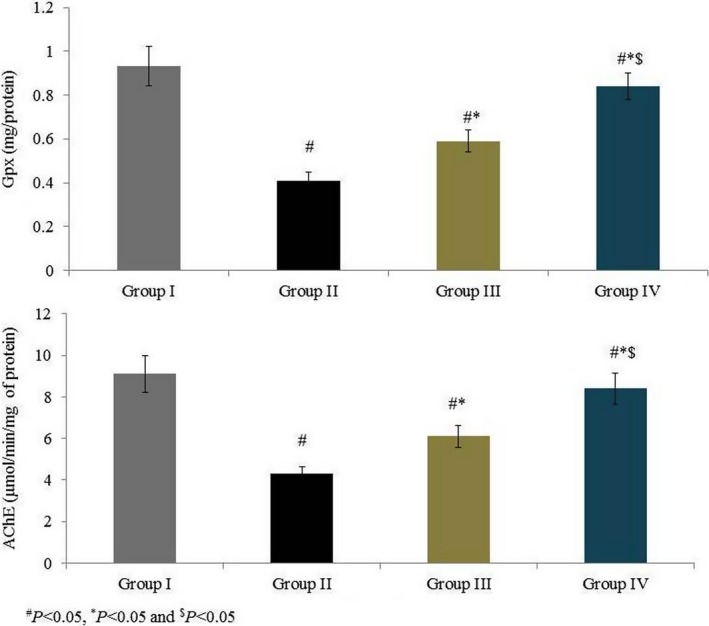
Effects of omega‐6 fatty acids on glutathione peroxidase (Gpx) and acetylcholinesterase (AChE) activities in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 5.
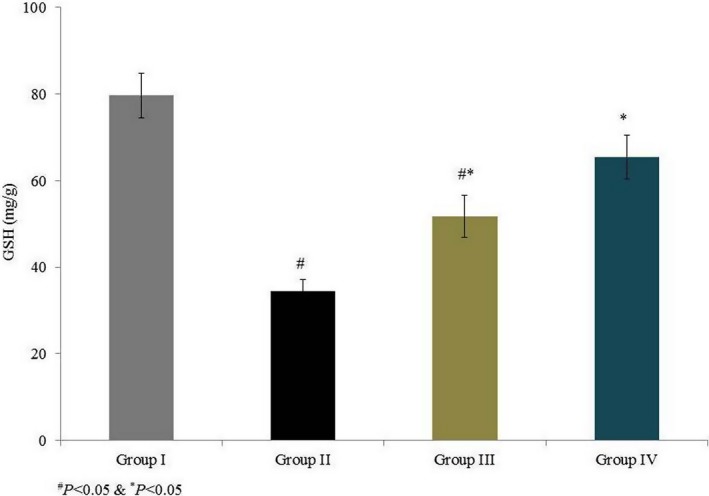
Effects of omega‐6 fatty acids on reduced glutathione (GSH) content in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
MMP‐2 mRNA expression was drastically increased, by 95%, in group II. Treatment significantly reduced MMP‐2 mRNA expression by 12.3% and 26.7% in groups III and IV respectively (P < 0.05 Figure 6). MMP‐9 mRNA expression drastically increased, by 121%, in group II. Treatment significantly reduced MMP‐9 mRNA expression by 22.6% and 29.4% in groups III and IV respectively (P < 0.05; Figure 6). MMP‐2 protein expression was drastically increased, by 81%, in group II. Treatment significantly reduced MMP‐2 protein expression by 9.4% and 26% in groups III and IV respectively (P < 0.05; Figure 7). MMP‐9 protein expression was drastically increased, by 100%, in group II. Treatment significantly reduced MMP‐9 protein expression by 18.9% and 26.9% in groups III and IV respectively (P < 0.05; Figure 7). Immunohistochemical analysis of MMP‐2 and MMP‐9 showed similar results to Western blot analysis (Figures 8 and 9).
Figure 6.
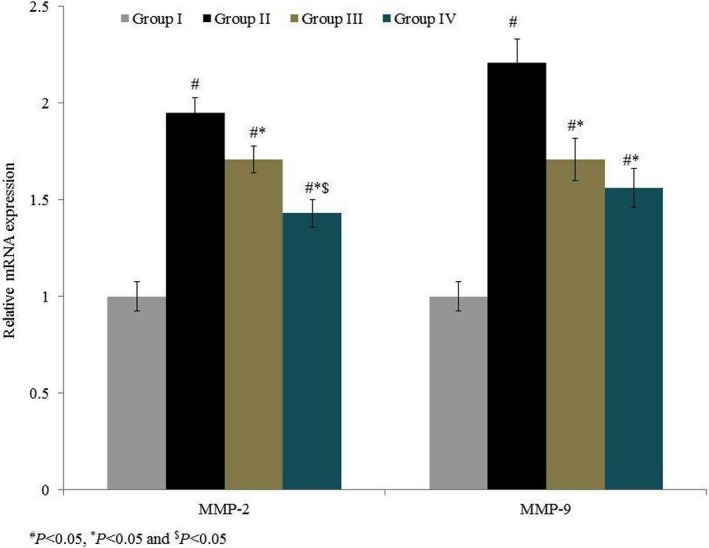
Effects of omega‐6 fatty acids on the mRNA expression of matrix metalloproteinase (MMP)‐2 and MMP‐9 in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 7.
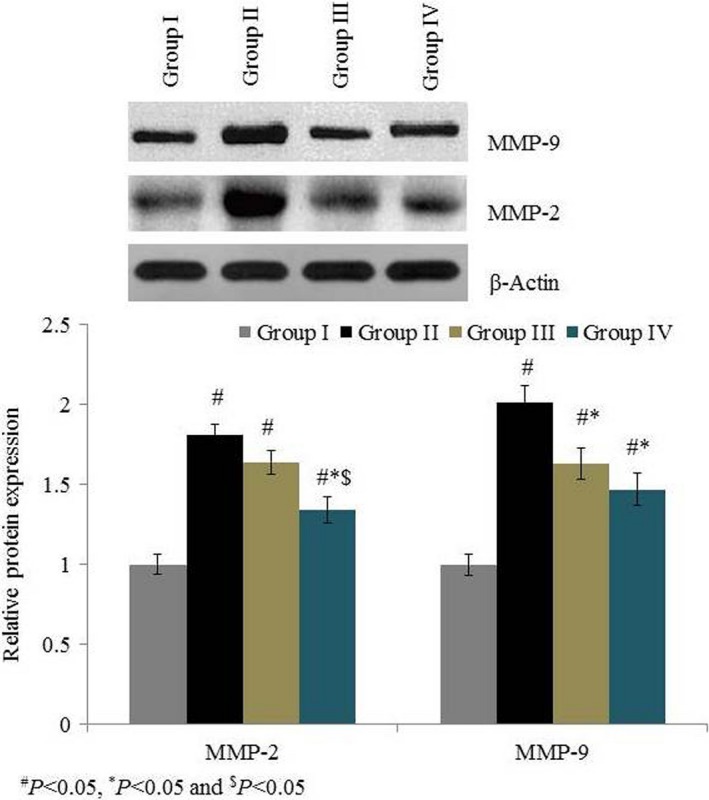
Effects of omega‐6 fatty acids on the protein expression of MMP‐2 and MMP‐9 in the heart tissue homogenate of coronary heart disease‐induced rat model. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 8.
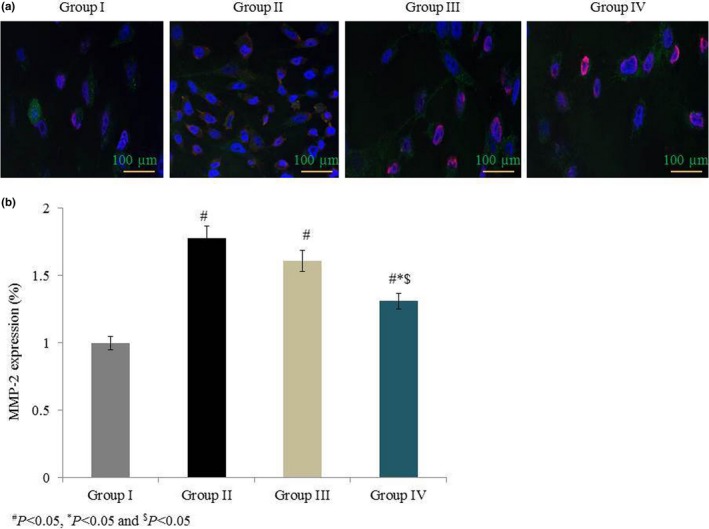
Effects of omega‐6 fatty acids on MMP‐2 protein expression in the heart tissue of coronary heart disease‐induced rat model. In A, immunohistochemistry labelling shows the MMP‐2 expression in green colour. MMP‐2 expression (green), DAPI staining (blue) and AO staining (purple). In B, quantitative analysis shows the MMP‐2 protein expression. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 9.
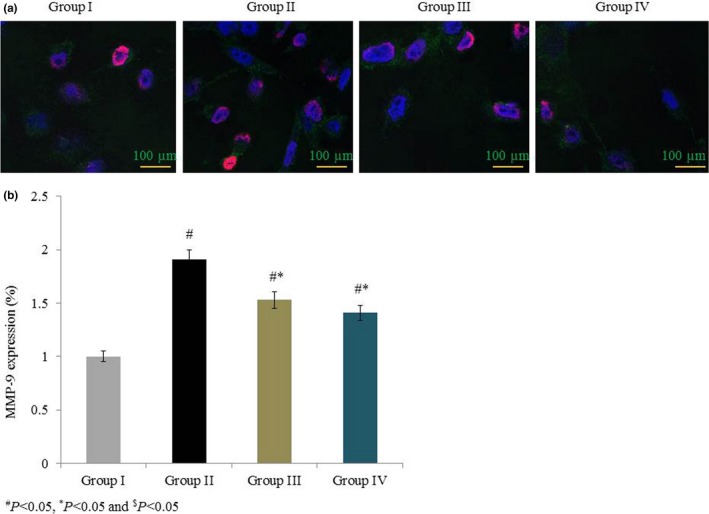
Effects of omega‐6 fatty acids on MMP‐9 protein expression in the heart tissue of coronary heart disease‐induced rat model. In A, immunohistochemistry labelling shows the MMP‐9 expression in green colour. MMP‐9 expression (green), DAPI staining (blue) and AO staining (purple). In B, quantitative analysis shows the MMP‐9 protein expression. # P < 0.05 vs group I, *P < 0.05 vs group II and $ P < 0.05 vs group III. n = 6. Values are shown as means ± standard error of the mean (SEM) [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Our study investigated the therapeutic potential of omega‐6 fatty acids, according to their effects on antioxidant markers and MMPs, in a coronary heart disease‐induced rat model. Several researchers have reported beneficial effects of omega‐6 fatty acids. Merendino et al18 have reported the consumption of polyunsaturated fatty acids and fish oil associated with anti‐proliferative, anti‐inflammatory and anti‐apoptotic effects. The balanced ratio of omega‐3 and omega‐6 fatty acids was important in male rat reproduction system.19 Compare to omega‐3 fatty acids rich diet, the diet rich in omega‐6 fatty acids, docosahexaenoic and eicosapentaenoic fatty acids improved the circulating lipid profile.20
Salvati et al8 reported the functional impact of essential fatty acids on membrane fluidity, inflammatory eicosanoids and neural membrane oxidation. Bas et al7 reported the beneficial effects of fish oil on lipid peroxidation in neural tissues. Infarction size was reduced in ischaemia/reperfusion‐induced rats following supplementation with polyunsaturated fatty acids.21 Other researchers have reported that polyunsaturated fatty acids are incorporated into neuron membranes following uptake by the brain.22 Oxidative burden and neurological deficits are reduced in ischaemia/reperfusion‐induced rats following essential fatty acid supplementation.23
In this study, we noted increased levels of SOD, catalase, GSH, Gpx and AChE, whereas MDA and ROS were reduced, following supplementation with omega‐6 fatty acids. Supplementation with fish oil significantly reduced MDA content in ischaemia/reperfusion‐induced rats.24 Atherosclerotic plaque can be identified by elevated MMP‐2 and MMP‐9 in the blood (2014). Several researchers have attempted to modulate MMP function in coronary heart disease for therapeutic interventions.
Omega‐6 fatty acids are essential polyunsaturated fatty acids that provide several biological and therapeutic effects. Several researchers have reported that MMPs are reduced following supplementation with omega‐3 fatty acids.25, 26 Renier et al27 reported that dietary factors play an important role in the prevention and treatment of coronary heart diseases. Protective effects of polyunsaturated fatty acids against coronary heart diseases have been reported. Research has indicated a negative role for MMPs in vascular smooth muscle cell migration, cardiovascular remodelling and the formation of atherosclerotic plaque, which can lead to the development of coronary heart disease and heart failure (2014).
5. CONCLUSION
In summary, we conclude that consumption of omega‐6 fatty acids significantly decreases MDA and ROS levels, while those of SOD, catalase, GHS, Gpx and AChE increase. Furthermore, omega‐6 fatty acids significantly downregulated MMP‐2 and MMP‐9 in our coronary heart disease‐induced rat model.
6. CONFLICT OF INTEREST
The authors declare to have no conflict of interest.
7. FUNDING SOURCE
This study was supported by Project of Yunnan Science and Technology Department (No: 2017FB147).
Lu N, Du Y, Li H, et al. Omega‐6 fatty acids down‐regulate matrix metalloproteinase expression in a coronary heart disease‐induced rat model. Int J Exp Path. 2018;99:210–217. 10.1111/iep.12293
Funding information
This study was supported by Project of Yunnan Science and Technology Department (2017FB147)
REFERENCES
- 1. Patel SP, Katyare SS. A comparative study of reactive oxygen species (ROS) related parameters in rat tissues. Indian J Clin Biochem. 2006;21(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hammamieh R, Chakraborty N, Miller SA, et al. Differential effects of omega‐3 and omega‐6 Fatty acids on gene expression in breast cancer cells. Breast Cancer Res Treat. 2007;101(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 3. Khandelwal S, Kelly L, Malik R, Prabhakaran D, Reddy S. Impact of omega‐6 fatty acids on cardiovascular outcomes: a review. J Prev Cardiol. 2013;2(3):325‐336. [PMC free article] [PubMed] [Google Scholar]
- 4. Popović T, Borozan S, Arsić A, et al. Fish oil supplementation improved liver phospholipids fatty acid composition and parameters of oxidative stress in male Wistar rats. J Anim Physiol Anim Nutr (Berl). 2011;96(6):1020‐1029. [DOI] [PubMed] [Google Scholar]
- 5. Verschuren L, Wielinga PY, van Duyvenvoorde W, et al. A dietary mixture containing fish oil, resveratrol, lycopene, catechins, and vitamins E and C reduces atherosclerosis in transgenic mice. J Nutr. 2011;141:863‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr. 2005;72(3):239‐242. [PubMed] [Google Scholar]
- 7. Bas O, Songur A, Sahin O, et al. The protective effect of fish n‐3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int. 2007;50:548‐554. [DOI] [PubMed] [Google Scholar]
- 8. Salvati S, Attorri L, Di Benedetto R, Di Biase A, Leonardi F. Polyunsaturated fatty acids and neurological diseases. Mini Rev Med Chem. 2006;6:1201‐1211. [DOI] [PubMed] [Google Scholar]
- 9. Mittal B, Mishra A, Srivastava A, Kumar S, Garg N. Matrix metalloproteinases in coronary artery disease. Adv Clin Chem. 2014;64:1‐72. [DOI] [PubMed] [Google Scholar]
- 10. Simopoulos AP. An Increase in the omega‐6/omega‐3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin G, Mingming G, Yunan W, et al. LDL receptor gene‐ablated hamsters: a rodent model of familial hypercholesterolemia with dominant inheritance and diet‐induced coronary atherosclerosis. EBioMedicine. 2018;27:214‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arutyunyan TV, Korystova AF, Kublik LN, Levitman MKh, Shaposhnikova VV, Korystov YN. Taxifolin and fucoidin abolish the irradiation‐induced increase in the production of reactive oxygen species in rat aorta. Bull Exp Biol Med. 2016;160:635‐638. [DOI] [PubMed] [Google Scholar]
- 13. Kaddour T, Omar K, Oussama AT, Nouria H, Iméne B, Abdelkader A. Aluminium‐induced acute neurotoxicity in rats: treatment with aqueous extract of Arthrophytum (Hammada scoparia). J Acute Dis. 2016;5(6):470‐482. [Google Scholar]
- 14. Erden Inal M, Akgün A, Kahraman A. The effects of exogenous glutathione on reduced glutathione level, glutathione peroxidase and glutathione reductase activities of rats with different ages and gender after whole‐body Γ‐irradiation. J Am Aging Assoc. 2003;26:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zurmanová J, Půta F, Stopková R, Soukup T. Real‐time RT‐PCR with a newly designed set of primers confirmed the presence of 2b and 2x/d myosin heavy chain mRNAs in the rat slow soleus muscle. Physiol Res. 2008;57(6):973‐978. [DOI] [PubMed] [Google Scholar]
- 16. Dmitriev AD, Factor MI, Segal OL, et al. Western blot analysis of human and rat serotonin transporter in platelets and brain using site‐specific antibodies: evidence that transporter undergoes endoproteolytic cleavage. Clin Chim Acta. 2005;356(1–2):76‐94. [DOI] [PubMed] [Google Scholar]
- 17. Evilsizor MN, Ray‐Jones HF, Lifshitz J, Ziebell J. Primer for immunohistochemistry on cryosectioned rat brain tissue: example staining for microglia and neurons. J Vis Exp. 2015;99:52293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merendino N, Costantini L, Manzi L, Molinari R, D'Eliseo D, Velotti F. Dietary ω ‐3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan L, Bai XL, Fang ZF, Che LQ, Xu SY, Wu D. Effect of different dietary omega‐3/omega‐6 fatty acid ratios on reproduction in male rats. Lipids Health Dis. 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaíva MH, Couto RC, Oyama LM, et al. Diets rich in polyunsaturated fatty acids: effect on hepatic metabolism in rats. Nutrition. 2013;19(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 21. Choikwon S. Temporal changes in cerebral antioxidant enzyme activities after ischemia and reperfusion in a rat focal brain ischemia model: effect of dietary fish oil. Brain Res Dev Brain Res. 2004;152:11‐18. [DOI] [PubMed] [Google Scholar]
- 22. Songur A, Sarsilmaz M, Sogut S, et al. Hypothalamic superoxide dismutase, xanthine oxidase, nitric oxide, and malondialdehyde in rats fed with fish omega‐3 fatty acids. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:693‐698. [DOI] [PubMed] [Google Scholar]
- 23. Yang D, Pan H, Yen Y, et al. Detrimental effects of post‐treatment with fatty acids on brain injury in ischemic rats. NeuroToxicology. 2007;28:1220‐1229. [DOI] [PubMed] [Google Scholar]
- 24. Ozen OA, Cosar M, Sahin O, et al. The protective effect of fish n‐3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurol Sci. 2008;29:147‐152. [DOI] [PubMed] [Google Scholar]
- 25. Harris MA, Hansen RA, Vidsudhiphan P, et al. Effects of conjugated linoleic acids and docosahexaenoic acid on rat liver and reproductive tissue fatty acids, prostaglandins and matrix metalloproteinase production. Prostaglandins Leukot Essent Fatty Acids. 2001;65(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 26. McCabe AJ, Wallace J, Gilmore WS, Strain JJ, McGlynn H. The effect of eicosapentanoic acid on matrix metalloproteinase gene expression. Lipids. 1999;34(6):S217‐S218. [DOI] [PubMed] [Google Scholar]
- 27. Renier G, Skamene E, DeSanctis J, Radzioch D. Dietary n‐3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler Thromb. 1993;13:1515‐1524. [DOI] [PubMed] [Google Scholar]


