Abstract
Background and Aims
Extraintestinal manifestations [EIMs] such as arthritis/arthralgia are common in inflammatory bowel disease. We performed post hoc analyses of data from the GEMINI studies to evaluate the effect of vedolizumab, a gut-selective anti-trafficking agent, on arthritis/arthralgia.
Methods
Sustained resolution of baseline arthritis/arthralgia, worsening of baseline arthritis/arthralgia, the occurrence of new arthritis/arthralgia, and the composite of new/worsening arthritis/arthralgia were evaluated. Cox modelling was used for time-to-event analysis. The influence of corticosteroid-tapering was also investigated.
Results
In Crohn’s disease [CD] patients, vedolizumab was significantly less likely than placebo to be associated with new/worsening arthritis/arthralgia (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.44–0.89). Similar incidences of sustained resolution of arthritis/arthralgia occurred with vedolizumab and placebo. In CD patients on corticosteroids at baseline, a decrease in corticosteroid dose increased the risk of new/worsening arthritis/arthralgia (odds ratio [OR], 7.49; 95% CI, 3.50–15.97) regardless of treatment; and in those achieving corticosteroid-free status, arthritis/arthralgia was less likely with vedolizumab than with placebo [HR, 0.14; 95% CI, 0.05–0.35]. In ulcerative colitis [UC] patients, vedolizumab and placebo showed a similar incidence of new/worsening of arthritis/arthralgia. In UC patients on corticosteroid at baseline, arthritis/arthralgia was more likely in those achieving corticosteroid-free status than in those continuing corticosteroids (HR 2.63 [95% CI 1.13–6.11]); and in those achieving corticosteroid-free status, the incidence of arthritis/arthralgia was similar with vedolizumab and placebo.
Conclusions
Vedolizumab therapy was associated with a reduced likelihood of new/worsening arthritis/arthralgia in CD and no increased incidence of these events in UC.
Studies included [ClincialTrials.gov, number]
GEMINI 1 [NCT00783718]; GEMINI 2 [NCT00783692]; GEMINI 3 [NCT01224171].
Keywords: Vedolizumab, extraintestinal manifestations, inflammatory bowel disease
1. Introduction
Extraintestinal manifestations [EIMs] of inflammatory bowel disease [IBD] occur in up to 55% of patients with Crohn’s disease [CD] and 35% of those with ulcerative colitis [UC].1–3 Although arthritis/arthralgia is the most common EIM in both disorders,2,3 multiple organs may be affected including skin, eye and liver. While the pathophysiology of EIMs is poorly understood, current theories indicate an immune response to antigens entering the systemic circulation from an inflamed gastrointestinal mucosa or migration of pro-inflammatory leucocytes from the gut to target organs.4,5
Effective management of EIMs is an important component of patient care. Given that corticosteroids and tumour necrosis factor [TNF] antagonists act systemically, treatment with these agents usually controls arthritis/arthralgia and other EIMs.6–8 However, TNF antagonists are not uniformly effective for either bowel-related symptoms or EIMs. Approximately one-third of patients fail to respond to induction therapy with these agents, and relapse in responders is common.9 Furthermore, TNF antagonists are associated with an increased risk of serious infection, drug-induced arthralgias and psoriasiform skin lesions.10–12 Consequently, a need exists for treatments that provide sustained clinical remission, are safe and well-tolerated, and control EIMs.
Vedolizumab [Entyvio®, Takeda Pharmaceuticals America, Inc., Deerfield, IL, USA], a monoclonal antibody targeting the α4β7 integrin that selectively blocks lymphocyte trafficking to the gastrointestinal tract, is approved for the treatment of patients with moderately to severely active IBD.13–15 Vedolizumab has demonstrated long-term effectiveness16–20 and has a favourable safety profile with a low risk of serious infections.21 However, it is currently unknown whether a gut-selective mechanism of action is optimal for controlling EIMs. On the one hand, if the pathophysiology of these conditions is driven by mucosal inflammation, treatment directed to that process should be effective. Alternatively, if EIMs are the result of systemic immune activation, then a gut-selective therapy might be less effective than corticosteroids or TNF antagonists. To investigate this question, we evaluated the effect of vedolizumab treatment on new and existing EIMs using data from the pivotal GEMINI clinical trials.
2. Methods
2.1. Study populations and treatment groups
GEMINI 1 [UC] and GEMINI 2 [CD] were phase 3, randomized, placebo-controlled trials of long-term vedolizumab treatment [52 weeks] with separate induction and maintenance phases [details published previously].16,17 GEMINI 3 [CD] was a phase 3, randomized, placebo-controlled 10-week induction study predominantly in patients refractory to TNF antagonists.18 For all three studies, the protocols were approved by an investigational review board at each centre, and all patients gave written informed consent.16–18
The current analyses investigated three treatment groups from GEMINI 1 and 2 [Supplementary Figure 1]. The PLA group comprised patients receiving placebo during induction and maintenance phases. The VDZ/PLA group comprised vedolizumab responders at Week 6 after two induction doses at Weeks 0 and 2, who were subsequently randomized to placebo during maintenance. The VDZ group comprised patients receiving vedolizumab during induction and maintenance, and included both vedolizumab responders [both Q4W and Q8W dosing regimens during maintenance] and non-responders [Q4W during maintenance] at Week 6. GEMINI 3 included VDZ and PLA groups only; patients received three induction doses at Weeks 0, 2 and 6.
2.2. EIM data and endpoints
The EIMs evaluated were arthritis/arthralgia, aphthous stomatitis, erythema nodosum, iritis/uveitis and pyoderma gangrenosum.
In GEMINI 2 and 3, EIM source data included the Crohn’s Disease Activity Index [CDAI] and reported adverse events [AEs]. Baseline prevalence and distribution of these outcomes were documented using the CDAI diary card, which captured the presence of each EIM [yes/no] at screening, baseline and every 4 weeks during treatment until the end of the study. AE report forms captured events that were new/worsening in severity during treatment.
The following endpoints were defined for GEMINI 2 and 3: [1] sustained resolution of a baseline EIM [defined as a report of a baseline EIM and two or more subsequent study visits with no EIM reports, without recurrence until study completion; captured using the CDAI]; [2] occurrence of a new EIM [no report at baseline using CDAI, and captured using CDAI or AE report forms at any subsequent study visit]; [3] worsening of a baseline EIM [reported at baseline using CDAI, and reported as an AE at any follow-up visit]; and [4] composite of occurrence of a new EIM or worsening of a baseline EIM [capturing the overall worsening aspect of EIMs; subsequently referred to as ‘new/worsening EIM’].
In GEMINI 1, only AE report forms were available for analysis and it was not possible to distinguish between new or worsening of baseline EIMs. Therefore, only the composite endpoint of a new/worsening EIM was captured. Unlike the CDAI, the Mayo score, which is used to assess clinical disease activity in UC, does not include EIMs as a measure of disease activity.
2.3. Statistical methods
Endpoints were evaluated in the overall safety population and in the subgroup of patients receiving concomitant corticosteroids at baseline. Kaplan–Meier plots and Cox regression models were generated to describe the time-to-event course for the designated endpoints. Results were based upon actuarial risk estimates, and expressed as hazard ratios [HRs] with 95% confidence intervals [CIs] with adjustment for covariation using the Cox model. Supplementary Table 1 lists all covariates tested and the significant covariates included in each Cox model.
In the corticosteroid subgroup, the endpoint of occurrence of new/worsening arthritis was analysed for those who achieved or did not achieve corticosteroid-free status. In addition, the effect of a decrease in corticosteroid dose in the 4 weeks prior to this endpoint was assessed using a generalized estimating equations model for repeated measures with treatment group, prior exposure to TNF antagonist, baseline corticosteroid dose and time-dependent corticosteroid dose decrease as explanatory covariates.
The potential associations between sustained resolution of baseline arthritis/arthralgia or new/worsening arthritis/arthralgia and clinical remission or response [CDAI score ≤150 or ≥150-point decrease in CDAI score, respectively] were explored using a chi-square test at weeks 6 and 52 in GEMINI 1 and 2, and at weeks 6 and 10 in GEMINI 3.
3. Results
3.1. Baseline characteristics
Baseline characteristics of CD and UC patients with baseline EIMs included in these analyses were generally similar between treatment groups in all three GEMINI studies [Table 1]. Over half of the patients in each study received concomitant corticosteroids, while approximately 50% had failed prior TNF antagonist therapy in GEMINI 1 and 2, compared with 76% of those in GEMINI 3. Given the high rate of arthritis/arthralgia and the low occurrence of other EIMs, the analysis of endpoints focused exclusively on arthritis/arthralgia.
Table 1.
Baseline characteristics of patients with a historya of EIMs in UC and baselineb EIMs in CD
| Characteristic | UC [GEMINI 1]a | CD [GEMINI 2]b | CD [GEMINI 3]b | |||||
|---|---|---|---|---|---|---|---|---|
| VDZ n = 199 |
VDZ/PLA n = 36 |
PLA n = 38 |
VDZ n = 394 |
VDZ/PLA n = 77 |
PLA n = 82 |
VDZ n = 99 |
PLA n = 107 |
|
| Disease duration, mean [SD] years | 7.6 [6.6] | 10.6 [8.0] | 9.0 [9.8] | 9.8 [7.7] | 10.0 [8.7] | 7.9 [7.5] | 10.8 [8.5] | 11.5 [9.0] |
| Disease duration ≥7 years, n [%] | 83 [42] | 23 [64] | 15 [39] | 220 [56] | 42 [55] | 33 [40] | 61 [62] | 66 [62] |
| Disease activityc | ||||||||
| CDAI, mean [SD] | – | – | – | 319.1 [69.5] | 323.3 [66.9] | 325.4 [78.3] | 313.9 [51.4] | 298.8 [48.7] |
| CDAI >330, n [%] | – | – | – | 168 [43] | 30 [39] | 36 [44] | 38 [38] | 27 [25] |
| MS, mean [SD] | 8.7 [1.7] | 8.7 [1.7] | 8.7 [2.0] | – | – | – | – | – |
| MS <6, n [%] | 4 [2] | 2 [6] | 1 [3] | – | – | – | – | – |
| MS 6–8, n [%] | 90 [45] | 16 [44] | 16 [42] | – | – | – | – | – |
| MS 9–12, n [%] | 105 [53] | 18 [50] | 21 [55] | – | – | – | – | – |
| CRP, mean [SD] mg/l | – | – | – | 19.3 [25.8] | 17.4 [20.6] | 23.3 [28.4] | 18.6 [26.0] | 17.3 [21.9] |
| CRP >10 mg/l, n [%] | – | – | – | 191 [48] | 38 [49] | 43 [52] | 42 [42] | 49 [46] |
| Concomitant therapy, n [%] | ||||||||
| CS and IM | 27 [14] | 5 [14] | 4 [11] | 64 [16] | 11 [14] | 10 [12] | 19 [19] | 19 [18] |
| CS only | 73 [37] | 18 [50] | 16 [42] | 132 [34] | 32 [42] | 29 [35] | 31 [31] | 33 [31] |
| IM only | 31 [16] | 6 [17] | 4 [11] | 62 [16] | 12 [16] | 15 [18] | 14 [14] | 13 [12] |
| Prior TNF antagonist, n [%]d | ||||||||
| Use | 118 [59] | 17 [47] | 25 [66] | 265 [67] | 41 [53] | 42 [51] | 71 [72] | 85 [79] |
| Failure | 103 [52] | 15 [42] | 22 [58] | 213 [54] | 38 [49] | 39 [48] | 70 [71] | 84 [79] |
| Smoking status, n [%]e | ||||||||
| Current smoker | 16 [8] | 3 [8] | 3 [8] | 118 [30] | 28 [36] | 19 [23] | 39 [39] | 37 [35] |
| Former smoker | 71 [36] | 13 [36] | 15 [39] | 94 [24] | 19 [25] | 15 [18] | 22 [22] | 28 [26] |
| Prior surgery for CD, n [%] | – | – | – | 194 [49] | 26 [34] | 31 [38] | 51 [52] | 50 [47] |
CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; CS, corticosteroid; EIM, extraintestinal manifestation; IM, immunomodulator; PLA, placebo; SD, standard deviation; TNF, tumour necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab.
aData collected using case report forms during screening visit asking patients about their UC history over last 12 months. Patients could have either experienced an EIM within the past 12 months or had an active EIM at baseline.
bData collected using CDAI and recorded at screening, baseline and Q4W. Patients had active EIMs at baseline.
cData missing for one patient in the GEMINI 2 PLA group.
dGEMINI 1 and 2 enrolled patients with prior TNF antagonist use, while GEMINI 3 enrolled patients with prior TNF antagonist failure.
eData missing for one patient in the GEMINI 2 VDZ group.
3.2. Crohn’s disease: GEMINI 2 and 3
In GEMINI 2, a total of 554 EIM events were recorded at any time between baseline and the end of treatment. Most of these events were captured by the CDAI [75% vs 43% as AEs]; this comprised 317 [57%] events captured by CDAI alone, 139 [25%] events by AE reports alone, and 98 [18%] events by both CDAI and AE reports. In GEMINI 3, a total of 70 events were recorded. Similar to GEMINI 2, most events were captured on the CDAI assessment [64%] rather than on AE reports [46%]: 38 events [54%] on CDAI alone, 25 events [36%] on AE reports alone and seven events [10%] on both.
3.2.1. Sustained resolution of baseline arthritis/arthralgia
In the CD patients in these trials, the prevalence and distribution of EIMs at baseline were similar for all treatment groups [Table 2].
Table 2.
Prevalence and distribution of baseline EIMs in CD patients
| EIM, n [%] | CD [GEMINI 2] | CD [GEMINI 3] | |||
|---|---|---|---|---|---|
| VDZ n = 814 |
VDZ/PLA n = 153 |
PLA n = 148 |
VDZ n = 209 |
PLA n = 207 |
|
| Prevalence of baseline EIMs | |||||
| Arthritis/arthralgia | 367 [45] | 71 [46] | 78 [53] | 94 [45] | 98 [47] |
| Aphthous stomatitis | 39 [5] | 6 [4] | 6 [4] | 8 [4] | 14 [7] |
| Erythema nodosum | 21 [3] | 6 [4] | 4 [3] | 8 [4] | 12 [6] |
| Iritis/uveitis | 14 [2] | 0 [0] | 3 [2] | 3 [1] | 2 [<1] |
| Pyoderma gangrenosum | 4 [<1] | 1 [<1] | 0 [0] | 1 [<1] | 1 [<1] |
| EIM, n [%] | CD [GEMINI 2] | CD [GEMINI 3] | |||
|---|---|---|---|---|---|
| VDZ na = 394 |
VDZ/PLA na = 77 |
PLA na = 82 |
VDZ na = 99 |
PLA na = 107 |
|
| Distribution of baseline EIMs | |||||
| Arthritis/arthralgia | 367 [93] | 71 [92] | 78 [95] | 94 [95] | 98 [92] |
| Aphthous stomatitis | 39 [10] | 6 [8] | 6 [7] | 8 [8] | 14 [13] |
| Erythema nodosum | 21 [5] | 6 [8] | 4 [5] | 8 [8] | 12 [11] |
| Iritis/uveitis | 14 [4] | 0 [0] | 3 [4] | 3 [3] | 2 [2] |
| Pyoderma gangrenosum | 4 [1] | 1 [1] | 0 [0] | 1 [1] | 1 [1] |
CD, Crohn’s disease; EIM, extraintestinal manifestation; PLA, placebo; VDZ, vedolizumab.
aTotal patients reporting at least one baseline EIM.
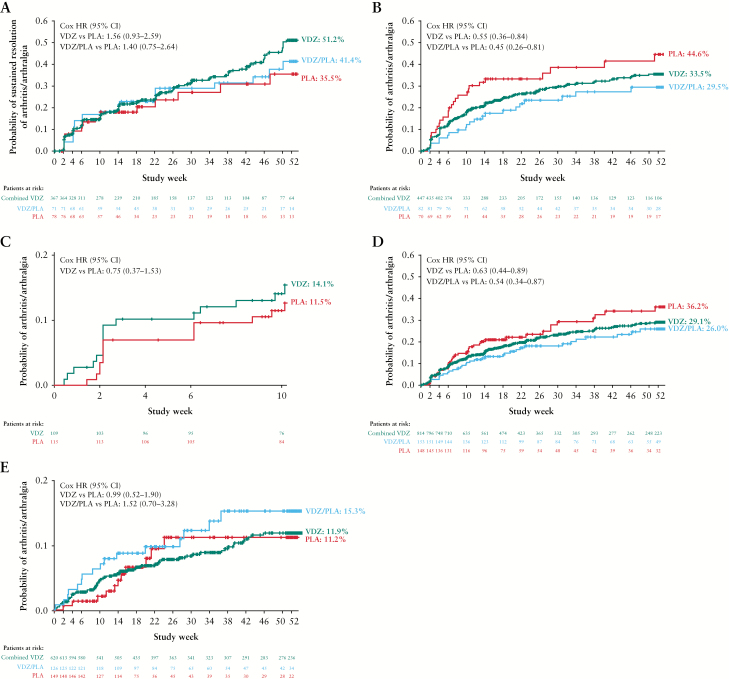
The Kaplan–Meier plots of time to sustained resolution of arthritis/arthralgia in GEMINI 2 are shown in Figure 1A. Although the results of the Cox analysis favoured patients assigned to vedolizumab over placebo, the differences were not statistically significant [VDZ vs PLA: HR, 1.56; 95% CI, 0.93–2.59; VDZ/PLA vs PLA: HR, 1.40; 95% CI, 0.75–2.64] [Supplementary Figure 2A].
Figure 1.
Kaplan–Meier analyses for [A] time to sustained resolution of baseline arthritis/arthralgia in CD [GEMINI 2], [B and C] time to occurrence of new arthritis/arthralgia in CD [GEMINI 2 and 3 respectively], [D] time to new/worsening arthritis/arthralgia in CD [GEMINI 2], and [E] time to new/worsening of arthritis/arthralgia in UC [GEMINI 1]. In GEMINI 1 and 2, the VDZ group includes both induction responders and non-responders, the VDZ/PLA group is responders only, and the PLA group is both responders and non-responders. CD, Crohn’s disease; PLA, placebo; VDZ, vedolizumab; UC, ulcerative colitis.
In the short-term GEMINI 3 study, the rates of sustained resolution of baseline arthritis/arthralgia at Week 10 were 22% [n = 21/94] and 16% [n = 16/98] in the VDZ and PLA groups, respectively. Cox analysis showed no significant difference in the relative likelihood of sustained resolution with VDZ or PLA [HR, 1.40; 95% CI, 0.73–2.67] [Supplementary Figure 2B].
In GEMINI 2, lower rates of early withdrawal for any cause were reported in CD patients with sustained resolution of arthritis/arthralgia compared to those without sustained resolution across all treatment groups [36.1% vs 72.0% for the overall population].
3.2.2. New and new/worsening arthritis/arthralgia
The incidence of all new EIMs, except for arthritis/arthralgia, in GEMINI 2 was low and not substantially different between treatment groups [Table 3 and Figure 1B]. Cox analysis results indicated that the VDZ groups were significantly less likely than PLA groups to have a new occurrence of arthritis/arthralgia [VDZ vs PLA: HR, 0.55; 95% CI 0.36–0.84; and VDZ/PLA vs PLA: HR, 0.45; 95% CI, 0.26–0.81] [Supplementary Figure 3A]. Patients with prior TNF antagonist exposure were more likely to experience a new occurrence of arthritis/arthralgia than patients naive to TNF antagonists [HR, 2.20; 95% CI, 1.56–3.11].
Table 3.
New EIMs in CD patients [GEMINI 2 and 3]a
| EIM, n [%] | GEMINI 2a | GEMINI 3a | |||
|---|---|---|---|---|---|
| VDZ | VDZ/PLA | PLA | VDZ | PLA | |
| Arthritis/arthralgia | n = 447 | n = 82 | n = 70 | n = 115 | n = 109 |
| 125 [28] | 21 [26] | 27 [39] | 14 [12] | 17 [16] | |
| Aphthous stomatitis | n = 775 | n = 147 | n = 142 | n = 201 | n = 193 |
| 41 [5] | 4 [3] | 11 [8] | 10 [5] | 5 [3] | |
| Erythema nodosum | n = 793 | n = 147 | n = 144 | n = 201 | n = 195 |
| 11 [1] | 2 [1] | 6 [4] | 3 [1] | 1 [<1] | |
| Iritis/uveitis | n = 800 | n = 153 | n = 145 | n = 206 | n = 205 |
| 11 [1] | 4 [3] | 2 [1] | 3 [1] | 2 [<1] | |
| Pyoderma gangrenosum | n = 810 | n = 152 | n = 148 | n = 208 | n = 206 |
| 1 [<1] | 1 [<1] | 0 [0] | 0 [0] | 0 [0] | |
CD, Crohn’s disease; EIM, extraintestinal manifestation; PLA, placebo; VDZ, vedolizumab.
aFor each EIM, incidence is expressed as the percentage of patients without that EIM at baseline.
In GEMINI 3, the incidence of new EIMs was low and similar between treatment groups, with new arthritis/arthralgia as the most common EIM [Table 3]. The Kaplan–Meier plot of time to new arthritis/arthralgia showed low and similar event probability in both treatment groups [Figure 1C]. Cox analysis found no difference in the likelihood of new arthritis/arthralgia in the VDZ than the PLA group [HR, 0.75; 95% CI, 0.37–1.53] [Supplementary Figure 3B].
In GEMINI 2, survival analysis showed a significant difference in new/worsening arthritis/arthralgia between treatment groups. The Kaplan-Meier plot of time to new/worsening arthritis/arthralgia is shown in Figure 1D. Cox analysis of GEMINI 2 indicated that the VDZ and VDZ/PLA groups were significantly less likely to have new/worsening arthritis/arthralgia compared with those assigned to PLA [VDZ vs PLA: HR, 0.63; 95% CI, 0.44–0.89; and VDZ/PLA vs PLA: HR, 0.54; 95% CI, 0.34–0.87] [Supplementary Figure 4]. Patients with prior TNF antagonist exposure were more likely to experience new/worsening arthritis/arthralgia than patients naive to TNF antagonists [HR, 1.81; 95% CI, 1.37–2.38].
In GEMINI 3, 9% [n = 19/209] in the VDZ group and 11% [n = 23/207] in the PLA group experienced new/worsening arthritis/arthralgia. Kaplan–Meier and Cox analyses were not performed due to insufficient numbers of events.
There were no notable differences in any-cause withdrawal before study end between CD patients with or without new/worsening arthritis/arthralgia in GEMINI 2 [59.4% vs 62.7%] or GEMINI 3 [5.7% vs 12.9%].
3.2.3. Influence of corticosteroid therapy
In GEMINI 2, in the 50% [n = 278/553] of patients with baseline EIM on concomitant corticosteroids, Cox time-dependent analysis indicated that the VDZ group was significantly more likely to achieve sustained resolution of baseline arthritis/arthralgia compared with PLA [HR, 2.66; 95% CI, 1.03–6.88] but not with VDZ/PLA compared with PLA [HR, 1.91; 95% CI, 0.61–6.01]. In the 50% [n = 552/1115] of all patients with or without EIMs who received concomitant corticosteroids, the likelihood of developing new arthritis/arthralgia was also significantly lower in the VDZ than the PLA group [HR, 0.51; 95% CI, 0.26–1.00] but was similar with VDZ/PLA compared with PLA [HR, 0.51; 95% CI, 0.22–1.20]. Patients who achieved corticosteroid-free status in the VDZ and VDZ/PLA groups were also significantly less likely to have new/worsening arthritis/arthralgia than the PLA group [VDZ: HR, 0.14; 95% CI, 0.05–0.35; VDZ/PLA: HR, 0.33; 95% CI, 0.12–0.92].
Importantly, an increase in the incidence of arthritis/arthralgia in the 4 weeks after corticosteroid dose-decrease was observed in patients receiving corticosteroids at baseline in GEMINI 2 [Supplementary Figure 5]. Repeated-measures analysis showed an increased rate of new/worsening arthritis/arthralgia events when the corticosteroid dose was decreased (odds ratio [OR], 7.49; 95% CI, 3.50–15.97), with no significant difference across the treatment groups [VDZ vs PLA: OR, 0.46; 95% CI, 0.18–1.15; and VDZ/PLA vs PLA: OR, 0.64; 95% CI, 0.30–1.35]. Consistent results were found when analysing the same data in a Cox regression for recurrent events with the same covariates.
3.3. Ulcerative colitis: GEMINI 1
3.3.1. New/worsening arthritis/arthralgia
With the exception of arthritis/arthralgia, as in CD patients, the incidence of new/worsening of EIMs was low and did not differ between treatment groups [Table 4]. Kaplan–Meier plots of time to arthritis/arthralgia showed low and similar event probabilities in all treatment groups [Figure 1E]. Cox analysis showed that there was no significant difference in the relative likelihood of events [VDZ vs PLA: HR, 0.99; 95% CI, 0.52–1.90; and VDZ/PLA vs PLA: HR, 1.52; 95% CI, 0.70–3.28] [Supplementary Figure 6].
Table 4.
New/worsening EIMs in UC patients [GEMINI 1]a
| EIM, n [%] | GEMINI 1 [UC] | ||
|---|---|---|---|
| VDZ n = 620 |
VDZ/PLA n = 126 |
PLA n = 149 |
|
| Arthritis/arthralgia | 58 [9] | 16 [13] | 11 [7] |
| Aphthous stomatitis | 2 [<1] | 0 [0] | 0 [0] |
| Erythema nodosum | 5 [<1] | 1 [<1] | 0 [0] |
| Iritis/uveitis | 4 [<1] | 0 [0] | 0 [0] |
| Pyoderma gangrenosum | 1 [<1] | 0 [0] | 0 [0] |
EIM, extraintestinal manifestation; PLA, placebo; UC, ulcerative colitis; VDZ, vedolizumab.
aIncidence expressed as the percentage of all patients in each treatment group.
In GEMINI 1, the rates of any-cause withdrawals before study end were generally similar in patients with, versus those without, new/worsening arthritis/arthralgia across treatment groups [51.8% vs 58.9% in the overall population].
3.3.2. Influence of corticosteroid therapy
Among the 51% [n = 460/895] of all GEMINI 1 patients [regardless of baseline EIM status] who received concomitant corticosteroids at baseline, the likelihood of new/worsening arthritis/arthralgia was similar in the VDZ group compared to the PLA group [HR 0.89; 95% CI, 0.37–2.16] and with VDZ/PLA compared with PLA [HR 1.26; 95% CI, 0.43–3.66]. Achieving corticosteroid-free status increased the likelihood of new/worsening arthritis/arthralgia compared with continuing concomitant corticosteroids, irrespective of treatment group [HR, 2.63; 95% CI, 1.13–6.11]. There were too few events to conduct a repeated-measures logistic regression analysis as was performed for GEMINI 2. [Supplementary Figure 5].
3.4. Potential association between clinical response/remission status and arthritis/arthralgia
In CD patients in GEMINI 2, both clinical response and clinical remission at weeks 6 and 52 were significantly associated with better sustained resolution of baseline arthritis/arthralgia [p < 0.05]. In all three GEMINI studies in both CD and UC patients, no association was observed between clinical response/remission status and new/worsening EIMs.
4. Discussion
These analyses of GEMINI 2 and 3 trial data show that the rate of sustained resolution of baseline arthritis/arthralgia was nominally higher in patients with CD receiving vedolizumab than those assigned to placebo. Furthermore, patients who received vedolizumab during the maintenance phase of GEMINI 2 were 45% less likely to develop new arthritis/arthralgia than patients who received placebo [HR, 0.55; 95% CI, 0.36–0.84] and 37% less likely to develop either new/worsening arthritis/arthralgia [HR, 0.63; 95% CI, 0.44–0.89]. In GEMINI 2, clinical response and remission at weeks 6 and 52 were associated with sustained resolution of arthritis/arthralgia. These effects were not evident in GEMINI 3; this was a short-term induction study and the lower number of events observed may have precluded identification of this treatment benefit.
In GEMINI 1, patients with UC developed new/worsening arthritis/arthralgia with a similar incidence across the treatment groups. It could be speculated that differences in underlying pathophysiology between CD and UC might account for these disparate results; however, another important explanation lies in the methods used for EIM data collection in the clinical trials. Both baseline and monthly CDAI records plus continuous AE reports throughout the study were used for CD, while AE reports alone were used for data collection for UC.
Collectively, these data indicate that treatment with vedolizumab for up to 52 weeks is not associated with new/worsening arthritis/arthralgia compared with placebo in CD or UC patients. Moreover, vedolizumab treatment was associated with a lower risk of new or worsening of baseline arthritis/arthralgia in CD patients. Prior TNF antagonist exposure in both UC and CD patients was associated with a greater likelihood of experiencing new/worsening arthritis/arthralgia compared with TNF antagonist-naive status, independent of whether patients were randomized to vedolizumab or placebo. The explanation for this finding is not obvious; however, these patients had longer disease durations and, consistent with other studies, were less likely to respond to treatment, thereby suggesting more severe underlying disease.
EIMs as an adverse event associated with vedolizumab treatment have been reported in the pivotal GEMINI studies.13,14 Pooled clinical data showed a higher incidence of arthralgia with vedolizumab than with placebo [11.6% vs 9.8%] but lower incidence of other objective joint-related events, such as arthropathies, joint effusions and joint swelling [3.1% vs 5.4%]; few of these events led to discontinuation.22 In post-marketing safety studies, joint-related events, including mostly arthralgia, were infrequent non-serious events [526 events during 46798 patient-years of vedolizumab exposure; 75% did not discontinue treatment].22 Moreover, published reports have described ‘paradoxical worsening’ of arthralgias following treatment with vedolizumab.23,24 However, we believe that the existing literature regarding the efficacy of vedolizumab in controlling EIMs is biased due to the usual limitations of observational studies—a lack of controls and an absence of blinding,23 coupled with the notion that mucosal targeted therapy cannot control non-intestinal manifestations of systemic diseases. The latter hypothesis is not supported by our data. Furthermore, one of the striking findings of our study was the strength of the association between corticosteroid withdrawal and worsening of EIMs, a factor that to our knowledge was not considered in any of the previous studies reporting a putative association between treatment with vedolizumab and worsening of EIMs.22,23
In this respect, our results revealed two interesting insights into the use of concomitant corticosteroid therapy in these patients. First, CD patients receiving concomitant corticosteroids at baseline had a more than two-fold, significantly greater likelihood of sustained resolution of arthritis/arthralgia and fewer new/worsening events when receiving vedolizumab compared with placebo. This contrasts with results in the overall population, which favoured vedolizumab over placebo, but were not significantly different. Although this subgroup analysis should be interpreted cautiously, it suggests a potential beneficial interaction for combined vedolizumab and corticosteroid therapy in controlling EIMs. This observation is potentially relevant to clinical practice. However, it is important to note that a similar relationship was not observed in UC patients.
Second, to our knowledge this study is the first to quantitatively evaluate the association between withdrawal of corticosteroids and arthritic symptoms. Although the development of arthralgia following withdrawal of corticosteroid therapy is generally accepted by clinicians, this phenomenon has not been well studied.6 In the current analysis, a reduction in corticosteroid dose in CD patients using concomitant corticosteroids at baseline was associated with a seven-fold increased risk of new/worsening arthritis/arthralgia within a 4-week time frame, regardless of whether patients were receiving vedolizumab or placebo. This observation underlines that short-term worsening or new onset of arthritis/arthralgia following corticosteroid tapering is a common occurrence and should not necessarily be causally attributed to corticosteroid-sparing agents such as vedolizumab,20–22 TNF antagonists25 or ustekinumab.26 It could be speculated that the co-administration of a systemically active anti-inflammatory therapy, such as a TNF antagonist, in this context might mask clinical symptoms of arthralgia related to corticosteroid withdrawal.
The finding that vedolizumab treatment reduced the likelihood of new and new/worsening arthritis/arthralgia has potential implications regarding the pathophysiology of EIMs in IBD. Some EIMs, such as peripheral arthritis, have been associated with bowel inflammation; others, such as pyoderma gangrenosum or ankylosing spondylitis, are proposed to occur independent of intestinal inflammation.27 Our observations are consistent with the theory that control of inflammation in the gut compartment alone is effective in reducing systemic inflammation and support development of orally administered antibodies or peptides targeting cytokines or adhesion molecules within the gut compartment with the intent of minimizing systemic immunosuppression.28 Previous real-world studies have shown that vedolizumab can reduce inflammatory EIMs that occur in parallel with intestinal inflammation while also showing that paradoxical inflammation may arise in some patients,23,24 similar to what has been reported previously in clinical studies of TNF antagonists.11,12
Our study had several limitations. First, validated instruments to evaluate IBD-related EIMs were not available. We used CDAI diary cards and clinical standardized reporting of AEs to assess these events, which is a relatively insensitive approach, rather than more specific measures of EIMs, which were not part of the GEMINI protocols. Accordingly, the study was not specifically designed or powered to evaluate EIMs. Thus, EIMs were under-reported in both CD and UC patient populations, and the low frequency of most EIMs except arthritis/arthralgia precluded analysis of skin, eye and liver disorders. This under-reporting was less likely in CD patients who, unlike UC patients, were asked directly about EIM occurrence as part of the CDAI assessment. The incidence of EIMs in the GEMINI 2 CD population was approximately three-fold that observed in the GEMINI 1 UC population. Notwithstanding the difference in reporting, we believe that these results are consistent with the literature, which shows the reported incidence of EIMs is higher in CD than in UC.1–3
Second, it was necessary to deconstruct the original randomized designs of GEMINI 1 and 2 to assess patient cohorts receiving continuous vedolizumab, vedolizumab induction followed by placebo maintenance [VDZ/PLA group], and those receiving continuous placebo. Lack of true randomization makes the findings susceptible to bias. However, despite these limitations, this study evaluated a large number of patients in whom outcomes were collected using standardized methods with both patients and investigators unaware of treatment assignment.
In conclusion, analyses of the GEMINI studies indicate that long-term treatment with vedolizumab is not associated with new occurrences or worsening of arthritis/arthralgia in UC patients and suggest potential benefits in reducing the likelihood of new occurrences or worsening of these events in CD patients even after corticosteroid withdrawal.
Funding
This study was supported by Takeda. Editorial assistance and medical writing support were sponsored by Takeda.
Conflict of Interest
BGF: Grant/Research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd, Atlantic Pharmaceuticals Ltd, Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc./Hoffmann-La Roche Ltd, Gilead Sciences Inc., GlaxoSmithKline [GSK], Janssen Research & Development LLC., Pfizer Inc., Receptos Inc. /Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, UCB. Consultant for Abbott/AbbVie, Actogenix, Akros, Albireo Pharma, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Avir Pharma, Baxter Healthcare Corp., Biogen Idec, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Inception IBD Inc, Ironwood Pharma, Janssen Biotech [Centocor], JnJ/Janssen, Kyowa Kakko Kirin Co Ltd, Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Roche/Genentech, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd, Warner-Chilcott, Wyeth, Zealand, Zyngenia. Speaker bureau for Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, UCB Pharma. Scientific advisory board Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, TiGenix, Tillotts Pharma AG, UCB Pharma. Board of directors Robarts Clinical Trials. WJS: Personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune [AstraZeneca], Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals; personal fees from Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, University of Western Ontario [owner of Robarts Clinical Trials]; grants and personal fees from Prometheus Laboratories, AbbVie, Gilead Sciences, Boehringer Ingelheim, Amgen, Takeda, Atlantic Pharmaceuticals, Bristol-Myers Squibb Genentech, GlaxoSmithKline, Pfizer, Nutrition Science Partners, Receptos, Amgen; grants, personal fees and non-financial support from Janssen; grants from Broad Foundation, American College of Gastroenterology, Exact Sciences. JFC: Consultancy/advisory board membership: AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Pfizer, Protagonist, Second Genome, Seres, Takeda, Theradiag; Speaker: AbbVie, Ferring, Takeda, Shire; Research support: AbbVie, Janssen and Janssen, Genentech, Takeda; Stock options: Intestinal Biotech Development, Genfit. SOB, CK, PG: Employees of Takeda Pharmaceuticals International AG. JMK: Employee of Takeda International - UK Branch. FB: Employee of Takeda Pharmaceuticals International Co. DTR: Consultancy: AbbVie, AbGenomics, Allergan, Inc., Amgen, Celgene Corporation, Forward Pharma, Genentech/Roche, Janssen, Merck, Miraca Life Sciences, Napo Pharmaceuticals, Pfizer, Salix Pharmaceuticals, Samsung Bioepis, Sandoz Pharmaceuticals, Shire, and Takeda; grant support from AbbVie, Genentech/Roche, Janssen, Prometheus Laboratories, Shire, Takeda, and UCB Pharma; has served on Amgen Board of Trustees; and is Co-Founder/CFO of Cornerstones Health, Inc., and Co-Founder of GoD uRn, LLC.
Author Contributions
BGF: concept of the post hoc analysis; data acquisition as a study investigator; review and interpretation of data; drafting and critical review of the manuscript; approval of the final draft. WJS, JFC, DTR: data acquisition as a study investigator; review and interpretation of data; drafting and critical review of the manuscript; approval of the final draft. SOB: concept of the post hoc analysis; review and interpretation of data; drafting and critical review of the manuscript; approval of the final draft. JMK, PG, FB: review and interpretation of data; drafting and critical review of the manuscript; approval of the final draft. CK: analysis of the data as statistician; review and interpretation of data; critical review of manuscript drafts; approval of the final draft.
Supplementary Material
Acknowledgments
We thank Nigel Bradshaw for his statistical support during early development of the analyses. Editorial assistance and medical writing support on initial drafts were provided by Chameleon Communications International Ltd, UK [a Healthcare Consultancy Group Company].
Writing assistance: Editorial assistance [medical writing] with initial drafts was provided by Claudia Wiedemann of The Healthcare Consultancy Group, funded by Takeda.
References
- 1. Zippi M, Corrado C, Pica R, et al. . Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol 2014;20:17463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol 2013;10:585–95. [DOI] [PubMed] [Google Scholar]
- 3. Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome?World J Gastroenterol 2011;17:2702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arvikar SL, Fisher MC. Inflammatory bowel disease associated arthropathy. Curr Rev Musculoskelet Med 2011;4:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harbord M, Annese V, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vavricka SR, Gubler M, Gantenbein C, et al. ; Swiss IBD Cohort Study Group Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis 2017;23:1174–81. [DOI] [PubMed] [Google Scholar]
- 8. Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, et al. . Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol 2017;15:25–36.e27. [DOI] [PubMed] [Google Scholar]
- 9. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016;7:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nyboe Andersen N, Pasternak B, Friis-Møller N, Andersson M, Jess T. Association between tumour necrosis factor-α inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ 2015;350:h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiebault H, Boyard-Lasselin P, Guignant C, et al. . Paradoxical articular manifestations in patients with inflammatory bowel diseases treated with infliximab. Eur J Gastroenterol Hepatol 2016;28:876–81. [DOI] [PubMed] [Google Scholar]
- 12. Rahier JF, Buche S, Peyrin-Biroulet L, et al. ; Groupe d’Etude Thérapeutique des Affections Inflammatoires du Tube Digestif [GETAID] Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol 2010;8:1048–55. [DOI] [PubMed] [Google Scholar]
- 13. Entyvio® [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2014. Available at: https://general.takedapharm.com/ENTYVIOPI. Accessed December 18, 2017. [Google Scholar]
- 14. Entyvio® [Summary of Product Characteristics]. Zurich, Switzerland: Takeda Pharma A/S; 2014. Available at: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002782/WC500168528.pdf. Accessed December 18, 2017. [Google Scholar]
- 15. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016;10:1437–44. [DOI] [PubMed] [Google Scholar]
- 16. Feagan BG, Rutgeerts P, Sands BE, et al. . Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 18. Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014;147:618–627.e3. [DOI] [PubMed] [Google Scholar]
- 19. Loftus EV Jr, Colombel JF, Feagan BG, et al. . Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
- 20. Vermeire S, Loftus EV Jr, Colombel JF, et al. . Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017;11:412–24. [DOI] [PubMed] [Google Scholar]
- 21. Colombel JF, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ungaro R, Blake A, Bhayat F, et al. . A description of joint-related events in patients receiving vedolizumab from clinical and post-marketing safety experience. Inflamm Bowel Dis 2017;23:S47–S48. Abstract Poster P-135. [Google Scholar]
- 23. Dulai PS, Singh S, Jiang X, et al. . The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY Consortium. Am J Gastroenterol 2016;111:1147–55. [DOI] [PubMed] [Google Scholar]
- 24. Tadbiri S, Peyrin-Biroulet L, Serrero M, et al. ; GETAID OBSERV-IBD study group Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther 2018;47:485–93. [DOI] [PubMed] [Google Scholar]
- 25. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ; ACCENT I Study Group Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 26. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 27. Juillerat P, Mottet C, Pittet V, et al. . Extraintestinal manifestations of Crohn’s disease. Digestion 2007;76:141–8. [DOI] [PubMed] [Google Scholar]
- 28. Mattheakis L, Bhandari A, Bai L, et al. . PTG-100, an oral peptide antagonist of integrin α4β7 that alters trafficking of gut homing T cells in preclinical animal models. Inflamm Bowel Dis 2016;22[suppl 1]:S48. Abstract P-126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.