Abstract
Estimates of the minimal clinically important difference (MCID) for physical activity (PA) in chronic obstructive pulmonary disease (COPD) are needed. The objective is to provide an anchor-based estimate of the MCID for daily step count. PA was promoted in persons with COPD using a pedometer (Omron HJ-720ITC) alone or a pedometer plus interactive website for 3 months. Participants wore the pedometer daily and received phone calls monthly to ascertain medical events. Medical events were counted when a participant self-reported that he/she had (1) worsening of breathing, (2) change to breathing medications, (3) medical care from an emergency room for any reason, or (4) hospitalization for any reason. Generalized linear regression models assessed daily step count as change at the end of study and averaged over the 15, 31, or 61 days centered on the event, in those with an event compared to those without one. All categories of events carried equal weight in the analyses. We studied 93 persons, 46 of whom had an event. Participants who experienced an event had a decrease of 1086 (95% confidence interval (CI): −2124 to −48) or 887 (95% CI: −2030 to 257) steps/day in the pedometer plus website or pedometer alone groups, respectively, compared to those without one. In the days centered on an event, participants who had an event experienced a decrease of 882–983 steps/day (pedometer plus website) or a decrease of 351–495 steps/day (pedometer alone), compared to those without one. The MCID for PA in COPD ranges from 350 steps/day to 1100 steps/day.
Keywords: Anchor-based method, daily step count, exercise, physical activity, COPD
Introduction
Increasing physical activity (PA) is recommended for all patients with chronic obstructive pulmonary disease (COPD).1 Lower levels of PA are associated with increased risk of adverse medical events including acute exacerbations (AEs), hospital (re)admissions, and death, independent of lung function.2–5 Studies to date have examined PA in patients with stable disease.2–5 However, the clinical course of COPD is punctuated with AEs, and patients have multiple comorbidities resulting in clinically significant medical events.1,6,7
Little is known about changes in directly measured PA when a person with COPD gets sick—immediately before, during, and after the onset of either an AE or a medical event. Studies have been limited by lack of direct comparison to persons who have not had an event and by inability to quantify PA immediately preceding the occurrence of an event.8–11 Knowledge of these acute changes would provide an estimate for the minimal clinically important difference (MCID) of PA, anchored to a significant clinical event. Estimates for the MCID for PA would allow interpretation of changes in PA in clinical and research settings, as for other COPD outcomes.12,13 The MCID can be used when assessing PA change in individuals in a clinical setting in response to a treatment intervention, for counseling after a medical event has occurred, and for sample size calculations in designing a trial for which PA is the primary outcome.
The only published study to date used distribution-based approaches to estimate the minimal important difference for PA in a cohort undergoing pulmonary rehabilitation with expected improvements in PA.14 However, anchor-based methods are recommended as the primary means to obtain estimates of MCID.15 Distribution-based methods rely solely on the statistical properties of the measure in a population. Therefore, these thresholds may not be considered clinically important. Anchor-based methods incorporate the patient’s perspective regarding the change in their status or medical events (AEs, emergency room (ER) visits, and hospitalizations) that are of indisputable clinical importance. Furthermore, no study to date has examined changes in directly measured PA in COPD associated with worsening health status or used a comparison group of persons with no events or no expected changes.
PA interventions that ask participants to wear a monitoring device every day provide a unique opportunity to prospectively and objectively measure PA immediately before, during, and after the onset of medical events. We developed a PA intervention that combines the Omron HJ-720ITC pedometer (Omron Healthcare, Inc., Bannockburn, Illinois, USA) and a website that provides goal setting, feedback, motivational and educational content, and social support to promote PA in persons with COPD. In two studies, we have shown its efficacy to increase daily step counts16,17 and improve health-related quality of life (HRQL)16 in persons with stable COPD. In one of the studies,17 participants wore the Omron pedometer every day for 3 months, and the occurrence of clinically significant medical events was ascertained monthly (Clinical Trials.gov NCT 01772082). In this secondary analysis, our goal is to characterize the magnitude of change in PA around the time of a medical event and provide an anchor-based estimate of the MCID for PA.
Methods
Participant selection and study design
Details on participant selection and study design have been previously published.17 Briefly, eligible participants were at least 40 years of age, had COPD defined as a smoking history of >10 pack-years and a ratio of forced expiratory volume in one second (FEV1) to forced vital capacity <0.70 or emphysema on chest computed tomography, had a health-care provider who provided medical clearance, and had access to a personal computer with an Internet connection and a USB port.17 Exclusion criteria included inability to ambulate and unstable cardiovascular disease.17 The protocol (#2328) was approved by the VA Boston Healthcare System Committee on Human Research, and written informed consent obtained from each participant.
PA promotion groups
Participants in both groups, pedometer plus website or pedometer alone, were instructed to wear the Omron pedometer every day for 3 months when stable and during clinically significant medical events.17 They were asked to upload step-count data via the study website. The pedometer plus website group received weekly step-count goals and access to motivational and educational content on the website.16,17 At study entry, persons in the pedometer alone group received written materials about exercise in COPD. During the study, they received no instructions to exercise and were not assigned step-count goals.
Clinically significant medical events
In semi-structured telephone interviews conducted monthly for 3 months, participants in both groups were queried if they (1) had experienced worsening of their breathing, (2) had changes to their breathing medications, (3) had sought medical care from an ER for any reason, or (4) had been hospitalized for any reason (see Online Supplementary Material). All four categories of events carried equal weight in the analyses. Worsening of breathing was determined by the participant and self-reported at the monthly phone contacts. If the worsening of breathing resulted in health-care utilization such as an ER visit or hospitalization, we confirmed these events through medical record review. Changes in breathing medication referred to the following: corticosteroids, antibiotics, inhaled beta agonist, or any bronchodilator. If medication changes for breathing problems were reported, participants were asked about the use of antibiotics or systemic corticosteroids to determine whether the event was an AE.2,6 Two investigators, blinded to baseline characteristics, determined the nature of each event.2 For each medical event, the anchor date was the onset date for the event. If a subject reported more than one medical event, the date of the first event was used whenever possible. For subjects with no events, the anchor date was the date of the month-2 phone call. An event was included in these analyses if valid step-count data were available for more than half the days before and for more than half the days after the anchor date.
Assessment of daily step counts
At baseline and 3 months, daily step count was averaged over at least 5 wear days of 7 days. Participants were instructed to wear the pedometer every day during awake hours for 7 days. Participants were blinded to their step counts during the collection period since the face of the pedometer was covered with a sticker. They were instructed to go about their usual physical activities. After 7 days, participants uploaded their step-count data to the study server. Baseline daily step count was averaged over at least 5 wear days of 7 days. A wear day was defined as one with >200 steps and >8 hours of wear time.18,19 To comprehensively and accurately reflect the time periods of baseline, the onset of a medical event and deterioration in clinical status, and recovery,20 daily step counts were also averaged over the 15 (7 days before and 7 days after the onset of event), 31 (15 days before and 15 days after), or 61 (30 days before and 30 days after) days centered on the anchor date.
Clinical assessments
Participants were characterized at baseline. We obtained a medical history of cigarette use, comorbidities, medication use, current oxygen use, prior participation in pulmonary rehabilitation, and occurrence of an AE (i.e. if they had received treatment with prednisone for breathing problems) or hospitalization in the year prior to enrollment. FEV1 was measured using an Eaglet spirometer (nSpire Health, Inc., Longmont, CO, USA).22 Six-minute walk test (6MWT) was performed according to ATS guidelines21; a practice walk was not completed. HRQL was assessed with the St. George’s Respiratory Questionnaire Total Score (SGRQ-TS).23 Scores range from 0 to 100 with lower scores indicating better HRQL. Dyspnea was assessed with the Modified Medical Research Council (MMRC) scale24 and depression with the Beck Depression Inventory-II (BDI-II).25 BDI-II items are rated on a four-point scale ranging from 0 to 3 based on severity of each item. The maximum total score is 63. Total score of 0–13 is considered minimal range, 14–19 is mild, 20–28 is moderate, and 29–63 is severe. In this study, participants with a BDI-II total score >14, with the patient’s permission, had their primary health-care providers informed of the results, with the suggestion that they consider evaluation and/or treatment of depression.
Statistical analysis
Descriptive results are reported as mean ± standard deviation (SD) or frequency (percentage). Two-sample t tests or Wilcoxon rank sum tests, as appropriate, compared baseline characteristics between those with an event versus those with no event. The MCID was calculated using anchor-based approaches.15 Daily step count was examined in two ways: (1) the difference in step count at the end of study compared to baseline and (2) averaged over the 15, 31, or 61 days centered on the anchor date. Generalized linear regression models (PROC GLM, SAS 9.4; SAS Institute, Cary, North Carolina, USA) assessed daily step count in those who had a medical event compared to those who did not have an event, adjusting for baseline variables that were significantly different between groups. Distribution-based estimates, including empirical rule effect size, effect size, and 0.5 times SD, were also calculated.13–15 Analyses were conducted separately in the pedometer plus website and the pedometer alone groups.
Results
Subject characteristics
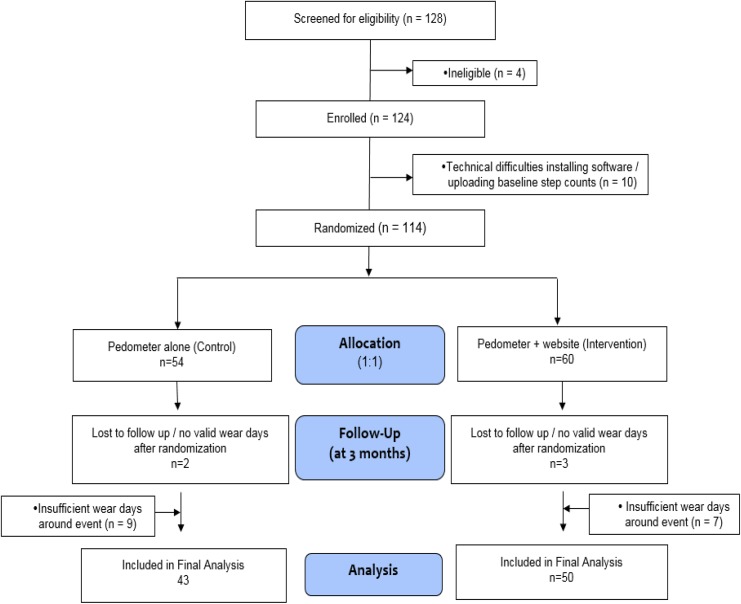
Figure 1 shows the CONSORT for participants analyzed for this secondary analysis compared to the participants randomized in the primary study.17 Forty-six participants had a medical event and sufficient step-count data for analyses. Forty-seven persons reported no medical event and had sufficient step-count data. Thus, 93 persons were included in the analyses, 50 of whom were in the pedometer plus website group (30 of whom had an event and 20 had no event), and 43 were in the pedometer alone group (16 of whom had an event and 27 had no event). Baseline subject characteristics for those randomized and analyzed were no different from those randomized but not analyzed (see Online Supplementary Table S1).
Figure 1.
CONSORT.
For the cohort, mean age was 69 ± 8 years and mean FEV1 1.86 ± 0.6 L, 63 ± 22% predicted. Table 1 shows that those with an event had a significantly higher BDI-II depression score than those with no event, p = 0.03. There were no baseline differences between those with or without events with respect to baseline daily step count, age, FEV1% predicted, 6MWT distance, SGRQ-TS, MMRC dyspnea score, and season of enrollment. There was no difference between those with or without events in terms of number of participants who had an AE or any cause hospitalization in the year prior to enrollment (Table 1). There was no difference in baseline characteristics between those assigned to the pedometer plus website group versus pedometer alone group (see Online Supplementary Table S2).
Table 1.
Baseline subject characteristics.a
| Characteristic | Total (N = 93) | Event (N = 46) | No event (N = 47) | Between group (p valueb) |
|---|---|---|---|---|
| Age (years) | 69 ± 8 | 70 ± 10 | 69 ± 6 | 0.49 |
| Male (sex) | 91 (97.9) | 45 (97.8) | 46 (97.9) | 0.99c |
| Race (White) | 85 (91.4) | 42 (91.3) | 43 (91.5) | 1.0c |
| BMI (kg/m2) | 29 ± 5.3 | 28.5 ± 4.7 | 29.5 ± 5.9 | 0.33 |
| Pack-years | 61.5 ± 40.9 | 60.8 ± 42 | 62.2 ± 40.3 | 0.73d |
| Current smoker | 33 (35.5) | 18 (39.1) | 15 (31.9) | 0.47 |
| Current oxygen use | 22 (23.7) | 8 (17.4) | 14 (29.8) | 0.16 |
| Prior pulmonary rehabilitation | 11 (11.8) | 4 (8.7) | 7 (14.9) | 0.52c |
| Baseline daily step count | 3127 ± 1830 | 3437 ± 1995 | 2824 ± 1618 | 0.13d |
| FEV1 (L) | 1.86 ± 0.6 | 1.8 ± 0.6 | 1.9 ± 0.7 | 0.34d |
| FEV1% predicted | 62.7 ± 22.3 | 61.9 ± 20.8 | 63.4 ± 24 | 0.88d |
| GOLD stage | 0.55c | |||
| I | 18 (19.4) | 10 (21.7) | 8 (17.0) | |
| II | 50 (53.8) | 25 (54.4) | 25 (53.2) | |
| III | 19 (20.4) | 7 (15.2) | 12 (25.5) | |
| IV | 6 (6.5) | 4 (8.7) | 2 (4.3) | |
| Comorbidities | ||||
| CAD | 19 (20.4) | 11 (23.9) | 8 (17) | 0.41 |
| Hypertension | 51 (54.8) | 24 (52.2) | 27 (57.5) | 0.61 |
| CHF | 5 (5.4) | 1 (2.2) | 4 (8.5) | 0.36c |
| Arthritis | 36 (38.7) | 14 (30.4) | 22 (46.8) | 0.105 |
| Diabetes | 22 (23.7) | 8 (17.4) | 14 (29.8) | 0.16 |
| Depression | 34 (36.6) | 19 (41.3) | 15 (31.9) | 0.35 |
| Back pain | 38 (40.9) | 22 (47.8) | 16 (34) | 0.18 |
| 6MWT distance (m) | 390 ± 82 | 384 ± 86 | 396 ± 77 | 0.49 |
| SGRQ-TS | 34 ± 16 | 37 ± 16 | 31 ± 16 | 0.05 |
| MMRC dyspnea score | 0.21 | |||
| 0–2 | 70 (75.3) | 32 (69.6) | 38 (80.9) | |
| 3–4 | 23 (24.7) | 14 (30.4) | 9 (19.2) | |
| BDI-II depression score | 8.9 ± 9.3 | 10.7 ± 9.6 | 7.2 ± 8.8 | 0.03d |
| AE in the past year | 15 (16.1) | 11 (23.9) | 4 (8.5) | 0.05c |
| Hospitalized for any reason in past year | 10 (10.8) | 6 (13) | 4 (8.5) | 0.52c |
BMI: body mass index; FEV1: forced expiratory volume in one second; GOLD: global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; CAD: coronary artery disease; CHF: congestive heart failure; 6MWT: 6-minute walk test; SGRQ-TS: St. George’s Respiratory Questionnaire-Total Score; MMRC: Modified Medical Research Council; AE: acute exacerbation; SD: standard deviation.
a Values are means ± SD or frequency (percentages).
b Unpaired t-test for continuous and χ 2 test for categorical variables, unless otherwise noted.
c Fisher’s exact test.
d Wilcoxon rank-sum test.
Clinically significant medical events
There was a total of 46 events, 33 (72%) of which were pulmonary ones (Table 2). Thirty-two (70%) of the 46 events were confirmed with medical records, including 18 of the 25 (72%) with worsened breathing, 8 of the 13 (62%) ER visits, and 2 of the 2 (100%) hospitalizations. Over the 3-month study, 16, 17, and 13 events occurred in month 1, 2, and 3, respectively. The first reported event was used for all but five participants, in whom insufficient step-count data were available before and after the first event, so a subsequent event served as the anchor date. For those with no events, the date of the month-2 phone call was used as the anchor date for 41 participants, and the date of the month-1 phone call was used for 6 participants in whom insufficient step-count data were available at month 2.
Table 2.
Characteristics of clinically significant medical events.a
| Type of medical event | Total (N = 46) | Pedometer plus website (N = 30) | Pedometer alone (N = 16) |
|---|---|---|---|
| Self-reported worsened breathing | 25 | 19 | 6 |
| Required new medications for breathing | 3 | 1 | 2 |
| Required antibiotic and/or systemic corticosteroid | 3 | 1 | 2 |
| ER visit for non-pulmonary reasonb | 10 | 6 | 4 |
| ER visit for a breathing problem | 3 | 2 | 1 |
| Hospitalization for non-pulmonary reasonb | 1 | 0 | 1 |
| Hospitalization for a breathing problem | 1 | 1 | 0 |
| Study month of occurrence | |||
| Month 1 | 16 | 10 | 6 |
| Month 2 | 17 | 11 | 6 |
| Month 3 | 13 | 9 | 4 |
ER: emergency room.
a Values in cells are Ns.
b Non-pulmonary reasons include back pain, car accident, knee pain, fever and viral illness, growth near eye, neck pain, thumb trauma, atrial fibrillation, urinary tract infection, earache, and external iliac artery stent placement.
Daily step count: MCID for clinically significant medical event
Generalized linear regression models, adjusted for BDI-II depression score which differed significantly at baseline between those with an event and those with no event, showed that participants in the pedometer plus website group who had an event had a decrease of 1086 (95% confidence interval (CI): −2124 to −48) steps/day at the end of study, compared to those with no events (Table 3). Similarly, those in the pedometer alone group with an event had a decrease of 887 (95% CI: −2030 to 257) steps/day compared to those with no event (Table 3).
Table 3.
Multivariate analysis of the effect of having an event on change in daily step count.
| Pedometer plus website (N = 50)a | Pedometer alone (N = 41)a | |||||
|---|---|---|---|---|---|---|
| Effect on change in daily step countb | 95% CI | p Value | Effect on change in daily step countb | 95% CI | p Value | |
| Event | −1086 | −2124 to −48 | 0.04 | −887 | −2030 to 257 | 0.12 |
BDI-II: Beck Depression Inventory-II; CI: confidence interval.
a Ns do not sum to 93 because step-count data at the end of study were not available for two subjects.
b Effects on change in daily step count are the model coefficients as determined by generalized linear regression models, adjusted for BDI-II depression score. Event/no event is the independent variable (reference group = no event). Change in daily step count (3-month minus baseline values) is the dependent variable.
Examining daily step counts averaged over the 15 days around the anchor date in the pedometer plus website group, generalized linear regression models showed that those with an event walked on average 983 fewer (95% CI: −1898 to −67) steps per day than those with no event, adjusting for baseline step count and BDI-II depression score (Table 4). Similar declines of −973 (95% CI: −1794 to −153) and −882 (95% CI: −1697 to −67) were observed when daily step count was averaged over 31 and 61 days, respectively, around the anchor date. Results in the pedometer alone group comparing those with an event to those with no event showed decreases in step count by 351–495 (p values = 0.41, 0.44, and 0.54) steps/day (Table 4).
Table 4.
Multivariate analysis of the effect of having an event on daily step counts averaged over different time windows around the anchor date.
| Pedometer plus website | Pedometer alone | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N a | Effect on daily step countb | 95% CI | p Value | N a | Effect on daily step countb | 95% CI | p Value |
| 15 days around datec | 47 | −983 | −1898 to −67 | 0.04 | 42 | −351 | −1490 to 789 | 0.54 |
| 31 days around datec | 46 | −973 | −1794 to −153 | 0.02 | 41 | −468 | −1683 to 747 | 0.44 |
| 61 days around datec | 44 | −882 | −1697 to −67 | 0.03 | 39 | −495 | −1692 to 702 | 0.41 |
BDI-II: Beck Depression Inventory-II; CI: confidence interval.
a Events were included if step-count data were available for more than half the days before and for more than half the days after the anchor date. Ns vary because not every event met this criterion.
b Effects on daily step count are the model coefficients as determined by generalized linear regression models, adjusted for baseline daily step count and BDI-II depression score. Event/no event is the independent variable (reference group = no event). Daily step count averaged 15, 31, or 61 days around the anchor date is the dependent variable.
c Anchor date of medical event or month-2 telephone call.
Distribution-based methods provided estimates to support the anchor-based calculations (Table 5). At baseline in participants with an event, daily step count in the pedometer plus website group versus the pedometer alone group was 2992 ± 1684 and 4272 ± 2306, respectively. Using these SD values, in the pedometer plus website group, the MCID estimates ranged from 842 steps/day to 906 steps/day. In the pedometer alone group, the MCID estimates ranged from 939 steps/day to 1153 steps/day.
Table 5.
Distribution-based estimates of the MCID.
| Method | MCID calculation | MCID estimate (steps/day); pedometer plus websitea | MCID estimate (steps/day); pedometer aloneb |
|---|---|---|---|
| Empirical rule effect size | 0.08 × 6 × SDΔ | 869 | 939 |
| Effect size | 0.5 × SDbaseline | 842 | 1153 |
| 0.5 times SD | 0.5 × SDΔ | 906 | 979 |
MCID: minimal clinically important difference; SD: standard deviation.
a SDΔ of 1811 and SDbaseline of 1684 were used in calculations.
b SDΔ of 1957 and SDbaseline of 2306 were used in calculations.
Discussion
Persons with COPD who have a clinically significant medical event experience a decrease in PA in the range of 350 steps/day to 1100 steps/day compared to persons with no events.
To the best of our knowledge, these results represent the first estimates of the MCID of PA using an anchor-based approach, with clear deteriorations in clinical status and a comparison group of persons who did not experience a medical event. Since anchor-based methods are recommended as the primary means to obtain clinically meaningful estimates of the MCID,15 our results significantly extend the current literature. Daily step count was examined in two ways—as the difference at the end of study compared to baseline and over three different time intervals around the anchor date of the medical event—both of which provided similar estimates. Although estimates of the MCID are not defined by statistical significance, our values in the pedometer plus website group were also statistically significant.
Using distribution-based methods, our values further support the range of MCID estimates obtained by an anchor-based method. Our values from both methods are strikingly similar to the range for the minimal important difference in PA from 599 steps/day to 1131 steps/day reported by Demeyer et al. using distribution-based approaches in a population undergoing pulmonary rehabilitation.14 Taken together, these results support that the MCID of daily step count defined by a clinical deterioration is very similar to that defined by an improvement in health status. It is a strength that we have examined MCID during clinical deterioration, providing a complementary and corroborating result that extends the Demeyer et al. findings. To be robust in the estimates, the MCID should be evaluated from both perspectives of clinically significant improvements and significant decrements.26 It should not be assumed that they are the same. Our current results contribute to the interpretation of PA and support its use as a clinical and research outcome in COPD. Our estimated MCID can aid power calculations when designing research studies and interpretation of changes in PA in clinical settings.
Strengths of the current study include the ability to directly monitor PA before the occurrence of a medical event and the use of a comparison group of persons with no events. It has been shown that 86% of AEs recover to baseline symptoms within 35 days, but there is a wide range in recovery time20 using 7-, 15-, and 30-day time windows around the date of events in our study provide accurate and detailed PA assessments at baseline, during deterioration, and recovery from an event. In the current study, the evaluation of two groups undergoing active PA promotion, that is, pedometer plus website or pedometer alone, is a strength since our results demonstrate changes in daily step count during a medical event at a time when patients are encouraged to increase PA. Thus, our estimates likely reflect the “minimum” change in daily step count that can be regarded as important and are unlikely to overestimate the MCID.15
Our study has several limitations. This is a single center study in a cohort of predominantly White male participants. Only 70% of the self-reported events were confirmed by medical records, but it is specifically patient report that identifies the changes in medical status needed for MCID calculations.15 We acknowledge that the various types of medical events assessed may have different impacts on PA, but all events were meaningful to the patient, and the overall sample size limited analyses by subtype of medical event. There may have been a Hawthorne effect as all participants received a pedometer. Since they received the pedometer at study entry, it is unlikely that they changed walking behavior because of the pedometer at the time of an event during the study. Participants were enrolled and monitored in an ongoing walking study so their perception of clinical change was not influenced by the initiation of PA monitoring at the immediate time of an event. We believe that the nonsignificant finding in the pedometer alone group is related to the small sample size, and larger studies are needed to corroborate our findings. Although not statistically significant, the change in step counts is clinically relevant.
The confidence intervals around the MCID estimates are wide demonstrating the difficulty of producing an MCID for such a varied outcome measure as daily step count. We considered combining the participants into one cohort, but the two groups received very different interventions that most likely differentially impacted the change of daily step counts around the occurrence of medical events. Participants were instructed to wear the pedometer upon awakening and to remove it upon going to bed. Although we excluded days with < 200 steps and < 8 hours of wear time, we did not objectively assess and adjust for actual hours of wear time.
In conclusion, persons with COPD who have a clinically significant adverse medical event experience a decrease in PA in the range of 350 steps/day to 1100 steps/day compared to persons with no events, providing an anchor-based estimate for the MCID. Additional evidence using different clinical anchors is needed to strengthen confidence in these MCID values.
Supplemental material
Supplement_Moy_10.9.18 for Physical activity in COPD: Minimal clinically important difference for medical events by Merilee Teylan, Ana Kantorowski, Diana Homsy, Reema Kadri, Caroline Richardson, and Marilyn Moy in Chronic Respiratory Disease
Acknowledgement
The authors thank the Veterans who participated in this study.
Footnotes
Author contributions: MLM and CRR conceived and designed all stages of the study. DH and RK collected study data. MAT and AK conducted study analyses. All authors contributed to writing the manuscript, read, and approved the final manuscript.
Trial registration: Clinical Trials.gov NCT01772082.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Moy reports receiving an honorarium for consulting from AstraZeneca, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Veterans Affairs, Rehabilitation Research and Development Service (Career Development Award, F6847W, Dr. Moy).
ORCID iD: Marilyn Moy  https://orcid.org/0000-0002-2471-9218
https://orcid.org/0000-0002-2471-9218
Supplemental material: Supplemental material for this article is available online.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD, 2017. Available from: http://goldcopd.org. (accessed 1 June 2018).
- 2. Moy ML, Teylan M, Weston NA, et al. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One 2013; 8(4): e60400 PMID: 23593211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nguyen HQ, Chu L, Amy Liu IL, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11(5): 695–705. PMID: 24713094 [DOI] [PubMed] [Google Scholar]
- 4. Moy ML, Gould MK, Liu IA, et al. Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res 2016; 2(1): 00062–2015. PMID: 27730174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140(2): 331–342. PMID: 21273294 [DOI] [PubMed] [Google Scholar]
- 6. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12): 1128–1138. PMID: 20843247 [DOI] [PubMed] [Google Scholar]
- 7. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186(2): 155–161. PMID: 22561964 [DOI] [PubMed] [Google Scholar]
- 8. Donaldson GC, Wilkinson TM, Hurst JR, et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171(5): 446–452. PMID:15579723 [DOI] [PubMed] [Google Scholar]
- 9. Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129(3): 536–544. PMID: 16537849 [DOI] [PubMed] [Google Scholar]
- 10. Alahmari AD, Patel AR, Kowlessar BS, et al. Daily activity during stability and exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med 2014; 14: 98 DOI: 10.1186/1471-2466-14-98. PMID: 24885188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alahmari AD, Kowlessar BS, Patel AR, et al. Physical activity and exercise capacity in patients with moderate COPD exacerbations. Eur Respir J 2016; 48(2): 340–349. PMID:27126688 [DOI] [PubMed] [Google Scholar]
- 12. Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med 2013; 187(4): 382–386. PMID: 23262518 [DOI] [PubMed] [Google Scholar]
- 13. Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189(3): 250–255. PMID:24383418 [DOI] [PubMed] [Google Scholar]
- 14. Demeyer H, Burtin C, Hornikx M, et al. The minimal important difference in physical activity in patients with COPD. PloS One 2016; 11(4): e0154587 DOI: 10.1371/journal.pone.0154587. PMID:27124297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008; 61(2): 102–109. PMID:18177782 [DOI] [PubMed] [Google Scholar]
- 16. Moy ML, Collins RJ, Martinez CH, et al. An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest 2015; 148(1): 128–137. PMID: 25811395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan ES, Kantorowski A, Homsy D, et al. Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respir Med 2017; 130: 102–110. DOI: 10.1016/j.rmed.2017.07.057 Epub 2017 Jul 25. PMID:29206627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danilack VA, Okunbor O, Richardson CR, et al. Performance of a pedometer to measure physical activity in a U.S. cohort with chronic obstructive pulmonary disease. J Rehabil Res Dev 2015; 52(3): 333–342. PMID:26230737 [DOI] [PubMed] [Google Scholar]
- 19. Matthews CE, Hagstromer M, Pober DM, et al. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc 2012; 44: S68–S76. PMID:22157777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seemungal TA, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161(5): 1608–1613. PMID:10806163 [DOI] [PubMed] [Google Scholar]
- 21. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. PMID: 12091180 [DOI] [PubMed] [Google Scholar]
- 22. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–338. PMID:16055882 [DOI] [PubMed] [Google Scholar]
- 23. Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. PMID:1595997 [DOI] [PubMed] [Google Scholar]
- 24. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. PMID:3342669 [DOI] [PubMed] [Google Scholar]
- 25. Phan T, Carter O, Adams C, et al. Discriminant validity of the Hospital Anxiety and Depression Scale, Beck Depression Inventory (II) and Beck Anxiety Inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13(3): 220–228. PMID: 26944070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holland AE, Nici L. The return of the minimum clinically important difference for 6-minute-walk distance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187(4): 335–336. PMID:23418323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement_Moy_10.9.18 for Physical activity in COPD: Minimal clinically important difference for medical events by Merilee Teylan, Ana Kantorowski, Diana Homsy, Reema Kadri, Caroline Richardson, and Marilyn Moy in Chronic Respiratory Disease