Abstract
Non-cystic fibrosis (non-CF) bronchiectasis is a condition characterized by an airway inflammatory response to bacterial pathogens. Frequent exacerbations have a major influence on the quality of life. Macrolide antibiotics have not only antibacterial but also immune-regulation effects. It is proved that macrolides have a benefit in preventing exacerbations. However, it is still uncertain whether azithromycin or erythromycin is more effective and safe. The purpose of this study was to answer the following question: Which kind of macrolide antibiotic is more effective and safe in preventing non-CF bronchiectasis exacerbation? We conducted a systematic review to identify randomized clinical trials published up to May 2017 that reported on macrolides for non-CF bronchiectasis and an adjusted indirect treatment comparison (AITC) between macrolides to evaluate their efficacy and safety. The direct comparison meta-analysis found that macrolides decreased the rate of exacerbation of non-CF bronchiectasis (risk ratio (RR) = 0.45; 95% confidence interval (CI) 0.36–0.55) with heterogeneity (I 2 = 63.7%, p = 0.064). The AITC showed that azithromycin had a significantly lower bronchiectasis exacerbation rate than erythromycin (RR = 0.35; 95% CI: 0.403–0.947). Azithromycin increased the risk of diarrhea and abnormal pain. This meta-analysis suggested that long-term treatment with macrolides significantly reduced the incidence of non-CF bronchiectasis exacerbation. Moreover, azithromycin is more efficient than roxithromycin and erythromycin in preventing exacerbation.
Keywords: Bronchiectasis, macrolides, adjusted indirect treatment comparison, meta-analysis, erythromycin, azithromycin
Introduction
Non-cystic fibrosis (non-CF) bronchiectasis is a condition characterized by an airway inflammatory response to bacterial pathogens.1,2 Patients with non-CF bronchiectasis endure sputum production, recurrent exacerbations, and progressive airway destruction.3 Variable courses may lead to the disease,4 and frequent exacerbations have a major influence on the quality of life.5 The morbidity of bronchiectasis in the US was 1106 cases per 100,000 with an annual percentage increase of 8.74%.6
Bronchiectasis management is mainly to prevent disease exacerbation and improve quality of life.7 Physiotherapy,8 inhalation of hyperosmolar agents,9 long-term antibiotic treatment,10 macrolides,11 and inhaled antibiotics12 are effective treatments currently.
Macrolide antibiotics have not only antibacterial but also immune-regulation effects.13–16 Growing evidence included high-quality randomized controlled trials (RCTs)2,4,11 and systematic reviews10,17,18 support the finding that azithromycin, erythromycin, and roxithromycin have a benefit in preventing exacerbations of non-CF bronchiectasis. Long-term macrolides are the only treatment agents in bronchiectasis that have been proved in randomized double-blind placebo-controlled trials to reduce exacerbations to date.19
It is still uncertain which kind of macrolides will be more effective for preventing non-CF bronchiectasis from exacerbation. There are no head-to-head RCT comparisons between macrolides. This adjusted indirect treatment comparison (AITC)20 is performed to evaluate the effects between macrolides.
Materials and methods
This review was registered in PROSPERO (CRD42013004656) and performed adhering to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Literature search strategy
We conducted an online search up to December 2017 in PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Embase (http://www.embase.com), and the Cochrane Central Register of Controlled Trials (CENTRAL) (http://onlinelibrary.wiley.com/cochranelibrary/) using the expression “(erythromycin or azithromycin or clarithromycin or roxithromycin or macrolides) and bronchiectasis”. In addition, we used hand-searches of the references of all identified articles and relevant review. The results were restricted to human studies.
Study eligibility
Eligible clinical trials were defined based on the following criteria: (1) RCT, (2) patient age >18 years, and (3) intervention with macrolides compared with placebo or another macrolide. The outcome was the rate of exacerbation or the number of patients with exacerbation.
The exclusion criteria were the following: (1) animal research, (2) case control or prospective cohort studies, and (3) reviews, letters, or case reports.
Two authors (LW and CYZ) respectively reviewed the titles and abstracts. If there were discrepancies between the reviewers, then another author (QZ), as the third investigator, was consulted to reach a consensus.
Data extraction and quality assessment
We collected data from each eligible study, including the name of the first author, publication year, study design, inclusion and exclusion criteria, cases, PubMed ID, and intervention drugs. Recommendations for reporting were followed as recommended by the PRISMA guidelines (GYH).21,22 We evaluated the quality of individual records according to the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials,23 and the details are presented in Table 1.
Table 1.
Quality assessment.
| Author | Year | Design | PubMed ID | Study size | Treatment/ control | Dose | Duration |
|---|---|---|---|---|---|---|---|
| Altenburg et al.4 | 2013 | RCT | 23532241 | 83 | A/P | 250 mg once/day | 52 weeks |
| Cymbala et al.24 | 2005 | RCT | 15813663 | 22 | A/P | 500 mg twice/week | 6 months |
| Diego et al.25 | 2013 | RCT | 23714268 | 30 | A/P | 250 mg 3 times/week | 3 months |
| Liu et al.26 | 2014 | RCT | 25580060 | 43 | R/P | 150 mg once/day | 6 months |
| Serisier et al.2 | 2013 | RCT | 23532242 | 117 | E/P | 400 mg twice/day | 48 weeks |
| Tsang et al.27 | 1999 | RCT | 10065682 | 21 | E/P | 500 mg twice/day | 8 weeks |
| Wong et al.11 | 2012 | RCT | 22901887 | 141 | A/P | 500 mg 3 times/week | 6 months |
A: azithromycin; P: placebo; E: erythromycin; R: roxithromycin.
Statistical analysis
An intervention meta-analysis was conducted using STATA 14.0 software (Stata Corporation, College Station, Texas, USA). Risk ratios (RR) for dichotomous variables with 95% confidence intervals (95% CI) were calculated. We measured heterogeneity using the I 2 test. Effect size within subgroups was measured using the fixed effect model if I 2 is less than 40%.
AITC was conducted between different arms using STATA and the indirect package (st0325).28
Results
Literature review
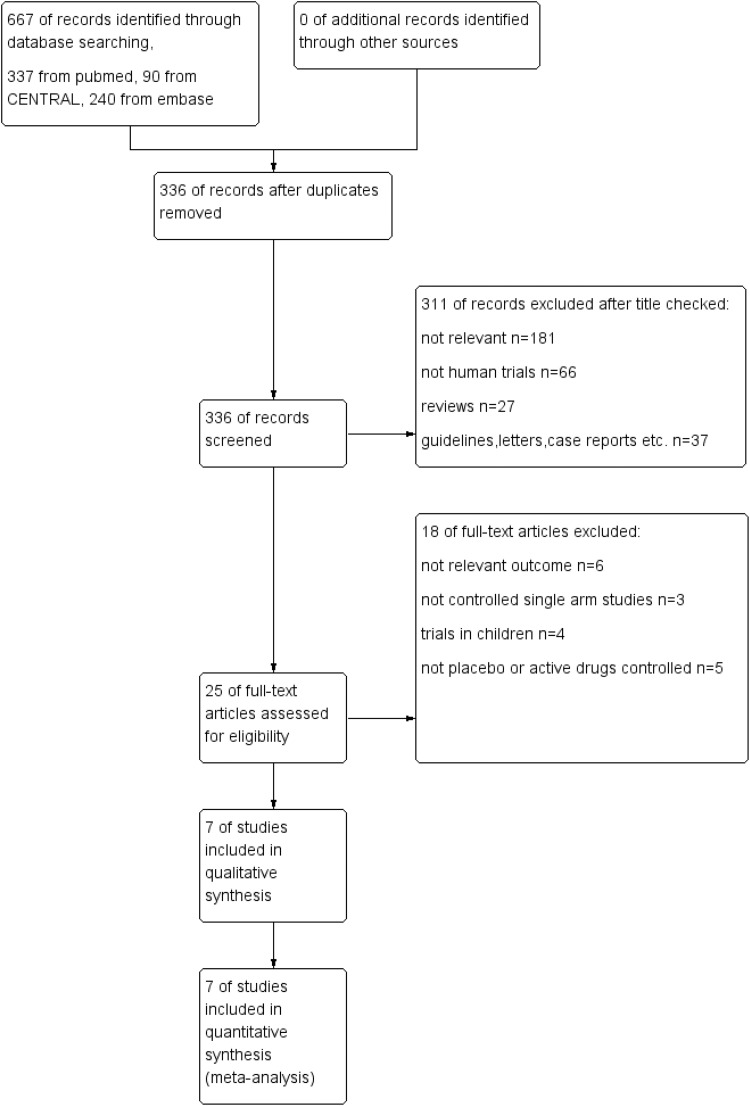
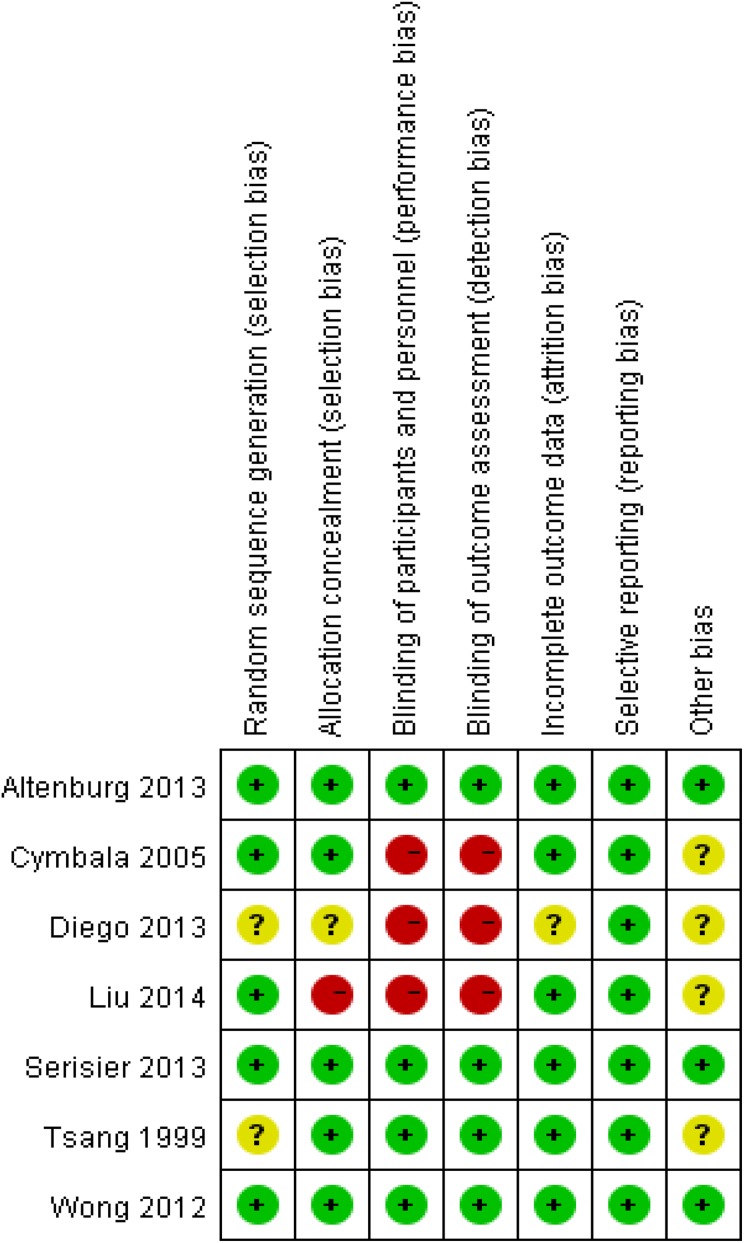
Using the search strategy mentioned above, a total of 336 records were identified after duplicates had been removed. Through screening of the titles and abstracts, we retrieved 25 records for their full text, of which 7 were ultimately included in our meta-analysis. Details of exclusions are shown in Figure 1. Seven studies with 457 participants were included in this meta-analysis. Risks of bias of included articles was shown in figure 2.
Figure 1.
Study flow diagram.
Figure 2.
Risk of bias.
Direct meta-analysis of the efficacy of macrolides for non-CF bronchiectasis
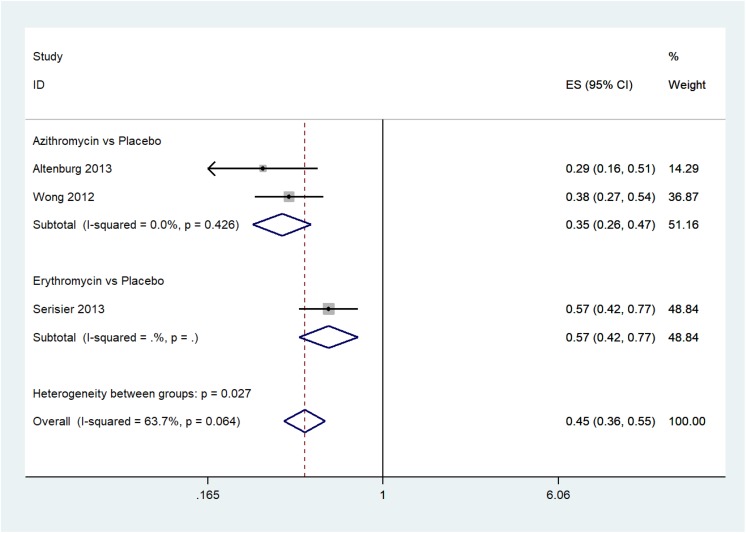
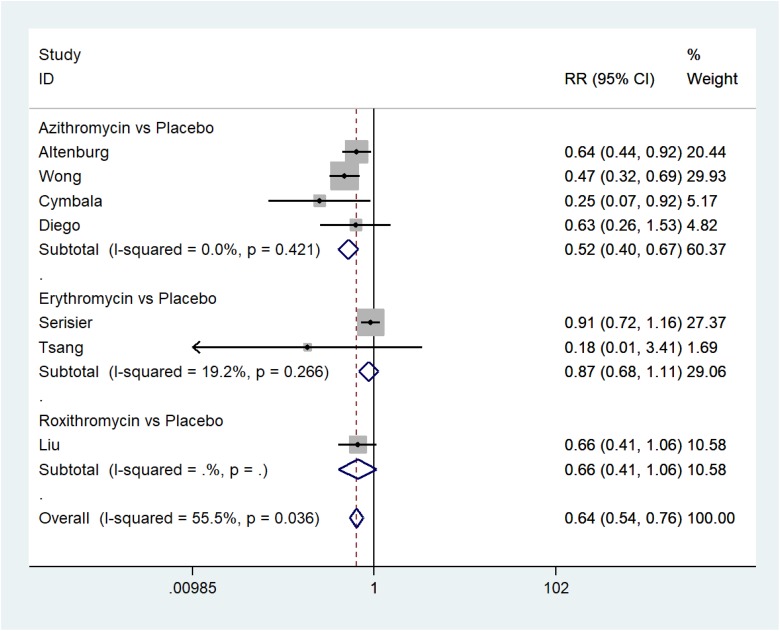
The pooled effect shows that macrolides decreased the rate of exacerbation of non-CF bronchiectasis (RR = 0.45; 95% CI: 0.36–0.55) with heterogeneity (I 2 = 63.7%; p = 0.064) (Figure 3), and the number of exacerbations (RR = 0.64; 95% CI: 0.54–0.76) with heterogeneity (I 2 = 55%; p = 0.036) (Figure 4).
Figure 3.
The rate of exacerbation of non-CF bronchiectasis. non-CF: non-cystic fibrosis.
Figure 4.
The number of exacerbations of non-CF bronchiectasis. non-CF: non-cystic fibrosis.
Subgroup analysis showed that azithromycin and erythromycin significantly decreased the rate of exacerbation of non-CF bronchiectasis (azithromycin: RR = 0.35; 95% CI: 0.26–0.47; p = 0.426; I 2 = 0.0%, erythromycin: RR = 0.0.57; 95% CI: 0.42–0.77; no heterogeneity test due to inclusion of only one RCT).
Macrolides significantly reduced the number of exacerbations (azithromycin: RR = 0.52, 95% CI: 0.40–0.67; p = 0.421, I 2 = 0.0%; Erythromycin: RR = 0.87, 95% CI: 0.68–1.11; p = 0.266, I 2 = 19.2%; roxithromycin: RR = 0.66, 95% CI: 0.41–1.06; no heterogeneity test due to inclusion of only one RCT). Based on the subgroup analysis results, we speculate that the heterogeneity may come from the different interventions.
Adjusted indirect meta-analysis of the efficacy of macrolides for non-CF bronchiectasis
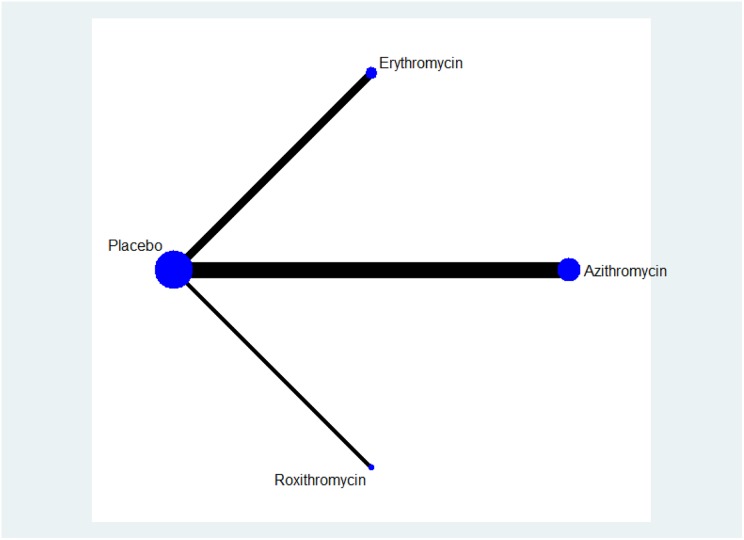
The network is shown in Figure 5. Compared with erythromycin, azithromycin showed a significantly lower exacerbation rate (RR: 0.35, 95% CI: 0.403–0.947) (Table 2). Compared with placebo, roxithromycin and erythromycin reduced the number of exacerbations (not statistically significant) (Table 3), whereas azithromycin showed better results, which were significant (RR: 0.52, 95% CI: 0.4–0.67).
Figure 5.
The network in the adjusted indirect analysis.
Table 2.
Indirect comparison exacerbation rate.
| Relative effect of exacerbation rate | ||
|---|---|---|
| Azithromycin | ||
| 0.618 (0.403, 0.947) | Erythromycin | |
| 0.35 (0.26, 0.47) | 0.57 (0.42, 0.77) | Placebo |
Bold:Stata with indirect meta-analysis package report the result as the format of side effect(95% CI).
Table 3.
Indirect comparison of number of exacerbations.
| Relative effect of exacerbation number | |||
|---|---|---|---|
| Azithromycin | |||
| 0.826 (0.48, 1.42) | Roxithromycin | ||
| 0.67 (0.286, 1.553) | 0.806 (0.314, 2.065) | Erythromycin | |
| 0.52 (0.4, 0.67) | 0.66 (0.41, 1.06) | 0.87 (0.68, 1.11) | Placebo |
Bold:Stata with indirect meta-analysis package report the result as the format of side effect(95% CI).
Safety evaluation
Diarrhea, nausea, and other gastrointestinal reactions are common adverse events of macrolides. In contrast to placebo, azithromycin increased the risk of diarrhea (Pooled RR = 4.18, 95% CI: 1.7–10.28; I 2 = 0.0%, p = 0.002) and abnormal pain (Pooled RR = 6.11, 95% CI: 1.41–26.52; I 2 = 0.0%, p = 0.02) (Table 4). However, macrolides did not show an effect of increasing nausea, rush, and headache. Additionally, cold, cough, chest pain,11 QT interval prolongation,2 and auditory problems4 were reported. Studies reported that macrolide antibiotics may be associated with myocardial infarction29,30; however, this adverse effect was not reported in the included articles.
Table 4.
Adverse events.
| Adverse events | No. of studies | RR (95% CI) | p Value | Heterogeneity | |
|---|---|---|---|---|---|
| Nausea | 4 | 1.29 (0.45, 3.67) | 0.63 | χ 2 = 5.12, p = 0.16, I 2 = 41% | |
| A versus P | 2 | 1.29 (0.61, 2.69) | 0.5 | χ 2 = 0.74, p = 0.39, I 2 = 0% | |
| E versus P | 1 | 0.14 (0.01, 2.66) | 0.19 | — | |
| R versus P | 1 | 11.00 (0.64, 189.31) | 0.1 | — | |
| Rash | 3 | 2.03 (0.75, 5.52) | 0.16 | χ 2 = 0.12, p = 0.94, I 2 = 0% | |
| A versus P | 1 | 1.86 (0.61, 5.70) | 0.28 | — | |
| E versus P | 1 | 2.75 (0.12, 60.70) | 0.52 | — | |
| R versus P | 1 | 3.00 (0.13, 70.42) | 0.5 | — | |
| Diarrhea | 3 | 4.18 (1.70, 10.28) | 0.002 | χ 2 = 0.84, p = 0.66, I 2 = 0% | |
| A versus P | 3 | 4.18 (1.70, 10.28) | 0.002 | χ 2 = 0.84, p = 0.66, I 2 = 0% | |
| Abdominal pain | 2 | 6.11 (1.41, 26.52) | 0.02 | χ 2 = 0.08, p = 0.78, I 2 = 0% | |
| A versus P | 2 | 6.11 (1.41, 26.52) | 0.02 | χ 2 = 0.08, p = 0.78, I 2 = 0% | |
| Headache | A versus P | 2 | 0.69 (0.17, 2.77) | 0.6 | χ 2 = 0.95, p = 0.33, I 2 = 0% |
| A versus P | 2 | 0.69 (0.17, 2.77) | 0.6 | χ2 = 0.95, p = 0.33, I2 = 0% |
A: azithromycin; P: placebo; E: erythromycin; R: roxithromycin.
Publication bias
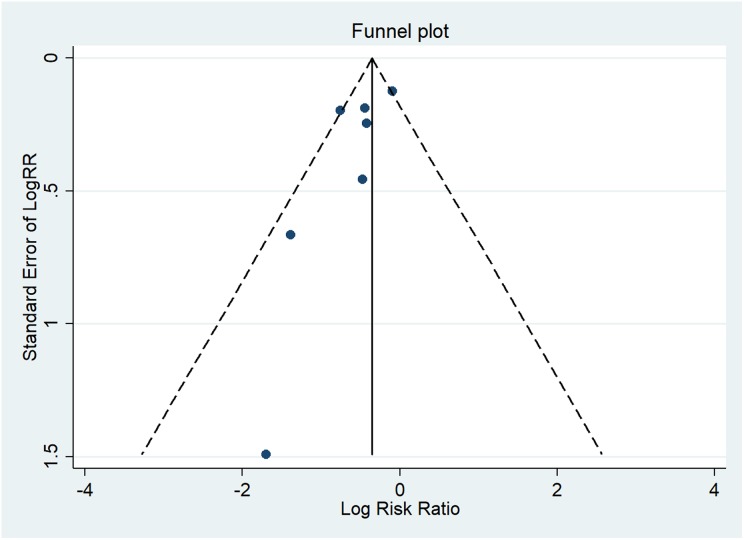
Egger’s test did not show a significant publication bias (p = 0.126). The funnel plot for the number of patients with bronchiectasis exacerbations appeared to be slightly asymmetrical (Figure 6). However, 7 studies with 457 participants were enrolled in this meta-analysis, which may be insufficient for the assessment of publication bias.
Figure 6.
Publication bias of included trials.
Discussion
This AITC28 suggested that based on the existing evidence, long-term treatment with macrolides significantly reduced the incidence of non-CF bronchiectasis exacerbations and that azithromycin in particular may be the most efficient intervention. Subgroup analysis showed that azithromycin and erythromycin but not roxithromycin significantly decreased the number of exacerbations of non-CF bronchiectasis. However, only one article compared the effectiveness of roxithromycin, which should be evaluated in the future.
The exact mechanism of macrolide clinical benefits in inflammatory lung diseases remains unclear.2 The benefit of macrolides may not be completely due to the antibiotic effect,31 as immunomodulatory effect might also played an important role.16 Modulation of host responses facilitates the long-term therapeutic benefit of macrolides in cystic fibrosis and non-CF bronchiectasis.
In the same subgroup, we found little heterogeneity (Figures 3 and 4); however, heterogeneity was significant between interventions. We speculate that the heterogeneity may come from the different drugs.
The limitations of this indirect comparison meta-analysis should be mentioned. First, macrolides could cause adverse effects, especially gastrointestinal complaints; however, we did not evaluate the indirect relative adverse effects due to inadequate data from the original research. Second, all included research chose the same placebo control design. Therefore, it is suboptimal that we did not conduct the inconsistency test between direct and indirect comparisons, which may decrease the reliability of this review. Third, 7 studies with 457 participants were enrolled in this meta-analysis, which may be insufficient. The relatively small sample size limited the effectiveness of the publication bias assessment. Additional high-quality RCTs and larger sample sizes may lead to more reliable results. Multicentered, large-sample, direct head-to-head comparisons should be conducted between macrolides. Fourth, the therapeutic duration and dose were not identical, which may lead to heterogeneity.
Understandably, erythromycin is easily destroyed by acid, and the oral absorption is low. Azithromycin is stable to gastric acid. The plasma concentration of azithromycin is low, and azithromycin is mainly concentrated in lung tissue and has a longer plasma half-life than erythromycin. Concentration in lung tissue may increase the effect of azithromycin.
Studies reported that macrolide antibiotics were associated with an increased risk of myocardial infarction but not arrhythmia or cardiovascular mortality,30 especially regarding erythromycin and clarithromycin. The risk of cardiovascular mortality between azithromycin and other antibiotics is not significantly different.29 It seems that azithromycin is safer than erythromycin. Additional clinical trials to evaluate the safety of macrolides are needed.
Conclusion
In summary, this meta-analysis suggested that long-term treatment with macrolides significantly reduced the incidence of non-CF exacerbation. Moreover, indirect treatment comparisons showed that azithromycin is more efficient than roxithromycin and erythromycin in preventing non-CF from exacerbation. Indirect evidence of adverse effects should be evaluated in the future.
Footnotes
Authors’ contribution: Li Wen and Chen Yunzhi contributed equally to this work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81373517, No. 81460694, No. 81760841), Project of Education Department of Guizhou Province (K[2017]041, KY[2017]172), Project of Administration of traditional Chinese medicine of Guizhou province (No. S201707260030), and Natural Science Foundation of Science and Technology Department of Guizhou Province (J[2012]2086).
ORCID iD: Wen Li  http://orcid.org/0000-0002-3975-3312
http://orcid.org/0000-0002-3975-3312
References
- 1. McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013; 188(6): 647–656. [DOI] [PubMed] [Google Scholar]
- 2. Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; 309(12): 1260–1267. [DOI] [PubMed] [Google Scholar]
- 3. Lee AL, Burge A, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2013; (5): Cd008351. [DOI] [PubMed] [Google Scholar]
- 4. Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309(12): 1251–1259. [DOI] [PubMed] [Google Scholar]
- 5. ten Hacken NH, Wijkstra PJ, Kerstjens HA. Treatment of bronchiectasis in adults. BMJ 2007; 335(7629): 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest 2012; 142(2): 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altenburg J, Wortel K, Van der Werf TS, et al. Non-cystic fibrosis bronchiectasis: clinical presentation, diagnosis and treatment, illustrated by data from a Dutch Teaching Hospital. Neth J Med 2015; 73(4): 147–154. [PubMed] [Google Scholar]
- 8. Mandal P, Sidhu MK, Kope L, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respir Med 2012; 106(12): 1647–1654. [DOI] [PubMed] [Google Scholar]
- 9. Wills P, Greenstone M. Inhaled hyperosmolar agents for bronchiectasis. Cochrane Database Syst Rev 2006; (2): CD002996. [DOI] [PubMed] [Google Scholar]
- 10. Hnin K, Nguyen C, Carson KV, et al. Prolonged antibiotics for non-cystic fibrosis bronchiectasis in children and adults. Cochrane Database Syst Rev 2015; (8): CD001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012; 380(9842): 660–667. [DOI] [PubMed] [Google Scholar]
- 12. Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J 2014; 44(2): 382–393. [DOI] [PubMed] [Google Scholar]
- 13. McDonald PJ, Pruul H. Macrolides and the immune system. Scand J Infect Dis Suppl 1992; 83: 34–40. [PubMed] [Google Scholar]
- 14. Rodriguez-Cerdeira C, Sanchez-Blanco E, Molares-Vila A. Clinical application of development of nonantibiotic macrolides that correct inflammation-driven immune dysfunction in inflammatory skin diseases. Med Inflamm 2012; 2012: 563709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura S, Izumikawa K, Yanagihara K, et al. New therapeutic strategies for pulmonary infection: the potency of immune activation by macrolides and Toll-like receptor agonist. Jpn J Antibiot 2016; 69(2): 91–100. [PubMed] [Google Scholar]
- 16. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014; 143(2): 225–245. [DOI] [PubMed] [Google Scholar]
- 17. Gao YH, Guan WJ, Xu G, et al. Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. PLoS One 2014; 9(3): e90047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan LC, Lu HW, Wei P, et al. Effects of long-term use of macrolides in patients with non-cystic fibrosis bronchiectasis: a meta-analysis of randomized controlled trials. BMC Infect Dis 2015; 15: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill AT. Macrolides for clinically significant bronchiectasis in adults. Chest 2016; 150(6): 1187–1193. [DOI] [PubMed] [Google Scholar]
- 20. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23(20): 3105–3124. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 22. Cornell JE. The PRISMA extension for network meta-analysis: bringing clarity and guidance to the reporting of systematic reviews incorporating network meta-analyses. Ann Intern Med 2015; 162(11): 797–798. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cymbala AA, Edmonds LC, Bauer MA, et al. The disease-modifying effects of twice-weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med 2005; 4(2): 117–122. [DOI] [PubMed] [Google Scholar]
- 25. Diego AD, Milara J, Martinez-Moragon E, et al. Effects of long-term azithromycin therapy on airway oxidative stress markers in non-cystic fibrosis bronchiectasis. Respirology (Carlton, Vic) 2013; 18(7): 1056–1062. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Zhong X, He Z, et al. Effect of low-dose, long-term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediators Inflamm 2014; 2014: 708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsang KW, Ho PI, Chan KN, et al. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J 1999; 13(2): 361–364. [DOI] [PubMed] [Google Scholar]
- 28. Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health 2011; 14(4): 417–428. [DOI] [PubMed] [Google Scholar]
- 29. Sutton SS, Hyche S, Magagnoli J, et al. Appraisal of the cardiovascular risks of azithromycin: an observational analysis. J Comp Eff Res 2017; 6(6): 509–517. [DOI] [PubMed] [Google Scholar]
- 30. Gorelik E, Masarwa R, Perlman A, et al. The cardiovascular safety of macrolides: a systematic review, meta-analysis and network meta-analysis. Antimicrob Agents Chemother 2018; 62(6): e00438–e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen AC, Martin MM, Burr L, et al. Clinical benefits of long-term, low-dose erythromycin in bronchiectasis are not due to anti-inflammatory effects. Am J Respir Crit Care Med 2013; 187: A5970. [Google Scholar]