Abstract
Long-term oxygen therapy (LTOT) has beneficial effects on survival in patients with chronic obstructive pulmonary disease (COPD) and severe hypoxemia at rest. Two landmark trials suggested that these benefits depend on the time of exposure to oxygen. Patients are usually prescribed LTOT for at least 15–18 hours/day. The primary objective of this study was to determine the average daily exposure to supplemental oxygen in patients with severely hypoxemic COPD who were newly prescribed LTOT and the proportion of patients who were adherent to their prescription. The secondary objective was to identify predictors of compliance to LTOT. We performed a retrospective observational study of patients newly registered in a regional home oxygen program in Quebec, Canada, between July 1, 2013, and December 31, 2014. Daily exposure to oxygen was objectively measured from the concentrator’s counter clock. From 196 patients registered in the program during the study period, 115 contributed to the analysis. Most patients (n = 84; 73%) were prescribed oxygen for ≥18 hours/day. Overall, the 115 patients were exposed to home oxygen for 17.8 hours/day; 60% of the patients were compliant according to our definition. Increasing age and ambulatory oxygen utilization predicted adherence to oxygen therapy. Adherence to home oxygen therapy is suboptimal. Behavioral and psychological interventions to improve compliance to LTOT should be investigated.
Keywords: COPD, chronic hypoxemia, long-term oxygen therapy, compliance, adherence
Introduction
Two randomized controlled trials of long-term oxygen therapy (LTOT) in chronic obstructive pulmonary disease (COPD) clearly demonstrated that low-flow domiciliary oxygen increases survival in severely hypoxemic patients.1,2 The British study randomly assigned patients to receive 15 hours of oxygen therapy (including hours of sleep) per day versus no oxygen therapy at all. At 5-year follow-up, the oxygen therapy group had improved survival: 19 of 42 (42%) oxygen therapy patients had died, compared to 30 of the 45 (66%) control patients.1 The American trial randomly assigned patients to receive oxygen for either 12 hours a day (nocturnal group) or 24 hours a day (continuous group). The continuous group actually received oxygen for an average of 19 hours a day. At 24 months, the overall mortality in the continuous group was 22.4%, whereas it was 40.8% in the nocturnal group.2 Survival of patients who were only referred for nocturnal oxygen in the American trial was greater than survival of those who were allocated to the control group in the British trial. This indirect comparison provided some evidence that the benefits of oxygen depend on the duration of daily exposure to therapy, at least in severely hypoxemic patients with COPD.
Following the introduction of the technology of oxygen concentrator, several studies have focused on patients’ compliance to home oxygen therapy.3 In this context, “compliance” refers to the extent to which the patient’s behavior coincides with the clinical prescription. It has been suggested that “compliance” is synonymous with “adherence,” and both terms may be used interchangeably.4 The evaluation of patients’ compliance to home oxygen often relied on patients’ reports based on interviews or administration of questionnaires to patients or medical staff. Other studies measured exposure to home oxygen more objectively, using the clock counter now available on most concentrators.5–13 Most reports were from cross-sectional studies, that is, studies of compliance over short periods of time in patients who had been on oxygen therapy for varying duration and suggested that, in general, adherence to home oxygen therapy is poor.
Given the costs incurred and the presumed relation between time of exposure to oxygen and its benefits on survival, the issue of compliance is of outmost interest. Also, a better understanding of adherence to home oxygen therapy and its determinants may help physicians and allied health professionals to improve patient care and cost-effectiveness. The primary objective of this study was, therefore, to determine the average daily use of LTOT in patients with severely hypoxemic COPD who were newly prescribed LTOT and the proportion of patients who were adherent to therapy. The secondary objective was to identify predictors of compliance to oxygen therapy.
Methods
Setting and patients
The study took place in the respiratory home care program of the Quebec City area (province of Quebec, Canada). This program is funded by the Quebec universal medical insurance plan and delivers home care (mainly LTOT and related services) to patients with any chronic lung disease. Every patient with LTOT living in this area is registered to the program, with the only exception of those living in a long-term care facility. This study received approval from the internal review board of our institution (approval number: 2016-2625, 21295).
All patients with COPD newly prescribed with LTOT who were registered between July 1, 2013, and December 31, 2014, were included in the study. To be admitted to the program, patients must meet the widely accepted criteria for LTOT (arterial partial pressure of oxygen (PaO2) ≤55 mmHg; or PaO2 ≤59 mmHg with clinical evidence of at least one of the following: pulmonary hypertension, right ventricular hypertrophy, cor pulmonale, or hematocrit ≥55%).2 No patient is admitted on the basis of severe disability alone or on the basis of resting oxygen desaturation only; arterial blood gas measurement is therefore mandatory. Patients are provided with a stationary, electrically powered oxygen concentrator for home-based oxygen therapy and are visited by the program’s staff (nurse and respiratory therapist, in turn) according to a predefined schedule.
Measures and outcomes
We conducted a retrospective observational study from chart review. The daily duration of oxygen use was objectively measured from the concentrators’ counter clock that records the cumulative number of hours of utilization. This information is routinely recorded by respiratory therapists during regular home visits. In addition, we collected data regarding patient’s gender, age, number of hours of utilization per day on the initial LTOT prescription, arterial blood gases (PaO2 and arterial partial pressure of carbon dioxide) on program admission, forced expiratory volume in 1 second (FEV1), smoking history, living environment (i.e. place of residence and whether they lived alone or not) as well as the use of portable cylinders. Comorbidities were also noted and summarized using the Charlson index,14 a measure of comorbidities in older adults that predicts mortality. Prior diagnoses of depression or anxiety were also noted. Hospital admissions during the study period and length of stay were recorded.
Statistics
From time 0 and after each home visit, daily exposure to oxygen was calculated from the number of hours recorded on the concentrator counter clock (i.e. the numerator) divided by the number of days since the previous visit (i.e. the denominator). For each patient, we, therefore, obtained a series of means from varying periods of time from which we calculated a weighted mean number of hours of exposure to oxygen through the study period.
We adjusted the number of hours of utilization for all patients who owned an ambulatory oxygen system and also took into account hospitalizations. Because portable systems are not necessarily provided by the home care program, we could not collect this information from chart review for all patients. In order to estimate the ambulatory oxygen utilization, we accessed administrative data available at the home care program where a log of all cylinders provided by the program is kept. Small pressurized 2.5-kg cylinders containing 160–180 liters of oxygen that are coupled with an oxygen-conserving device are still currently used.15 At a flow of 2 liters/minute, use time per cylinder is around 4 hours. During the 2013–2015 period, all patients with COPD who were provided portable oxygen by the program (whatever the time since the introduction of LTOT) used on average 23 cylinders per year (or 2 cylinders per month). From this statistics, we estimated that patients who owned an ambulatory oxygen system, whatever the provider, used it 8 hours/month (2 cylinders/month × 4 hours/cylinder) or 0.25 hour/day. This roughly corroborates our findings from a randomized trial of ambulatory oxygen in patients recently put on LTOT (as those included in the current study) where the mean daily use of portable oxygen was 0.5 hour/day.15 We, therefore, assumed that patients who owned an ambulatory oxygen system used it 0.5 hour/day. We also took into account hospitalizations that were recorded in our files by subtracting from the denominator the total number of days in hospital during each period.
Using the weighted mean number of hours of exposure to oxygen corrected for ambulatory oxygen utilization and hospitalizations, we then categorized each patient as “adherent” or “non-adherent.” Patients were considered as adherent to oxygen if they achieved oxygen treatment for a duration equivalent (±1 hour) to that prescribed by the physician.9
We compared the proportions using Fisher’s exact tests and continuous variables using one-way analyses of variance. In order to illustrate the changes in exposure to oxygen over time, we constructed B-spline curves using piecewise third-order regression models.16 In univariate analyses, baseline characteristics (including age, gender, FEV1, smoking status, blood gas results upon admission to the program, living environment, portable oxygen utilization, anxiety, depression, and comorbidities) were investigated to identify predictors of compliance. Only variables with p values <0.20 were retained for inclusion in multivariate logistic regression analyses. The results were considered significant with p values <0.05.
Results
Patients
One hundred and ninety-six patients with COPD were registered for LTOT between July 1, 2013, and December 31, 2014. Eighty-one patients were excluded from the study for the following reasons: patients prescribed nocturnal oxygen only (n = 41), insufficient information available (n = 23), insufficient number of home visits to calculate mean daily use of oxygen (n = 13), PaO2 unknown upon program admission (n = 2), LTOT initiated in another area (n = 1), and oxygen therapy prescribed but finally refused by the patient (n = 1). One hundred and fifteen patients were therefore included in the compliance study (Table 1). Altogether, they cumulated 529,334 hours of exposure to oxygen over 30,601 days of recording. Median follow-up duration was 358 days. Forty-four (38%) patients owned a portable oxygen system. Twenty-five (22%) patients were hospitalized at least once over the study period (total 47 hospitalizations); the median length of stay was 15 days.
Table 1.
Baseline characteristics (n = 115 patients).
| Male patients, % | 45 |
|---|---|
| Age (years), mean (SD) | 74 (9) |
| FEV1% pred, mean (SD) | 43 (18) |
| Current smokers, % | 17 |
| PaO2 (mmHg) mean (SD) | 50 (5) |
| PaCO2 (mmHg) mean (SD) | 48 (10) |
| Living in a seniors’ residence, % | 27 |
| Living alone, % | 37 |
| Use of ambulatory oxygen, % | 38 |
| Anxiety, % | 23 |
| Depression, % | 11 |
| Charlson score, mean (SD) | 5.6 (2.1) |
SD: standard deviation; FEV1: forced expiratory volume in 1 second; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide.
Oxygen prescriptions
Although most patients were prescribed oxygen for ≥18 hours/day, the prescriptions varied from ≥12 to 24 hours/day (Table 2). The severity of hypoxemia upon admission did not determine the prescriptions.
Table 2.
Prescription and arterial blood gas results upon admission (n = 115 patients).
| Prescription | Number of patients (%) | PaO2, mean (SD) | PaCO2, mean (SD) |
|---|---|---|---|
| ≥15 hours/day | 12 (10) | 50 (4) | 46 (9) |
| ≥16 hours/day | 3 (3) | 45 (8) | 40 (13) |
| ≥18 hours/day | 84 (73) | 51 (5) | 48 (10) |
| 24 hours/day | 16 (14) | 49 (5) | 48 (11) |
| Between-group comparisons | p = 0.3 | p = 0.7 | |
PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; SD: standard deviation.
Oxygen utilization and adherence
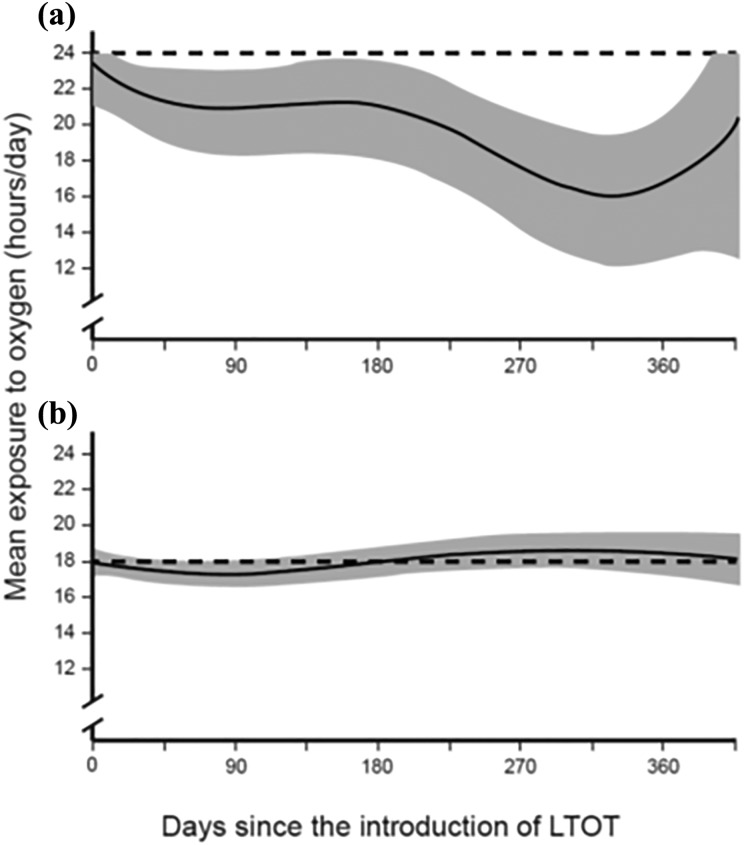
On average, the 115 patients were exposed to home oxygen for 17.8 hours/day (Table 3). On average, for all four categories of prescription (i.e. ≥15, ≥16, ≥18, and 24 hours/day), the mean daily exposure to oxygen correlated with prescription but was inferior to it. Table 3 also presents the proportion of adherent patients according to the prescription. Overall, 60% of the patients were compliant according to our definition. Although the mean daily exposure to oxygen remained stable during the study period in those who were prescribed it for ≥18 hours/day, it tended to decrease over time in those who were prescribed 24 hours/day (Figure 1). Spline curves for those who were prescribed oxygen for ≥15 and ≥16 hours/day are not shown.
Table 3.
Daily exposure and compliance to home oxygen therapy.
| Prescription | Number of patients | Daily exposure to oxygen (hours/day), mean (SD)a | Number of adherent patients (%) |
|---|---|---|---|
| All patients | 115 | 17.8 (5.5) | 69 (60) |
| ≥15 hours/day | 12 | 11.9 (5.4) | 2 (17) |
| ≥16 hours/day | 3 | 12.4 (2.3) | 0 (0) |
| ≥18 hours/day | 84 | 18.1 (5.1) | 57 (68) |
| 24 hours/day | 16 | 21.4 (4.1) | 10 (63) |
| Between-group comparison | p < 0.0001 | p = 0.0007 | |
SD: Standard deviation.
aAdjusted for portable oxygen utilizations and hospitalizations.
Figure 1.
Spline curves illustrating changes in patient compliance to home oxygen therapy over time: (a) prescription of oxygen for 24 hours/day in 16 patients and (b) prescription of oxygen for ≥18 hours/day in 84 patients. Dashed line: prescription; shaded area: 95% confidence interval.
Predictors of adherence
In the univariate analyses, we did not find any significant difference between adherent and non-adherent patients, with the only exception that the former patients were older (Table 4). After incorporating age, smoking status, ambulatory oxygen utilization, and whether patients still live at home (the only variables with p values <0.20 in the univariate analyses) into a multivariate logistic regression model, the only significant predictors of adherence were age (odds ratio per 1-year increment: 1.1; 95% confidence interval: 1.0–1.1) and ambulatory oxygen utilization (odds ratio: 2.5; 95% confidence interval: 1.1–6.0).
Table 4.
Comparison of adherent versus non-adherent patients.
| Compliant (n = 69) | Noncompliant (n = 46) | p Value | |
|---|---|---|---|
| Male patients, n (%) | 31 (45) | 21 (46) | 1.0 |
| Age (years), mean (SD) | 75 (9) | 71 (9) | 0.03 |
| FEV1% pred, mean (SD) | 40 (16) | 47 (21) | 0.12 |
| Current smokers, n (%) | 8 (12) | 12 (26) | 0.08 |
| PaO2, mean (SD) | 50 (5) | 51 (5) | 0.44 |
| PaCO2, mean (SD) | 48 (10) | 47 (10) | 0.83 |
| Living in a seniors’ residence, n (%) | 22 (33) | 8 (18) | 0.08 |
| Living alone, n (%) | 28 (41) | 14 (31) | 0.32 |
| Ambulatory oxygen, n (%) | 29 (45) | 12 (28) | 0.10 |
| Anxiety, n (%) | 16 (23) | 10 (22) | 1.0 |
| Depression, n (%) | 8 (12) | 5 (11) | 1.0 |
| Charlson score, mean (SD) | 5.7 (2.0) | 5.4 (2.1) | 0.56 |
PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; FEV1: forced expiratory volume in 1 second; SD: standard deviation.
Discussion
The results of this study indicated that, overall, compliance to home oxygen therapy is suboptimal. Forty percent of our patients did not use oxygen according to their medical prescription.
We found only a few clinical characteristics at treatment initiation that could predict adherence to LTOT. This result may be related to the small sample size and the consequent lack of power. The increased adherence with aging may reflect increased perception of benefits or diminished mobility with more opportunities to use oxygen at home. Exposure to oxygen was better in those who own a portable oxygen delivery system. We interpret this finding as an indication of high motivation to adherence to home oxygen through concentrator, as we have already demonstrated, in a randomized placebo-controlled trial, that the use of ambulatory oxygen is very limited and has little effect on dyspnea, quality of life, and exercise capacity.15 Contrary to what one could have expected, smoking status and living in a seniors’ residence did not clearly predict compliance to home oxygen, although both variables approached statistical significance in the univariate analyses (Table 4).
Several other studies have investigated the issue of adherence to home oxygen in COPD (Table 5). Their results are remarkably homogeneous. In the three studies that included, as we did, patients from the initiation of oxygen therapy,5,10,13 exposure to home oxygen was similar when compared to the other cross-sectional studies. Among them, one study investigated the change in adherence over time. In this small study that included only 23 patients with COPD, 48% used LTOT for ≥15 hours/day in the first month of treatment; this proportion decreased to 25–33% in the following months.13 This is in contrast with our finding that the mean daily exposure to oxygen remained stable throughout the study period in those who were prescribed it for ≥18 hours/day. However, it decreased over time in those who were prescribed 24 hours/day, demonstrating that permanent oxygen therapy cannot be achieved.
Table 5.
Studies of compliance to oxygen therapy in COPD that used objective measures.
| Reference | Population/sample size | Eligibility criteria to LTOT | Time since the introduction of LTOTa | Method for compliance measurement | Results |
|---|---|---|---|---|---|
| Evans et al.5 | 14 patients with COPD; 10 (71%) current smokers | Not specified | All patients presumably studied from prescription over 12 months | Every 3 months technical staff visited the patients’ home to read the hidden clock | Mean daily use of concentrator: 13.3 hours (SD: 2.0); 14% used LTOT for ≥15 hours/day |
| Walshaw et al.6 | 61 patients, including 55 (90%) with COPD; 12 patients (20%) current smokers | Not specified | 12 months (SD: 6.4; range: 4–22) | Oxygen concentrator meter readings | Mean daily use of concentrator: 14.7 hours (SD: 5.3); 28 patients (46%) ran their concentrator for at least 15 hours a day |
| Restrick et al.7 | 176 patients, including 148 (84%) with COPD; 34 patients (19%) current smokers | Absolute indications: FEV1 < 1.5 liters; FVC < 2 liters; PaO2 < 7.3 kPa; PaCO2 > 6.0 kPa; peripheral edema | Median: 19 months (range: 1–64) | Meter readings | Median daily use of concentrator: 15 hours; 74% used LTOT for >12 hours/day |
| Morrison et al.8 | 519 patients, including 410 (79%) with COPD; 14% current smokers at prescription | Absolute indications: FEV1 < 1.5 liters; PaO2 < 7.3 kPa; PaCO2 > 6.0 kPa; edema, clinically stable, repeated measures, optimal therapy, no smoking | Not specified | Three monthly oxygen concentrator meter readings | Mean daily use of concentrator: 14.9 hours (SD: 6.0); 56% used LTOT for ≥15 hours/day |
| Pépin et al.9 | 930 patients, all with COPD; 121 patients (13%) current smokers | Based on measured blood gas results in 72%; 23% were prescribed oxygen on the basis of severe disability | 36 months (SD: 24; range: 6–144) | Two readings of the concentrator clock counter over a 3-month period | Mean daily use of concentrator: 14.5 hours (SD: 5); 419 (45%) used LTOT for ≥15 hours/day; 230 (25%) used LTOT for ≥12 hours/day |
| Peckham et al.10 | Non-randomized comparison of two groups: total of 86 patients, including 75 (87%) with COPD; 7 (8%) current smokers; 45 patients received practical teaching about the use of oxygen; 41 served as controls | Teaching group: stable hypoxia with PaO2 <7.3 kPa with stable spirometry on two separate occasions at least 3 weeks apart; control group: not specified | All patients studied from prescription over 6 months | Electrical meter recordings from the concentrator | 82% of those who received practical teaching used LTOT for ≥15 hours/day (vs. 44% in the control group; p < 0.0002) |
| Ringbaek et al.11 | 182 patients, including 153 (84.1%) with COPD; 14.1% current smokers | Not specified | Median: 6.2 months (range: 0–112) | Oxygen concentrator meter readings and calculation of oxygen cylinders delivered | 65% used LTOT for >15 hours/day |
| Katsenos et al.12 | 249 patients, including 186 (74.7%) with COPD; 25.7% current smokers | Not specified | 22.3 months (SD: 30.11; range: 1–300) | Time counter attached to the concentrator | Mean daily use of concentrator: 9.7 hours (SD: 6.1); 27% used LTOT for ≥15 hours/day |
| Nasilowski et al.13 | 30 patients, including 23 (77%) with COPD; no data about smoking provided | PaO2 < 55 mmHg or between 55 mmHg and 59 mmHg when radiological or electrocardiographical signs of pulmonary hypertension and/or secondary polycythemia (hematocrit > 55%) were present | All patients studied from prescription over 14 months | Concentrator counter reading performed by visiting nurses | Mean daily use of concentrator: 12.5 hours (SD: 4.6); 37% used LTOT for ≥15 hours/day; compliance diminished over time: 48% used LTOT for ≥15 hours/day in the first month of treatment; this proportion decreased to 25–33% in the following months |
COPD: chronic obstructive pulmonary disease; LTOT: Long-term oxygen therapy; SD: standard deviation; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity.
aMean, unless otherwise specified.
The problem of adherence to home oxygen is complex. Predictors of the effective use of oxygen have been identified, including supplemental education on home oxygen therapy by a nurse or physiotherapist, smoking cessation, and the absence of side effect from oxygen treatment.9 Several qualitative studies have demonstrated that patients’ perception of oxygen therapy may be positive, but is often negative,17 a situation that does not foster treatment adherence. However, little is known about interventions aiming at improving patients’ compliance to LTOT. Although formal assessment at baseline and training as well as close follow-up may be effective in maximizing exposure to oxygen,10 behavioral and psychological interventions have not been investigated.
Does strict adherence to home oxygen really matter to improve survival? It probably does, because the nocturnal oxygen therapy trial (NOTT) demonstrated that 18 hours/day of exposure to oxygen is better than 12. What about 15 versus 20 hours/day or 18 versus 20? The comparison of the American and the British trials provided only indirect evidence that the benefit of oxygen is “dose dependent.” It is noteworthy that adherence to oxygen was not measured in the British trial and its “15-hour” arm did not necessarily represent the actual exposure to oxygen but only its prescription. In this trial, 500 days elapsed before any effect of continuous oxygen therapy appeared, when compared to no oxygen therapy at all.1 This suggests that the benefits of LTOT are slow to reveal and that the dose–response relationship is difficult to uncover. This demonstration will not come from randomized trials. Such evidence can only be derived from large population-based cohorts with careful and extended prospective follow-up with serial measurements of time of exposure.
Our study has obvious limitations. It is a retrospective study based on chart review. We may have overestimated the average daily use of oxygen if patients let their oxygen concentrator running while not wearing their oxygen cannula. Time of exposure to portable oxygen was only estimated from administrative data. It nevertheless corresponds to the results of a randomized trial that were prospectively and carefully measured.15 Also, hospitalizations may have occurred between home visits without being recorded. This situation would impact our measure of compliance by both underestimating the number of hours of oxygen exposure (the numerator) and falsely inflating the number of days at home (the denominator). For comparison, we used the results of a Canadian study of resource use in COPD that included patients with severe COPD (mean age: 68 ± 9; mean FEV1: 44 ± 14%).18 In this study, a subgroup of patients on LTOT was not at higher risk of hospitalization than those who were not oxygen dependent. We were reassured by our finding that the proportion of patients hospitalized and the total number of hospitalizations in our study was actually higher than expected from this Canadian study. We interpret this as an indication that the number of missed hospitalizations, if any, is probably small. Also, our study did not examine the patients’ experience with LTOT.
Our results have implications for practice and for research. Compliance issues must be discussed with the patients and their families at treatment initiation. The same questions regarding compliance to oxygen certainly apply to patients who receive oxygen therapy for conditions other than COPD (such as lung fibrosis) or for COPD patients who receive oxygen for other indications (such as nocturnal oxygen for isolated nocturnal desaturation). This is currently being investigated in a randomized trial of nocturnal oxygen therapy in COPD (the INOX trial; http://ClinicalTrials.gov NCT01044628). Further research should explore quality of life and clinical outcomes, treatment and health-care barrier, education improvement as well as the psychosocial, emotional, and behavioral domains.3
Acknowledgements
This work was supported by the Groupe de Recherche en Santé Respiratoire de l’Université Laval (GESER); however, the GESER was not involved in study design; in the collection, analysis, and interpretation of data; in the writing of the report; in the decision to submit the article for publication.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Medical_Research_Council_Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet 1981; 1: 681–686. [PubMed] [Google Scholar]
- 2. Nocturnal_Oxygen_Therapy_Trial_Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med 1980; 93: 391–398. [DOI] [PubMed] [Google Scholar]
- 3. Cullen DL. Long term oxygen therapy adherence and COPD: what we don’t know. Chron Respir Dis 2006; 3: 217–222. [DOI] [PubMed] [Google Scholar]
- 4. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans TW, Waterhouse J, Howard P. Clinical experience with the oxygen concentrator. Br Med J (Clin Res Ed) 1983; 287: 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walshaw MJ, Lim R, Evans CC, et al. Prescription of oxygen concentrators for long term oxygen treatment: reassessment in one district. BMJ 1988; 297: 1030–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Restrick LJ, Paul EA, Braid GM, et al. Assessment and follow up of patients prescribed long term oxygen treatment. Thorax 1993; 48: 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison D, Skwarski K, MacNee W. Review of the prescription of domiciliary long term oxygen therapy in Scotland. Thorax 1995; 50: 1103–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pépin JL, Barjhoux CE, Deschaux C, et al. Long-term oxygen therapy at home. Compliance with medical prescription and effective use of therapy. ANTADIR Working Group on Oxygen Therapy. Association Nationale de Traitement a Domicile des Insuffisants Respiratories. Chest 1996; 109: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 10. Peckham DG, McGibbon K, Tonkinson J, et al. Improvement in patient compliance with long-term oxygen therapy following formal assessment with training. Respir Med 1998; 92: 1203–1206. [DOI] [PubMed] [Google Scholar]
- 11. Ringbaek T, Lange P, Viskum K. Compliance with LTOT and consumption of mobile oxygen. Respir Med 1999; 93: 333–337. [DOI] [PubMed] [Google Scholar]
- 12. Katsenos S, Froudarakis ME, Charisis A, et al. Long-term oxygen therapy in Ioannina. Respiration 2004; 71: 619–624. [DOI] [PubMed] [Google Scholar]
- 13. Nasilowski J, Przybylowski T, Klimiuk J, et al. The effects of frequent nurse visits on patient’s compliance with long-term oxygen therapy (LTOT). A 14-month follow-up. Pneumonol Alergol Pol 2009; 77: 363–370. [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 15. Lacasse Y, Lecours R, Pelletier C, et al. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J 2005; 25: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 16. Smith PL. Splines as a useful and convenient statistical tool. Am Stat 1979; 33: 57–62. [Google Scholar]
- 17. Kelly CA, Maden M. How do respiratory patients perceive oxygen therapy? A critical interpretative synthesis of the literature. Chron Respir Dis 2014; 11: 209–228. [DOI] [PubMed] [Google Scholar]
- 18. FitzGerald JM, Haddon JM, Bradly-Kennedy C, et al. Resource use study in COPD (RUSIC): a prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J 2007; 14: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]