Abstract
Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) share similar quality of life impairment. The aim of the present study was to investigate health-related quality of life (HRQoL) and its relation to the perception of treatment and psychosocial support among PAH and CTEPH patients. All adult PAH or CTEPH patients in the Swedish Pulmonary Arterial Hypertension Register were invited to participate in a national cohort survey. The survey included the EuroQol 5-dimensions (EQ-5D) instrument that measures an individual’s HRQoL; the Beliefs about Medicines Questionnaire-Specific Scale that assesses the perception of PAH-specific treatment; the Mastery scale that evaluates the feeling of control and ability to cope with the disease; and the Social Network and Support Scale that maps the social support network. Of the 440 invited patients, 74% responded. Mean age was 66 ± 14 years, 58% were female and 69% diagnosed with PAH. Patients with PAH were younger, more often female and had a lower EQ-5D index (0.67 ± 0.29 vs. 0.73 ± 0.25, p = 0.050) than patients with CTEPH. Patients with a low EQ-5D index had more concerns about treatment (p = 0.004), lower coping ability (p < 0.001), less emotional support (p = 0.003) and less accessible social network (p = 0.002). In conclusion, patients with an impaired HRQoL also reported negative effects on their social support network, ability to handle stressors and concerns about treatment.
Keywords: Chronic disease, coping, pulmonary hypertension, patient preference, quality of life, social support
Introduction
Both pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are debilitating diseases that share a similar symptom burden and quality-of-life impairment.1 In both diseases, the small pulmonary arteries undergo structural changes leading to an increased pulmonary vascular resistance, for example, vessels that cannot expand as normal to an increased blood flow.1 Thus, at rest, the patient might be relatively or entirely asymptomatic, while even a low level of activity or exercise might cause severe dyspnoea, fatigue and even fainting. This will impact on the ability to participate in daily activities such as work, exercise and socialize with family and friends, that is, it affects the health-related quality of life (HRQoL).2–4 Both PAH and CTEPH are rare diseases with a prevalence in Sweden estimated to be 58 and 25 patients per million inhabitants, respectively.5
New PAH-targeted treatments have improved the outcome for PAH patients immensely1,5 and have helped patients to maintain more activities of a normal life. CTEPH is potentially curable if the patient can undergo a pulmonary endarterectomy surgery.1,6 However, a third are considered inoperable, and a similar number have remaining or recurrent pulmonary hypertension after surgery.6 Off-label PAH-targeted therapy has been used; however, a new drug approved for patients with inoperable or persistent CTEPH has shown reduced mortality rates as well as decreased symptom burden for these patients.7 Still, despite all these positive developments for both PAH and CTEPH, mortality remains high and quality of life impaired.1 Strong social support and taking medications as prescribed are assumed to provide a good HRQoL, while a low HRQoL might be linked to lack of social support and not adhering to prescribed drug treatment. However, this association has not been studied previously among patients with PAH and CTEPH.
The aim of the present study was to investigate HRQoL among Swedish PAH and CTEPH patients and its relation to the underlying psychosocial support, drug beliefs and drug adherence.
Materials and methods
Design and participants
The project was administered from the PAH centre at Skåne University Hospital in Lund in collaboration with all other Swedish PAH centres, located at the one of the seven university hospitals in Sweden. The study was conducted from May through July 2015. To be included, the patient had to be 18 years or older, diagnosed with PAH or CTEPH, registered in the Swedish National PAH Register (SPAHR) and able to understand Swedish. Exclusion criteria were severe mental or medical reason or being on the waiting list for lung transplantation. The full setting and sample in this study are described elsewhere.8
A quantitative methodology was employed where all eligible patients in SPAHR received an invitation to participate in the study by regular mail. The invitation included an introductory letter, an informed consent to be signed, a return envelope and questions about socio-demographic information and self-assessment questionnaires concerning the patient’s perspective on health, medication belief, adherence and medical treatments. One reminding letter was sent out to non-responders.
Questionnaires
For the purpose of this study, information from five questionnaires was used. HRQoL was measured by the EuroQol 5-dimensions (EQ-5D), the perception of PAH-specific treatment by the Morisky Medication Adherence Scale (MMAS-8) and the Beliefs about Medicines Questionnaire-Specific Scale (BMQ-S) and psychosocial support by the Mastery scale and the Social Network and Support Scale (SNASS). Necessary permissions and licences were obtained for all the questionnaires used in this study.
The EQ-5D-3L 9,10 is a well-established instrument, frequently used in both research and clinical practice. It consists of five questions representing five health dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension can be rated from 1 to 3 and will give a measure of an individual’s HRQoL. The EQ-5D can be converted into a single summary index, where the highest possible index for HRQoL, meaning best possible health, is 1.00.11,12 A previously determined mean score for a general Swedish population was 0.82,12 and this was used as a reference value in the present study. The EQ-5D also includes a self-rated health status scale (EQ-visual analogue scale) assigning a numerical value for a certain health state in relation to perfect or worst health status. It consists of a 20-cm vertical scale anchored with 100 at the top (best imaginable health state) and with 0 at the bottom (worst imaginable health state). Patients were asked to mark their current state of health on the vertical line in relation to perfect and worst health.
The MMAS-8 13 assesses attitudes and behaviours associated with the use of a specific medical treatment. It consists of eight items where the first seven items use a Yes (=0) and No (=1) scoring system, while the last item uses a five-point Likert-type scale. A maximum sum score of 8 is considered high adherence, 6–7 medium adherence and <6 low adherence. The MMAS-8 has been proved reliable and in this study, Cronbach’s α was 0.69.
The BMQ-S 14 assesses the patients’ cognitive perception of their treatment. The BMQ-S consists of two scales where one assesses patients’ beliefs about the necessity of prescribed medication for controlling their illness (five items) and the other assesses the patients’ concerns about potential adverse consequences of taking their medication (five items). All statements are answered on a five-point Likert-type scale where 1 = strongly disagree, 2 = disagree, 3 = uncertain, 4 = agree and 5 = strongly agree. A total sum was calculated. A higher mean score indicated a stronger belief in the described concept. The instrument has demonstrated acceptable reliability. In the present study, Cronbach’s α for the specific-necessity scale was 0.86 and for the specific-concerns scale 0.82.
The Mastery scale questionnaire consists of seven items that evaluate the measure of extent to which a person feels he/she is in control of his/her own lives and the patient’s ability to cope with his/her disease.15,16 The questionnaire uses a four-point Likert-type scale. The possible total score ranges from 7 to 28, where a higher score indicates higher ability to cope with the effects of the disease. In the present study, Cronbach’s α was 0.82.
The SNASS17 includes 19 items that attempt to map the patient’s social support network by assessing the dimensions of emotional (six items) and practical support (four items) as well as homogeneity (five items) and approachability (two items) a patient perceives. Seventeen of the 19 items use a point score where 1 point = Yes, absolutely; 2 point = Yes, partly; and 3 = No, and where a lower score indicates a stronger support, a sum of the items included in each dimension was calculated. In the two remaining items, patients were asked to answer the following questions: ‘Which person is most important in your social network?’ and ‘What person gives you the best support?’ Cronbach’s α for the 17 SNASS point score questions was 0.89.
PAH disease subgroups: A post hoc analysis
PAH includes several subgroups based on the cause of the increased pulmonary pressures.1 Of these subgroups, idiopathic PAH (IPAH) and connective tissue disease (PAH-CTD) are the two largest groups accounting for 50% and 30% of patients with PAH, respectively. While IPAH has an unknown origin, PAH-CTD occurs secondary to connective tissue disease.
Due to the underlying disease, patients with PAH-CTD can be expected to have both different and more symptoms than patients with IPAH.5 Thus, it was decided to, as a post hoc analysis, study if there was a difference between these PAH groups. This has not been reported on earlier.
Ethical considerations
This study was in accordance with the Declaration of Helsinki and was approved by the directors of participating PAH centres. Approval by the regional research ethics committee was obtained (LU 2015/112). A counsellor at each PAH centre was appointed to provide counselling to study participants as required.
Statistical methods
Descriptive statistics were used to characterize the data. To study the relation of HRQoL to psychosocial support, drug beliefs and drug adherence, patients were divided into two groups, those with an EQ-5D index below the national average12 and those with an EQ-5D index above the national average were compared. Statistical comparisons included Student’s t-test for continuous variables and χ 2 tests, or when applicable, Kruskal–Wallis test, for ordinal variables. A p value of <0.05 was considered significant. Data are presented as mean ± SD or number (%). Internal consistency was assessed using Cronbach’s α, and a score above 0.7 was considered acceptable. All analyses were carried out by use of the SAS statistical software (SAS 9.4).
Results
In total, 325 of the 440 approached patients (74%) completed and returned the questionnaire. The mean age of those who responded was 66 ± 14 years, 58% were female, 69% were diagnosed with PAH and 31% with CTEPH. Average time from diagnosis was 4.7 ± 4.2 years, and 95% were treated with PAH-specific drugs at the time of the study.
PAH and CTEPH
Overall, patients with PAH were younger, more often female and more often treated with PAH-specific drugs than patients with CTEPH. There was no difference in time from diagnosis, marital status or levels of education between the two diagnosed groups (Table 1).
Table 1.
Patient characteristics, socio-economic factors and HRQoL (EQ-5D).a
| PAH (n = 224) | CTEPH (n = 101) | p Value | |
|---|---|---|---|
| Age, years | 64 ± 15 | 71 ± 12 | <0.001 |
| Gender, female | 152 (68) | 38 (38) | <0.001 |
| Time from diagnosis, years | 4.9 ± 4.9 | 4.4 ± 3.3 | 0.364 |
| Treatment (n = 299) | |||
| PAH-specific drug | 217 (99) | 82 (85) | <0.001 |
| Pulmonary endarterectomy | − | 18 (19) | − |
| Marital status | |||
| Married/living with partner | 149 (67) | 68 (67) | 0.886 |
| Single/divorced/widowed | 75 (33) | 33 (33) | |
| Education | |||
| Low, ≤9 years | 70 (32) | 30 (30) | |
| Medium, 10–12 years | 92 (41) | 38 (38) | 0.690 |
| High, university | 61 (27) | 32 (32) | |
| EQ-5D | |||
| Index (score 0–1) | 0.67 ± 0.29 | 0.73 ± 0.25 | 0.050 |
| <0.82 | 165 (74) | 65 (65) | 0.088 |
| EQ-VAS scale (score 0–100) | 62 ± 21 | 64 ± 20 | 0.261 |
HRQoL: health-related quality of life; SD: standard deviation; PAH: pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; EQ-5D: EuroQol 5-dimensions; VAS: visual analogue scale.
a Data are shown as mean ± SD or number (%).
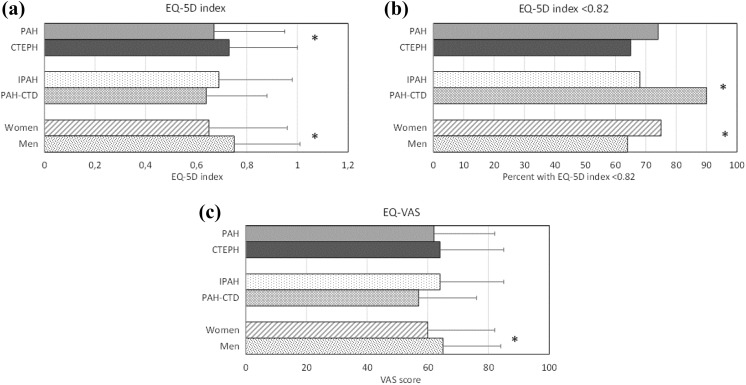
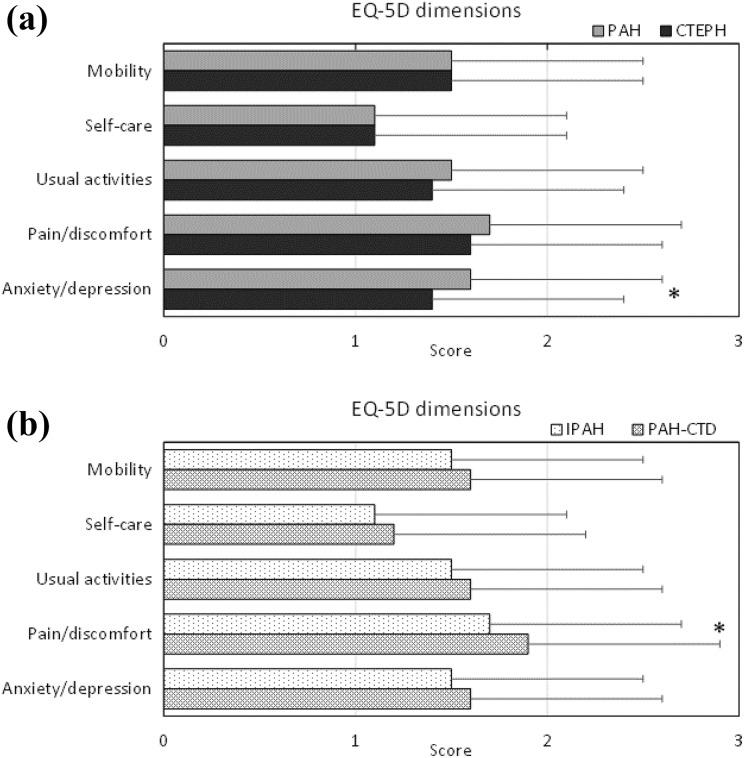
Patients with PAH had a lower EQ-5D index, that is, worse HRQoL, than patients with CTEPH (Table 1). Within PAH-group analysis showed that patients diagnosed with PAH-CTD more often had an EQ-5D index below the national average than those diagnosed with IPAH (90 vs. 68%, p = 0.002; Figure 1), and they also reported more pain and discomfort (Figure 2). There were no differences in the EQ-5D index (p = 0.225) between those diagnosed with IPAH and those diagnosed with CTEPH. Women had a lower EQ-5D index than men (Figure 1).
Figure 1.
The mean ± SD for EQ-5D index (a), percent of the study population with an EQ-5D index <0.82 (b) and mean ± SD for EQ-VAS (c). Data are shown for PAH (n = 224) versus CTEPH (n = 101); PAH subgroups IPAH (n = 133) versus PAH-CTD (n = 50); gender (women, n = 190 vs. men, n = 135). *Statistically significant difference. SD: standard deviation; EQ-5D: EuroQol 5-dimensions; VAS: visual analogue scale; PAH: pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; IPAH: idiopathic PAH; CTD: connective tissue disease.
Figure 2.
EQ-5D dimensions shown for PAH (n = 224) versus CTEPH (n = 101) in panel (a) and for PAH subgroups IPAH (n = 133) versus PAH-CTD (n = 50) in (b). Data are shown as mean ± SD. *Statistically significant difference. SD: standard deviation; EQ-5D: EuroQol 5-dimensions; PAH: pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; IPAH: idiopathic PAH; CTD: connective tissue disease.
Impaired HRQoL
Patients with a low EQ-5D index (i.e. below the national average,12) reported a better understanding about the necessity of treatment but also more concerns, a lower coping ability, less emotional support and less accessible social network than those with a high index (Table 2). There was no difference in drug adherence between those with low or high EQ-5D index.
Table 2.
Mean EQ-5D index divided for those below and above 0.82, the average score in a general Swedish population.12,a
| EQ-5D index <0.82 (n = 231) | EQ-5D index ≥0.82 (n = 94) | p Value | |
|---|---|---|---|
| Age, years | 68 ± 13 | 62 ± 15 | 0.001 |
| Gender, female | 144 (62) | 46 (49) | 0.026 |
| Diagnosis PAH/CTEPH | 165/66 (71/29) | 59/35 (63/37) | |
| Time from diagnosis, years | 4.4 ± 4.5 | 5.4 ± 4.4 | 0.081 |
| Treatment (n = 299) | |||
| PAH-specific drug | 216 (97) | 84 (90) | 0.016 |
| Marital status | |||
| Married/living with partner | 148 (64) | 69 (73) | 0.105 |
| Single/divorced/widowed | 83 (36) | 25 (27) | |
| Education | |||
| Low, ≤9 years | 75 (33) | 25 (27) | |
| Medium, 10–12 years | 94 (41) | 36 (38) | 0.250 |
| High, university | 60 (26) | 33 (35) | |
| BMQ-S | |||
| Necessity (score 5–25) | 22 ± 3 | 21 ± 4 | 0.021 |
| Concern (score 5–25) | 13 ± 5 | 11 ± 5 | 0.006 |
| MMAS-8 | |||
| High adherence (score 8) | 128 (56) | 57 (57) | |
| Medium adherence (score 6–7) | 71 (31) | 27 (27) | 0.762 |
| Low adherence (score <6) | 31 (13) | 11 (11) | |
| Mastery scale | |||
| Coping ability (score 7–28b) | 18 ± 4.3 | 23 ± 4.3 | <0.001 |
| SNASS | |||
| Emotional support (score 6–18c) | 8.0 ± 2.7 | 7.1 ± 2.1 | 0.004 |
| Homogeneity (score 5–15c) | 8.7 ± 2.0 | 9.1 ± 2.0 | 0.085 |
| Approachability (score 2–6b) | 3.2 ± 1.5 | 2.8 ± 0.9 | 0.002 |
| Practical support (score 4–12c) | 6.3 ± 1.9 | 6.1 ± 1.7 | 0.361 |
EQ-5D: EuroQol 5-dimensions; SD: standard deviation; PAH: pulmonary arterial hypertension; CTEPH: chronic thromboembolic pulmonary hypertension; BMQ-S: Beliefs about Medicines Questionnaire-Specific Scale; MMAS-8: Morisky Medication Adherence Scale; SNASS: Social Network and Support Scale.
a Data are shown as mean ± SD or number (%).
b High score indicates high coping ability.
c Low score indicates a strong support.
Discussion
Patients with PAH or CTEPH in Sweden have only a moderately impaired HRQoL compared to the general population. The HRQoL did affect the patient’s perception of psychosocial support, but not the understanding of the necessity of their PAH-specific treatment.
PAH and CTEPH
Patients with PAH reported worse HRQoL than patients with CTEPH. In a subgroup analysis, it was identified that the poor HRQoL in patients with PAH was mainly driven by the severely impaired HRQoL among PAH-CTD patients, a PAH subgroup. There was no difference in HRQoL index between IPAH and CTEPH patients; however, IPAH patients had more symptoms of anxiety and depression. These results are not surprising, HRQoL is negatively affected by physical and psychological symptoms, a shorter life expectancy and the grave human and social suffering that these patients experience.3,18,19
In the present study, a vast majority, 90%, of the PAH-CTD patients reported an EQ-5D index below the Swedish national average.12 Corresponding numbers for IPAH and CTEPH patients were 74% and 65%, respectively. These findings are in accordance with other studies.20,21 The worse HRQoL among PAH-CTD patients might be explained by the PAH being added on in a late stage of the index disease itself when it has already reached an advanced and disabling point.22 Thus, it is of particular importance that the care of this patient group, in addition to the rheumatology specialist, involves the multidisciplinary PAH team including a clinical nurse and physician specialist, physiotherapists, occupational therapist and social workers. The specialist nurse in the team may often work as the primary contact for the patients and their next of kin as well as be the person who will initiate contacts with the other members of the team.
Impaired HRQoL
Patients with an impaired HRQoL, measured as an HRQoL index below that in a general Swedish population,12 reported a good and even better understanding of the necessity of taking their disease-specific treatment than those with an ordinary HRQoL, but also expressed more concerns about their treatment. In addition, they also reported a lower coping ability, less social support network and less ability to handle stressors than those with a better HRQoL.
In the present study population, the understanding of why they should take their drugs were generally good, indicated by a mean score above 20 on a 25 point scale. This implies that the given information was relevant and well received, but as the concerns about treatment were high, the given information did not meet all the needs of the patients.8 However, it is worth noticing that, as previously reported from the same cohort,23 a majority of the patients reported high or moderate treatment adherence. In addition, the present study showed that there was no difference in adherence related to HRQoL index below or above that in a general Swedish population.12 The group with impaired HRQoL was older, more often diagnosed with PAH and as such more often female and on PAH-specific treatment. This might have affected the results, but Matura et al. showed that even if those 40 years or younger had better HRQoL than those above 40 years, there was no difference in HRQoL between the middle-aged or older patients,18 and that is the age span where the majority of patients in the present study are to be found. Although factors such as age and disease might affect how the care is delivered, this should not diminish the importance of measuring HRQoL among these patients to reflect on the results of the measures and use them to provide the fullest of care.2
Patients with an impaired HRQoL were more often living alone which might be reflected in the lack of social network and lower ability to deal with the disease and to handle stress. In the meeting with the patient, it is therefore important to initiate the discussion about their social network and include this in the discussions with social and healthcare systems as well as in the private sphere. Involving an existing network or support, the building of a new network should aim to improve the patients coping ability and capacity and thus provide an opportunity to improve many aspects of a patient’s quality of life. Networks outside family and friends could be a patient association, groups on social media or patient group forums for mutual sharing of experiences. Using HRQoL instruments in the clinical practice will give an opportunity to discuss the aspects of life that the instrument covers and also follow changes over time and measure the effect of interventions in this area.
In the care of these patients, responsiveness from the PAH team is a key component. It is necessary that the PAH teams develop their ability to both understand and integrate measures of HRQoL as well as of the disease-specific quality of life in their work to provide an accurate and effective care for these patients. An improved understanding in the PAH team of the benefits of how to integrate the instruments in the care will very likely increase the use. Also, utilizing digital versions of the instrument and thus simplifying its use and interpretation would likely be beneficial. In addition to the use of instruments, networks in the social and healthcare systems and in the private sphere should be encouraged, established and maintained.
Strengths and limitations
A major strength of the present study is the high response rate that implies a high interest among the patients for the questions asked. It also suggests that the questionnaires were easy to understand and complete. Another strength is that patients from all Swedish PAH centres were included, allowing us to interpret and generalize the results to the whole PAH and CTEPH population in Sweden. A limitation of the study was that EQ-5D is a rather crude measurement and might not show the real health limitations in these patient groups. The analysis of PAH subgroups (IPAH and PAH-CTD) was not planned initially but that the PAH-CTDs low HRQoL affected the whole PAH group has not been reported before and is of value for those working at the PAH clinics. Thus, these post hoc results were included in the manuscript. The work at PAH clinics or by the PAH teams was not included in the study, and suggestions for improvements can only be speculated on. More studies in this area are warranted.
Conclusion
Patients with an impaired HRQoL also reported more concerns about treatment, lower coping ability, negative effects on their social support network and ability to handle stressors. Patients diagnosed with IPAH and CTEPH have a moderately affected HRQoL. A subgroup analysis revealed that those diagnosed with PAH-CTD were more severely affected.
Acknowledgements
We would like to thank all patients who participated in the study. We also want to thank the staff at the PAH centres and those who register the patients in SPAHR, and their help to find eligible patients for the study is greatly acknowledged. We also thank Uppsala Clinical Research Centre (UCR) for developing and administering the platform for SPAHR.
Authors’ note: Permission to use the MMAS scales is required. Reproduction and distribution of the MMAS is protected by US copyright laws. A licensing agreement to use the Swedish translation of the scale is available from Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772, dmorisky@gmail.com.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Medicine Service University Trust, Region Skåne and by unrestricted research grants from the Swedish Society of Pulmonary Hypertension (Bayer AB, Actelion Pharmaceuticals Sverige AB) and The Swedish Heart and Lung Association.
ORCID iD: Bodil Ivarsson  http://orcid.org/0000-0002-5647-3929
http://orcid.org/0000-0002-5647-3929
References
- 1. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2. Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev 2015; 24: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urushibara T, Tanabe N, Suda R, et al. Effects of surgical and medical treatment on quality of life for patients with chronic thromboembolic pulmonary hypertension. Circ J 2015; 79: 2696–2702. [DOI] [PubMed] [Google Scholar]
- 4. Guillevin L, Armstrong I, Aldrighetti R, et al. Understanding the impact of pulmonary arterial hypertension on patients’ and carers’ lives. Eur Respir Rev 2013; 22: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rådegran G, Kjellström B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000-2014. Scand Cardiovasc J 2016; 50: 243–250. [DOI] [PubMed] [Google Scholar]
- 6. Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015; 24: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonneau G, D’Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 8. Ivarsson B, Rådegran G, Hesselstrand R, et al. Information, social support and coping in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. A nationwide population-based study. Patient Educ Couns 2017; 100: 936–942. [DOI] [PubMed] [Google Scholar]
- 9. The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 10. Rabin R, Oemar M, Oppe M. EQ-5D-3L user guide-basic information on how to use the EQ-5D-3L instrument. 2011. http://euroqol.org. [Google Scholar]
- 11. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 12. Burström K, Rehnberg C. Hälsorelaterad livskvalitet i Stockholmslän 2002. Resultat per åldersgrupp och kön, utbildningsnivå, födelseland samt syssel-sättningsgrupp. Rapport 2006:1. Stockholm: Enheten för Socialmedicin och Hälsoekonomi. Centrum för Folkhälsa. FORUM för kunskap och gemensamutveckling. Stockholms läns landsting, 2006, p. 26 (in Swedish). [Google Scholar]
- 13. Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008; 10: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999; 47: 555–567. [DOI] [PubMed] [Google Scholar]
- 15. Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978; 19: 2–21. [PubMed] [Google Scholar]
- 16. Eklund M, Erlandsson LK, Hagell P. Psychometric properties of a Swedish version of the Pearlin Mastery Scale in people with mental illness and healthy people. Nord J Psychiatry 2012; 66: 380–388. [DOI] [PubMed] [Google Scholar]
- 17. Hildingh C, Fridlund B, Baigi A. Sense of coherence and experiences of social support and mastery in the early discharge period after an acute cardiac event. J Clin Nurs 2008; 17: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 18. Matura LA, McDonough A, Carroll DL. Symptom prevalence, symptom severity, and health-related quality of life among young, middle, and older adults with pulmonary arterial hypertension. Am J Hosp Palliat Care 2016; 33: 214–221. [DOI] [PubMed] [Google Scholar]
- 19. Harzheim D, Klose H, Pinado FP, et al. Anxiety and depression disorders in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir Res 2013; 14: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kukkonen M, Puhakka A, Halme M. Quality of life among pulmonary hypertension patients in Finland. Eur Clin Respir J 2016; 3: 26405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zlupko M, Harhay MO, Gallop R, et al. Evaluation of disease-specific health-related quality of life in patients with pulmonary arterial hypertension. Resp Med 2008; 102: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 22. Hesselstrand R, Wildt M, Ekmehag B, et al. Survival in patients with pulmonary arterial hypertension associated with systemic sclerosis from a Swedish single centre: prognosis still poor and prediction difficult. Scand J Rheumatol 2011; 40: 127–132. [DOI] [PubMed] [Google Scholar]
- 23. Ivarsson B, Hesselstrand R, Rådegran G, et al. Adherence and medication belief in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension: a nationwide population-based cohort survey. Clin Respir J 2018. Epub ahead of print 22 January 2018 DOI: 10.1111/crj.12770. [DOI] [PubMed] [Google Scholar]