Abstract
BACKGROUND. Matrix metalloprotease 9 (MMP-9) is associated with inflammation and lung remodeling in chronic obstructive pulmonary disease (COPD). We hypothesized that elevated circulating MMP-9 represents a potentially novel biomarker that identifies a subset of individuals with COPD with an inflammatory phenotype who are at increased risk for acute exacerbation (AECOPD).
METHODS. We analyzed Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) and Genetic Epidemiology of COPD (COPDGene) cohorts for which baseline and prospective data were available. Elevated MMP-9 was defined based on >95th percentile plasma values from control (non-COPD) sample in SPIROMICS. COPD subjects were classified as having elevated or nonelevated MMP-9. Logistic, Poisson, and Kaplan-Meier analyses were used to identify associations with prospective AECOPD in both cohorts.
RESULTS. Elevated MMP-9 was present in 95/1,053 (9%) of SPIROMICS and 41/140 (29%) of COPDGene participants with COPD. COPD subjects with elevated MMP-9 had a 13%–16% increased absolute risk for AECOPD and a higher median (interquartile range; IQR) annual AECOPD rate (0.33 [0–0.74] versus 0 [0–0.80] events/year and 0.9 [0.5–2] versus 0.5 [0–1.4] events/year for SPIROMICS and COPDGene, respectively). In adjusted models within each cohort, elevated MMP-9 was associated with increased odds (odds ratio [OR], 1.71; 95%CI, 1.00–2.90; and OR, 3.03; 95%CI, 1.02–9.01), frequency (incidence rate ratio [IRR], 1.45; 95%CI, 1.23–1.7; and IRR, 1.24; 95%CI, 1.03–1.49), and shorter time-to-first AECOPD (21.7 versus 31.7 months and 14 versus 21 months) in SPIROMICS and COPDGene, respectively.
CONCLUSIONS. Elevated MMP-9 was independently associated with AECOPD risk in 2 well-characterized COPD cohorts. These findings provide evidence for MMP-9 as a prognostic biomarker and potential therapeutic target in COPD.
TRIAL REGISTRATION. ClinicalTrials.gov: NCT01969344 (SPIROMICS) and NCT00608764 (COPDGene).
FUNDING. This work was funded by K08 HL123940 to JMW; R01HL124233 to PJC; Merit Review I01 CX000911 to JLC; R01 (R01HL102371, R01HL126596) and VA Merit (I01BX001756) to AG. SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study) is funded by contracts from the NHLBI (HHSN268200900013C, HHSN268200900014C,HHSN268200900015C HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C) and a grant from the NIH/NHLBI (U01 HL137880), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals Inc.; Chiesi Farmaceutici; Forest Research Institute Inc.; GlaxoSmithKline; Grifols Therapeutics Inc.; Ikaria Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan. COPDGene is funded by the NHLBI (R01 HL089897 and R01 HL089856) and by the COPD Foundation through contributions made to an Industry Advisory Board composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Keywords: Pulmonology
Keywords: COPD, Proteases
MMP-9 is an independent risk factor for the development and frequency of acute pulmonary exacerbation in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disorder characterized by progressive airflow limitation leading to functional impairment. Acute exacerbations of COPD (AECOPD) are critical events in the natural history of COPD, affecting quality of life, trajectory of lung function loss, and mortality (1–3). However, the biologic pathways conveying increased risk for these events are poorly understood, and a biomarker of risk remains elusive. Recent studies analyzing large panels of biomarkers as predictors for AECOPD risk have found various markers were associated with AECOPD within individual cohorts, but replication across COPD populations is poor (4). The identification of a reproducible biomarker for AECOPD would provide key prognostic value and, if causally linked to pathogenesis, could present a new therapeutic target for AECOPD prevention or treatment.
Among the many inflammatory pathways and mediators implicated in COPD development, proteases are particularly relevant to the pathophysiology of the disease. Inflammation alters protease/antiprotease balance, leading to progressive airway destruction and remodeling. The matrix metalloproteases (MMPs) have been connected to the development of emphysema through direct and indirect mechanisms (5–8). MMP-9 uniquely mediates pulmonary inflammation through extracellular matrix degradation, neutrophil chemotaxis, and augmentation of inflammation (7, 9) — key features of AECOPD. To date, no clinical study has evaluated the impact of elevated MMP-9 on important events including risk of COPD exacerbation.
We sought to understand if there were associations between MMP-9 and AECOPD, thus establishing its relevance as a COPD biomarker. As opposed to prior work investigating mean MMP-9 values, we used the approach of defining elevated MMP-9 based on >95th percentile values derived from non-COPD Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) subjects and then applied this threshold in predicting risk for AECOPD, a well-validated approach to defining biomarkers in many disorders (10–12). We hypothesized that elevated systemic MMP-9 identifies a subset of individuals with COPD with an inflammatory phenotype who are at increased risk for AECOPD.
Results
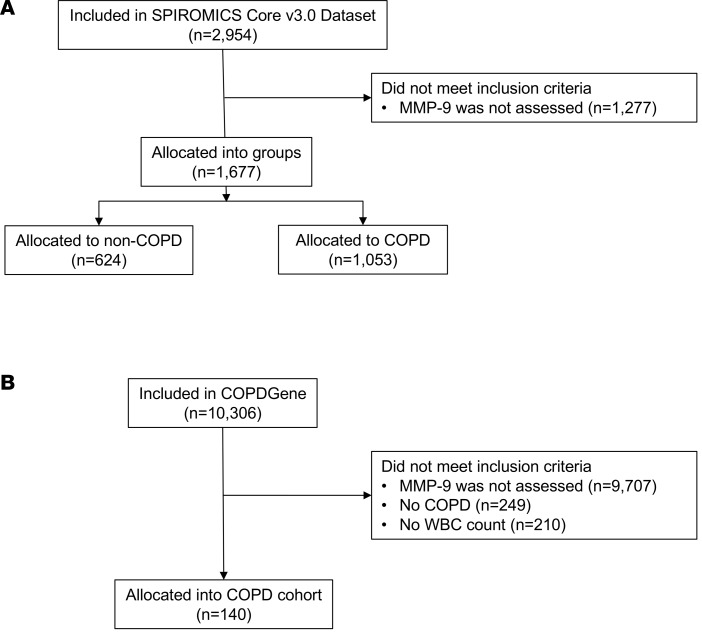
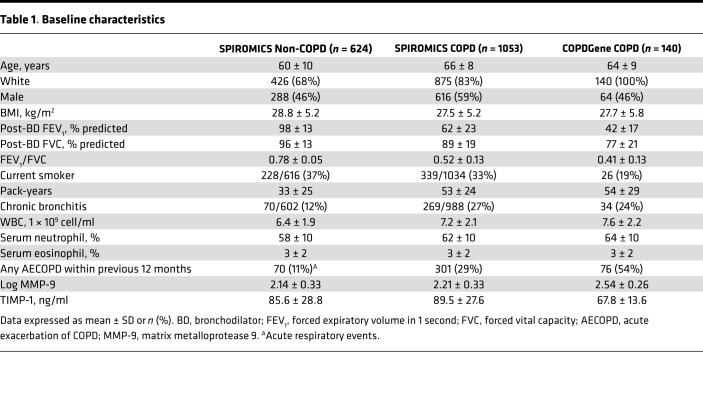
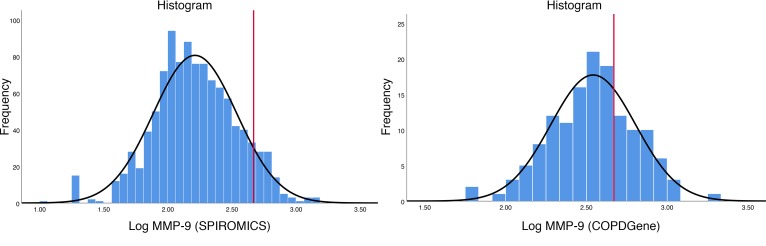
Ascertainmentofelevated MMP-9. Flow diagrams for SPIROMICS and Genetic Epidemiology of COPD (COPDGene) are shown in Figure 1. In SPIROMICS, there were 624 individuals in strata 1 and 2 (non-COPD controls) who had plasma MMP-9 measured. These non-COPD study participants were 60 ± 10 years old, 68% White, and 46% male (Table 1). Plasma MMP-9 was 2.14 ± 0.33 ng/ml (log-transformed) for non-COPD controls, and the 95th percentile value of 2.67 ng/ml log-transformed was used to indicate elevated MMP-9. The distributions of MMP-9 in the COPD cohorts are shown in Figure 2. In SPIROMICS, the 1,053 individuals with COPD and with plasma MMP-9 values were 66 ± 8 years old, 83% White, 59% male, and 33% current smokers, and they had a mean post-bronchodilator forced expiratory volume in 1 second (FEV1) of 62% ± 23% predicted (Table 1). In the SPIROMICS COPD cohort, the average plasma level of MMP-9 was 2.21 ± 0.33 ng/ml log-transformed, and 95 individuals (9%) had elevated MMP-9 using the 2.67 ng/ml log-transformed threshold. Among the participants in SPIROMICS with COPD who were included in the primary analysis, 290 had a second plasma MMP-9 measurement performed after 1 year of follow-up, and the variation of MMP-9 over time is shown in Supplemental Figure 1 (supplemental material available online with this article; https://doi.org/10.1172/jci.insight.123614DS1). The coefficient of variation of logMMP-9 was 14.7% for Visit 1 and 14.8% for Visit 2.
Figure 1. Flow diagrams for the participants in the study.
(A) SPIROMICS and (B) COPDGene. All allocated subjects in both cohorts were included in analysis. COPD, chronic obstructive pulmonary disease; MMP, matrix metalloprotease.
Table 1. Baseline characteristics.
Figure 2. Plasma MMP-9 distribution in the 2 cohorts.
(A) The distribution of log-transformed MMP-9 values in 1,053 individuals with COPD in SPIROMICS. (B) The distribution of log-transformed MMP-9 values in 140 individuals with COPD in COPDGene. The red line corresponds to elevated MMP-9 (MMP-9 >2.67 ng/ml, log transformed; defined as the 95th percentile value in the non-COPD cohort). COPD, chronic obstructive pulmonary disease; MMP, matrix metalloprotease.
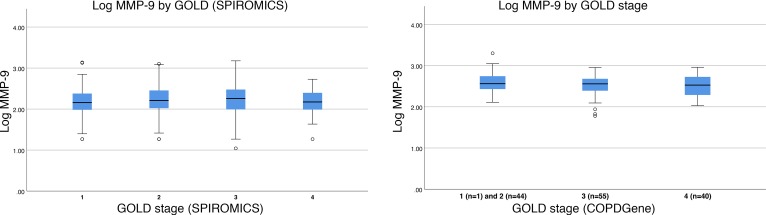
Within COPDGene, 140 individuals had COPD and plasma MMP-9 measurements available. Participants in this validation cohort were 64 ± 9 years old, 100% White, 46% male, and 19% current smokers and had a mean postbronchodilator FEV1 of 42% ± 17% predicted (Table 1). The average plasma MMP-9 was 2.54 ± 0.26 ng/ml log-transformed, with 41 (29%) classified as elevated MMP-9. The distributions of MMP-9 values across Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages for both cohorts are shown in Figure 3.
Figure 3. MMP-9 values across GOLD stages.
MMP-9 (ng/ml log transformed) values were different across GOLD stage in (A) SPIROMICS (P = 0.023) but not in (B) COPDGene (P = 0.48) by 1-way ANOVA. In COPDGene, GOLD stages 1 and 2 were combined due to the presence of a single GOLD stage 1 participant. The values included within the box(es) represent the interquartile range, the horizontal line the median, and the whiskers the 95% CI; outliers are represented by circles. GOLD, Global initiative for Obstructive Lung Disease; MMP, matrix metalloproteinase.
Characteristics of individuals with COPD and elevated MMP-9.
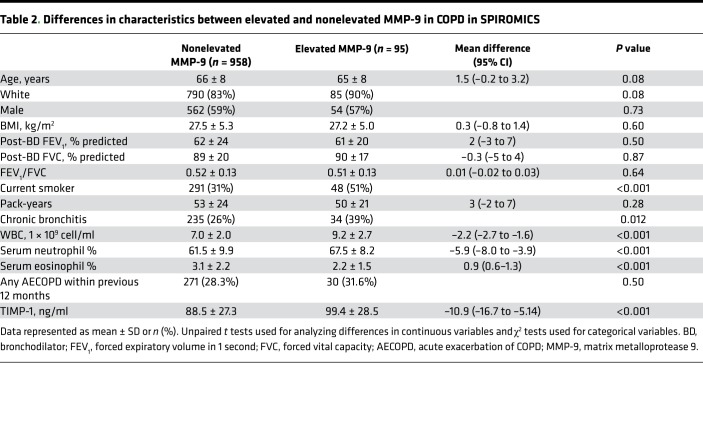
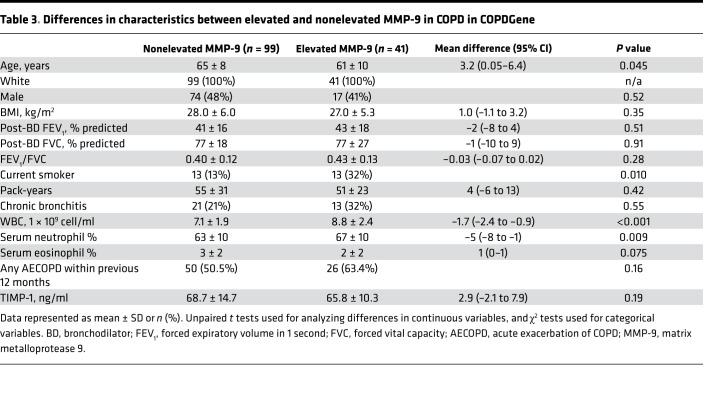
Compared with participants with nonelevated MMP-9, SPIROMICS participants with elevated MMP-9 were more often current smokers (51% vs. 31%, P < 0.001) and had a higher proportion of chronic bronchitis (39% vs. 26%, P = 0.012), higher WBC count, higher neutrophil percent, and lower eosinophil percent (Table 2). In COPDGene, participants with elevated MMP-9 were younger, more likely current smokers (32% vs. 13%, P < 0.001), and had higher WBC count and neutrophil percent as compared with the nonelevated MMP-9 group (Table 3). However, in both cohorts, we found no significant differences between individuals with elevated MMP-9 and those with normal MMP-9 values in race (in SPIROMICS only), sex, BMI, lung function, pack-year history of smoking, comorbid conditions, or proportion of who had a previous AECOPD within the 12 months before baseline evaluation. While we observed ~12% higher tissue inhibitor of metalloproteinase 1 (TIMP-1) levels in the elevated MMP-9 group compared with the nonelevated MMP-9 group in SPIROMICS (Table 2), there were no intergroup differences observed in COPDGene (Table 3).
Table 2. Differences in characteristics between elevated and nonelevated MMP-9 in COPD in SPIROMICS.
Table 3. Differences in characteristics between elevated and nonelevated MMP-9 in COPD in COPDGene.
Associations between elevated MMP-9 and prospective AECOPD in SPIROMICS.
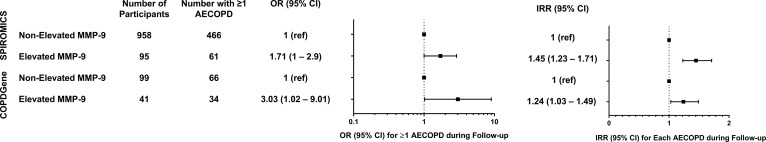
Participants with COPD in SPIROMICS were followed for a median of 34 months (IQR25–75 25–37). During this time, 527 (50.5%) developed at least 1 AECOPD (median 1; IQR25–75 0–2). Participants in the elevated MMP-9 group had a 16% higher absolute risk for having ≥1 AECOPD during follow-up compared with the group without elevated MMP-9 (65% vs. 49%, P = 0.003). In unadjusted logistic regression models, elevated MMP-9 was associated with prospective AECOPD (OR, 1.92; 95% CI, 1.23–2.99; P = 0.004), as were age, sex, lower FEV1 percent predicted, chronic bronchitis, WBC, and an AECOPD in the year before the baseline evaluation (Supplemental Table 1). Moreover, elevated MMP-9 was independently associated with AECOPD (OR, 1.71; 95% CI, 1.00–2.90; P = 0.049) in a logistic regression model adjusting for age, sex, race, FEV1 percent predicted, current smoking, chronic bronchitis, WBC count, and previous AECOPD (Figure 4A and Supplemental Table 2). There was no significant interaction between elevated MMP-9 and WBC (P = 0.81) or elevated MMP-9 and PMN counts (P = 0.78) for AECOPD. The area under the receiver operating curve was 0.74 (95% CI, 0.71–0.77; P < 0.001).
Figure 4. Associations between elevated MMP-9 prospective exacerbations.
Models were adjusted for age, race, sex, FEV1 percent predicted, smoking status, chronic bronchitis, leukocyte count, and history of previous exacerbations. (A) Logistic regression was used to measure associations with ≥1 AECOPD, and (B) Poisson regression was used for exacerbation frequency. Boxes represent the point estimates (OR or IRR), and the horizontal bars represent the 95% CI. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; MMP, matrix metalloprotease; OR, odds ratio; IRR, incidence rate ratio.
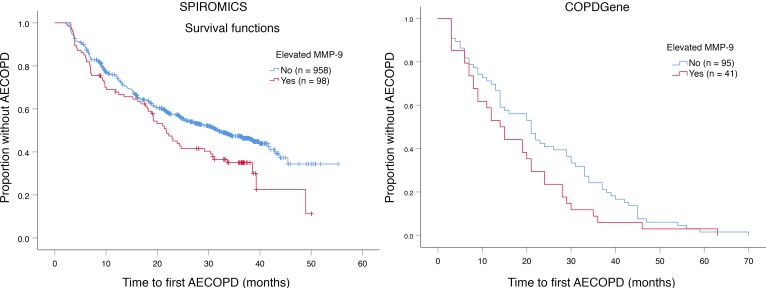
In SPIROMICS, the median [IQR25–75] annual AECOPD rate was higher in the elevated MMP-9 group compared with the nonelevated MMP-9 group (0.33 [0–0.74] events/year versus 0 [0–0.80] events/year, P = 0.013). In Poisson regression models adjusted for age, sex, FEV1 percent predicted, chronic bronchitis, WBC count, and previous AECOPD, elevated MMP-9 was independently associated with increased AECOPD frequency (incidence rate ratio [IRR], 1.45; 95% CI, 1.23–1.71; P < 0.001) (Figure 4B and Supplemental Table 3). In SPIROMICS, elevated MMP-9 was associated with a shorter median time-to-first AECOPD (21.7 vs. 31.7 months, P = 0.015) as shown in Figure 5A.
Figure 5. Kaplan-Meier plots for the probability of remaining free of AECOPD.
(A) In SPIROMICS, the median time-to-first event was 10 months shorter in individuals with elevated MMP-9 compared with those without MMP-9 elevation (21.7 months [95% CI, 17.5–25.9] versus 31.7 months [95% CI, 27.2–36.2], P = 0.015). (B) In COPDGene, the median time-to-first AECOPD was also shorter among the elevated MMP-9 group (14 months [95% CI, 9.4–18.6] versus 21 months [95% CI, 15–27], P = 0.065). AECOPD, acute exacerbation of chronic obstructive pulmonary disease; MMP, matrix metalloprotease.
Associations between elevated MMP-9 and prospective AECOPD in COPDGene.
We next aimed to replicate our findings using data from COPDGene, in which participants were followed for 64 (IQR25–75 45–73) months and 100 (74%) developed at least 1 AECOPD (median 3; IQR25–75 0–6). In this cohort, participants with elevated MMP-9 had a 13% higher absolute risk (83% vs. 70%, P = 0.103) for having ≥1 AECOPD during follow-up compared with the group without elevated MMP-9. Elevated MMP-9 was independently associated with prospective AECOPD (OR, 3.03; 95% CI, 1.02–9.01; P = 0.046) in adjusted logistic regression models (Figure 4A and Supplemental Table 2). There was no significant interaction between elevated MMP-9 and WBC (P = 0.081) or elevated MMP-9 and PMN counts (P = 0.26) for AECOPD. In COPDGene, the median (IQR25–75) annualized AECOPD rate was higher in the elevated MMP-9 group compared with the nonelevated group (0.9 [0.5–2] events/year versus 0.5 [0–1.4] events/year, P = 0.029). In Poisson models adjusted for age, sex, race, FEV1 percent predicted, current smoking, chronic bronchitis, WBC count, and previous AECOPD, MMP-9 elevation was associated with increased AECOPD IRR (IRR, 1.24; 95% CI, 1.03–1.49; P = 0.024; Figure 4B and Supplemental Table 3). Unadjusted Poisson models for both cohorts are reported in Supplemental Table 4. Elevated MMP-9 was associated with a shorter median time-to-first AECOPD in COPDGene (14 versus 21 months, P = 0.065), as shown in Figure 5B. There were no associations between TIMP-1 and AECOPD in either cohort (Supplemental Tables 1 and 4).
Discussion
Elevated plasma MMP-9 was associated with the risk of prospective acute exacerbations in 2 well-characterized COPD populations, even when adjusted for established clinical risk factors for exacerbations, including lung function; symptoms including chronic bronchitis; leukocyte counts; and previous exacerbations (13). These findings help advance our understanding of the potential role for MMP-9 in the natural history of COPD, providing insight about the stability of MMP-9 measurement over time and measuring the prognostic significance of elevated circulating MMP-9. A major strength of our study was replicating the associations between MMP-9 elevation and AECOPD in 2 different clinical cohorts. Our findings address a key first step in establishing evidentiary criteria suggested by the Foundation for the NIH (FNIH) in qualifying MMP-9 elevation as a blood-based biomarker with clinical utility in COPD (14).
These results extend the few studies evaluating the association of MMP-9 in COPD populations by linking systemic levels of the protease to a clinically meaningful prospective outcome. In contrast to our study, most of the published literature about MMP-9 focuses on sputum or bronchoalveolar lavage (BAL) MMP-9 in COPD. These studies have shown associations between MMP-9 and lower lung function, metrics of small airways disease or emphysema on CT imaging (15, 16), and elevated MMP-9 levels during AECOPD (17–20). Although these studies have provided a rationale for the importance of MMP-9 in COPD, they have been limited by relatively small sample sizes and reliance on BAL or sputum measurements, samples that are impractical for deploying into routine clinical care. To our knowledge, the largest longitudinal study of plasma MMP-9 in non–α-1 anti-trypsin–related COPD included 101 participants (21). In that study, D’Armiento and colleagues did not show relationships with disease progression, but they did not assess exacerbation risk. In α-1 anti-trypsin deficiency, plasma MMP-9 is associated with greater exacerbation frequency (22), supporting our current findings. Our study addressed gaps in our understanding of the prognostic importance of MMP-9 in COPD through analyzing 2 of the largest, well-characterized, prospective cohorts of COPD patients to date.
The use of an empirically defined biomarker values has been used previously in COPD (23, 24). In the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort, Agustí and colleagues described an endotype of persistent systemic inflammation by measuring a panel of 6 nonspecific inflammatory biomarkers, including WBC, c-reactive protein (CRP), IL-6, IL-8, fibrinogen, and TNF-α (23). Participants with ≥2 elevated biomarkers compared with those with no biomarker elevations had higher rates of AECOPD the year prior to enrollment, higher rates of smoking, and more symptoms. In an analysis of 2 general population–based studies from Copenhagen, Thomsen et al. found associations between a panel of inflammatory biomarkers (WBC, CRP, and fibrinogen) and exacerbation risk (24). The authors found that the risk of AECOPD increased as the number of elevated biomarkers increased in a dose-dependent manner, albeit the overall magnitude of effects (5%, 12%, or 20% absolute difference for elevations in 1, 2, or 3 markers, respectively) were similar to our current observations with elevated MMP-9 (13%–16% absolute difference) as a single biomarker. As these panels of nonspecific biomarker elevations aid in defining an inflammatory phenotype, elevated MMP-9 alone identified a distinct COPD endotype characterized by current smokers with chronic bronchitis and evidence of increased systemic inflammation, exacerbation risk, and shorter time-to-first event. Unlike the analytes included in the panels mentioned above, MMP-9 may be a more biologically relevant marker, given its close relationship with the pathophysiology of COPD, including tissue degradation, release of matrikines, and its role in inflammation. Further, these observations of MMP-9’s relation to AECOPD highlight the potential of examining other proteases (such as other MMPs and serine proteases; refs. 25, 26) as rational biomarkers for studies in large COPD cohorts. One may even envision a panel of proteases and antiproteases that may further delineate risk in a population for loss of lung function and/or symptom progression.
We found that elevated MMP-9 was present in a modest proportion of the overall COPD population. This observation was not surprising, given the heterogeneity of COPD, and supports the notion that precision-based approaches to managing COPD are of paramount importance. Placing this into context, COPD affects approximately 15 million patients in the US (27). Logically, one could estimate that between 1.3 and 4.3 million individuals in the US may have elevated MMP-9 and, thus, may be at increased risk for AECOPD and healthcare utilization. It is worth noting that MMP-9 elevations were predictive of exacerbation frequency in both groups, despite differences in overall exacerbation rates and severity of lung function impairment within the individual cohorts and in models adjusted for FEV1 percent predicted. Further, there was not a dose-dependent relationship between MMP-9 levels and GOLD stages, suggesting that MMP-9 does not reflect the degree of airflow limitation associated with disease severity. Hence, the information provided by MMP-9 may have a role in risk stratification in the general COPD population — findings that warrant validation.
There are several important limitations to consider for this study. First, although the populations of both cohorts are largely similar, there are some notable differences between cohorts, including lung function, smoking status, and MMP-9 values. We believe these differences may be due to population differences or batch effects. For example, participants at all SPIROMICS study sites had MMP-9 values, including representation from Black and White races. Of these, a lower proportion of Black subjects were in the elevated MMP-9 group compared with the nonelevated MMP-9 group, as shown in Table 2, though this was not statistically significant. Conversely, all participants from the COPDGene cohort were exclusively White, somewhat limiting the generalizability from COPDGene alone. Additionally, the Myriad-RBM assays were measured in 2 batches in SPIROMICS and in 1 batch in COPDGene, potentially adding variability. We did adjust analyses for batch to account for these potential differences. Despite these differences, it is intriguing that the use of a threshold level to define elevated MMP-9 performed well in both cohorts. This approach of using an extreme MMP-9 value, as opposed to an unbiased approach examining multiple biomarkers that require adjustment for multiple comparisons (4), provides unique information and may be practical in implementing in future studies. Additionally, our results were based on a single time-point measure of MMP-9 in both cohorts, and there is a lack of understanding of the stability of the elevated MMP-9 phenotype. However, our analysis of the participants in SPIROMICS with follow-up MMP-9 measurements shown in Supplemental Figure 1 suggests that MMP-9 elevation may persist over time and, thus, may be a reliable biomarker for AECOPD risk. Finally, these analyses rely on circulating MMP-9 levels in the blood. While this has obvious advantages as utilization in clinical practice, future studies should also focus on concomitant measurements of both MMP-9 levels and activity in both airway secretions (i.e., sputum or BAL) and blood to inform our understanding of MMP-9 in local versus systemic compartments in COPD.
In conclusion, this study provides evidence that MMP-9 elevation is associated with distinct clinical features and increased risk for COPD exacerbations, and it may aid in risk prediction. In addition to its potential role as a blood-based prognostic biomarker for COPD, MMP-9 elevation may also serve as a precision medicine–based therapeutic target as novel interventions directed at MMP-9 modulation are developed.
Methods
Study populations.
The SPIROMICS is a multicenter prospective observational study aimed at subclassifying COPD participants into groups through identification of biomarkers and phenotypes that can be used as intermediate outcomes to reliably predict clinical benefit in future clinical trials (ClinicalTrials.gov, NCT01969344) (28). SPIROMICS enrolled 2,982 individuals between November 2011 and January 2015. Participants were categorized into 4 distinct strata: Strata 1, never smokers; Strata 2, current and former smokers without airflow obstruction; and Stratas 3–4, which comprised current and former smokers with COPD, defined as a postbronchodilator FEV1/forced vital capacity (FVC) < 0.70. Participants in Strata 3 had an FEV1 > 50% and Strata 4 had an FEV1 < 50% predicted (29). Participants underwent baseline and annual in-person follow-up visits plus quarterly telephone calls. We used SPIROMICS participants in Stratas 1 and 2 (non-COPD) to define elevated MMP-9, and participants in Stratas 3 and 4 (COPD) were used for analysis of COPD exacerbations. Data reported here include results from the SPIROMICS Core3 dataset (n = 2,954 subjects). For these studies, we report data from subjects with COPD, complete clinical information, MMP-9 measurements, WBC count, and appropriate longitudinal follow-up AECOPD ascertainment.
As a replication cohort, we used the COPDGene study (ClinicalTrials.gov, NCT00608764; ref. 30). Participants with COPD, MMP-9 and WBC count measurements, and prospective AECOPD information were included in the analysis. These participants were followed longitudinally at 6-month intervals via an automated telephony system, via web-based survey, or by telephone contact (31).
Plasma MMP-9 measurement.
Plasma was collected from participants in both studies at baseline. MMP-9 and TIMP-1 were measured using a commercially available Myriad-RBM multiplex assay in SPIROMICS (32) and via a custom Myriad-RBM multiplex assay in COPDGene. MMP-9 values were log-transformed to fit a normal distribution. We defined elevated MMP-9 a priori by plasma MMP-9 >95th percentile (MMP-9 >2.67 ng/ml, log transformed) as measured in non-COPD SPIROMICS participants. For SPIROMICS, MMP-9 was measured in 2 separate batches due to sample availability (batch 1 was run in May 2013, and batch 2 was run in February 2014). A subset of participants in SPIROMCIS had plasma MMP-9 measured at a subsequent visit that occurred 1 year after the baseline visit.
Phenotypic measurements.
Demographics included age, White or Black race, and sex; comorbidities were self-reported; smoking status was defined as current or former; and smoking history was reported in pack-years. Chronic bronchitis was defined by chronic cough and phlegm definitions (33). Pulmonary function testing was performed according to American Thoracic Society/ European Respiratory Society (ATS/ERS) criteria (29); postbronchodilator spirometry was recorded using a KoKo spirometer in SPIROMICS and an ndd EasyOne spirometer in COPDGene. Participants were stratified according to GOLD stage (34).
COPD exacerbation assessment.
In both cohorts, AECOPD were self-reported and defined as a worsening of respiratory symptoms lasting longer than 48 hours that warranted treatment with antibiotics and/or systemic corticosteroids, irrespective of treatment location (35).
Statistics.
Descriptive statistics, including means and SDs for continuous data and frequencies and percentages for categorical data, were calculated for all study variables of interest. Bivariate analyses were conducted by using the unpaired t test or Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables. ANOVA was used to compare logMMP-9 values across GOLD stages. Logistic regression models were used to identify associations with having ≥1 AECOPD during follow-up. The frequency of prospective AECOPD followed a Poisson distribution in both cohorts (Supplemental Figure 2); thus, Poisson regression models were used to measure associations with AECOPD frequency by calculating IRR and their corresponding 95% CIs.
Variables that were known to have relevance with AECOPD risk; plus, variables statistically significant in univariate analyses were included in the multivariable logistic regression and Poisson models, with the same set of variables being included in all multivariable analyses. Variables in the final adjusted logistic and Poisson regression models included age, sex, race, postbronchodilator FEV1 percent predicted, current smoking status, chronic bronchitis, WBC count, having ≥1 prior exacerbation in the previous year, and elevated MMP-9, as well as the batch analysis (in SPIROMICS only). An interaction term was added to the multivariable models for both study cohorts in order to determine whether there was a statistically meaningful interaction between MMP-9 and WBC count. Pearson’s correlation analyses were performed to assess the correlation between the log-transformed MMP-9 values and the frequency of exacerbations for both study cohorts. Kaplan-Meier survival analysis was used to identify time-to-first AECOPD based on the presence or absence of elevated MMP-9. All statistical tests were 2-sided and were performed using a significance level of P < 0.05. Statistical analyses were conducted using SAS software (version 9.4; SAS Institute).
Study approval.
The study protocols for SPIROMICS and COPDGene were approved by the IRBs at all participating sites. All participants in both studies provided written informed consent prior to inclusion in the respective study.
Author contributions
JMW had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis. JMW and AG contributed to the conception and design of the study. JMW, MMP, RAO, RPB, MHC, WO, EKS, JDC, PW, and PJC contributed to the acquisition of the data. JMW, MMP, RAO, MTD, SPB, PJC, and AG contributed to the drafting of the manuscript. JMW, MMP, RAO, RPB, MTD, SPB, MHC, VK, JLC, FJM, RP, WO, WWL, RJK, IB, MKH, EKS, JDC, RGB, PW, PJC, and AG contributed to revisions of the manuscript for critically important intellectual content. All of the authors approved this version of the manuscript to be published.
Supplementary Material
Acknowledgments
This work was supported by COPDGene grants U01 HL089897 and U01 HL089856. See Supplemental Acknowledgments for SPIROMICS and COPDGene investigators details.
Version 1. 11/15/2018
Electronic publication
Funding Statement
K08 HL123940 to JMW; R01HL124233 to PJC; VA Merit Review I01 CX000911 to JLC; R01 (R01HL102371, R01HL126596) and VA Merit (I01BX001756) to AG. SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study) is funded by contracts from the NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, and HHSN268200900020C), supplemented by contributions made through the Foundation for the National Institutes for Health COPDGene is funded by the NHLBI (R01 HL089897 and R01 HL089856) and by the COPD Foundation
Footnotes
Conflict of interest: JMW has received grant support and consulting fees from GSK, AZ, Gilead, Bayer, Quintiles, Mylan, and Mereo BioPharma. MTD has received grants from; Consulting fees from AstraZeneca, BI, GSK, PneummRx/BTG, and Quark; and contracted clinical trial support from AstraZeneca, BI, Boston Scientific, GSK, Novartis PneumRx/BTG, Pulmonx, and Yungjin. JLC has received grants from MedImmune Corp. Ltd. IB has received grant support and consulting fees from AZ, Grifols, CSL Behring, and Amgen. EKS has received honoraria from Novartis for Continuing Medical Education Seminars and grant and travel support from GSK. PJC has received grant support and consulting fees from GSK. AG has received grant support from the and consulting fees from Gilead Sciences, Grifols Inc., and Celtaxsys Inc.
License: Copyright 2018, American Society for Clinical Investigation.
Reference information: JCI Insight. 2018;3(22):e123614. https://doi.org/10.1172/jci.insight.123614.
Contributor Information
Michael H. Cho, Email: remhc@channing.harvard.edu.
Victor Kim, Email: victor.kim@tuhs.temple.edu.
Jeffrey L. Curtis, Email: jlcurtis@med.umich.edu.
Robert J. Kaner, Email: rkaner@med.cornell.edu.
Igor Barjaktarevic, Email: ibarjaktarevic@mednet.ucla.edu.
Amit Gaggar, Email: agaggar1@uab.edu.
References
- 1.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 2.Dransfield MT, et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748–1755. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 4.Keene JD, et al. Biomarkers Predictive of Exacerbations in the SPIROMICS and COPDGene Cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J. 2012;39(1):197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 6.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61(3):259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44-46:122–129. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, et al. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One. 2011;6(1):e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an Essential Constituent of Antigenic Determinants Recognized by Rheumatoid Arthritis-specific Autoantibodies. 1998. J Immunol. 2015;195(1):8–16. [PubMed] [Google Scholar]
- 12.Omland T, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JR, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 14.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker Qualification: Toward a Multiple Stakeholder Framework for Biomarker Development, Regulatory Acceptance, and Utilization. Clin Pharmacol Ther. 2015;98(1):34–46. doi: 10.1002/cpt.136. [DOI] [PubMed] [Google Scholar]
- 15.Ostridge K, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71(2):126–132. doi: 10.1136/thoraxjnl-2015-207428. [DOI] [PubMed] [Google Scholar]
- 16.Koo HK, Hong Y, Lim MN, Yim JJ, Kim WJ. Relationship between plasma matrix metalloproteinase levels, pulmonary function, bronchodilator response, and emphysema severity. Int J Chron Obstruct Pulmon Dis. 2016;11:1129–1137. doi: 10.2147/COPD.S103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cane JL, Mallia-Millanes B, Forrester DL, Knox AJ, Bolton CE, Johnson SR. Matrix metalloproteinases -8 and -9 in the Airways, Blood and Urine During Exacerbations of COPD. COPD. 2016;13(1):26–34. doi: 10.3109/15412555.2015.1043522. [DOI] [PubMed] [Google Scholar]
- 18.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol. 2009;86(4):913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papakonstantinou E, et al. Acute exacerbations of COPD are associated with significant activation of matrix metalloproteinase 9 irrespectively of airway obstruction, emphysema and infection. Respir Res. 2015;16:78. doi: 10.1186/s12931-015-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Armiento JM, et al. Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema. PLoS ONE. 2013;8(2):e56352. doi: 10.1371/journal.pone.0056352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omachi TA, Eisner MD, Rames A, Markovtsova L, Blanc PD. Matrix metalloproteinase-9 predicts pulmonary status declines in α1-antitrypsin deficiency. Respir Res. 2011;12:35. doi: 10.1186/1465-9921-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agustí A, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen M, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 25.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandhaus RA, Turino G. Neutrophil elastase-mediated lung disease. COPD. 2013;10 Suppl 1:60–63. doi: 10.3109/15412555.2013.764403. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control Prevention (CDC) Chronic obstructive pulmonary disease among adults--United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(46):938–943. [PubMed] [Google Scholar]
- 28.Couper D, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-204992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 30.Regan EA, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart JI, et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neal WK, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9. doi: 10.1186/1479-5876-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 34.Vogelmeier CF, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 35.Han MK, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.