Abstract
Glucocorticoids (GCs) are essential for stress adaptation, acting centrally and in the periphery. Corticotropin-releasing factor (CRF), a major regulator of adrenal GC synthesis, is produced in the paraventricular nucleus of the hypothalamus (PVH), which contains multiple neuroendocrine and preautonomic neurons. GCs may be involved in diverse regulatory mechanisms in the PVH, but the target genes of GCs are largely unexplored except for the CRF gene (Crh), a well-known target for GC negative feedback. Using a genome-wide RNA-sequencing analysis, we identified transcripts that changed in response to either high-dose corticosterone (Cort) exposure for 12 days (12-day high Cort), corticoid deprivation for 7 days (7-day ADX), or acute Cort administration. Among others, canonical GC target genes were upregulated prominently by 12-day high Cort. Crh was upregulated or downregulated most prominently by either 7-day ADX or 12-day high Cort, emphasizing the recognized feedback effects of GC on the hypothalamic-pituitary-adrenal (HPA) axis. Concomitant changes in vasopressin and apelin receptor gene expression are likely to contribute to HPA repression. In keeping with the pleotropic cellular actions of GCs, 7-day ADX downregulated numerous genes of a broad functional spectrum. The transcriptome response signature differed markedly between acute Cort injection and 12-day high Cort. Remarkably, six immediate early genes were upregulated 1 hour after Cort injection, which was confirmed by quantitative reverse transcription PCR and semiquantitative in situ hybridization. This study may provide a useful database for studying the regulatory mechanisms of GC-dependent gene expression and repression in the PVH.
The internal milieu of the living organism is maintained in a steady state (homeostasis) despite continuous challenges by interoceptive and exteroceptive stimuli (stressors) (1). A major player in protecting the organism from stressful stimuli and securing homeostasis is the paraventricular nucleus of the hypothalamus (PVH), a complex structure of neuroendocrine and preautonomic neurons (2, 3). Stress signals, triggered by physical or psychogenic stressors, are conveyed to the PVH via neural inputs and/or humoral factors (2). The PVH, in turn, functions as a relay center for integrating the stress signals and converts them to hormonal and autonomic outputs, which activate a number of peripheral and central effector sites that are involved in stress adaptation (2).
The PVH comprises functionally distinct populations of neurons, including corticotropin-releasing factor (CRF)-, thyrotropin-releasing hormone (TRH)-, somatostatin-, vasopressin (AVP)-, and oxytocin (OXT)-expressing neurons (3). CRF, TRH, and somatostatin are produced in the parvocellular neurons and function as hypophysiotrophic hormones, whereas AVP and OXT are produced mainly in the magnocellular neurons and secreted into the peripheral circulation from nerve endings in the posterior pituitary. Part of the parvocellular CRF neurons coexpress AVP (4); these two peptides are cosecreted into capillaries in the median eminence (4) and stimulate ACTH secretion synergistically in the pituitary corticotrophs (5). OXT- (6) or AVP-containing preautonomic neurons (7) are present in the lateral, dorsal, and ventromedial parvocellular divisions; these neurons project to the brainstem and spinal cord and influence the autonomic outflow. Thus, the PVH is involved in multiple endocrine and autonomic functions, participating in cardiovascular and fluid homeostasis, metabolism, and reproductive functions (3, 8).
Regulation of the hypothalamic-pituitary-adrenal (HPA) axis is one of the most important functions of the PVH. CRF neurons in the PVH play a central role in stimulating the production of adrenal glucocorticoids (GCs), which are essential for stress coping and homeostasis (9). Whereas basal GC secretion, following circadian and ultradian rhythms, is important for normal cell function in resting conditions (10), rapid but transient increases during stress are critical for adaptation and survival (9). Although GC actions, mediated through diverse mechanisms in the periphery and in the central nervous system (9), are essential for life, excessive and sustained production of GC has adverse effects and predisposes to illness (11). Thus, a prompt termination of stress-induced GC synthesis is necessary. Negative GC feedback, repressing CRF gene expression in the PVH, as well as proopiomelanocortin gene expression and ACTH release in the pituitary, plays a critical role in this process (12). Considerable progress has been made in our understanding of the mechanism of GC-induced CRF gene repression in the PVH (12). However, little is known about other GC target genes and how their expression is regulated by GC in the PVH.
Genome-wide RNA-sequencing (RNA-seq) analysis, based on high-throughput sequencing techniques, allowed us to reveal changes in transcriptome signatures induced by GC (13–15). Whereas genome-wide approaches have been undertaken in tumor cell lines (13, 14) or immune cells (15, 16), transcriptome analysis by RNA-seq has not been carried out in the PVH for exploring the mechanism of GC actions. In the current study, we searched for genes that are transcribed or repressed in a GC-dependent manner in the PVH region of male rats. We performed an unbiased quantification of the abundance of DNA transcripts provided by RNA-seq and identified genes whose expression is enhanced or suppressed by the long-term excess of corticosterone (Cort) or deprivation of corticoids, as well as by acute Cort increase. The results uncovered a broad spectrum of Cort-responsive genes potentially involved in the regulation of HPA-axis and other neuroendocrine and preautonomic functions of the PVH.
Materials and Methods
Animals
Male Wistar rats (body weight, 280 to 300 g) were maintained under standard conditions in the animal facility. Rats were housed three to four per cage, with free access to water and rat chow pellets, on a 12-hour light/dark cycle (lights on from 7:00 am to 7:00 pm) at a constant room temperature (23°C). All animal procedures were approved by the Animal Care and Use Committees at the National Institute of Child Health and Human Development of the US National Institutes of Health, and Tohoku University.
Surgery and euthanasia
Bilateral adrenalectomy (ADX) was performed via the dorsal route under ketamine and xylazine anesthesia, as described previously (17). Sham-operated animals underwent the same surgical procedure except that the adrenals were exposed bilaterally but not removed. For sustained administration of high dose Cort, a 10 × 10 mm implant of grade GF/B Whatman glass microfiber filter, impregnated with 100% Cort (Sigma-Aldrich, St Louis, MO), was placed subcutaneously in the interscapular region at the time of the sham-ADX surgery. For preparation of the implants, Cort powder was melted in 20-mL scintillation vials on a hot plate at 200°C before immersing the precut filters and letting them cool and solidify.
After recovery from anesthesia, rats were returned to their home cages to recuperate from surgery. Adrenalectomized rats were given free access to 0.9% NaCl and tap water, both containing 25 μg/mL of Cort, before initiation of the experimental protocols. Because anesthesia activates the HPA axis, euthanasia was performed by decapitation without sedation.
Experimental protocols
Experiment 1 was designed for examining the sustained effect of a high dose of Cort or of corticoid deprivation. Experiment 2 was designed for studying the effect of acute increase in Cort. Both experiments were performed simultaneously and all samples processed together to allow comparisons between groups across both experiments. Six experimental groups, each comprising six rats, were used for the RNA-seq study. After surgery, rats in each group were kept three to four per cage, as before surgery, until the day of the experiments.
Experiment 1: effect of long-term Cort excess or of corticoid deprivation
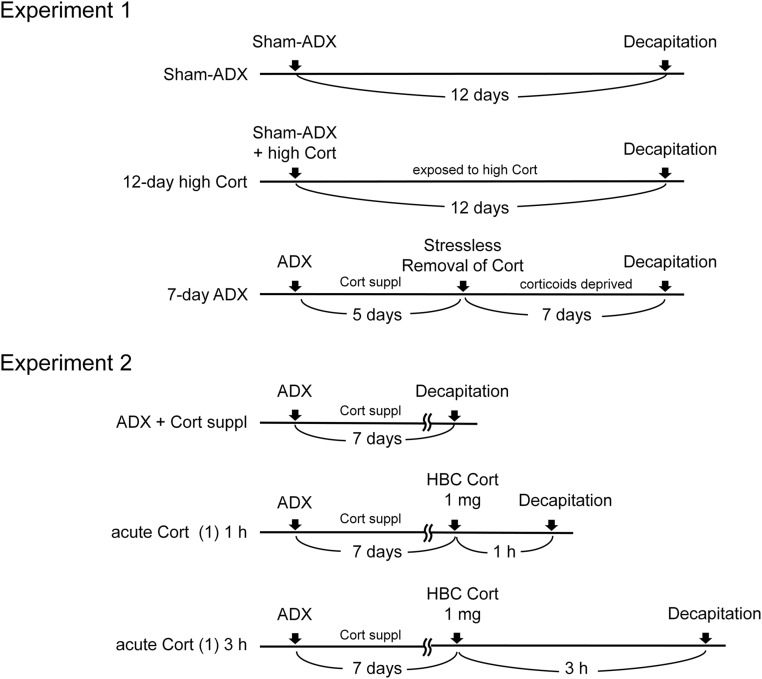
Three groups of rats were used for the experiment (Fig. 1): a sham-ADX control group, a 12-day high Cort group, and a 7-day ADX group. The sham-ADX control group had free access to chow and water. The rats in this group were euthanized at day 13 (12 days after sham-ADX) by decapitation. Rats in the 12-day high Cort group underwent sham-ADX, and Cort implants were placed subcutaneously at the time of sham-ADX. They were allowed free access to chow and water in their home cages before decapitation on day 13 (12 days after sham-ADX). Rats in the 7-day ADX group underwent bilateral ADX, followed by a 5-day recovery period with Cort replacement (25 μg/mL) in drinking fluids (0.9% saline and water); a bottle of saline and also a bottle of water were provided in each cage.
Figure 1.
Experimental protocols. Experiment 1: effect of long-term Cort excess or corticoids deprivation. Three groups of rats were used: sham-ADX group, 12-day high Cort group, and 7-day ADX group. Experiment 2: effect of acute Cort administration. Three groups of rats were used: ADX + Cort suppl, acute Cort (1) 1 h, and acute Cort 3 h. Rats in each group underwent ADX and Cort supplementation in drinking fluids, and 7 days were allowed before the experiment. Complete details are reported in the text.
Five days after ADX, rats were deprived of corticoids by removing Cort from the drinking fluid (stressless removal of Cort) (18, 19) and subjected to daily handling. After 7 days of total deprivation of corticoids (day 13), rats were decapitated. All rats in experiment 1 were decapitated between 8:00 am and 11:30 am for blood collection and PVH dissection.
Experiment 2: effect of acute Cort administration
Adrenalectomized rats underwent Cort replacement (25 μg/mL) in drinking fluids (i.e., water and 0.9% saline) for 7 days. They were handled daily and divided into three groups (six rats in each group Fig. 1). One group served as a control (ADX + Cort supplementation). At the beginning of the experiment, rats in this group were euthanized without injection. A second group received 1 mg of 2-hydroxypropyl-β-cyclodextrin (HBC)–encapsulated Cort (containing 67 μg of Cort; Sigma-Aldrich) that was dissolved in 300 μL of sterile saline and injected intraperitoneally. Rats were euthanized 1 hour after injection. This is referred to hereafter as the acute Cort (1) 1 h group. In the third group, hereafter as the acute Cort (1) 3 h group, HBC Cort (1 mg) was injected as in the acute Cort (1) 1 h group, and rats were euthanized 3 hours after injection.
Control and Cort-injected rats were euthanized by decapitation for blood collection and PVH dissection between 8:00 am and 11:30 am. To inject Cort promptly and minimize stress during injection, rats were labeled in the tail for identification, and the cages were placed in a separate room, adjacent to the experimental room, on the night before the experiment. On the following day, injections were performed by an experienced investigator within approximately 5 seconds of opening the cage.
Dissection of the PVH-containing hypothalamic tissue
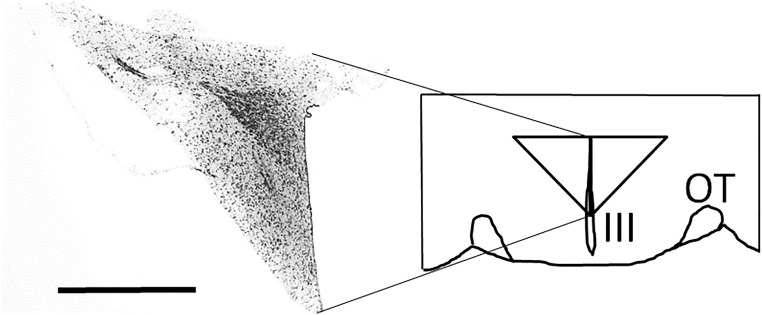
Immediately after decapitation, the brain was removed from the skull and placed on a rat-brain slicer (MK-RC-01; Muromachi, Tokyo, Japan). A 2-mm coronal section was cut on ice with a stainless steel blade. The rostral plane of the section was at the level of the rostral-most border of the optic chiasm. The section was placed on a cooled plate, and a tissue block containing the PVH region was removed from each side of the section (Fig. 2). Immediately after dissection, the tissue block was frozen and kept at −80°C until processed for RNA preparation. A preliminary experiment was carried out using three rats to verify that the tissue block contained the whole PVH region. Dissected tissue blocks were fixed in 4% paraformaldehyde, which was replaced with 20% sucrose. Coronal sections (30 μm) were cut serially by a cryostat, and every section was stained with cresyl violet. The entire PVH was contained in all six tissue blocks removed bilaterally from three animals. A representative photomicrograph taken at the midpoint of the rostro-caudal extent of the tissue block is shown in Fig. 2.
Figure 2.
A diagram showing the dissected region containing the PVH. Immediately after decapitation, the brain was removed from the skull and placed on a rat-brain slicer. A 2-mm coronal section containing the hypothalamic region was cut on ice with a stainless steel blade. A triangular tissue block, containing the entire PVH, was removed from each side of the section on ice, as indicated in the diagram. A representative photomicrograph, taken at the midpoint of the rostro-caudal extent of the tissue block, is shown in the diagram. Scale bar, 1 mm. III, third ventricle; OT, optic tract.
RNA extraction and quality control
Dissected PVH regions from individual rats were used for RNA extraction using Trizol (Thermo Fisher Scientific, Waltham, MA) followed by purification with RNeasy mini kit reagents (Qiagen, Valencia, CA) and column DNase digestion (Qiagen). After quantification in a Nanodrop 2000 (Thermo Fisher Scientific), RNA quality was assessed using a BioAnalyzer (Agilent Technologies, Santa Clara, CA); all the samples had an RNA integrity number >9.
RNA sequencing
RNA sequencing was performed by the National Institutes of Health Sequencing Center, National Human Genome Research Institute (Bethesda, MD). Oligo(dT) columns (Thermo Fisher Scientific) were used to isolate poly(A)+ RNAs from individual samples. Poly(A)+ RNA aliquots from three rats in each experimental group were pooled, and two samples per group were prepared. Messenger RNA libraries were constructed from 1000 ng of high-quality total RNA, using the Illumina TruSeq RNA Sample Prep Kits, version 2 (San Diego, CA). The resulting cDNA was fragmented using a Covaris E210 (Covaris, Woburn, MA). Library amplification was performed using 10 cycles to minimize the risk of overamplification. Unique barcode adapters were applied to each library. Library insert sizes averaged 275 bp. Individual libraries (a total of 12 libraries: 2 libraries from 6 groups) were quantitated by quantitative PCR and then pooled in an equimolar ratio before sequencing as a pool on three lanes of an Illumina HiSeq2000 machine with version 3 flow cells and sequencing reagents. At least 30 million 100-base read pairs were generated for each individual library. Data were processed using RTA 1.12.4.3 and CASAVA 1.8.2 software (Illumina). The sequenced reads were mapped to the rat genome (version rn6) using Tophat (version 2.1.1), and BAM files were obtained (20). Remaining RNA from each sample was used for verification by quantitative RT-PCR (qRT-PCR; as explained later in Methods).
Plasma ACTH and Cort assay
After decapitation, trunk blood was collected in an ice-cooled tube containing EDTA 2Na (Sigma-Aldrich), centrifuged at 3000 rpm, and the plasma was separated and kept frozen at −80°C until use. Plasma concentrations of ACTH and Cort were measured using kit reagents from the ACTH Immunoradiometric Assay (DiaSorin, Stillwater, MN) and the Rat Cort Coat-A-Count kit (Siemens, Los Angeles, CA), respectively. The sensitivity of the assay for ACTH was 5 pg/mL, and the intraassay and interassay coefficients of variation were 3.0% and 8.3%, respectively. The sensitivity of the assay for Cort was 6 ng/mL, and the intraassay and interassay coefficients of variation were 3.1% and 8.5%, respectively.
qRT-PCR
Remaining RNA samples, after removing aliquots for poly(A)+ RNA preparation, were used for qRT-PCR for selected genes. cDNA was reverse transcribed from 0.3 to 0.75 μg of total RNA, and mRNA levels were quantitated using primers designed to amplify the respective RNA specifically. Power SYBR Green PCR mix (Applied Biosystems, Foster City, CA) was used for the amplification mixture with each primer at a final concentration of 200 nM and 1.5 μL of cDNA, for a total reaction volume of 12.5 μL. PCRs were performed on a spectrofluorometric thermal cycler (7900 HT Fast Real-Time PCR System; Applied Biosystems), as described previously (17). Levels of mRNA were normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA as determined in separate real-time quantitative PCR reactions. The respective sequences of the PCR primers for Crh (corticotropin-releasing factor; the gene symbol is italicized, and corresponding gene product is indicated in the parentheses hereafter), Per1 (period circadian protein homolog 1), Egr1 (early growth response protein 1), Fos (proto-oncogene cFos), and Nr4a3 (nuclear receptor subfamily 4 group A member 3) were as follows: forward, 5′–CTCTCTGGATCTCACCTTCCAC–3′, reverse, 5′–CTAAATGCAGAATCGTTTTGGC–3′; forward, 5′–GAAGCCCCCGGAGTCGGACAT–3′, reverse, 5′–GGACAGCACGGCCTTGGTCA–3′; forward, 5′–ACGAGCACCTGACCACAGAGTC–3′, reverse, 5′–TCCAGGGAGAAGCGGCCAGT–3′; forward, 5′–AGAGCGCAGAGCATCGGCAG–3′, reverse, 5′–TGATCTGTCTCCGCTTGGAGCGT–3′; and forward, 5′–CGGCGCTCTCCCATGAGTTGG–3′, reverse, 5′–CCCGCAGTGGGCTTTGGGTT–3′.
Semiquantitative in situ hybridization
The effects of acute Cort injection on immediate early gene (IEG) expression were studied in a separate experiment using semiquantitative in situ hybridization. The purpose of this part of our study was to validate the RNA-seq data and to determine the localization of IEG mRNAs. Adrenalectomized rats underwent Cort replacement for 7 days in drinking fluids (water and 0.9% saline) as in experiment 2, and they were divided into three groups (n = 6 for each group). The first group received vehicle (300 μL of sterile saline) intraperitoneally (hereafter, the acute vehicle 1 h group). The second group, acute Cort (1) 1 h, received a 1-mg intraperitoneal injection of HBC Cort (67 μg Cort) in 300 μL of saline. The third group, hereafter referred to as acute Cort (10) 1 h, received a high dose (10 mg) of HBC Cort (670 μg Cort). At 1 hour after injection of Cort or its vehicle, rats were decapitated, and the brain was removed immediately, frozen, and kept at −80°C until being processed further. Serial sections (15 μm) were cut by a cryostat (HM505N; Microm, Heidelberg, Germany), and semiquantitative in situ hybridization using [35S]-labeled RNA probes was carried out as was described elsewhere (21). The plasmids containing the rat Fos and Egr1 were kindly donated by Dr. Stanley Watson at the University of Michigan and Dr. Jeffrey Milbrandt at Washington University, respectively. The X-ray films (Kodak Biomax MR; Sigma-Aldrich) were captured by a digital camera and quantitated using ImageJ software (https://imagej.nih.gov/ij/).
Data analysis
Cuffdiff in Cufflinks, version 2.2.1 (http://cole-trapnell-lab.github.io/cufflinks/) (22), was used to identify differentially expressed genes. Differential gene expression was calculated as the mean fragments per kilobase of transcript per million mapped reads. The Benjamini and Hochberg method was applied to correct the unavoidable false-positive data from the multiple tests, and a q value was calculated as the false discovery rate–adjusted P value. A q value <0.05 was used as the cutoff criterion. Pearson correlation analysis was carried out for grouping genes that showed similar expression patterns. We categorized the genes that were upregulated or downregulated by Cort by manual annotations based on the UniProt functions (http://www.uniprot.org/). Cluster analysis was done for the genes, the expression of which was significantly different between any two groups out of the three experimental groups in either experiment 1 (sham-ADX, 12-day high Cort, and 7-day ADX) or experiment 2 [ADX + Cort suppl, acute Cort (1) 1 h, and acute Cort (1) 3 h].
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses (https://www.genome.jp/kegg/) were carried out to identify important biological processes and pathways, and the identified pathways were visualized using Pathview package of R (23). The similarity of expression (i.e., the gene coexpression) was defined from the pattern of gene expression changes between two genes of interest and calculated from available data obtained from Gene Coexpression Database, version 6.0 (http://coxpresdb.jp/) (24). KEGG and Gene Coexpression Database analyses were done for the genes whose expression was significantly different between any two groups out of the three experimental groups in either experiment.
The data of the qRT-PCR and semiquantitative in situ hybridization were analyzed by Fisher protected least significant difference test and expressed as the mean ± SEM. P < 0.05 was regarded as statistically significant.
Results
Effects of long-term changes in circulating Cort levels on the transcriptomes in the hypothalamic PVH region
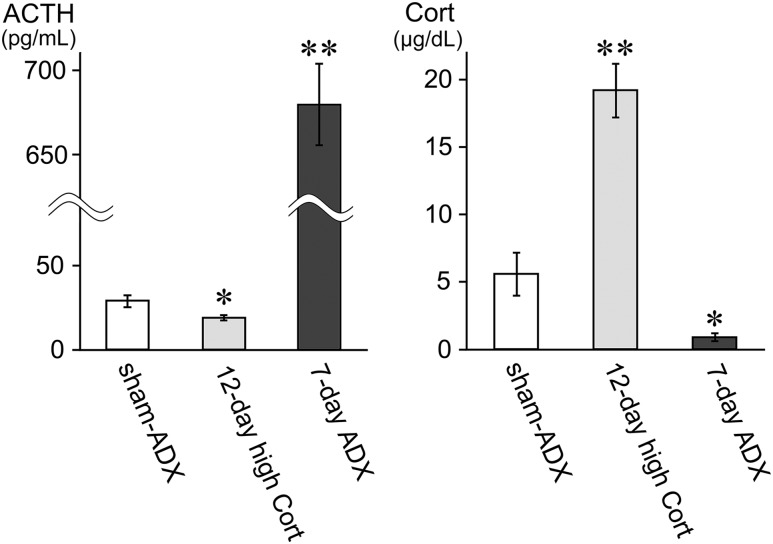
Sustained exposure to a high dose of Cort for 12 days resulted in a significant increase in early morning plasma Cort levels (19.2 ± 2.0 μg/dL; n = 6) compared with those in sham-ADX controls (5.6 ± 1.6 μg/dL; n = 6; P < 0.01), and a significant decrease in plasma ACTH levels (19.1 ± 1.9 pg/mL; n = 6) vs sham-ADX controls (28.9 ± 3.6 pg/mL; n = 6; P < 0.05; Fig. 3).
Figure 3.
Plasma ACTH and Cort levels in experiment 1. (Left panel) Plasma ACTH levels are shown for the sham-ADX group, the 12-day high Cort group, and the 7-day ADX group. ACTH levels were reduced in 12-day high Cort and elevated in 7-day ADX significantly compared with sham-ADX. (Right panel) Plasma Cort levels are shown as for plasma ACTH levels. Cort levels were elevated in 12-day high Cort and reduced in 7-day ADX significantly compared with sham-ADX. Data are reported as mean ± SEM (n = 6 for each group). *P < 0.05, **P < 0.01 vs sham-ADX group.
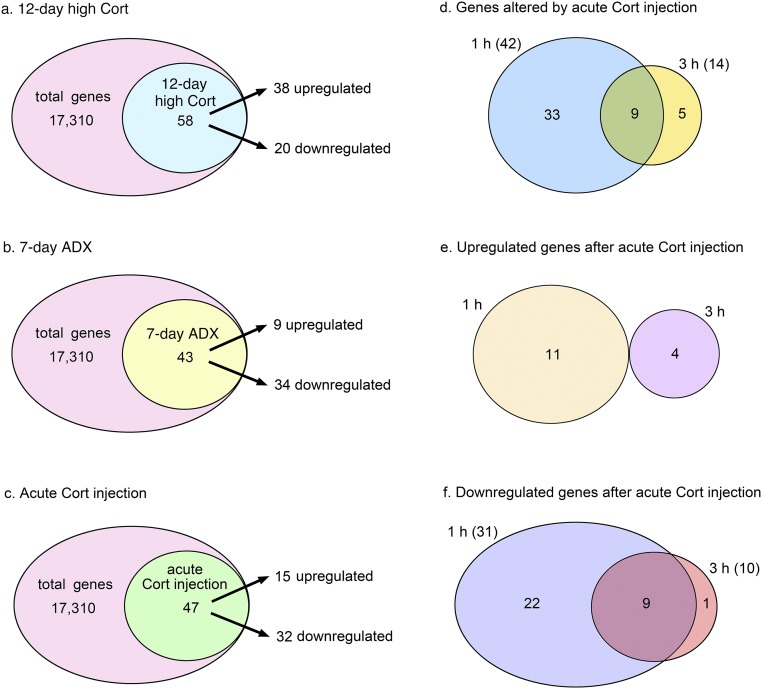
Exposure to a high plasma concentration of Cort for 12 days caused significant changes in the levels of 58 transcripts (n = 38 upregulated and n = 20 downregulated) compared with the sham-ADX group (Fig. 4a). The genes whose expression was affected significantly by the 12-day high Cort treatment represented a broad spectrum of cellular functions: neuroendocrine function, gene expression, signal transduction, neurotransmission, metabolism, immune function, protein degradation, and circadian clock (Supplemental Tables 1 and 2). The list of genes, the expression of which changed most prominently after 12-day high Cort, is shown in Table 1.
Figure 4.
Venn diagrams showing the number of genes upregulated or downregulated significantly (q < 0.05) compared with the respective controls. (a) Of the 17,310 transcripts, 58 were changed significantly in the 12-day high Cort group compared with the sham-ADX group; 38 transcripts were upregulated, and 20 were downregulated. (b) In the 7-day ADX group, 43 transcripts were changed significantly; 9 were upregulated and 34 were downregulated. (c) Forty-seven transcripts were changed significantly either in the acute Cort (1) 1 h or acute Cort (1) 3 h groups compared with the noninjected control group (ADX + Cort suppl); 15 genes were upregulated and 32 were downregulated. (d) Forty-two transcripts were changed significantly in the acute Cort (1) 1 h group, and 14 were changed significantly in the acute Cort (1) 3 h group. Nine genes were affected at both 1 and 3 hours. (e) Of the 15 transcripts upregulated significantly by acute Cort injection, 11 were affected at 1 hour and 4 at 3 hours. (f) Of the 32 transcripts downregulated significantly by acute Cort injection, 31 were affected at 1 hour and 10 at 3 hours. Nine genes were affected at both 1 hour and 3 hours.
Table 1.
Transcripts Most Prominently Affected by Either the Long-Term Changes in Cort or Acute Cort Injection
| Upregulated | Downregulated | |||||
|---|---|---|---|---|---|---|
| Gene Symbol | Gene Product | Fold Change | Gene Symbol | Gene Product | Fold Change | |
| Experiment 1 | ||||||
| 12-day high Cort | Hif3a | Hypoxia-inducible factor 3-α | 7.00 | Crh | Corticotropin-releasing factor | 0.27 |
| Pla2g3 | Group 3 secretory phospholipase A2 | 4.61 | Fcrl2 | Fc receptor-like protein 2 | 0.29 | |
| Gpd1 | Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic | 3.73 | Pcdh12 | Protocadherin-12 | 0.30 | |
| LOC102723236 | 3.32 | Polq | DNA polymerase θ | 0.32 | ||
| Fmo2 | Dimethyaniline monooxygenase [N-oxide-forming] 2 | 3.16 | Mmp14 | Matrix metalloproteinase-14 | 0.40 | |
| Sgk1 | Serine/threonine-protein kinase Sgk1 | 2.77 | Cd93 | Compliment component C1q receptor | 0.45 | |
| Rn5-8s | 5.8S ribosomal RNA | 2.73 | Igf2 | Insulin-like growth factor 2 | 0.47 | |
| Ctgf | Connective tissue growth factor | 2.64 | Fabp7 | Fatty acid-binding protein, brain | 0.48 | |
| Sult1a1 | Sulfotransferase 1A1 | 2.56 | ||||
| Cd163 | Scavenger receptor cysteine-rich type 1 protein M130 | 2.52 | ||||
| Fst | Follistatin | 2.47 | ||||
| Gjb2 | Gap junction β-2 protein | 2.39 | ||||
| Slc19a3 | Thiamine transporter 2 | 2.24 | ||||
| Zbtb16 | Zinc finger and BTB domain-containing protein 16 | 2.12 | ||||
| LOC310926 | 2.12 | |||||
| Rn18s,Rn45s | 18S ribosomal RNA | 2.11 | ||||
| 7-day ADX | Crh | Corticotropin-releasing factor | 2.21 | RT1-N2 | 0.25 | |
| Clca1 | Calcium-activated chloride channel regulator 1 | 2.10 | Vip | Vasoactive intestinal polypeptide | 0.34 | |
| Ifit1 | Interferon-induced protein with tetratricopeptide repeats 1 | 0.38 | ||||
| Acsm5 | Acyl-coenzyme A synthetase ACSM5, mitochondrial | 0.39 | ||||
| Sult1a1 | Sulfotransferase 1A1 | 0.45 | ||||
| Gabrd | Gamma-aminobutyric acid receptor subunit δ | 0.49 | ||||
| Experiment 2 | ||||||
| Acute Cort (1) 1 h | Bsx | Brain-specific homeobox protein homolog | 2.60 | Rn5-8s | 5.8S ribosomal RNA | 0.18 |
| Nr4a3 | Nuclear receptor subfamily 4 group A member 3 | 2.58 | Mybpc1 | Myosin-binding protein C, slow-type | 0.27 | |
| Fos | Proto-oncogene c-Fos | 2.03 | LOC102723236 | 0.27 | ||
| Fosb | Protein fosB | 2.03 | Slc17a7 | Vesicular glutamate transporter 1 | 0.35 | |
| LOC310926 | 0.39 | |||||
| Rn18s,Rn45s | 18S ribosomal RNA | 0.42 | ||||
| Rn28s | 28S ribosomal RNA | 0.45 | ||||
| Wnt9b | Protein Wnt-9b | 0.46 | ||||
| Prkcd | Protein kinase C, δ type | 0.48 | ||||
| RGD1560028 | 0.48 | |||||
| Acute Cort (1) 3 h | LOC102723236 | 0.28 | ||||
| Rn5-8s | 5.8S ribosomal RNA | 0.29 | ||||
| LOC310926 | 0.38 | |||||
| Rn18s | 18S ribosomal RNA | 0.40 | ||||
| Slc17a7 | Vesicular glutamate transporter 1 | 0.42 | ||||
| Rn28s | 28S ribosomal RNA | 0.44 | ||||
| Mybpc | Myosin-binding protein C, slow-type | 0.49 | ||||
Transcripts significantly upregulated (more than twofold) or significantly downregulated (<0.5-fold) are listed in this table. See text for experimental procedures.
High Cort for 12 days upregulated the expression of Hif3a (hypoxia-inducible factor 3-α) most prominently (a sevenfold increase compared with the sham-ADX controls). The expression of Pla2g3 (group 3 secretory phospholipase A2), Gpd1 (glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic), and Fmo2 (dimethylaniline monooxygenase [N-oxide–forming] 2) was also upregulated markedly (more than threefold; Table 1). Fst (follistatin), Slc19a3 (thiamine transporter 2; Table 1), Per1, Sgk1 (serine/threonine protein kinase Sgk1), Fkbp5 (FK506-binding protein 5), and Dusp1 (dual-specificity protein phosphatase 1), among other genes, were also upregulated significantly by 12-day high Cort (Supplemental Table 1).
The expression of Crh was downregulated most prominently (0.27-fold) by 12-day high Cort (Table 1). The expression of other neuroendocrine peptides or receptors [i.e., Avp, Oxt, and Aplnr (apelin receptor)], among other genes, was also downregulated significantly (Supplemental Table 2).
The 7-day ADX treatment resulted in plasma Cort levels that were near detection limits of the assay (0.9 ± 0.3 μg/dL; P < 0.05 vs sham-ADX group) and marked increase in plasma ACTH levels (680 ± 24 pg/mL; n = 6) compared with the sham-ADX controls (28.9 ± 3.6 pg/mL; n = 6; P < 0.01; Fig. 3). The 7-day ADX treatment caused significant changes in the levels of 43 transcripts (n = 9 upregulated and n = 34 downregulated; Fig. 4b). The genes affected significantly by 7-day ADX have a broad spectrum of functions, including neuroendocrine function, gene expression, signal transduction, neurotransmission, metabolism, and immune function (Supplemental Tables 3 and 4). Table 1 lists the most markedly affected genes.
Crh expression was most prominently enhanced by 7-day ADX (2.2-fold). The read mapping to the CRF genomic sequence in sham ADX, as well as an influence of 7-day ADX on its profile, together with that of 12-day high Cort, is shown in Supplemental Figure 1. The upregulated genes by 7-day ADX included, among others, Clca1 (calcium-activated chloride channel receptor 1; Table 1), Avp, Aplr, and Kcng1 (voltage-gated potassium channel subfamily G member 1) (Supplemental Table 3).
Vip (vasoactive intestinal polypeptide) expression was decreased most prominently (0.34-fold) by 7-day ADX (Table 1). The expression of the Gabrd [γ-aminobutyric acid receptor A (GABAA) subunit δ; Table 1] and Gabra4 (GABAA receptor subunit α-4), among other genes, was also downregulated significantly by 7-day ADX(Supplemental Table 4). In addition, a protein-protein interaction was identified between the Gabra4 and Gabrd by the coexpression network analysis (Supplemental Fig. 3). Slc17a7 (vesicular glutamate transporter 1), Wnt9b (protein Wnt-9b), and Lef1 (lymphoid enhancer-binding factor 1), which are involved in the Wnt signaling pathway, and Cck (cholecystokinin) were also downregulated by 7-day ADX (Supplemental Table 4).
By the KEGG pathway mapping, we could identify the genes affected by 12-day high Cort or 7-day ADX. They are associated with the glycerophospholipid metabolism (Gpd1, Pla2g3), chemokine signaling pathway [Ptk2b (protein-tyrosine kinase 2-β), Prkcd (protein kinase C δ type)], vascular smooth muscle contraction [Ramp3 (receptor activity-modifying protein), Pla2g3, Prkcd], and focal adhesion [Ptk2b, Col3a1 (collagen α-1(III) chain), Itga4 (integrin α-4), Itgal (integrin α-L), Vav3 (guanine nucleotide exchange factor Vav3), Pkcd] (Supplemental Fig. 5a–5d).
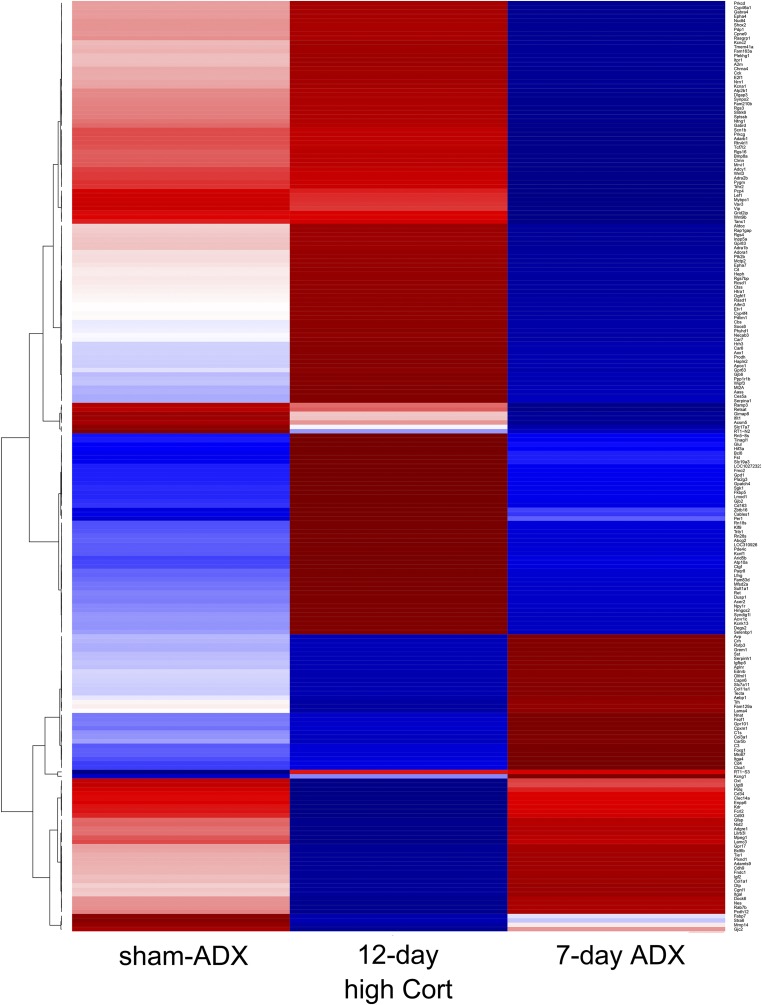
Most of the genes that were upregulated by 12-day high Cort were downregulated by 7-day ADX. Conversely, the genes that were downregulated by 12-day high Cort were mostly upregulated by 7-day ADX (see heatmap in Fig. 5). However, the expression of a limited number of genes was affected only by either the high Cort or ADX (Fig. 5).
Figure 5.
The clustering analysis of the profiles of transcripts in experiment 1. A hierarchical clustering analysis (correlation/ward D2) was done for the transcripts identified in experiment 1, and they are presented as a heatmap. The mean values of duplicate RNA-seq data for each experimental group were used. Most transcripts upregulated in the 12-day high Cort group were downregulated in the 7-day ADX group and vice versa, but there were a limited number of genes that responded unidirectionally, either to the 12-day high Cort or to the 7-day ADX. Note that the number of genes presented in the heatmap is greater than that shown in Venn diagrams in Fig. 4a and 4b and Supplemental Tables 1–4 because cluster analysis was done for the genes whose expression was significantly different between any two groups out of the three experimental groups (i.e., sham-ADX, 12-day high Cort, and 7-day ADX).
In a population of genes, transcript levels were significantly different between the 12-day high Cort group and the 7-day ADX group, although the levels were not significantly different in either 12-day high Cort or 7-day ADX compared with the sham-ADX group. These genes also appeared in the heatmap (Fig. 5), because clustering was done for genes that were differentially expressed between any two groups out of the three. Therefore, the number of genes contained in the heatmap was greater than that categorized as Cort-responsive genes (Fig. 4a and 4b, Supplemental Tables 1–4). For example, Trh and Sst (somatostatin) are shown in the heatmap (Fig. 5) but not in Supplemental Tables 1–4.
Effects of transient increases in circulating Cort on the transcriptomes in the hypothalamic PVH region
The effects of increased circulating Cort in the range of a stress surge on the transcriptomes of the PVH region were examined at 1 and 3 hours after an acute injection of Cort. According to previously reported protocols (18, 19), Cort replacement was initiated immediately after ADX and maintained until the experiments were performed. However, Cort levels at the time of euthanasia in the ADX + Cort suppl group (2.5 ± 0.3 μg/dL; n = 6) were lower than those in sham-ADX animals in experiment 1 (5.6 ± 1.6 μg/dL; n = 6), although the difference was not significant statistically. Despite the Cort replacement, plasma ACTH levels were elevated (568 ± 80 pg/mL; n = 6; P < 0.05 vs sham-ADX controls), suggesting that the diurnal plasma Cort levels were lower than those mimicking the natural circadian variation in previous studies (18, 19).
For acute Cort injection, Cort-supplemented adrenalectomized rats (euthanized at either 1 or 3 hours) received 1 mg of HBC Cort, a dose that elicits a surge in plasma Cort (approximately 30 μg/dL by 30 minutes), which was comparable to that observed during acute stress (25). Plasma Cort levels at 1 or 3 hours after acute Cort injection had already returned to the levels of ADX + Cort suppl group, and plasma ACTH levels at either time point were not significantly different from those in the ADX + Cort suppl group (data not shown).
Acute Cort injection [acute Cort (1) 1 h and acute Cort (1) 3 h groups] significantly changed the levels of 47 transcripts compared with ADX + Cort suppl, which were partially Cort deficient. Of these genes, 32 were downregulated and 15 upregulated (Fig. 4c). The genes affected by acute Cort injection belonged to functional categories including gene expression, neurotransmission, signal transduction, neuroendocrine function, and circadian clock (Supplemental Tables 5–8). Table 1 lists the most markedly changed genes.
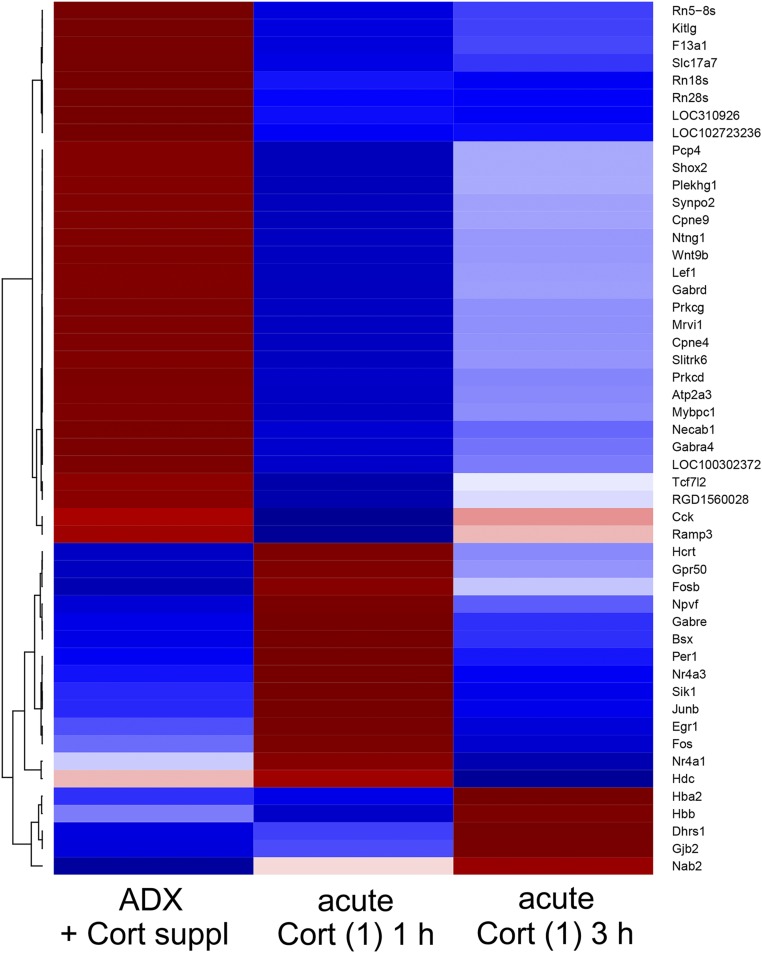
Most of these genes (n = 33 transcripts) were significantly affected only at 1 hour after Cort injection. Nine transcripts were affected at 1 and 3 hours, and five were affects at 3 hours only (Fig. 4d). Of the 15 transcripts upregulated by acute Cort injection, 11 were affected significantly at 1 hour only, and four were significantly elevated at 3 hours only (Fig. 4e). Of the 32 transcripts downregulated significantly by acute Cort injection, 22 were only transiently affected at 1 hour, and nine were affected at 1 and 3 hours (Fig. 4f). Clustering was done for genes that were differentially expressed between any two groups out of the three, and the results are shown as a heatmap in Fig. 6 (note: the number of transcripts shown in Fig. 6 was slightly greater than that in Fig. 4c and Supplemental Tables 5–8 because of the clustering method).
Figure 6.
The clustering analysis of the profiles of transcripts in experiment 2. A hierarchical clustering analysis was done for the transcripts identified in experiment 2, following the same procedure as for experiment 1. The transcripts that were downregulated in the acute Cort (1) 1 h group outnumbered the upregulated ones. The upregulation of transcripts was short lived, and the expression levels of most transcripts, which were upregulated in the acute Cort (1) 1 h group compared with the ADX + Cort suppl group, recovered to the control levels in the acute Cort (1) 3 h group. The expression levels of most of the transcripts that were downregulated at 1 hour tended to recover at 3 hours, but most of them remained at lower levels than in the control. Fewer transcripts remained downregulated at 3 hours to the same degree as at 1 hour. A small number of transcripts, including Nab2, were “slow responders” and were upregulated at 3 hours but not at 1 hour. Note that the number of genes presented in the heatmap is slightly greater than that shown in Venn diagram (Fig. 4c) because of the clustering procedure (see “Materials and Methods”).
It is noteworthy that the expression of a group of IEGs, i.e.,Bsx (brain-specific homeobox protein homolog), Nr4a3, Fos, Fosb (protein fosB) (Table 1), Egr1, and Junb (transcription factor jun-B) (Supplemental Table 5), was upregulated significantly in the acute Cort (1) 1 h group (see also heatmap in Fig. 6). In the acute Cort (1) 3 h group, the expression of all these genes declined, returning to near the levels of the ADX + Cort suppl group (namely, Bsx, Junb and Nr4a3) or becoming somewhat lower than the control levels (namely, Egr1 and Fos; Fig. 6, see also read-mapping profiles to the genomic sequences in Supplemental Fig. 2a and 2b). Per1, Gabre (GABAA receptor subunit ε), and Hcrt (orexin), among others, were upregulated significantly in the acute Cort (1) 1 h group compared with the ADX + Cort suppl group (Supplemental Table 5).
Among the genes prominently suppressed in the acute Cort (1) 1 h group were Mybpc1 (myosin-binding protein C, slow-type), Slc17a7 (Table 1), and Cck (Supplemental Table 6).
In the acute Cort (1) 3 h group, a relatively small number of genes were upregulated or downregulated significantly (Supplemental Tables 7 and 8). Nab2 (NGFI-A-binding protein 2) was one of the four genes upregulated significantly in the acute Cort (1) 3 h group. Most of the genes that were downregulated significantly in the acute Cort (1) 3 h group coincided with those downregulated in acute Cort (1) 1 h group (Supplemental Tables 6 and 8).
The KEGG pathway mapping revealed the acutely Cort-regulated genes, which are associated with the calcium signaling pathway [Atp2a3 (sarcoplasmic/endoplasmic reticulum calcium ATPase 3), Prkcd, and Prkcg (protein kinase C gamma type)], the vascular smooth muscle contraction [Ramp3, Mrvi1 (protein MRVI1), Prkcd, and Prkcg], and the Wnt signaling pathway [Wnt9b, Lef1, Prkcd, Prkcg, and Tcf7l2 (transcription factor 7-like 2)] (Supplemental Fig. 6a–6c). By the coexpression network analysis, we could identify protein-protein interactions between the Gabra4 and Gabrd (Supplemental Fig. 4).
Unexpectedly, 14 (including Cck, Wnt9b, Lef1, Slc17a7, Gabra4, and Gabrd) of the 31 genes downregulated in the acute Cort (1) 1 h group were also downregulated by 7-day ADX) (Supplemental Tables 4 and 6).
Validation by qRT-PCR and in situ hybridization
qRT-PCR
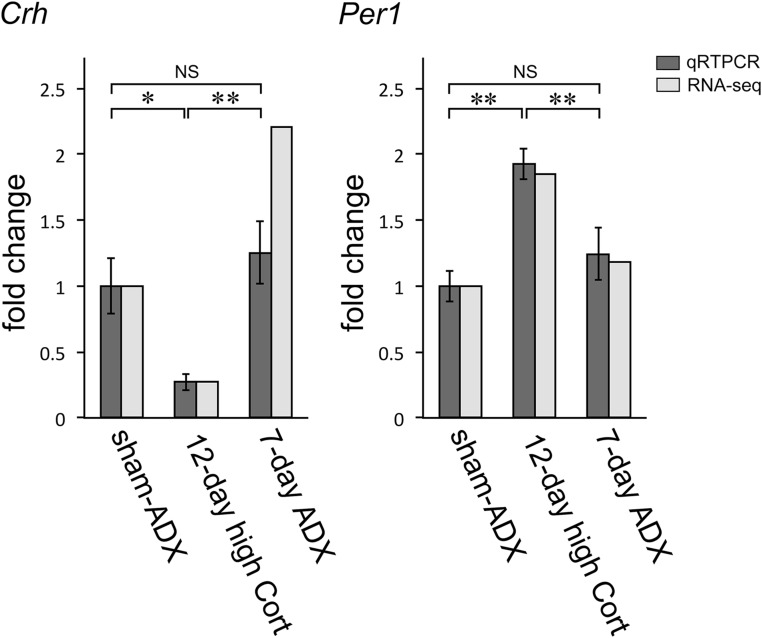
A comparison was made between the RNA-seq data and qRT-PCR data in a subset of representative transcripts. As a result, the expression profiles of the former were mostly reproducible by the latter in either experiment 1 (Fig. 7) or experiment 2 (Fig. 8). For example, the fold changes in qRT-PCR values of Per1 mRNA were similar to those of RNA-seq values in either the 12-day high Cort group or the sham-ADX group (experiment 1) (Fig. 7): They were significantly greater in the 12-day high Cort group compared with the sham-ADX group. In the 7-day ADX group, however, they were not different from those in the sham-ADX group significantly by either assay. The CRF mRNA levels, as examined by qRT-PCR, changed in parallel with those evaluated by RNA-seq, although the fold-change values were somewhat different between the two assays in the 7-day ADX group.
Figure 7.
Comparison of the values between the RNA-seq and qRT-PCR in experiment 1. The RNA-seq data of representative genes (Crh and Per1) were compared with the respective qRT-PCR data. Overall, the RNA-seq profiles were similar to the qRT-PCR profiles. The RNA-seq data are presented as the means of duplicates, and qRT-PCR data as the mean ± SEM (n = 6 for each group). *P < 0.05; **P < 0.01. NS, not significant.
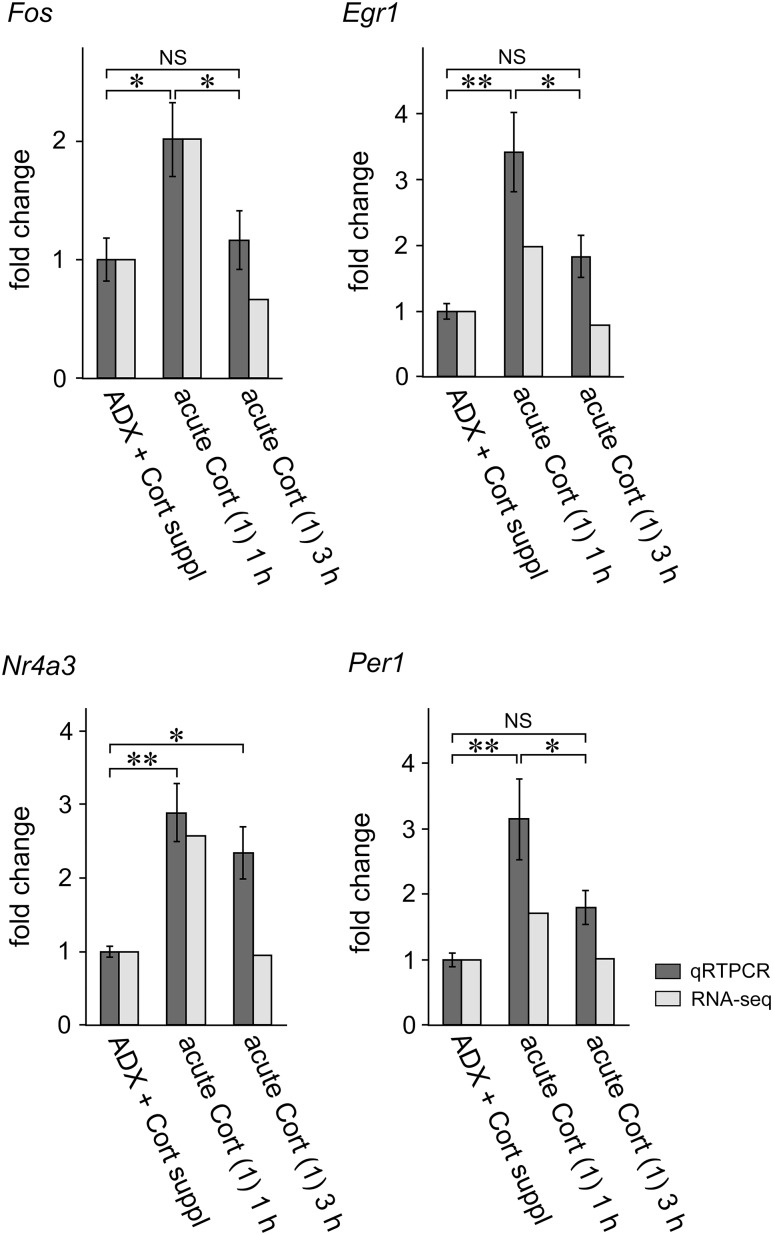
Figure 8.
Comparison of the values between the RNA-seq and qRT-PCR in experiment 2. The RNA-seq data of representative genes (Fos, Egr1, Nr4a3, and Per1) were compared with the respective qRT-PCR data. Overall, the RNA-seq profiles were similar to the qRT-PCR profiles. The RNA-seq data are presented as the means of duplicates, and qRT-PCR data as the mean ± SEM (n = 6 for each group). *P < 0.05; **P < 0.01. NS, not significant.
Fos, Egr1, Nr4a3, or Per1 mRNA levels were significantly greater in the acute Cort (1) 1 h group compared with those in the ADX + Cort suppl group in either the qRT-PCR or RNA-seq (experiment 2; Fig. 8). Fos, Egr1, and Per1 mRNA levels were not significantly different between the acute Cort (1) 3 h group and ADX + Cort suppl group in either the qRT-PCR or RNA-seq. Nr4a3 mRNA expression levels, as examined by qRT-PCR, were significantly greater in the acute Cort (1) 3 h group compared with those in the ADX + Cort suppl group, although the expression levels in the former group were comparable to those in the latter by RNA-seq (Fig. 8).
Semiquantitative in situ hybridization
Fos and Egr1 were representative genes upregulated at 1 hour after Cort injection in experiment 2. To validate RNA-seq data and determine their localization in the hypothalamus, the effects of acute Cort injection on Fos and Egr1 mRNA expression were examined by semiquantitative in situ hybridization in a separate experiment. In this experiment, the acute vehicle 1 h group received vehicle injection to account for any stress related to the injection.
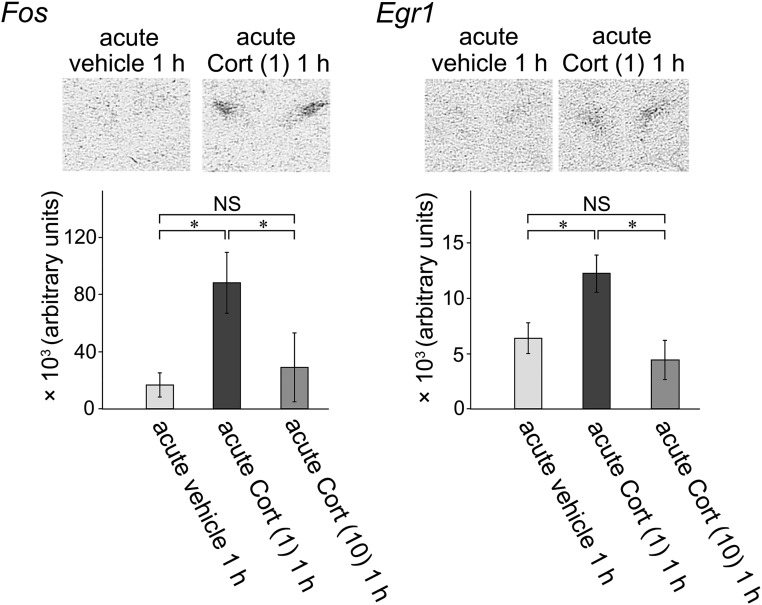
Representative autoradiograms of Fos and Egr1 mRNA in the acute vehicle 1 h group and acute Cort (1) 1 h group are shown in Fig. 9 (upper panels). Fos and Egr1 mRNA expression levels were very low in the PVH in the acute vehicle 1 h group, and the autoradiographic signals were hardly detectable. Fos and Egr1 mRNA levels became increased markedly and could be visualized clearly in the acute Cort (1) 1 h group. Fos and Egr1 mRNA signals were localized in a region that was identified as the PVH by its characteristic shape and location (Fig. 9). Semiquantitative data by autoradiographic analyses are shown in Fig. 9 (lower panels). Fos and Egr1 mRNA expression levels were significantly greater in the acute Cort (1) 1 h group than those in the acute vehicle 1 h group. In contrast, neither Fos nor Egr1 mRNA levels were affected significantly in the acute Cort (10) 1 h group compared with those in the acute vehicle 1 h group.
Figure 9.
Results of in situ hybridization to validate the changes in IEG mRNAs after acute Cort injection and to localize mRNA expression in the PVH. The effects of acute Cort injection on Fos and Egr1 mRNA expression were examined by semiquantitative in situ hybridization. (Upper panels) Autoradiograms show either Fos or Egr1 mRNA could hardly be observed in the PVH in the acute vehicle 1 h group. However, Fos and Egr1 mRNA levels became increased marked and observed clearly in the acute Cort (1) 1 h group. Fos and Egr1 mRNA were localized clearly in the PVH. (Lower panels) Densitometry analysis. By quantification of the autoradiograms, Fos and Egr1 mRNA expression levels were significantly greater in the acute Cort (1) 1 h group than those in the acute vehicle 1 h group. In contrast, in the acute Cort (10) 1 h group, neither Fos nor Egr1 mRNA levels were affected significantly compared with those in the acute vehicle 1 h group. *P < 0.05. NS, not significant.
Discussion
We carried out a genome-wide RNA-seq analysis of transcriptomes in the PVH region of male rats and identified transcripts whose expression levels changed after either long-term (12 days) high Cort treatment, 7-day deprivation of corticoids, or acute Cort injection. Because plasma Cort levels were manipulated systemically in the experiments, the induced changes in Cort could affect the PVH region either directly or indirectly by modifying the activity of afferent inputs to the PVH.
Long-term high Cort or deprivation of corticoids
The expression levels of a number of genes were changed in response to the 12-day high Cort and 7-day ADX treatments. It should be noted that most of the genes upregulated significantly by the high Cort treatment were downregulated by ADX and vice versa, and that only a limited number of genes responded unidirectionally, either to long-term elevation of circulating GC (12-day high Cort) or corticoid deprivation (7-day ADX).
Upregulated genes by long-term high Cort
The genes upregulated by 12-day high Cort included canonical GC target genes such as Hif3a, Sgk1, Sult1a1, Per1, Fkbp5, and Dusp1; these genes are known to contain canonical glucocorticoid response element sequences in either their 5′-flanking or intragenic regions and may most likely be activated by direct binding of dimeric GC receptors (GRs) to the DNA motifs (15, 16).
The clock gene, Per1, was upregulated not only by 12-day high Cort treatment but also by acute Cort injection. This is a unique characteristic of Per1, because no other genes that were affected significantly by 12-day high Cort treatment were affected significantly after acute Cort injection. It may be related to the fact that GCs act as strong entraining signals for the circadian oscillators; the clock genes in virtually all peripheral organs (26), and at least in some regions in the central nervous system, including the PVH, are under the control of circulating GCs (27).
Dusp 1 encodes a phosphatase that inactivates the mitogen-activated protein kinases (MAPKs). Consistent with this action of Dusp1, downregulation of Dusp 1 gene expression was shown to enhance MAPK activity in hypothalamic explants (28). Dusp 1 is upregulated by GCs in macrophages and other peripheral cells and has been proposed to mediate anti-inflammatory actions of GCs (29). MAPK may confer stimulatory signals to the CRF gene (30), so the Cort-induced upregulation of Dusp 1, observed in the current study, could counteract MAPK-mediated CRF gene expression.
Fkbp5 was upregulated significantly by 12-day high Cort treatment, consistent with the results of a previous report showing that Cort treatment in mice increases Fkbp5 expression in the hypothalamus (31). FKBP5, encoded by Fkbp5, is a cochaperone to heat shock protein 90. The GC-induced upregulation of Fkbp5 is regarded as the ultra-short feedback of GC action, because binding of FKBP5 to the GR complex not only reduces its affinity to GC (32) but also impairs nuclear translocation of the GR complex (33). Counteraction of GR activity by FKBP5 induced by GCs may be part of a feedback mechanism to limit overactivation or repression of GC-responsive genes in the PVH in conditions of chronic elevation of circulating GCs.
Sgk1 was reported to be induced by GC (34), and it was also upregulated markedly in the current study. This serine/threonine protein kinase is involved in the regulation of various ionic channels, carriers, and Na+/K+ ATPase, and may have multiple physiological functions (35), but its role in the hypothalamus is not clear yet.
Slc19a3, which encodes a class of thiamine transporters, was also upregulated markedly by 12-day high Cort. Thiamine is a major factor in the metabolism of glucose, but it also changes the neural excitability in the hippocampus (36), so GC may also be capable of changing the activity of hypothalamic neurons by facilitating thiamine transport into the cells. Because thiamine transporter gene expression was also upregulated by GC in murine macrophages (16), the GC-induced thiamine recruitment may not be specific for the hypothalamus.
Fmo2, one of the most markedly upregulated genes, encodes an enzyme that catalyzes oxidation of xenobiotics. The Fmo2 product, dimethylaniline monooxygenase [N-oxide-forming] 2, is induced by GC also in the lung and kidney (37), although its physiological implication is not known.
The changes in transcript profiles induced by either 12-day high Cort or acute Cort injection in the current study differ markedly from those observed in immune cells (15, 16) or tumors (13, 14), with the exception of a small number of canonical GC target genes, emphasizing the high tissue specificity of the effects of GCs (13–16). However, caution is required when comparing the present results to previous ones, because pharmacological doses of the synthetic GC dexamethasone, with different exposure times, were used in most previous studies in vitro (13–16).
Downregulated genes by 12-day high Cort
Crh was downregulated most prominently by 12-day high Cort, which can be readily understood in view of the biological significance of GC negative feedback in the HPA regulation. In addition, the expression of other neuroendocrine peptides and receptors (i.e.,Avp, Oxt, and Aplnr) was also downregulated significantly. AVP is synthesized in the parvocellular CRF neurons in the PVH, as well as in the magnocellular AVP neurons (38). The parvocellular AVP is a physiological secretagogue of ACTH in the pituitary, and its secretion is stimulated by stress (4, 5). In addition, the parvocellular AVP synergizes CRF-induced ACTH secretion from the pituitary (5). The parvocellular CRF neurons express GRs, and AVP gene expression within the parvocellular CRF neurons is highly sensitive to GC (38–42). In addition, it has been reported that mineralocorticoid receptors and 11β−hydroxysteroid dehydrogenase 2 (43), and not GRs (44), are present in magnocellular neurons, suggesting that these neurons are responsive to mineralocorticoids rather than GCs. Therefore, the Cort-induced downregulation of Avp observed in the current study is likely to reflect mainly the changes in parvocellular AVP mRNA coexpressed in CRF neurons. However, it is not clear whether magnocellular neurons, the major source of AVP in the PVH, contributed to the observed changes in AVP expression, because part of the Cort-dependent Avp downregulation may have resulted secondarily from the cardiovascular effects of long-term GC excess, the signals of which are transmitted via inhibitory neural afferents to the magnocellular PVH (2).
Oxytocin is not regarded primarily as a partner in the HPA regulation, and the suppressive effect of GC on Oxt gene expression has not been described in the previous literature. Because OXT is also secreted during stress (45), Cort-induced downregulation of Oxt may represent negative feedback action of Cort on stress-induced activation of oxytocin neurons. Apelin receptors are expressed in the PVH (46), and their activation elicits secretion of CRF and AVP, which results in elevation of circulating GC levels (47). Therefore, the Cort-induced suppression of the Aplr may also counteract the apelin-derived HPA activation.
Upregulated or downregulated genes by corticoid deprivation
Crh expression was most prominently enhanced by 7-day ADX, in an opposite direction to the effect of the long-term high Cort treatment. This reiterates the importance of the GC negative feedback in maintaining the appropriate levels of CRF expression required for basal activity and for coping with stress challenges. Avp and Aplr were also upregulated significantly by 7-day ADX, which is in the opposite direction of the significant downregulation of these genes by 12-day high Cort treatment.
On the other hand, Vip was downregulated most prominently by 7-day ADX. This finding is consistent with the suppression of VIP gene expression in the suprachiasmatic nucleus of adrenalectomized animals (48). However, the GC message must reach the clock through an indirect mechanism because the suprachiasmatic nucleus does not express significant levels of GRs (49). VIP is reported to be colocalized in CRF neurons in the PVH (50), but the physiological implication of the GC-induced downregulation of Vip is yet to be examined.
Gabra4 and Gabrd were also downregulated significantly by 7-day ADX; these genes encode the α4 and δ subunits of the GABAA channels, respectively. The GABAA receptors containing δ subunits in combination with α4 subunits, among other subunits, generate uninterrupted conductance referred to as “tonic,” and these GABAA receptors are characterized by their extrasynaptic localization (51). Therefore, the simultaneous downregulation of Gabra4 and Gabrd may be evidence of the permissive role of Cort in maintaining the extrasynaptic GABAA receptor–mediated tonic inhibition. Slc17a7, a subtype of the vesicular glutamate transporters, was also downregulated by 7-day ADX. These results suggest that GC may be necessary for maintaining the glutamatergic and GABAergic transmission in the PVH. Indeed, glutamatergic and GABAergic interneurons directly innervate the neuroendocrine neurons, including CRF (52, 53), AVP, and OXT neurons, in the PVH (54, 55).
It should also be noted that Per1, which was upregulated by either 12-day high Cort treatment or acute Cort injection, was not downregulated by 7-day ADX. Therefore, the basal expression of Per1 may not require GCs, whereas its induction is GC-dependent.
The Cort-regulated genes were associated with canonical biological pathways such as glycerophospholipid metabolism, chemokine signaling, vascular smooth muscle contraction, and focal adhesion, but the roles of these pathways in the regulation of hypothalamic functions remain elusive and need to be examined further. Some immune-related genes were either upregulated or downregulated by 12-day high Cort or 7-day ADX, but their functional implications in the PVH remain to be elucidated. Because glial cells represent a considerable proportion of cells in the PVH, part of the GC-dependent transcriptome changes observed in the current study may be attributable to the GC actions in glia. It is also tempting to speculate that the immune-related genes affected by Cort may be relevant to the functions of resident microglia and astrocytes, which are innate immune cells in the central nervous system (56).
Acute Cort injection
After acute Cort injection, the changes in transcriptome signature in the hypothalamic PVH region were totally different from those after the 12-day treatment with the high-dose Cort. Indeed, Per1 was the only gene that was upregulated by acute Cort injection, among the genes upregulated by the 12-day high Cort treatment. We could not find any gene that was suppressed significantly by both acute Cort injection and 12-day high Cort treatments. It is well recognized that GC action depends on its concentration and duration of action (13, 14), and the striking difference in transcriptome profiles resulted from the two experimental conditions (i.e., acute Cort increase mimicking the stress-induced Cort surge and sustained exposure to high Cort for a long time) is consistent with this characteristic of GCs. This should be taken into consideration when designing studies or drawing conclusions from experiments performed with nonphysiological doses of GC.
Upregulated genes by acute Cort injection
Unexpectedly, six of the 11 genes upregulated in the acute Cort (1) 1 h group [i.e.,Bsx, Egr1, Fos, Fosb, Junb, and Nr4a3 (Nor1)] were members of the IEG families. To our knowledge, this is the first report that acute Cort administration–induced gene expression of the members of the Fos and Jun families, as well as Bsx, Egr1, and Nr4a3, in the hypothalamus. These findings obtained by the RNA-seq analysis were reproduced by qRT-PCR.
Because the control rats did not receive vehicle injections in experiment 2, the injection stress, and not the Cort itself, might have been responsible for the changes in gene expression observed after acute Cort injection, especially for the upregulation of the IEGs, which are highly responsive to stress. However, this is unlikely, because the semiquantitative in situ hybridization showed minimal autoradiographic signals in vehicle-injected controls, and significant increases in Fos and Egr1 mRNA levels were observed after Cort injection compared with the vehicle-injected controls. Previous reports have also shown negligible effects of intraperitoneal injection of normal saline to male rats on mRNA expression of IEGs, including Fos, Junb, and Egr1, compared with noninjected rats (57, 58). Therefore, upregulation of IEG expression observed in the current study may have been the consequence of Cort actions, although we still cannot rule out the possibility that the changes in transcript levels of other genes were related to injection. Interestingly, injection of a higher (pharmacological) dose of Cort did not increase Fos and Egr1 mRNA levels in the PVH, indicating that an optimal Cort concentration is required for the induction of the IEGs. This finding again emphasizes that the concentration of GCs is critical for their cellular effects.
Activator protein-1 (AP-1), which is the heterodimer of the Fos/Jun family IEG products, has been regarded as one of the transcription factors that positively regulate the promoter activity of target genes. However, it is still controversial whether AP-1 enhances CRF and other neuroendocrine peptide gene transcription (59–62).
Recent advances in genome-wide searches for transcription-factor binding sites, as well as the “open chromatin” loci, have revealed that that the AP-1 facilitates GR access to target gene promoters by keeping the chromatin configuration in an open state (63, 64). In this regard, the prior binding of AP1 to the AP-1 site is required to preprogram and maintain chromatin structure permissive for GR binding in a heterologous cell line (63, 64). Furthermore, both positive and negative GR cistromes are predominantly composed of the canonical GC response elements that are near the AP-1 binding sites in macrophages (16). Thus, AP-1 plays a critical role in facilitating GC-induced transactivation or repression of target genes in peripheral tissues. It is tempting to speculate that acute Cort-induced upregulation of the Fos/Jun family transcription factors observed in the current study may contribute to opening the chromatin and allowing access of GR to promoters of target genes in the hypothalamus.
Consistent with the present demonstration of Egr1 mRNA induction by acute Cort, GCs have been reported to induce Egr1 expression via GRs in the AtT20 cell line (65). Egr1 expression was also induced in the hippocampus by acute restraint stress, an effect mediated by the GRs (65). The mechanisms by which Egr1 regulates hypothalamic gene expression are not clear, so the exact promoter targets of Egr1 in the PVH region remain to be elucidated. Nab2 is one of the few genes that were upregulated only at 3 hours after Cort injection. Because Nab2 products are known to repress Egr1-induced transcriptional activation (66), they may counteract the action of Egr1 in a delayed fashion. The induction of IEGs by acute elevations of GC may be part of the mechanisms by which GCs modulate gene transcription according to biological demands, but additional studies are required to elucidate the functional implication of the observed phenomena.
Sato et al. (34) conducted a pilot study for screening the dexamethasone-responsive genes in the rat hypothalamus by DNA microarray. They examined the effect of a large dose (2 mg) of dexamethasone 2 hours after intraperitoneal injection and identified ∼20 genes, but only four [Sgk1, Dusp1, Bcl6 (B-cell lymphoma 6 protein), and Gpd1] of the 15 upregulated genes in their study were also upregulated significantly in the current study, and not by the acute Cort injection but by the 12-day high Cort treatment. None of the seven downregulated genes identified in their study was identified in the current study. This discrepancy is probably due to the differences in the chemical property of the GCs used (Cort vs dexamethasone), as well as their doses, again reinforcing the idea that the mode of action of GC is different depending on its dose and exposure time.
Downregulated genes by acute Cort injection
The suppressive effect of acute Cort administration tended to be rapid and long lasting: whereas most genes that were suppressed significantly at 1 hour after acute Cort injection were suppressed to a certain extent at 3 hours, almost all genes, the expression of which was suppressed significantly at 3 hours after Cort injection, coincided with those whose expression was already suppressed at 1 hour.
In contrast to the prominent changes in Crh expression under the 12-day high Cort treatment and 7-day ADX, it is remarkable that the transient increase in Cort, which is comparable to the stress surge, had no significant effect on Crh expression. It is well recognized that stress increases CRF mRNA levels in the PVH, despite the rapid increases in GCs, which has been attributed to the ability of stimulatory pathways to overcome the GC feedback (12). However, the present results suggest that the transient increase in circulating Cort within a stress range may not repress CRF gene expression in the PVH, at least in the short term. This is also consistent with the observation that Cort injection in adrenalectomized rats rapidly decreases AVP but not CRF heterogeneous nuclear RNA levels (67).
Expression of Atp2a3, Prkcd, and Prkcg, which are involved in the calcium-signaling pathway, were suppressed significantly by acute Cort injection. Because calcium signaling has been implicated in transcriptional activation of Avp (60), these changes may contribute to the GC-induced suppression of Avp.
Unexpectedly, the expression of 14 genes (including Prkcd, Wnt9b, Wnt9b, Lef1, Gabra4, Gabrd, and Slc17a) of the 34 genes whose expression was downregulated at 1 hour after Cort injection was also suppressed by 7-day ADX. This group of genes may require GC for basal expression, while being downregulated by acute elevation of circulating GCs. These observations could also be explained by differential GC actions on target genes, depending on the dose and exposure time.
In conclusion, using genome-wide RNA-seq analysis, we identified genes in the hypothalamic region containing the PVH, the expression of which was upregulated or downregulated depending on the circulating levels of Cort and time of exposure to the steroid. These genes may take part in a broad spectrum of functions in the hypothalamic region and play roles in the central regulation of endocrine and autonomic nervous systems for securing homeostasis. Along with the suppression of Crh and Avp expression by long-term high-dose Cort, the downregulation of Aplr and upregulation of Dusp1 may also contribute to the downregulation of the HPA activity. Remarkably, the IEGs (Bsx, Egr1, Fos, Fosb, Junb, and Nr4a3) were upregulated transiently by acute Cort administration. The roles of the IEG products are still elusive, but they may possibly participate in transcriptional activation or repression of GC target genes by interacting with GRs or modulating chromatin configuration to facilitate GR access to the promoter of target genes. An important unanswered question is which of the GC-sensitive genes identified in the current study are regulated directly by GCs and which of them are regulated indirectly via neural inputs from other nuclei that are activated primarily by GCs. Although additional studies are required to determine the functional roles of the uncovered genes, the current study may provide a useful database for studying the mechanisms of action of GCs in regulating the functions of the PVH.
Supplementary Material
Acknowledgments
We thank Drs. Keiko Ozato, Yoshi Yamada, and members of National Institutes of Health (NIH) Japanese Scientists Association for supporting K.I. after the Great East Japan Earthquake in 2011. We thank Dr. Stanley Watson, University of Michigan, and Dr. Jeffrey Milbrandt, Washington University, for providing us with the DNA plasmids for rat Fos and rat Egr1, respectively. We thank Dr. David Klein, National Institute of Child Health and Human Development, NIH, for helpful discussions and encouragement for accomplishing this work.
The Gene Expression Omnibus accession number for the RNA-seq data reported in this paper is GSE120002.
Financial Support: This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development of the National Institutes of Health (to G.A.), and Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant JP18K08499 to K.I.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 7-day ADX
corticoid deprivation for 7 days
- 12-day high Cort
high-dose corticosterone exposure for 12 days
- ADX
adrenalectomy
- AP-1
activator protein-1
- AVP
vasopressin
- Bsx
brain-specific homeobox protein homolog
- Cck
cholecystokinin
- Cort
corticosterone
- CRF
corticotropin-releasing factor
- Dusp1
dual-specificity protein phosphatase 1
- Egr1
early growth-response protein 1
- Fkbp5
FK506-binding protein 5
- Fmo2
dimethylaniline monooxygenase [N-oxide–forming] 2
- Fst
follistatin
- Gabra4
γ-aminobutyric acid receptor A (GABAA) subunit α-4
- Gabrd
GABAA subunit δ
- GC
glucocorticoid
- Gpd1
glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic
- GR
glucocorticoid receptor
- HBC
hydroxypropyl-β-cyclodextrin
- Hif3a
hypoxia-inducible factor 3-α
- HPA
hypothalamic-pituitary-adrenal
- IEG
immediate early gene
- Junb
transcription factor jun-B
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Lef1
lymphoid enhancer-binding factor 1
- MAPK
mitogen-activated protein kinase
- Nab2
NGFI-A-binding protein 2
- Nr4a3
nuclear receptor subfamily 4 group A member 3
- OXT
oxytocin
- Per1
period circadian protein homolog 1
- Pla2g3
group 3 secretory phospholipase A2
- Prkcd
protein kinase C δ type
- Prkcg
protein kinase C γ type
- PVH
paraventricular nucleus of the hypothalamus
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- RNA-seq
RNA sequencing
- Sgk1
serine/threonine protein kinase Sgk1
- Slc17a7
vesicular glutamate transporter 1
- Slc19a3
thiamine transporter 2
- TRH
thyrotropin-releasing hormone
- Vip
vasoactive intestinal polypeptide
- Wnt9b
protein Wnt-9b
References
- 1. Cooper SJ. From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite. 2008;51(3):419–427. [DOI] [PubMed] [Google Scholar]
- 2. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6(1):269–324. [DOI] [PubMed] [Google Scholar]
- 4. Whitnall MH, Mezey E, Gainer H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature. 1985;317(6034):248–250. [DOI] [PubMed] [Google Scholar]
- 5. Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299(5881):355–357. [DOI] [PubMed] [Google Scholar]
- 6. Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433(2):222–238. [DOI] [PubMed] [Google Scholar]
- 7. Hallbeck M, Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J Comp Neurol. 1999;411(2):201–211. [PubMed] [Google Scholar]
- 8. Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516(5):423–441. [DOI] [PubMed] [Google Scholar]
- 9. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 10. Spiga F, Walker JJ, Gupta R, Terry JR, Lightman SL. 60 years of neuroendocrinology: glucocorticoid dynamics: insights from mathematical, experimental and clinical studies. J Endocrinol. 2015;226(2):T55–T66. [DOI] [PubMed] [Google Scholar]
- 11. Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006; 147(suppl 1):S258–S268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguilera G, Kiss A, Liu Y, Kamitakahara A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress. 2007;10(2):153–161. [DOI] [PubMed] [Google Scholar]
- 13. Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19(12):2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubbs FE, Birnie MT, Biddie SC, Lightman SL, Conway-Campbell BL. SKOV3 cells containing a truncated ARID1a protein have a restricted genome-wide response to glucocorticoids. Mol Cell Endocrinol. 2018;461:226–235. [DOI] [PubMed] [Google Scholar]
- 15. Cronk JC, Derecki NC, Ji E, Xu Y, Lampano AE, Smirnov I, Baker W, Norris GT, Marin I, Coddington N, Wolf Y, Turner SD, Aderem A, Klibanov AL, Harris TH, Jung S, Litvak V, Kipnis J. Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity. 2015;42(4):679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uhlenhaut NH, Barish GD, Yu RT, Downes M, Karunasiri M, Liddle C, Schwalie P, Hübner N, Evans RM. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell. 2013;49(1):158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25(16):4073–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacobson L, Akana SF, Cascio CS, Scribner K, Shinsako J, Dallman MF. The adrenocortical system responds slowly to removal of corticosterone in the absence of concurrent stress. Endocrinology. 1989;124(5):2144–2152. [DOI] [PubMed] [Google Scholar]
- 19. Das G, Uchida K, Kageyama K, Iwasaki Y, Suda T, Itoi K. Glucocorticoid dependency of surgical stress-induced FosB/DeltaFosB expression in the paraventricular and supraoptic nuclei of the rat hypothalamus. J Neuroendocrinol. 2009;21(10):822–831. [DOI] [PubMed] [Google Scholar]
- 20. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Itoi K, Helmreich DL, Lopez-Figueroa MO, Watson SJ. Differential regulation of corticotropin-releasing hormone and vasopressin gene transcription in the hypothalamus by norepinephrine. J Neurosci. 1999;19(13):5464–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks [published correction appears in Nat Protoc. 2014;9(10):2513] Nat Protoc. 2012;7(3):562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29(14):1830–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okamura Y, Aoki Y, Obayashi T, Tadaka S, Ito S, Narise T, Kinoshita K. COXPRESdb in 2015: coexpression database for animal species by DNA-microarray and RNAseq-based expression data with multiple quality assessment systems. Nucleic Acids Res. 2015;43(Database issue):D82–D86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans AN, Liu Y, Macgregor R, Huang V, Aguilera G. Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids. Mol Endocrinol. 2013;27(11):1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pezük P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chun LE, Christensen J, Woodruff ER, Morton SJ, Hinds LR, Spencer RL. Adrenal-dependent and -independent stress-induced Per1 mRNA in hypothalamic paraventricular nucleus and prefrontal cortex of male and female rats. Stress. 2018;21(1):69–83. [DOI] [PubMed] [Google Scholar]
- 28. Adachi K, Goto M, Onoue T, Tsunekawa T, Shibata M, Hagimoto S, Ito Y, Banno R, Suga H, Sugimura Y, Oiso Y, Arima H. Mitogen-activated protein kinase phosphatase 1 negatively regulates MAPK signaling in mouse hypothalamus. Neurosci Lett. 2014;569:49–54. [DOI] [PubMed] [Google Scholar]
- 29. Clark AR, Martins JR, Tchen CR. Role of dual specificity phosphatases in biological responses to glucocorticoids. J Biol Chem. 2008;283(38):25765–25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kageyama K, Hanada K, Takayasu S, Iwasaki Y, Sakihara S, Nigawara T, Suda T. Involvement of regulatory elements on corticotropin-releasing factor gene promoter in hypothalamic 4B cells. J Endocrinol Invest. 2008;31(12):1079–1085. [DOI] [PubMed] [Google Scholar]
- 31. Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, Huo Y, Rongione M, Wand GS, Potash JB. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151(9):4332–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141(11):4107–4113. [DOI] [PubMed] [Google Scholar]
- 33. Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280(6):4609–4616. [DOI] [PubMed] [Google Scholar]
- 34. Sato H, Horikawa Y, Iizuka K, Sakurai N, Tanaka T, Shihara N, Oshima A, Takeda J, Mikuni M. Large-scale analysis of glucocorticoid target genes in rat hypothalamus. J Neurochem. 2008;106(2):805–814. [DOI] [PubMed] [Google Scholar]
- 35. Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588(Pt 18):3349–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tallaksen CM, Taubøll E. Excitatory effect of thiamin on CA1 pyramidal neurones in rat hippocampal slices in vitro. Eur J Neurol. 2000;7(6):693–698. [DOI] [PubMed] [Google Scholar]
- 37. Lee MY, Clark JE, Williams DE. Induction of flavin-containing monooxygenase (FMO B) in rabbit lung and kidney by sex steroids and glucocorticoids. Arch Biochem Biophys. 1993;302(2):332–336. [DOI] [PubMed] [Google Scholar]
- 38. Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16(4):348–355. [DOI] [PubMed] [Google Scholar]
- 39. Ma XM, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140(12):5642–5650. [DOI] [PubMed] [Google Scholar]
- 40. Helmreich DL, Itoi K, Lopez-Figueroa MO, Akil H, Watson SJ. Norepinephrine-induced CRH and AVP gene transcription within the hypothalamus: differential regulation by corticosterone. Brain Res Mol Brain Res. 2001;88(1-2):62–73. [DOI] [PubMed] [Google Scholar]
- 41. Itoi K, Mouri T, Takahashi K, Murakami O, Imai Y, Sasaki S, Yoshinaga K, Sasano N. Suppression by glucocorticoid of the immunoreactivity of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of rat hypothalamus. Neurosci Lett. 1987;73(3):231–236. [DOI] [PubMed] [Google Scholar]
- 42. Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–3309. [DOI] [PubMed] [Google Scholar]
- 43. Haque M, Wilson R, Sharma K, Mills NJ, Teruyama R. Localisation of 11β-hydroxysteroid dehydrogenase type 2 in mineralocorticoid receptor expressing magnocellular neurosecretory neurones of the rat supraoptic and paraventricular nuclei. J Neuroendocrinol. 2015;27(11):835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berghorn KA, Knapp LT, Hoffman GE, Sherman TG. Induction of glucocorticoid receptor expression in hypothalamic magnocellular vasopressin neurons during chronic hypoosmolality. Endocrinology. 1995;136(2):804–807. [DOI] [PubMed] [Google Scholar]
- 45. Laczi F, Iványi T, Sarnyai Z, Vecsernyés M, Lengyel G, Szabó G, Bíró E, Gardi J, Julesz J, Telegdy G. The role of central corticoliberin in the ether stress-induced secretion of neurohypophyseal hormones and corticosterone in the rat. Neuropeptides. 1994;26(1):33–37. [DOI] [PubMed] [Google Scholar]
- 46. Pope GR, Roberts EM, Lolait SJ, O’Carroll AM. Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides. 2012;33(1):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Newson MJ, Roberts EM, Pope GR, Lolait SJ, O’Carroll AM. The effects of apelin on hypothalamic-pituitary-adrenal axis neuroendocrine function are mediated through corticotrophin-releasing factor- and vasopressin-dependent mechanisms. J Endocrinol. 2009;202(1):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gozes I, Avidor R, Giladi E, Shani Y, McEwen BS, Dussaillant M, Rostene W. Adrenalectomy decreases vasoactive intestinal peptide mRNA levels in the rat suprachiasmatic nucleus. Neurosci Lett. 1994;167(1-2):24–28. [DOI] [PubMed] [Google Scholar]
- 49. Girardet C, Becquet D, Blanchard MP, François-Bellan AM, Bosler O. Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. Eur J Neurosci. 2010;32(12):2133–2142. [DOI] [PubMed] [Google Scholar]
- 50. Hökfelt T, Fahrenkrug J, Ju G, Ceccatelli S, Tsuruo Y, Meister B, Mutt V, Rundgren M, Brodin E, Terenius L, Hulting A-L, Werner S, Björklund H, Vale W Analysis of peptide histidine-isoleucine/vasoactive intestinal polypeptide-immunoreactive neurons in the central nervous system with special reference to their relation to corticotropin releasing factor- and enkephalin-like immunoreactivities in the paraventricular hypothalamic nucleus. Neuroscience. 1987;23(3):827–857. [DOI] [PubMed] [Google Scholar]
- 51. Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56(5):763–770. [DOI] [PubMed] [Google Scholar]
- 52. Itoi K, Talukder AH, Fuse T, Kaneko T, Ozawa R, Sato T, Sugaya T, Uchida K, Yamazaki M, Abe M, Natsume R, Sakimura K. Visualization of corticotropin-releasing factor neurons by fluorescent proteins in the mouse brain and characterization of labeled neurons in the paraventricular nucleus of the hypothalamus. Endocrinology. 2014;155(10):4054–4060. [DOI] [PubMed] [Google Scholar]
- 53. Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci. 2012;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Daftary SS, Boudaba C, Szabó K, Tasker JG. Noradrenergic excitation of magnocellular neurons in the rat hypothalamic paraventricular nucleus via intranuclear glutamatergic circuits. J Neurosci. 1998;18(24):10619–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morton LA, Popescu IR, Haam J, Tasker JG. Short-term potentiation of GABAergic synaptic inputs to vasopressin and oxytocin neurones. J Physiol. 2014;592(19):4221–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. [DOI] [PubMed] [Google Scholar]
- 57. Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol. 2005;17(4):227–237. [DOI] [PubMed] [Google Scholar]
- 58. Hwang BH, Chang HM, Gu ZH, Suzuki R. c-fos gene expression is increased in the paraventricular hypothalamic nucleus of Sprague-Dawley rats with visceral pain induced by acetic acid without detectable changes of corticotrophin-releasing factor mRNA: a quantitative approach with an image analysis system. Anat Rec (Hoboken). 2007;290(4):406–413. [DOI] [PubMed] [Google Scholar]
- 59. Itoi K, Horiba N, Tozawa F, Sakai Y, Sakai K, Abe K, Demura H, Suda T. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137(6):2389–2396. [DOI] [PubMed] [Google Scholar]
- 60. Iwasaki Y, Oiso Y, Saito H, Majzoub JA. Positive and negative regulation of the rat vasopressin gene promoter. Endocrinology. 1997;138(12):5266–5274. [DOI] [PubMed] [Google Scholar]
- 61. Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149(7):3512–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kageyama K, Itoi K, Iwasaki Y, Niioka K, Watanuki Y, Yamagata S, Nakada Y, Das G, Suda T, Daimon M. Stimulation of corticotropin-releasing factor gene expression by FosB in rat hypothalamic 4B cells. Peptides. 2014;51:59–64. [DOI] [PubMed] [Google Scholar]
- 63. Biddie SC, John S, Sabo PJ, Thurman RE, Johnson TA, Schiltz RL, Miranda TB, Sung MH, Trump S, Lightman SL, Vinson C, Stamatoyannopoulos JA, Hager GL. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol Cell. 2011;43(1):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43(3):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Revest JM, Di Blasi F, Kitchener P, Rougé-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids [published correction appears in Nat Neurosci. 2005;8(6):835] Nat Neurosci. 2005;8(5):664–672. [DOI] [PubMed] [Google Scholar]
- 66. Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16(7):3545–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma XM, Levy A, Lightman SL. Rapid changes of heteronuclear RNA for arginine vasopressin but not for corticotropin releasing hormone in response to acute corticosterone administration. J Neuroendocrinol. 1997;9(10):723–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.