Abstract
Desert truffles are seasonal and important edible fungi that grow wild in many countries around the world. Truffles are natural food sources that have significant compositions. In this work, the antioxidant, chemical composition, anticancer, and antiangiogenesis properties of the Terfezia claveryi truffle were investigated. Solvent extractions of the T. claveryi were evaluated for antioxidant activities using (DPPH, FRAP and ABTS methods). The extracts cytotoxicity on the cancer cell lines (HT29, MCF-7, PC3 and U-87 MG) was determined by MTT assay, while the anti-angiogenic efficacy was tested using ex-vivo assay. All extracts showed moderate anticancer activities against all cancer cells (p < 0.05). The hexane extract inhibited the brain cell line (U-87 MG) with an IC50 of 50 μg/ml and significantly promoted cell apoptosis through the mitochondrial pathway and DNA fragmentation p < 0.001. The ethanol extract demonstrated potent antioxidants; DPPH, FRAP, and ABTS with an IC50 value of 52, 48.5 and 64.7 μg/ml, respectively. In addition, the hexane and ethyl acetate extract significantly (p < 0.001) inhibited the sprouting of microvessels by 100% and 81.2%, at 100 μg/ml, respectively. The GC analysis of the most active extract (hexane) showed the presence of several potent phytochemicals such as stigmasterol, beta-Sitosterol, squalene, lupeol, octadecadienoic acid, and oleic acid.
Keywords: Black truffle, Antioxidant, Cancer, Angiogenesis, T. claveryi
1. Introduction
The first reference of traditional medicinal plant use, dates back to 4000 years ago, where it was depicted on a Sumerian clay table that records remedies for various diseases (Kong et al., 2003). Desert truffles have been documented as medicinal food in Chinese, Greek and Egyptian civilization, and were called the miracle of nature in Mesopotamia (Wang and Marcone, 2011, Badalyan, 2012, Patel, 2012, Bahrani, 2006). Truffle is one of the oldest forms of food, it has been used as a meat substitute and consumed in large quantities due to their highly delicious taste and musky aroma (Mandeel and Al-laith, 2007, Al-Shabibi et al., 1982, Dundar et al., 2012). It has a unique nutritional profile of unsaturated fatty acid, vitamins, minerals, and protein (Patel, 2012) and has been used for eye treatment in folk medicine (Janakat and Nassar, 2010). Terfezia claveryi is among the various known edible truffles in the world, including Iraq where it grows naturally in the central, southern and western parts (Gutiérrez et al., 2003, Al-Laith, 2010). The quantity of truffles is usually varied from season to season depending on the amount of rainfall (Al-Shabibi et al., 1982). Several studies have reported the nutritional value of Iraqi truffles. Al-Delaimy (1977) investigated the amino acid compositions of T. claveryi. Al-Shabibi et al. (1982) reported that the black truffle T. claveryi contains 19.60% of saturated fatty acid with a high amount of linoleic acid. Different chemical constituents were reported in Iraqi truffles of the T. claveryi species as (17.6 2%) protein and (62% linoleic acid) (Al-Kaisey et al., 1996). Another study conducted by Bokhary and Parvez (1993) on the Saudi T. claveryi reported the presence of (16%) protein, (28%) carbohydrate and (78%) total moisture. Noteworthy, the same species of truffles from different regions may not exhibit the same chemical composition; the diversity of the chemical profile is probably controlled by many environmental factors, such as amount of rain and time, soil types and climatic changes (Hussain and Al-Ruqaie, 1999).
Despite its nutritional importance, the biological activities and phytochemicals of T. claveryi did not receive much attention. In addition, no reports on cytotoxicity, apoptosis, or antiangiogenic properties of the truffle T. claveryi were found. The phytochemical classes have a great role in the prevention and treatment of several diseases such as cancer, aging, and inflammation (Dahham et al., 2015). Therefore, the aim of this study was to investigate the antioxidant, anticancer and antiangiogenic properties of the T. claveryi truffle. In addition, the apoptosis mechanism and the main chemical constituents of the most active extracts using (GC–MS) will be identified.
2. Experimental section
2.1. Chemicals and reagents
RPMI 1640 medium, Dulbecco’s Modified Eagle Medium and F-12K medium were purchased from ATCC (American Type Culture Collection, from Rockville, MD, USA). All chemicals were purchased from Sigma–Aldrich, Darmstadt, Germany.
2.2. Cell lines and culture conditions
Human brain carcinoma cell line U-87 MG (HTB-14); human colorectal carcinoma cell line HT29 (CCL-247) and human hormone sensitive and invasive breast cancer cell line MCF-7 (HTB-22) were purchased from ScienCell USA. HT29 and U-87 MG cells were maintained in RPMI, whereas, MCF-7 was maintained in DMEM medium.
2.3. Sample extraction
T. claveryi truffles were collected from the western area of Iraq, cleaned, peeled, and sliced. The samples were dried in an oven at (35–40 °C) and ground mechanically. Approximately 100 g of the sample was extracted with 500 mL of solvents (hexane, ethyl acetate, ethanol, methanol and water) at 40 °C using the hot maceration method. The extracts were filtered and concentrated using a rotary evaporator (Buchi, USA). Extracts were stored at −20 °C for further analysis.
2.4. DPPH radical scavenging activity
DPPH assay was carried out to evaluate the scavenging activity of the extracts Bondet et al. (1997). The stock solution of DPPH was prepared at a concentration of 200 μM in absolute methanol while stock solutions of the extracts were prepared at a concentration of 10 mg/mL. DPPH was dispensed into a 96-well plate (100 μL/well) and 100 μL of the samples were immediately added at concentrations of (12.5, 25, 50, 100, 200) μg/mL. Methanol and ascorbic acid were used as a negative and a positive control respectively. The mixtures were incubated at 30 °C for 30 min in the dark and then the absorbance was measured at 517 nm. The obtained dose–response curves were used to calculate the median inhibitory concentration (IC50).
2.5. Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was conducted according to the method of (Ðordević et al., 2010) with minor modifications. Truffle extracts (50 μl) were added at concentrations of (3.25–100 μM) to 1.5 ml of freshly prepared FRAP reagent. After 45 min of incubation, the absorbance was measured at 593 nm using infinite®Pro200 TECAN (Switzerland). A standard curve was constructed using FeSO4 solution.
2.6. ABTS cation radical scavenging assay
ABTS radical scavenging activity was determined according to the method by Re et al. (1999). The ABTS+ solution was prepared and stored in the dark at room temperature for 16 h. Then, 1 mL of the solution was diluted with 40 mL deionized water to yield working ABTS+ solution with an absorbance equal to 0.70 ± 0.02 at 734 nm. To 180 μL ABTS+ working solution, 20 μL test samples at serial concentrations (3.1–200 μg/ml) were added. After 10 min, the absorbance of the plate was read at 734 nm. The scavenging capability of ABTS+ was calculated using the following equation:
where AS is the absorbance of ABTS+ in the presence of sample, AABTS+ is the initial concentration of the ABTS+.
2.7. Cytotoxicity assay
The MTT cytotoxicity assay was performed according to the method Ibrahim et al. (2011). Cells were seeded at 1.5 × 104 cells in each well of 96-well plate in 100 μL in fresh culture medium and were incubated at 37 °C in 5% CO2 overnight. Cells at 70–80% confluence were treated with the truffle extracts at a concentration range of (3.1–200 μg/mL). After a 48 h incubation, the medium was aspirated and MTT solution (5 mg/mL in sterile PBS) was added, and incubated for 4 h at 37 °C in 5% CO2. After incubation, the wells were solubilized with 200 μL DSMO/well and the absorbance was measured by infinite®Pro200 TECAN (Switzerland) at a primary wave length of 570 nm and reference wavelength of 620 nm. Each plate contained the samples, negative control and blank. DMSO (1% v/v) was used as a negative control. Tamoxifen, 5-fluorouracil, and butanilic acid were used as standard reference control for HT 29, MCF-7, PC3 and U-87 MG cell lines, respectively.
2.8. Detection of mitochondrial membrane potential loss
Detection of the changes in mitochondrial membrane potential (ΔΨm) in U-87 MG cells treated with hexane truffle extract was assessed by the retention of Rhodamine 123 fluorescent dye. Normally, the dye interacts with the inner mitochondrial negatively charged membrane and accumulates in normal mitochondria, but the reduction of ΔΨm leads to the dye leakage which will result in reducing the fluorescence intensity. In this study, the cells were separately seeded in 6 well plates and incubated overnight. The cells were treated with the active extract at a concentration of 50 μg, for 6 and 12 h intervals and then fixed using 4% paraformaldehyde for 20 min. DMSO (0.1%) and butanlic acid (10 μg/ml) were used as negative and positive controls, respectively. Rhodamine 123 was added to the cells at a final concentration of 5 μg/mL and incubated for 30 min to stain the mitochondria. The wells then were photographed using the inverted EVOS digital microscope (USA) at 20× magnification power to monitor the fluorescent signals.
2.9. Determination of nuclear condensation
The effect of the truffle active extract on nuclear chromatin condensation for the most sensitive cells (U-87 MG) was quantified using Hoechst 33258 stain according to the method (Ibrahim et al., 2013). The cell line was treated with the truffle extracts (50 μg) and analyzed at 6 and 12 h. DMSO (0.1%) and butanlic acid (50 μg/ml) were used as negative and positive controls, respectively. The cells were fixed in 4% paraformaldehyde for 20 min before staining with Hoechst stain 33342 (1 μg/mL in PBS) for another 20 min. Nuclear morphology was examined under a fluorescent microscope. Cells with brightly colored, condensed, or fragmented nuclei were considered as apoptotic. The number of cells with apoptotic morphology was counted in randomly selected fields per well. The cells were photographed at 20× magnification, using EVOS digital microscope (USA).
2.10. Antiangiogenic effect of T. claveryi extracts
The angiogenic (blood vessel formation) inhibition was investigated using the rat aortic ring assay. This experiment was done according to the guidelines of OECD, and in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for animal experiments. This assay was carried out as previously described by Al-Rawi et al. (2011). Thoracic aortas were removed from euthanized male Sprague–Dawley rats (12–14 week old), rinsed with serum free medium and cleaned from fibro adipose tissues. The aortas were cross sectioned into small rings (1 mm thickness) and seeded individually in a 48-well plate in 300 μl serum free M199 media containing 3 mg/ml fibrinogen and 5 mg/ml aprotinin. 10 μl of thrombin (50 NIH U/ml) was added into each well and incubated at 37 °C for 90 min. A second layer of M199 medium containing the truffle extracts was added at a concentration range of 6.25–200 μg/ml. Suramin and DMSO were used as positive and negative controls, respectively. After five days, rings were photographed using EVOS digital microscope (40× magnification). Subsequently, lengths of the outgrown blood vessels from the rings were measured using Leica Quin software. The inhibition of blood vessel formation was calculated using the formula:
where A0 = distance of blood vessel growth in treated rings in μm, A = distance of blood vessels growth in the control in μm.
The results are presented as mean percent inhibition ± SEM, (n = 8).
2.11. Gas chromatography – mass spectrometry (GC/MS) analysis
Quantitative chemical analysis of the most active extracts was carried out using GC/MS (Agilent Technologies – Hewlett Packard Model, Santa, CA, USA). The assay conditions were as follows: HP-5MS capillary column (30 m × 0.25 mm ID × 0.25 μm, film thickness); held at 70 °C for 2 min, raised to 285 °C at a rate of 20 °C/min and held for 20 min; 285 °C for MSD transfer line heater; carrier gas helium at a flow rate of 1.2 mL/min; 2:1 split ratio. About 1 μL solution of SF-1 in chloroform (10 mg/mL) was injected automatically. Scan parameter low mass: 35 and higher mass: 550. The constituents were identified by comparison with standards using NIST 2002. A total ion chromatogram (TIC) was used to compute the percentage of the identified constitutes.
2.12. Statistical analysis
Results are expressed as mean ± SD. Differences between groups were compared by a one-way analysis of variance (ANOVA) using GraphPad Prism6 and considered significant at p < 0.05.
3. Result and discussion
In this study, the antioxidant, cytotoxic and antiangiogenic properties of T. claveryi solvent extracts were evaluated. However, this is the first study that investigates the DPPH, FRAP, ABTS, cytotoxicity, apoptosis and angiogenesis properties of the black truffle T. claveryi from Iraq. In addition, the main chemical composition of the most active extract was analyzed using GCMS analysis. The extraction yields varied based on the different solvent polarities as shown in Table 1. The highest extracted yield was the water extraction with 2.89%, while the lowest extraction was the ethyl acetate extraction with 1.63%.
Table 1.
Yield and antioxidant activity of different extracts of T. claveryi.
| Sample | Solvent | Yield (%) | DPPH (IC50 μg/ml) | FRAP (μmol Fe2+/mg) | ABTS (IC50 μg/ml) |
|---|---|---|---|---|---|
| T. claveryi | Hexane | 1.91 | 87.21 | 105.8 | 96.28 |
| Ethyl acetate | 1.63 | 121.84 | 88.00 | 133.71 | |
| Ethanol | 2.11 | 52.10 | 48.53 | 64.76 | |
| Methanol | 1.85 | 69.50 | 66.04 | 92.63 | |
| Water | 2.89 | 57.91 | 83.24 | 102.50 | |
| Standard | Ascorbic acid | – | 49.3 | 39.7 | 58.4 |
The results are expressed as mean ± SEM of three independent experiments (n = 6).
3.1. DPPH radical scavenging activity
DPPH is considered as one of the most effective methods for evaluating the concentration of radical-scavenging materials (Gordon, 2001). The results were calculated and presented as IC50 values (the necessary amount of antioxidant that reduces 50% of the initial DPPH concentration). The DPPH radical scavenging activity results are shown in Table 1. It has been found that all extractions of the truffle T. claveryi have significant amounts of radical scavenging activity. However, ethanol extraction showed the strongest DPPH radical scavenging activity with an IC50 of 52 μg/ml, followed by water and methanol extract with an IC50 of 57.9 μg/ml and 69.50 μg/ml, respectively, while the positive control ascorbic acid showed 49 μg/ml. Ethyl acetate showed the lowest DPPH (IC50 121.84 μg/ml), whereas the hexane extraction was 87.21 μg/ml. These data showed that the DPPH activity was affected greatly by the extraction solvents. On the contrary from our result, ethyl acetate extracts of T. claveryi from Turkey exhibited the highest DPPH with an IC50 of 57.73 μg/mL (Kıvrak, 2014). Similar findings were obtained from Saddiq and Danial (2012) as they found that T. claveryi extract has a DPPH activity of 72. 6%. On the other hand, Al-laith (2010) stated that the Saudi truffle Tirmania nivea extract has an antioxidant between 24% and 69%, with a good EC50 of 0.55–0.38 mg/ml, while Akyüz (2013) found that T. boudieri has a DPPH activity of 22.24%.
3.2. Ferric reducing antioxidant power (FRAP) result
FRAP is considered as a direct assay to measure antioxidant properties in various samples (Halvorsen et al., 2002). The FRAP of T. claveryi extracts is shown in Table 1. It was found that the ethanol hexane extract has the highest FRAP property followed by methanol with an IC50 of 48.53 and 66 μmol Fe2+/mg, respectively. Ethyl acetate and water extract showed lower FRAP with an IC50 of 88 and 83.24 μmol Fe2+/mg, respectively. However, hexane extract showed the lowest FRAP IC50 of 105.8 μmol Fe2+/mg. Despite that some extracts showed similar trends in FRAP activity to DPPH activity, yet, other extracts did not show this trend activity such as the ethyl acetate and water extract. This contradictory finding was explained by Bondet et al. (1997), that the reaction of DPPH might be reversible with the presence of certain compounds which result in low readings of antioxidant activity. Therefore, other antioxidant assays are required to be done in order to evaluate the broad antioxidant activity of the samples. Moreover, the method of extraction and the extraction solvent has a great impact on the bioactive compounds presence and on the nutritional composition (Al-Rawi et al., 2013, Rahman et al., 2011). It has been reported that many truffle species have good FRAP value. According to Al-Laith (2010) desert truffle T. nivea have FRAP values ranging from 15.41 to 3.51 mmol/100 g dw. Andlauer and Heritier (2011) stated that the antioxidant power of tea is related to its FRAP antioxidant activity, they found that the FRAP values of green tea ranging from 1.053 ± 0.024 to 1.146 ± 0.016, yellow tea 0.709 ± 0.010, white tea 0.484 ± 0.002 mg TE/ml (Trolox Equivalents unit). According to this observation, it can be said that our samples have good FRAP values that lie within the range of yellow to white tea.
3.3. The ABTS scavenging assay result
This assay is used for determining antioxidant activity in various samples (Ozgen et al., 2006). Thus, ABTS+ was conducted to determine the total antioxidant capacity (TAC) of truffle T. claveryi. The result showed that the ethanol extracts of T. claveryi have the utmost inhibition activity with an IC50 of 64.76 μg/ml followed by methanol and hexane, 92.63 and 96.28 μg/ml, respectively as shown in Table 1. The water extract showed lower inhibition activity (IC50 133.71 μg/ml), whereas the lowest activity was 102.5 μg/ml for the ethyl acetate extract. On the contrary, ethyl acetate extracts of T. claveryi from Turkey showed strong ABTS⋅+ scavenging activity (IC50 17.34 μg/mL) (Buharalioglu et al., 2011, Kıvrak, 2014). On the other hand, the ethanol extracts exhibited very close antioxidant activity to the standard ascorbic acid which was 58.4 μg/ml, which is similar to the DPPH result. This result showed that the ABTS⋅+ antioxidant activity supports our DPPH result. This was confirmed previously by Awika et al. (2003), where they observed high correlation between ABTS and DPPH results. These findings indicate the significance of these antioxidants to the chemical profile properties of the truffle T. claveryi.
3.4. Cytotoxic effect of T. clavery extracts
In this study, the effect of the T. claveryi extractions on 4 types of cancer cell lines (U-87 MG, HT 29, MCF-7 and PC3) were investigated in 6 serial concentrations ranging from 3.125 to 100 μg/ml. The results are depicted in Table 2. The result showed that the hexane extract significantly (p < 0.05) inhibited the human brain carcinoma cell line (U-87 MG), and was the most potent extract with IC50 values of 50.3 ± 5.2 μg/ml, followed by ethyl acetate extract with an IC50 of 72.6 ± 6.3 μg/ml. Moreover, ethanol and methanol extracts showed a moderate cytotoxicity against U-87 MG with an IC50 value of 109.25 ± 9.51 and 136.2 ± 7.4 μg/ml, respectively. However, the hexane extract also inhibited the cell growth of PC3 and MCF7 cell line with an IC50 of 106.1 ± 7.3 and 125.8 ± 6.5 μg/ml, respectively. On the other hand, the ethyl acetate extract showed similar inhibition activity against PC3 and MCF7 cell lines with an IC50 of 121.6 ± 8.3 and 125.8 ± 6.5 μg/ml. While, the methanol extract showed a moderate activity toward the colon adenocarcinoma cell line (HT-29) with an IC50 of (145.2 ± 7.2 μg/ml), followed by ethyl acetate, methanol, and hexane extract with an IC50 of 163.5 ± 6.3, 210.84 ± 10.1 and 239.2 ± 14.3 μg/ml, respectively. Methanol extract also showed a moderate activity with an IC50 value of 111.2 ± 4.8 and 252.3 ± 8.6 toward the PC3 and MCF7 cell lines, respectively. However, the water extract showed the weakest inhibition effect with an IC50 of 242.4 ± 11.1 and 428.5 ± 19.3 μg/ml toward the PC3 and MCF7 cell lines, respectively.
Table 2.
Cytotoxic activities of T. claveryi extracts.
| IC50 values of cell proliferation inhibition of T. claveryi extract (μg/ml) | |||||
|---|---|---|---|---|---|
| Cells | Hexane | Ethyl acetate | Ethanol | Methanol | Water |
| U-87 MG | 50.3 ± 5.2 | 72.6 ± 6.3 | 109.2 ± 9.51 | 136.2 ± 7.4 | 428.5 ± 19.3 |
| PC3 | 106.1 ± 7.3 | 125.6 ± 8.3 | 154.1 ± 6.6 | 111.2 ± 4.8 | 242.4 ± 11.1 |
| MCF7 | 115.8 ± 6.5 | 125.8 ± 9.3 | 91.3 ± 5.9 | 252.3 ± 8.6 | 335.7 ± 11.1 |
| HT29 | 239.2 ± 14.3 | 163.5 ± 6.3 | 210.8 ± 10.1 | 145.2 ± 7.2 | 389.6 ± 11.3 |
The results are expressed as mean ± SEM of three independent experiments (n = 6).
In this study, antioxidant results showed a variety of antioxidant scavenging activities that varied from extract to another. However, the hexane extract of T. claveryi which shows the most potent inhibition against most of the tested cells, showed the highest FRAP activity, a moderate DPPH and ABTS antioxidant properties. Moreover, the inhibition of cell growth by the T. claveryi extracts might be due to the power of the solvent in surpassing the antioxidant properties, in addition to the synergistic effect of several bioactive constituents and some peptide antibiotics that are present in T. claveryi Badalyan (2012).
3.5. The effect of T. claveryi on the mitochondrial membrane
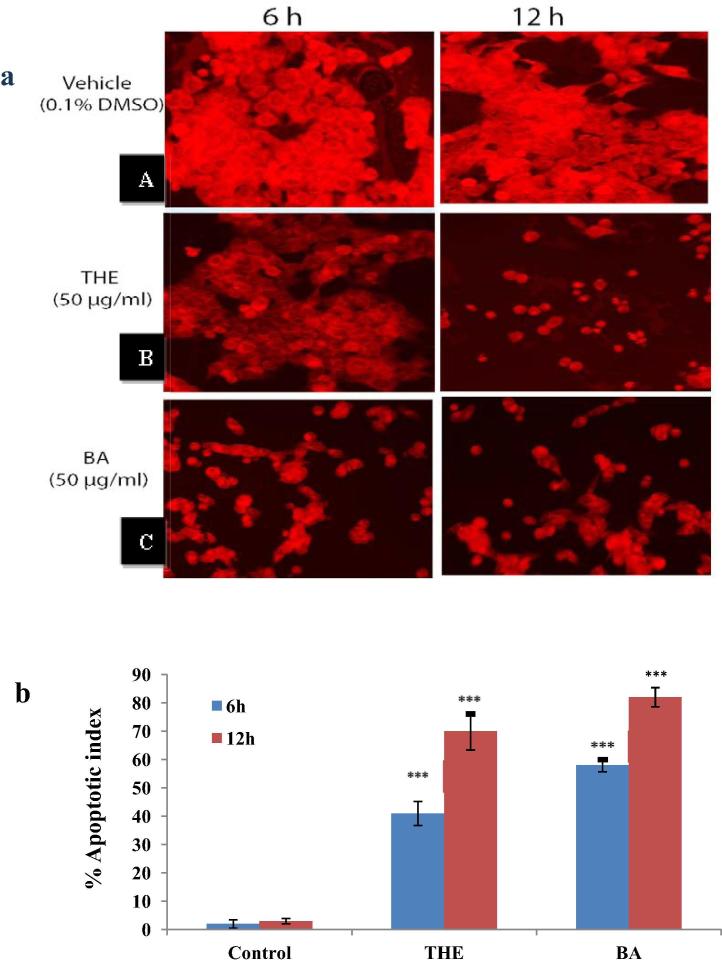
The loss of the mitochondrial membrane potential (ΔΨm) in cells is a pre-apoptotic process that induces the mitochondria to undergo a manifest permeability transition (Marchetti et al., 1996). Moreover, it has been well established that the apoptosis promoted by the mitochondrial pathway is accompanied by a decrease in the mitochondrial membrane potential (Li et al., 2015). Therefore, Rhodamine 123 was used to determine the mitochondrial membrane potential reduction in treated U-87 MG cancer cell line cells with T. claveryi. The result showed that the hexane extract of T. claveryi induced a reduction in the mitochondrial membrane potential of U-87 MG cells using 50 μg/ml after 6 and 12 h as shown in Fig.1a. Moreover, the treated cells looked brighter than the control (untreated cells). Furthermore, a strong intensity of fluorescence in the untreated cells indicates the aggressive growth and proliferation of the cells. Whereas, the fluorescent signal decreased in the cells treated with the hexane extract of T. claveryi as shown in Fig.1b. These findings suggest that T. claveryi truffles might have a great effect in promoting cell apoptosis through the mitochondrial pathway.
Figure 1.
(a) Shows the effect of hexane extract of T. claveryi on mitochondrial membrane potential (ΔΨm) in U-87 MG cancer cells. The cells were incubated with the extract and then stained with Rhod123 to detect the ΔΨm. (A) Control untreated group; (B) THE; T. claveryi hexane extract at 50 μg/ml; (C) BA; butanolic acid (positive control) at 50 μg/ml; (b) fluorescence intensity of ΔΨm, evaluated by Rhod123, and calculated based on the number of cells that lost the mitochondrial membrane potential. Values represent 3 experiment means ± SD. ∗∗∗p < 0.001 compared to the control group.
3.6. The result of nuclear condensation by Hoechst 33342 stain
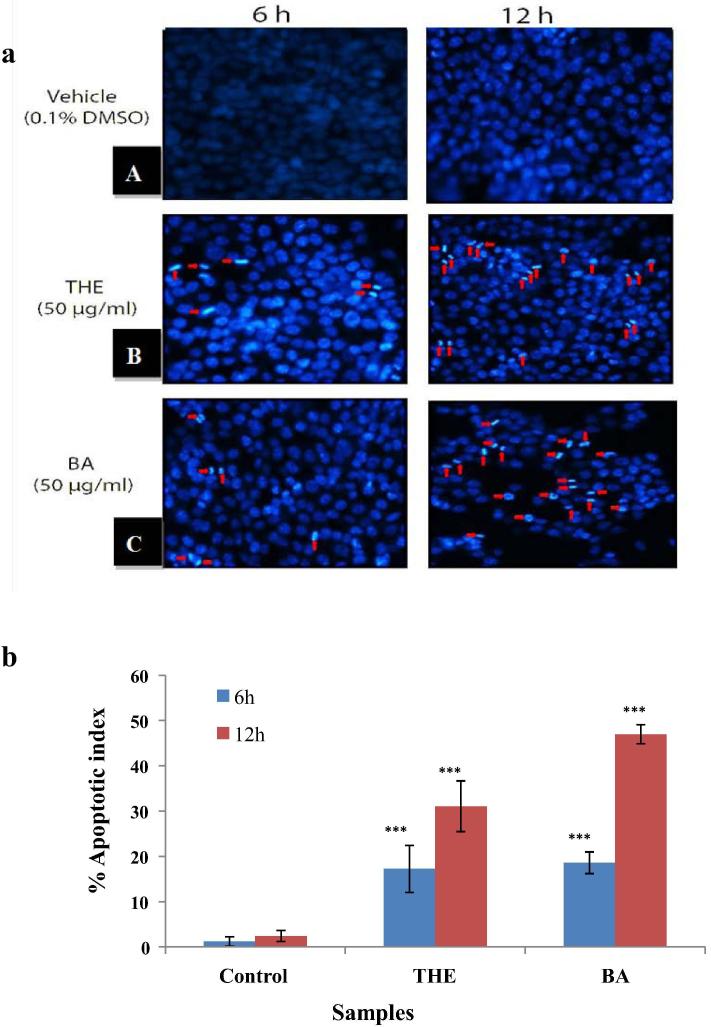
According to Marchetti et al. (1996), cells first lose their mitochondrial membrane potential as a response to tumor necrosis factor or cycloheximide, then these cells will undergo late apoptotic changes, such as; the generation of reactive oxygen species (ROS) and DNA fragmentation. Therefore, in this study, Hoechst 33342 stain, a DNA binding dye was used to investigate the morphological changes in the apoptotic cells. The result showed that the number of apoptotic cells increased significantly (p < 0.05) when the cells were exposed to the hexane extract of T. claveryi at a concentration of 50 μg/mL for 48 h. The apoptotic cells showed distinct morphological characteristics expressed by the condensed and fragmented nuclei that formed clusters against the nuclear periphery or/and crescent-like nuclei as shown in (Fig. 2). Furthermore, after a 6 and 12 h treatment with T. claveryi at a concentration of 50 μg/mL, apoptotic indexes were 17.2 ± 5.2, and 31.1 ± 5.6 for the T. claveryi and 18.6 ± 2.4 and 46.9 ± 2.1 for butanolic acid (positive control), respectively. This finding revealed that the hexane extract of T. claveryi has a potent apoptotic activity comparable and quite close to the butanolic acid (positive control) effect on the U-87 MG cancer cell line. These results indicate that the apoptotic pathway has a fundamental role on cancer cells inhibition effect using T. claveryi extracts.
Figure 2.
(a) Shows the effect of hexane extract of T. claveryi on nucleic morphology of U-87 MG cancer cells. Untreated and treated cells were stained with Hoechst 33342 to detect apoptotic morphology. (A) Control untreated cells. (B) Treated cells with 50 μg/ml of T. claveryi (C) Positive control, treated cells with 50 μg/ml of butanolic acid. Cells treated with the extract displayed characteristics of apoptosis with condensed and fragmented nuclei. Red arrows represent cells with DNA condensation. (b) Shows the Graphical percentage of apoptotic indices for U-87 MGcancer cells. The apoptotic index was expressed as a percentage ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are expressed as mean ± SD. ∗∗∗p < 0.001 compared to the control group.
3.7. Antiangiogenic of T. claveryi extracts
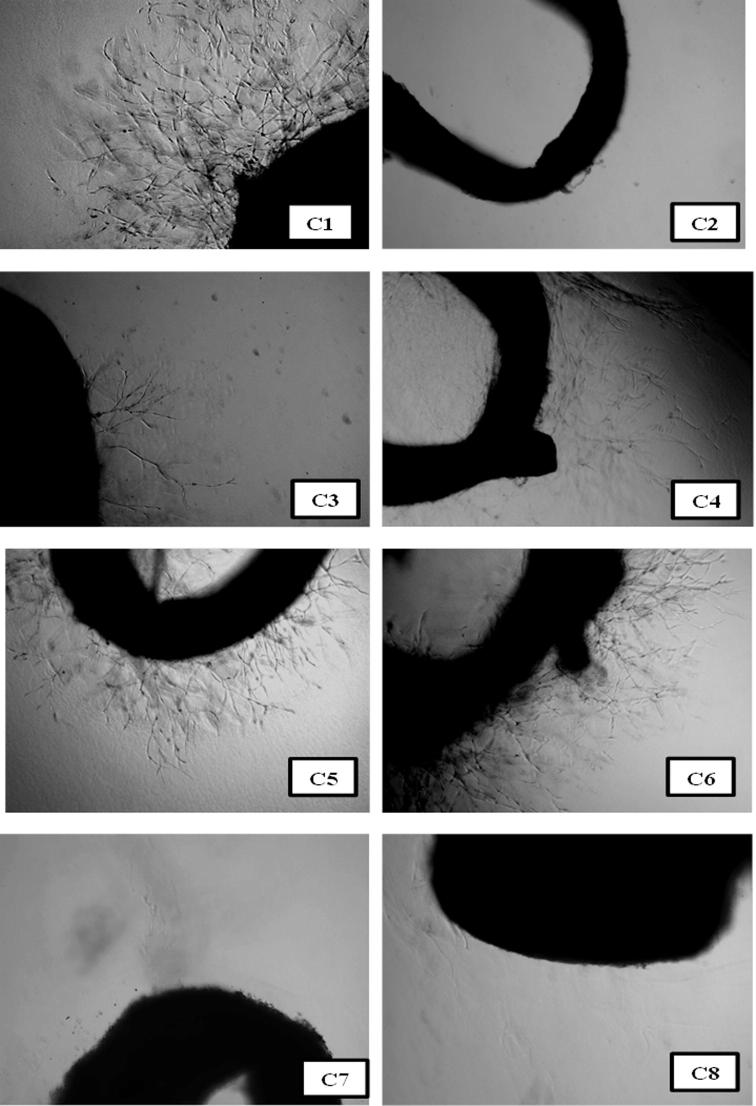
The anti-angiogenic property of the extracts was determined using rat aorta ring assay. The extracts of T. claveryi truffle were added to the isolated rat aorta rings at 6 different concentrations ranging from 3.125 to 100 μg/ml and incubated for 5 days. After the incubation, the new blood vessels outgrown from the seeded aorta rings were measured and quantified. The results of this experiment are shown in Table 3. The result showed that using the hexane extract, the outgrown blood vessels of rat aorta rings were significantly inhibited compared to the control (p < 0.001) as shown in Fig. 3. Moreover, the hexane extract inhibited 100% of new blood vessel development using the concentration of 100 μg/ml, and the IC50 of this extract was found to be 36.1 μg/ml, followed by the ethyl acetate extract with an IC50 value of 42.2 μg/ml. However, the methanol and ethanol extract inhibited the formation of new blood vessels, and showed a moderate and close value of anti-angiogenic activity, with an IC50 of 96.6 and 91.3 μg/ml, respectively. However, the weakest antiangiogenic activity was obtained using the water extract. Previous studies showed that antioxidants act as natural angiogenesis inhibitors. Oak et al. (2005) asserted that antioxidant compounds have an influence on cancer angiogenesis, as these compounds are able to hinder the angiogenesis process by inhibiting the blood vessel formation. Moreover, Ahn et al. (2009) demonstrate a correlation between the antioxidant and antiangiogenic activity. Therefore, it can be said that the extraction solvents significantly influenced the chemical composition of the extract and its antioxidant concentration which highlights its potential antiangiogenic activity.
Table 3.
Antiangiogenic activity of T. claveryi extracts on blood vessels of rat aortic explants.
| Sample | Extract | IC50 (μg/ml) |
|---|---|---|
| T. claveryi | Hexane | 36.1 ± 6.4 |
| Ethyl acetate | 42.2 ± 5.2 | |
| Ethanol | 96.6 ± 7.7 | |
| Methanol | 91.3 ± 6.2 | |
| Water | 117.2 ± 8.4 |
The results are expressed as mean ± SEM of three independent experiments (n = 6).
Figure 3.
Shows images of the angiogenesis inhibitory effect of the T. claveryi extracts. C1; negative control, a DMSO treated ring shows a full growth of blood vessel. C2: hexane extract, treated ring showed 100% inhibition using 10 μg/ml. C3; ethyl acetate extract showed 81% inhibition of blood vessel using 10 μg/ml, C4; methanol extract showed 55% inhibition of blood vessel using 10 μg/ml, C5; ethanol extract showed 49% inhibition of blood vessel using 10 μg/ml, C6; water extract showed 41% inhibition of blood vessel using 10 μg/ml. C7 and C8 Suramine showed 100% inhibition of blood vessel using 10 μg/ml. ∗∗∗p < 0.001 compared to the control group.
3.8. GC–MS result of T. claveryi extract
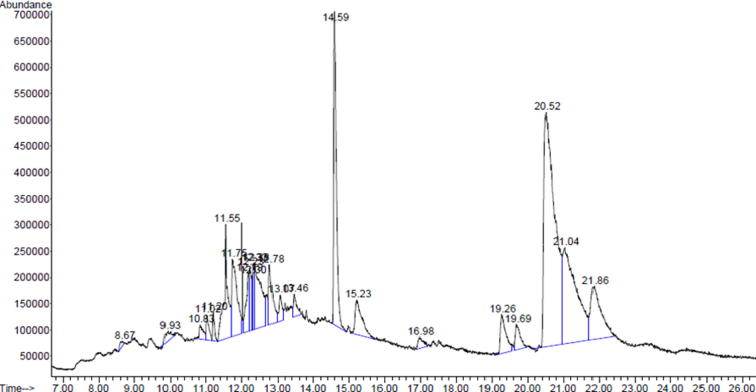
Quantitative chemical analysis of the most active extract of the T. claveryi (hexane extract) was carried out using GC–MS with an aim to identify the active compounds that might be responsible for its antioxidant, anti-proliferative, apoptosis and antiangiogenic properties. The results of the main chemical compositions of hexane extract of T. claveryi determined using GC/MS are shown in Table 4. The GC–MS analysis revealed that the hexane extract of the T. claveryi composed of various phytosterols, triterpene, vitamin and aromatic compounds. However, the quantitative analysis of the tested extract revealed that the major dominant peak corresponds to stigmasterol at the highest concentration of (31.10%), followed by beta-Sitosterol (15.73%), squalene (10.09%), lupeol (6.42%) as shown in Fig. 4. Interestingly, the entire detected chemical compounds in the hexane extract of T. claveryi were recognized as anticancer agents in many previous studies. Stigmasterols, beta-Sitosterol, and campestanol are plant sterols or phytosterol, which are structurally similar to cholesterol and exist in several forms in plants (Ostlund, 2002). According to Woyengo et al. (2009), phytosterols are involved in various mechanisms of action, such as inhibiting cancer-cell growth, angiogenesis and promotion of cancer cells apoptosis. Stigmasterol reduced the ovarian cancer risk (Bradford and Awad, 2007), and inhibited 83% of tumor size at a concentration of 10 mg/kg (Ghosh et al., 2011). In fact, many studies have confirmed the presence of stigmasterol in most of the nuts and seeds, as well as olive oil. According to Bradford and Awad (2007) the stigmasterol concentration in sesame oil is 28 mg/ml, and this concentration is quite close to the concentration of stigmasterol in our tested sample. Noteworthy, sesame oil dramatically inhibited the growth of the HT-29 malignant human colon cell line (Salerno and Smith, 1991). Another compound, squalene, which is a natural triterpene was also present in the hexane extract chemical analysis of T. claveryi. It has been reported that squalene has chemopreventive and antitumor effects on colon cancer cells contributing either directly or indirectly in treating cancer due to its potential effect (Rao et al., 1998, Smith, 2000, Reddy and Couvreur, 2009). On the other hand, the detected compound lupeol, is a triterpene present in diverse plant families e.g. grapes, mango, carrot, cucumber, soybean, melon, aloe and serve as major active constituents in several herbal medicines (Gallo and Sarachine, 2009). Lupeol is a novel anti-cancer dietary triterpene, it inhibits skin cancer in CD-1, and induces apoptosis in HL-60 human leukemia cells (Aratanechemuge et al., 2004, Zhang et al., 2009, Saleem, 2009, Saleem et al., 2004). On the other hand, it has been found that lupeol at 50 and 30 μg/mL showed a remarkable antiangiogenic activity (You et al., 2003). Therefore, it can be speculated that this compound might be behind the angiogenesis activity of T. claveryi hexane extract. Similarly, Carrillo and Cavia (2012) deduced that oleic acid has antitumor and antiproliferation effects on PC3, while, Hayshi et al. (1998) confirmed that octadecadienoic acid also has an antitumor activity. On the other hand, Wang and Marcone,(2011 stated that hexadecanoic acid is an aromatic compound found in many truffle Tuber species and is responsible for the aroma of truffle species. Likewise, Sawaya et al., 1985 said that the concentration of ascorbic acid in T. claveryi of Saudi Arabia is (5.10 mg/100) which is higher than the ascorbic acid concentration of our tested extract of T. claveryi. From the previous points, it can be speculated that the anticancer, apoptosis and antiangiogenic activity of T. claveryi is due to the presence of these main chemical constituents and the synergistic effect between them, as per only stigmasterol and beta-Sitosterol forms about 47% of the extract. In addition, the presence of antioxidants might also be behind its activity due to its significant role in disease prevention and treatment (Dillard and German, 2000, Lobo et al., 2010).
Table 4.
Shows the main chemical composition of Iraqi truffle T. claveryi.
| Peak | Ret time (Min) | Area% | Phytoconstituents | Mol. formula | Mol. weight |
|---|---|---|---|---|---|
| 1. | 11.02 | 2.89 | Hexadecanoic acid | C16H32O2 | 256 |
| 2. | 11.55 | 4.98 | Octadecadienoic acid | C18H32O2 | 280 |
| 3. | 11.74 | 4.86 | Oleic acid | C18H34O2 | 282 |
| 4. | 12.19 | 1.85 | Ascorbic acid | C6H8O6 | 176 |
| 5. | 14.59 | 10.09 | Squalene | C30H50 | 410 |
| 6. | 19.26 | 2.56 | Campestanol | C28H50O | 402 |
| 7. | 20.52 | 31.10 | Stigmasterol | C29H48O | 412 |
| 8. | 21.04 | 15.73 | beta-Sitosterol | C29H50O | 414 |
| 9. | 21.86 | 6.42 | Lupeol | C30H50O | 426 |
Figure 4.
The total ion chromatograms (TIC) of the hexane extract of T. claveryi (the most active extract) showing the peaks of the main active compounds.
4. Conclusion
This is the first study that shows the dynamic range of biological activities of Iraq truffle extracts. In this study, the extracts of T. claveryi were found to have a potent antiproliferation, antiangiogenic and antioxidant properties. The chemical analysis of T. claveryi exhibited the presence of some phytosterols, triterpenes and vitamins. The antioxidant, antiangiogenic and anticancer effects were emitted by the present of some bioactive components such as phytosterol, triterpenes, fatty acid and vitamins. Therefore, these findings provide evidences of truffle health benefits, owing it to its antioxidant and nutrient properties which may be used in nutraceutical or pharmaceutical industries. However, further studies on the mechanisms of the bioactive compounds of this extract could probably lead to the discovery of promising chemotherapeutic agents.
Acknowledgment
The author would like to thank University Sains Malaysia for the (USM) fellowship.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Saad Sabbar Dahham, Email: hawk_dijla@yahoo.com.
Sawsan S. Al-Rawi, Email: sawsan.hameed@uoz.ac.
Ahmad H. Ibrahim, Email: abraihimi@yahoo.com.
Aman Shah Abdul Majid, Email: aman@usm.com.
Amin Malik Shah Abdul Majid, Email: aminmalikshah@gmail.com.
References
- Ahn M.-R., Kunimasa K., Kumazawa S. Correlation between antiangiogenic activity and antioxidant activity of various components from propolis. Mol. Nutr. Food Res. 2009;53:643–651. doi: 10.1002/mnfr.200800021. [DOI] [PubMed] [Google Scholar]
- Akyüz M. Nutritive value, flavonoid content and radical scavenging activity of the truffle (Terfezia boudieri Chatin) J. Soil Sci. Plant Nutr. 2013;13:143–151. [Google Scholar]
- Al-Delaimy K.S. Protein and amino acid composition of Iraqi truffles. Can. Inst. Food Sci. Technol. J. 1977;10:221. [Google Scholar]
- Al-Kaisey M.T., Hadwan H.A., Abeed H.A., Taher E.J., Dhar B.L. Proximate analysis of Iraqi truffles. Mushroom Res. 1996;5:105–108. [Google Scholar]
- Al-Laith A.A.A. Antioxidant components and antioxidant/antiradical activities of desert truffle (Tirmania nivea) from various Middle Eastern origins. J. Food Compos. Anal. 2010;23:15–22. [Google Scholar]
- Al-Rawi S.S., Ibrahim A.H., Rahman N.A. The effect of supercritical fluid extraction parameters on the nutmeg oil extraction and its cytotoxic and antiangiogenic properties. Procedia Food Sci. 2011;1:1946–1952. [Google Scholar]
- Al-Rawi S.S., Ibrahim A.H., Majid A.S., Abdul Majid A.M.S., Kadir M.O.A. Comparison of yields and quality of nutmeg butter obtained by extraction of nutmeg rind by soxhlet and supercritical carbon dioxide (SC-CO2) J. Food Eng. 2013;119:595–601. [Google Scholar]
- Al-Shabibi M.M.A., Toma S.J., Haddad B.A. Studies on Iraqi truffles. I. Proximate analysis and characterization of lipids. Can. Inst. Food Sci. Technol. J. 1982;15:200–202. [Google Scholar]
- Andlauer W., Heritier J. Rapid electrochemical screening of antioxidant capacity (resAc) of selected tea samples. Food Chem. 2011;125:1517–1520. [Google Scholar]
- Aratanechemuge Y., Hibasami H., Sanpin K., Katsuzaki H., Imai K., Komiya T. Induction of apoptosis by lupeol isolated from mokumen (Gossampinus malabarica L. Merr) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004;11(2):289–292. [PubMed] [Google Scholar]
- Awika J.M., Rooney L.W., Wu X., Prior R.L., Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003;51:6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Badalyan S. Springer Berlin; Heidelberg: 2012. Medicinal aspects of edible ectomycorrhizal mushrooms; pp. 317–334. (Edible Ectomycorrhizal Mushrooms). [Google Scholar]
- Bahrani Z. Race and ethnicity in Mesopotamian antiquity 1. World Archaeology. 2006;38:48–59. [Google Scholar]
- Bokhary H.A., Parvez S. Chemical composition of desert truffles Terfezia claveryi. J. Food Compos. Anal. 1993;6:285–293. [Google Scholar]
- Bondet V., Brand-Williams W., Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT – Food Sci. Technol. 1997;30:609–615. [Google Scholar]
- Bradford P.G., Awad A.B. Review; Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007:161–170. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- Buharalioglu C.K., Song C.Y., Yaghini F.A., Ghafoor H.U., Motiwala M., Adris T., Malik K.U. Angiotensin II-induced process of angiogenesis is mediated by spleen tyrosine kinase via VEGF receptor-1 phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2011;301 doi: 10.1152/ajpheart.01018.2010. H1043-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo C., Cavia M.M. Antitumor effect of oleic acid; mechanisms of action: a review. Nutr. Hosp. 2012:1860–1865. doi: 10.3305/nh.2012.27.6.6010. [DOI] [PubMed] [Google Scholar]
- Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B., Ezzat M.O., Majid A.S., Majid A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20(7):11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard C.J., German J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. [Google Scholar]
- Đordević T.M., Slavica S., Šiler-Marinković S.I.D. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. [Google Scholar]
- Dundar A., Yesil O.F., Acay H., Okumus V., Ozdemir S., Yildiz A. Antioxidant properties, chemical composition and nutritional value of Terfezia boudieri (Chatin) from Turkey. Food Sci. Technol. Int. 2012;18:317–328. doi: 10.1177/1082013211427954. [DOI] [PubMed] [Google Scholar]
- Gallo M.B.C., Sarachine M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009;3:46–66. [Google Scholar]
- Ghosh T., Maity T.K., Singh J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011;11:41–49. [Google Scholar]
- Gordon M.H. Woodhead Publishing Ltd; NW, USA: 2001. The development of oxidative rancidity in foods. (Antioxidants in Food Practical Applications). [Google Scholar]
- Gutiérrez A., Morte A., Honrubia M. Morphological characterization of the mycorrhiza formed by Helianthemum almeriense Pau with Terfezia claveryi Chatin and Picoa lefebvrei (Pat.) Maire. Mycorrhiza. 2003;13:299–307. doi: 10.1007/s00572-003-0236-7. [DOI] [PubMed] [Google Scholar]
- Halvorsen B.L., Holte K., Myhrstad M.C. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002;132:461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- Hayshi Y., Nishikawa Y., Mori H., Tamura H., Matsushita Y.I., Matsui T. Antitumor activity of (10E, 12Z)-9-hydroxy-10, 12-octadecadienoic acid from rice bran. J. Ferment. Bioeng. 1998;86(2):149–153. [Google Scholar]
- Hussain G., Al-Ruqaie I.M. Occurrence, chemical composition, and nutritional value of truffles: an overview. Pak. J. Biol. Sci. 1999;2:510–514. [Google Scholar]
- Ibrahim A.H., Al-Rawi S.S., Majid, Rahman N.N.A., Salah K.M.A., Kadir M.O.A. Separation and fractionation of Aquilaria malaccensis oil using supercritical fluid extraction and the cytotoxic properties of the extracted oil. Procedia Food Sci. 2011;1:1953–1959. [Google Scholar]
- Ibrahim A.H., Al-Rawi S.S., Abdul Majid A.S., Al-Habib O., Abdul Majid A.M. Pro-angiogenic and wound healing potency of virgin coconut oil. Supp. Care Cancer (MASCC) 2013;21:235. [Google Scholar]
- Janakat S., Nassar M. Hepatoprotective activity of desert truffle (Terfezia claveryi) in comparison with the effect of Nigella sativa in the rat. Pak. J. Nutr. 2010;9:52–56. [Google Scholar]
- Kıvrak İ. Analytical methods applied to assess chemical composition, nutritional value and in vitro bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal. Methods. 2014:1–15. [Google Scholar]
- Kong J.M., Goh N.K., Chia L.S., Chia T.F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003;24:7–21. [PubMed] [Google Scholar]
- Li Q., Lu X.H., Cai L., Lu J.L., Wu J.S., Zhuge Q.C., Su Z.P. Antiproliferative and apoptosis-inducing activity of schisandrin B against human glioma cells. Cancer Cell Int. 2015;15(1):12. doi: 10.1186/s12935-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeel Q.A., Al-laith A.A.A. Ethnomycological aspects of the desert truffle among native Bahraini and non-Bahraini peoples of the Kingdom of Bahrain. J. Ethnopharmacol. 2007;110:118–129. doi: 10.1016/j.jep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Marchetti P., Susin S.A., Decaudin D., Gamen S., Castedo M., Hirsch T., Kroemer G. Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res. 1996;56(9):2033–2038. [PubMed] [Google Scholar]
- Oak M.H., El-Bedoui J., Schini-Kerth V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ostlund R.E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002;22:533–549. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- Ozgen M., Reese R.N., Tulio A.Z., Scheerens J.C., Miller A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2’-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006;54:1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- Patel S. Food, health and agricultural importance of truffles: a review of current scientific literature. Curr. Trends Biotechnol. Pharmacy. 2012;6:15–27. [Google Scholar]
- Rahman N.N.A., Nama M.M., Al.-Rawi Ben S.S., Ibrahim A.H., Kadir M.O.A. Comparison of nutritional composition between palm kernel fibre and the effect of the supercritical fluid extraction on its quality. Procedia Food Sci. 2011;1:1940–1945. [Google Scholar]
- Rao C.V., Newmark H.L., Reddy B.S. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–290. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reddy L.H., Couvreur P. Squalene: a natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009;61:1412–1426. doi: 10.1016/j.addr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Saddiq A.A., Danial E.N. Assessment of phenolic content, free radical-scavenging capacity and antimicrobial activities of Truffle claveryi. WULFENIA J. 2012;19:403–422. [Google Scholar]
- Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M., Afaq F., Adhami V.M., Mukhtar H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- Salerno J.W., Smith D.E. The use of sesame oil and other vegetable oils in the inhibition of human colon cancer growth in vitro. Anticancer Res. 1991;11:209–215. [PubMed] [Google Scholar]
- Sawaya W.N., Al-Shalhat A., Al-Sogair A., Mohammad M. Chemical composition and nutritive value of truffles of Saudi Arabia. J. Food Sci. 1985;50:450–453. [Google Scholar]
- Smith T.J. Squalene: potential chemopreventive agent. Expert Opin. Investig. Drugs. 2000;9:1841–1848. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- Wang S., Marcone M.F. The biochemistry and biological properties of the world’s most expensive underground edible mushroom: truffles. Food Res. Int. 2011;44:2567–2581. [Google Scholar]
- Woyengo T.A., Ramprasath V.R., Jones P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009;63:813–820. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- You Y.-J., Nam N.-H., Kim Y., Bae K.-H., Ahn B.-Z. Antiangiogenic activity of lupeol from Bombax ceiba. Phytotherapy Research, PTR. 2003;17:341–344. doi: 10.1002/ptr.1140. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang Y., Zhang L., Yang X., Lv Z. Cellular and molecular biology lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;9:163–170. doi: 10.1080/07357900802210745. [DOI] [PubMed] [Google Scholar]