Abstract
Primary pigmented nodular adrenal disease (PPNAD) accounts for <1% of ACTH-independent Cushing syndrome. We describe the case of twin female patients with PPNAD who both had sustainable disease control after unilateral adrenalectomy, which corroborates current evidence in favor of unilateral adrenalectomy for a subset of patients with PPNAD. Patient A presented with a 10-kg weight gain over the past year and facial plethora. Diagnostic evaluation revealed abolition of normal cortisol rhythm with suppressed ACTH levels, normal adrenal CT and MRI imaging and a slightly left-predominant adrenal uptake on 131I iodomethyl norcholesterol scintigraphy coupled with single-photon emission CT/CT. PPNAD was confirmed after genetic testing revealed a known pathogenic PRKA1A mutation (c.709 (-7-2) del6). At that time, her twin sister (patient B) was asymptomatic. Patient A underwent successful unilateral adrenalectomy and histology confirmed PPNAD. Two years after initial onset of symptoms in patient A, patient B was seen for the same subtle symptoms of progressive weight gain. Diagnostic test results were identical, revealing the same clinical features and mutational status as patient A. Patient B also underwent unilateral adrenalectomy with a favorable outcome. Follow-up 3 years after surgery for patient A and 18 months for patient B showed sustained disease control without recurrence and uncompromised quality of life, with no adrenal insufficiency having occurred. Unilateral adrenalectomy can be a successful therapeutic approach for patients with PPNAD with a mild phenotype without the risk and the inconvenience of subsequent adrenal insufficiency, which alters quality of life.
Keywords: twins, Cushing syndrome, PPNAD, unilateral adrenalectomy
Primary pigmented nodular adrenal disease (PPNAD) is a rare disease accounting for <1% of ACTH-independent Cushing syndrome (CS). PPNAD is often diagnosed in late childhood or early adulthood. Typical histological findings include multiple cortical pigmented nodules <1 cm with normal or even reduced total adrenal volume that explains the lack of major alteration of the adrenal gland morphology on imaging. PPNAD can be either isolated or associated with Carney complex [1]. Germline defects of regulatory subunit 1A of the protein kinase A (PRKAR1A) gene are detected in >60% of patients with PPNAD and/or Carney complex [2].
Unilateral adrenalectomy is very rarely performed for PPNAD [3–7]. We describe the case of monozygotic twins with CS due to PPNAD with longstanding symptoms remission of hypercortisolism after unilateral adrenalectomy. We also provide an updated literature review of PPNAD treatment options.
1. Clinical Case
We report the case of 28-year-old twin female patients [patients A and B; Fig. 1(a), 1(f), and 1(h)]. The study was approved by the Erasme Hôpital, Université Libre de Bruxelles, ethics committee and informed consent was obtained from both patients.
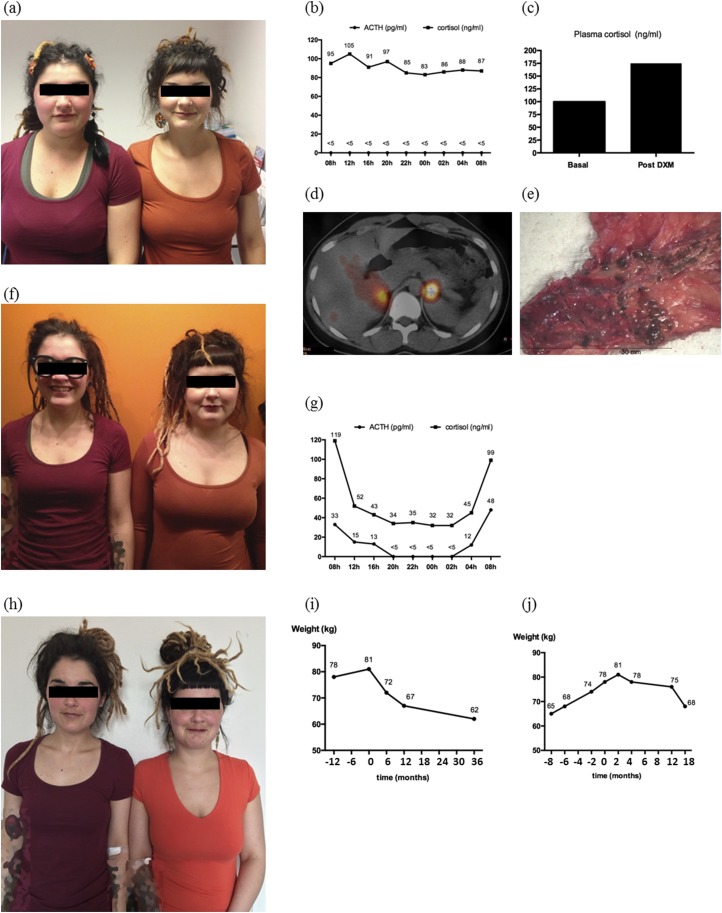
Figure 1.
Metamorphosis of twin sisters over time with corresponding biological and imaging findings. (a, f, h) Photographs of patient A (left) and patient B (right) over time. Pictures were taken at ∼18-month intervals: (a) at initial evaluation of patient A; (f) when patient B started to complain about weight gain; (h) ∼36 months after adrenalectomy for patient A and 18 months for patient B. (b) Abolition of normal circadian cortisol rhythm with a constant ACTH suppression in patient A at presentation [at time of photograph in (a)]. (c) Results of a suppression test during which 8 mg of DXM was given overnight to patient A at the time of the photograph in (a): Plasma cortisol increased paradoxically from 101 ng/mL to 178 ng/mL. (d) A fusion image from 131I iodomethyl norcholesterol scintigraphy coupled with SPECT/CT scanner in patient A at the time of the photograph in (a). Scintigraphy was performed after ACTH suppression by DXM and revealed a bilateral uptake with a slight left predominance. (e) The left adrenal gland of patient A. Numerous pigmented, 1- to 4-mm nodules are visible. (g) Graph shows the restoration of circadian cortisol rhythm after left-side adrenalectomy in patient A. (i, j) Evolution of both patients’ body weight over time. Time 0 corresponds to time of adrenalectomy. DXM, dexamethasone.
Patient A was referred to our outpatient clinic at the age of 25 years for a 10-kg weight gain over the past year, irregular menstruations, and acne. She had no medical history, no smoking habits, and took no medication. Physical examination revealed 78-kg body weight (body mass index, 23.7 kg/m2), normal blood pressure, and facial plethora and erythema.
Urinary free cortisol (UFC) measurement showed fluctuations but was mildly elevated (134 to 203 µg/24 hours; normal range is 10 to 110 µg/24 hours) on three subsequent occasions. Circadian cortisol rhythm was disrupted with constant ACTH suppression [Fig. 1(b)]. An 8-mg overnight dexamethasone suppression test showed a paradoxical response with plasma cortisol level rising [Fig. 1(c)]. Adrenal CT scan and MRI were both normal. 131I iodomethyl norcholesterol scintigraphy coupled with single-photon emission computed tomography (SPECT)/CT showed bilateral adrenal uptake with a slight left predominance [Fig. 1(d)].
PPNAD was suspected but no other Carney complex–related features were diagnosed except lentiginosis and a blue nevus (in particular, no cardiac myxomas). Genetic testing revealed a germline mutation (c. 709-7_709-2 del TTTTTA) of the PRKAR1A gene.
At that time, her twin sister (patient B) had no evidence of clinical CS and refused, as did the parents, to be tested for the mutation or biologically for subclinical CS. Patient A underwent a left laparoscopic adrenalectomy given the slightly higher left adrenal uptake. Histology confirmed the presence of numerous cortical adrenal pigmented nodules of 1 to 4 mm in the adrenal gland [Fig. 1(e)]. Four months later, clinical evolution was excellent, UFC level was normalized, and her body weight decreased. One year after surgery, patient A had normal UFC level (41 µg/24 hours), regained her initial normal body weight (67 kg), and her cortisol and ACTH circadian rhythm were almost restored [Fig. 1(g)].
At that time, with a 2-year delay, patient B presented with similar subtle symptoms of body weight gain (body mass index, 22.7 kg/m2) but no other feature of CS. She also had an unremarkable medical history and took no medication except oral contraception. UFC level was mildly elevated (151 µg/24 hours) and cortisol circadian rhythm abolished with consistently suppressed plasma ACTH levels. Diagnostic evaluation showed lentiginosis, normal adrenal CT scan, and bilateral 131I iodomethyl norcholesterol adrenal uptake. Genetic analysis revealed the same mutation of the PRKAR1A gene. Six months after symptom onset, patient B underwent left-side laparoscopic adrenalectomy with a successful outcome and rapid hospital discharge after 2 days. The left side was chosen because the left-side gland seemed slightly larger than right one on CT scan. Histologic examination also confirmed PPNAD.
Patient B had normal UFC level (65 µg/24 hours)18 months after surgery, and had regained her initial normal 68-kg body weight [Fig. 1(j)]. At that time, 3 years after her surgery, patient A still had normal body weight (62 kg) and normal UFC level (51 µg/24 hours), showing that our rather conservative unilateral adrenalectomy approach had a favorable clinical outcome with longstanding remission of symptoms and uncompromised quality of life without adrenal insufficiency or hydrocortisone supplementation [Fig. 1(i)].
2. Discussion
To our knowledge, this is one of the extremely rare cases of twins with PPNAD reported in the literature. As a general rule, bilateral adrenalectomy is the recommended surgical approach for PPNAD treatment with regard to the genetic origin of the disease that involves both adrenal glands [1, 3–7]. As suggested by Vezzosi et al. [8], imaging technologies including SPECT/CT and adrenal scintigraphy could be helpful in confirming the bilateral nature of the disease, suggesting the need for bilateral adrenalectomy. However, if unilateral adrenalectomy can at least temporarily relieve hypercortisolism symptoms without the inconvenience of adrenal insufficiency, a definite outcome after bilateral adrenal resection, it can be an attractive option for patients with mild symptoms. A conservative unilateral adrenalectomy has been proposed for patients with PPNAD with asymmetrical adrenal uptake in scintigraphy [8].
If a unilateral adrenalectomy is considered, two important elements should be taken into account: the chances of disease remission and which adrenal gland will be chosen to be removed. To date, only 24 patients have been reported in the literature, to our knowledge, who underwent unilateral or partial bilateral adrenalectomy for PPNAD; their follow-up ranged from 9 months to several years, as summarized in Table 1 [3–7]. Complete clinical data on the severity of initial PPNAD symptoms and degree of hypercortisolism are difficult to ascertain from the literature. In the largest of these series, by Xu et al. [4], the adrenal gland chosen to be removed was the largest in volume, as determined by preoperative CT and MRI scans of three patients; in the remaining nine patients with symmetrical adrenal glands, left- and right-side adrenalectomies were performed in an equal manner. Only one patient had recurrence of hypercortisolism. The other patients had complete remission of symptoms and had no need of hydrocortisone supplementation [4].
Table 1.
Reported Cases of PPNAD Treated With Unilateral or Partial Bilateral Adrenalectomy
| Study | No. of Patients | Mean Follow-Up (Range), Mo | Recurrence, No. |
|---|---|---|---|
| Powell et al. [3] | 3 | 80 (12–324) | 3 (two with better management of symptoms and no subsequent adrenalectomy; one contralateral adrenalectomy) |
| Xu et al. [4] | 13 | 47 (16–113) | 1 (contralateral adrenalectomy after 2 mo) |
| Guanà et al. [5] | 1 | 9 | No |
| Cohen et al. [6] | 1 | 9 | No |
| Lowe et al. [7] | 6 | 120 (12–792) | 3 (three contralateral adrenalectomies after 3, 10, and 25 y) |
Such data are reminiscent of the results of unilateral surgery in primary bilateral macronodular adrenal hyperplasia (PBMAH). PBMAH is a disease with a different genetic background than PPNAD but with a similar pathophysiological principle: Both adrenal glands are affected and contribute to the disease phenotype. Thus, PBMAH could provide grounds for clinical reflection of management of patients with PPNAD. To date, small series of patients (Table 2) [4, 9–13] and isolated cases have been reported to have undergone unilateral adrenalectomy for PBMAH with a long-term follow-up showing sustainable relief of clinical symptoms after intervention. These studies corroborate that unilateral adrenalectomy could be a solution in bilateral adrenal diseases to obtain either long-term remission or a milder and more controllable disease phenotype without the inconvenience of adrenal insufficiency. UFC values could theoretically help us select candidate patients for unilateral adrenalectomy. In the study by Debillon et al. [9], the median UFC level of patients was divided by a factor of 8 (from 2.19 times the upper limit of normal (ULN) at diagnosis to 0.27 times the ULN 1 week postoperatively). Therefore, if an upper limit of UFC values is to be used for patient selection, it is certainly greater than two times the ULN. In the cases we report here, the UFC level at 1 year was divided by 4.9 times for patient A and 2.9 for patient B.
Table 2.
Reported Cases of PBMAH Treated With Unilateral Adrenalectomy
| Study | No. of Patients | Mean Follow-Up (Range), Mo | Recurrence, No. |
|---|---|---|---|
| Debillon et al. [9] | 17 | 60 (39–105) | 2 (one treated with mitotane, one contralateral adrenalectomy 9 y later) |
| Albiger et al. [10] | 12 | 106 (80–135) | 8 (after 12–180 mo), one with persistent disease |
| Li et al. [11] | 15 | 36 | 3 (contralateral adrenalectomy) |
| Xu et al. [4] | 14 | 46.8 (12–108) | 1 (contralateral adrenalectomy) |
| Ito et al. [12] | 4 | 24–146 | 1 (subclinical CS, no contralateral adrenalectomy) |
| Lamas et al. [13] | 4 | 78.8 (30–137) | No |
The causal mutation is probably another important point to take into account to decide if unilateral or larger adrenal resection is indicated in patients with PPNAD. Both adrenal glands are affected in PPNAD, but unlike in PBMAH disease, they are mostly of normal size or minimally hyperplastic. In the large series of the Mayo Clinic, by Lowe et al. [7], six different PRKAR1A mutations were found in 13 tested patients, including four in the six patients who benefited from conservative adrenal surgery. Interestingly, the three patients who needed a second contralateral adrenalectomy had different mutations and a different delay between first and second adrenalectomy (3, 10 and 25 years). In the current cases, the causal mutation was different from those described by Lowe et al. [7]. More data are necessary to determine if genetics can predict the risk and the time of recurrence after conservative adrenal surgery. In the current report, the postoperative circadian cortisol rhythm was only partly restored, because plasma cortisol was too high for the midnight nadir.
In conclusion, this report of twin patients who did benefit from a safe and conservative surgical procedure can be added to the existing literature on clinical experience showing that a case-by-case personalized approach with close follow-up can result in a favorable clinical outcome after unilateral adrenalectomy for PPNAD without the risk and the inconvenience of subsequent adrenal insufficiency, which alters quality of life.
Acknowledgments
We thank our patients, their families, and their caring physicians and all the colleagues, clinicians, and geneticists at Cochin Hospital, Paris, for their valuable collaboration on this case.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CS
Cushing syndrome
- PBMAH
primary bilateral macronodular adrenal hyperplasia
- PPNAD
primary pigmented nodular adrenal disease
- SPECT
single-photon emission computed tomography
- UFC
urinary free cortisol
- ULN
upper limit of normal
References and Notes
- 1. Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86(9):4041–4046. [DOI] [PubMed] [Google Scholar]
- 2. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94(6):2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powell AC, Stratakis CA, Patronas NJ, Steinberg SM, Batista D, Alexander HR, Pingpank JF, Keil M, Bartlett DL, Libutti SK. Operative management of Cushing syndrome secondary to micronodular adrenal hyperplasia. Surgery. 2008;143(6):750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Y, Rui W, Qi Y, Zhang C, Zhao J, Wang X, Wu Y, Zhu Q, Shen Z, Ning G, Zhu Y. The role of unilateral adrenalectomy in corticotropin-independent bilateral adrenocortical hyperplasias. World J Surg. 2013;37(7):1626–1632. [DOI] [PubMed] [Google Scholar]
- 5. Guanà R, Gesmundo R, Morino M, Matarazzo P, Pucci A, Pasini B, Lala R, Fiore L, Repici M, Canavese F. Laparoscopic unilateral adrenalectomy in children for isolated primary pigmented nodular adrenocortical disease (PPNAD): case report and literature review. Eur J Pediatr Surg. 2010;20(4):273–275. [DOI] [PubMed] [Google Scholar]
- 6. Cohen O, Bogat S, Dolitzki M, Karasik A. Successful pregnancy after unilateral adrenalectomy in a case of primary pigmented adrenocortical disease. J Matern Fetal Neonatal Med. 2005;17(2):161–163. [DOI] [PubMed] [Google Scholar]
- 7. Lowe KM, Young WF Jr, Lyssikatos C, Stratakis CA, Carney JA. Cushing syndrome in Carney complex: clinical, pathologic, and molecular genetic findings in the 17 affected Mayo Clinic patients. Am J Surg Pathol. 2017;41(2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vezzosi D, Tenenbaum F, Cazabat L, Tissier F, Bienvenu M, Carrasco CA, Laloi-Michelin M, Barrande G, Lefebvre H, Hiéronimus S, Tabarin A, Bertagna X, Legmann P, Vantyghem MC, Bertherat J. Hormonal, radiological, NP-59 scintigraphy, and pathological correlations in patients with Cushing’s syndrome due to primary pigmented nodular adrenocortical disease (PPNAD). J Clin Endocrinol Metab. 2015;100(11):4332–4338. [DOI] [PubMed] [Google Scholar]
- 9. Debillon E, Velayoudom-Cephise FL, Salenave S, Caron P, Chaffanjon P, Wagner T, Massoutier M, Lambert B, Benoit M, Young J, Tabarin A, Chabre O. Unilateral adrenalectomy as a first-line treatment of Cushing’s syndrome in patients with primary bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(12):4417–4424. [DOI] [PubMed] [Google Scholar]
- 10. Albiger NM, Ceccato F, Zilio M, Barbot M, Occhi G, Rizzati S, Fassina A, Mantero F, Boscaro M, Iacobone M, Scaroni C. An analysis of different therapeutic options in patients with Cushing’s syndrome due to bilateral macronodular adrenal hyperplasia: a single-centre experience. Clin Endocrinol (Oxf). 2015;82(6):808–815. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Yang CH. Diagnosis and treatment of adrenocorticotrophic hormone-independent macronodular adrenocortical hyperplasia: a report of 23 cases in a single center. Exp Ther Med. 2015;9(2):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ito T, Kurita Y, Shinbo H, Otsuka A, Furuse H, Mugiya S, Ushiyama T, Ozono S, Oki Y, Suzuki K. Successful treatment for adrenocorticotropic hormone-independent macronodular adrenal hyperplasia with laparoscopic adrenalectomy: a case series. J Med Case Reports. 2012;6:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamas C, Alfaro JJ, Lucas T, Lecumberri B, Barceló B, Estrada J. Is unilateral adrenalectomy an alternative treatment for ACTH-independent macronodular adrenal hyperplasia? Long-term follow-up of four cases. Eur J Endocrinol. 2002;146(2):237–240. [DOI] [PubMed] [Google Scholar]