Abstract
BACKGROUND
Sodium-glucose cotransporter 2 inhibitors (SGLT2is, gliflozins) are associated with lower all-cause mortality than other anti-diabetic agents in patients with type 2 diabetes. In patients who may benefit from SGLT2is, but cannot add them to a dipeptidyl peptidase-4 inhibitor (DPP4i, gliptin) treatment for various reasons, replacement of the DPP4i with a SGLT2i may be considered.
OBJECTIVES
Evaluate changes in metabolic parameters in patients with diabetes after replacing a DPP4i with the SGLT2i empagliflozin.
DESIGN
Panel study (cross-sectional and cohort hybrid).
SETTING
The diabetes outpatient clinics of Chang Gung Memorial Hospital at Linkou, a university hospital in Taiwan.
PATIENTS AND METHODS
We reviewed the medical records of patients who had been treated with anti-diabetic agents including a DPP4i between May 2016 and May 2017. Patients who switched from DPP4is to empagliflozin (switched-to-empagliflozin group) without changes to other anti-diabetic agents for at least 6 months were compared patients who continued taking an original antidiabetic agent. The body weight (BW), body mass index (BMI), and glycated hemoglobin (HbA1c) level at baseline and after 3 and 6 months were collected for analysis.
MAIN OUTCOME MEASURES
BW, BMI, HbA1c at baseline, 3 and 6 months.
SAMPLE SIZE
236 patients.
RESULTS
The HbA1c level and BMI of 110 patients (71%) in the switched-to-empagliflozin group were significantly reduced at 6 months after the switch, and there was a significant negative correlation between baseline HbA1c and change in HbA1c (rho=-0.537, P <.001). In another 45 patients (29%) who switched to empagliflozin, HbA1c did not have improve, but BW decreased after the switch. No significant change in HbA1c occurred in the group that remained on DPP4is. In addition, BW and BMI decreased regardless of the degree of glucose reduction in the switched-to-empagliflozin group (P<.001 for both variables at 6 months vs baseline), but not in the patients who remained on DPP4is.
CONCLUSIONS
The study demonstrated the metabolic impact of switching from a DPP4i to a well-known SGLT2i, but further large-scale trials are needed to study the long-term effects of replacing a DPP4i with a SGLT2i.
LIMITATIONS
Retrospective design, a short observation period, a small number of patients, no evaluations of the quality of life, side effects, or the cost, and data limited to only empagliflozin.
The treatment of diabetes has evolved from glucose-centric to an event-driven strategy.1–3 As such, add-on to standard care or replacement therapies for anti-diabetic agents are now focused on long-term cardiac-renal and even hepatoprotective effects of add-on therapies.2,3 Increasing evidence supports an association between sodium-glucose cotransporter 2 inhibitors (SGLT2is, or gliflozins) and lower all-cause mortality than other antidiabetic agents in patients with type 2 diabetes.1 According to the Standard Medical Care of American Diabetes Association, SGLT2is are indicated after metformin to rescue heart tissue in patients with atherosclerotic cardiovascular diseases.4 Accordingly, adding SGLT2is to a dipeptidyl peptidase-4 inhibitor (DPP4i, or gliptin) in diabetic patients to reduce glycated hemoglobin (HbA1c) and for other specific clinical indications has been widely accepted. 5,6 Nevertheless, for diabetic patients who may benefit from SGLT2is, but cannot take both SGLT2is and DPP4is due to reasons such as DPP4i-related adverse effects, lack of insurance cover, prohibitive cost, or unwillingness to take more oral medication, replacement of DPP4is with SGLT2is should be considered. It is not yet known how replacing DPP4is with SGLT2is may influence metabolic parameters such as glycemic levels and body weight (BW). In this study, we evaluated glycemic and BW changes in diabetic patients who replaced a DPP4i with empagliflozin, a SGLT2i, while maintaining other anti-diabetic agents.
PATIENTS AND METHODS
This study was carried out at the diabetes outpatient clinics of Chang Gung Memorial Hospital at Linkou, a university teaching hospital in Taiwan. Ethical approval was given by the Chang Gung Medical Foundation Institutional Review Board (201701268B0). Patients with type 2 diabetes who had been treated with anti-diabetic agents including a DPP4i for at least half a year were enrolled. The study included patients that had switched from a DPP4i to empagliflozin during the period from 1 May 2016 to 31 May 2017, and had not changed other antidiabetic therapy for 6 months after the switch.
We defined these patients as the “switched-to-empagliflozin group”. The daily dosages of DPP4is and empagliflozin were prescribed according to regular practice. The remaining patients who kept taking their original anti-diabetic agents including DPP4is during the 6 months of observation were defined as the “remained- on-DPP4i group”. We excluded patients aged younger than 20 years, those with type 1 diabetes mellitus, those who had undergone an organ transplant, those who had previously used a SGLT2i, and those who stopped taking empagliflozin within 3 months of initiating therapy.
All of the participants received counselling for lifestyle modifications by certified diabetes educators and dietitians during the study period. This counselling included education on diet and how to record their diet, blood glucose self-monitoring, and proper exercise. Data on demographics, BW, body mass index (BMI), serum HbA1c level and creatinine level of all patients were collected from the medical records. The baseline data were defined as the data at the time of starting empagliflozin treatment in the switched-to-empagliflozin group, or as the first data point after 1 May 2016 in the remained-on-DPP4i group. The data at 3 and 6 months after baseline were recorded in both groups. We defined the change in HbA1c at 6 months as the difference in HbA1c level at 6 months minus the HbA1c level at baseline. In the switched-to-empagliflozin group, patients with a decline in HbA1c <0 at 6 months were defined as responders, and those with a no change or an increase in HbA1c ≥0 at 6 months as non-responders.
The demographic and clinical changes in HbA1c, BW, and BMI for the patients in each group were analyzed. The demographic and laboratory data between the groups (switched to empagliflozin or remained on DPP4i and responders and non-responders to empagliflozin) were compared using the Mann-Whitney test and chi-square test where appropriate. Within each group, differences in HbA1c level, BW, and BMI between baseline and 3 months, baseline and 6 months, and 3 months and 6 months were analyzed using the Wilcoxon signed-rank test. Correlations between baseline HbA1c and HbA1c at 6 months were evaluated using the Spearman rank correlation. All statistical tests were carried out at a two-tailed significance level of .05 using SPSS software version 22 (IBM SPSS Inc., Armonk, NY, USA).
RESULTS
Compared with the patients in the remained-on-DPP4i group, those in the switched-to-empagliflozin group were younger, had higher levels of baseline HbA1c, higher baseline BMI, and more insulin use (Table 1). More patients in the switched-to-empagliflozin group used the DPP4is saxagliptin and linagliptin than those in the remained-on-DPP4i group. There were no significant differences in baseline levels of serum creatinine and urinary albumin-creatinine ratio between the switched-to-empagliflozin and remained-on-DPP4i groups. Both the level of HbA1c and BMI of the patients in the switched-to-empagliflozin group were significantly reduced at 3 months after switching treatment, and then reached levels similar to the patients in the remained-on-DPP4i group at 6 months after the switch (Table 1). The serum creatinine levels of the patients in both groups did not change significantly during the 6-month follow-up period.
Table 1.
Demographic and clinical changes in patients who switched to empagliflozin treatment or who remained on DPP4i treatment.
| Switched-to-empagliflozin | Remained-on-DPP4i | P | ||
|---|---|---|---|---|
|
| ||||
| n | 155 | 81 | --- | |
| Age (years) | 60.4 (51.9, 67.0) | 65.0 (59.0, 70.0) | .001 | |
| Gender (male) | 99 (63.9) | 41 (50.6) | .049 | |
| Hypertension | 102 (65.8) | 58 (71.6) | .365 | |
| Dyslipidemia | 115 (74.2) | 71 (87.7) | .016 | |
|
| ||||
| DPP4i | Sitagliptin | 53 (34.2) | 39 (48.1) | .001 |
| Vildagliptin | 51 (32.9) | 35 (43.2) | ||
| Saxagliptin | 19 (12.3) | 1 (1.2) | ||
| Linagliptin | 31 (20.0) | 6 (7.4) | ||
|
| ||||
| Insulin (therapy) | 56 (36.1) | 17 (21.0) | .017 | |
| Sulfonylurea | 112 (72.2) | 61 (75.3) | .668 | |
| Metformin | 129 (83.2) | 74 (91.4) | .087 | |
| Pioglitazone | 20 (12.9) | 6 (7.4) | .200 | |
| Cancer history | 13 (8.4) | 5 (6.2) | .543 | |
| MACE history | 8 (5.2) | 5 (6.2) | .746 | |
|
| ||||
| HbA1c (%) | Baseline | 8.8 (8.0, 9.7) | 7.9 (7.3, 8.4) | <.001 |
| at 3 months | 8.2 (7.6, 9.1) | 7.7 (7.1, 8.2) | <.001 | |
| at 6 months | 8.2 (7.5, 8.9) | 7.9 (7.4, 8.4) | .054 | |
|
| ||||
| P value of HbA1c in the same group | Baseline vs 3 months | <.001 | <.001 | --- |
| Base vs 6 months | <.001 | .758 | ||
| 3 months vs 6 months | .001 | .025 | ||
|
| ||||
| Body weight (kg) | Baseline | 72.0 (65.0,83.0) | 67.0 (59.5, 76.0) | .003 |
| at 3 months | 71.0 (65.0,81.0) | 66.8 (59.3, 75.5) | .011 | |
| at 6 months | 70.5 (63.8,81.0) | 67.0 (59.6, 75.8) | .020 | |
|
| ||||
| P value of body weight in the same group | Baseline vs 3 months | <.001 | .432 | --- |
| Base vs 6 months | <.001 | .928 | ||
| 3 months vs 6 months | .021 | .940 | ||
|
| ||||
| Body mass index (kg/m2) | Baseline | 27.2 (24.7, 30.1) | 25.9 (24.0, 28.4) | .008 |
| at 3 months | 26.6 (23.9, 29.6) | 25.6 (23.8, 28.8) | .138 | |
| at 6 months | 26.3 (24.0, 29.4) | 25.9 (23.7, 28.6) | .132 | |
|
| ||||
| P value of body mass index in the same group | Baseline vs 3 months | <.001 | .717 | --- |
| Base vs 6 months | <.001 | .466 | ||
| 3 months vs 6 months | .005 | .494 | ||
|
| ||||
| Baseline urinary ACR (mg/g) | 27.5 (8.9, 106.2) | 23.4 (10.6, 100.6) | .780 | |
| Baseline serum creatinine (mg/dL) | 0.81 (0.68, 0.99) | 0.86 (0.69, 1.03) | .438 | |
| Serum creatinine at 3 months | 0.83 (0.69, 1.01) | 0.87 (0.68, 1.07) | .705 | |
| Serum creatinine at 6 months | 0.85 (0.69, 1.04) | 0.85 (0.69, 1.06) | .842 | |
Values are number (percentage) or median and interquartile ranges. DPP4i: Dipeptidyl peptidase-4 inhibitors; MACE: major adverse cardiovascular events; HbA1: glycated hemoglobin; ACR: albumin creatinine ratio
Among the 155 patients in the switched-to-empagliflozin group, 110 (71%) were categorized as responders, and 45 (29%) as non-responders to empagliflozin (Table 2). There were no significant differences in age, number of insulin users, types of DPP4i being replaced, baseline BMI, baseline levels of serum creatinine and urinary albumin-creatinine ratio between the responders and non-responders. Interestingly, the baseline HbA1c level of the responders was significantly higher than that of the non-responders. Both the level of HbA1c and BMI of the responders were significantly reduced at 3 and 6 months after the replacement (Table 2). In contrast, the level of HbA1c of the non-responders significantly increased after the replacement; however, their BMI was significantly reduced at 3 and 6 months after the replacement. In both the responders and non-responders, the changes in serum creatinine before and 3–6 months after replacement did not reach statistical significance.
Table 2.
Demographic and clinical changes in patients in the switched-to-empagliflozin group by HbA1c response to empagliflozin at 6 months.
| Responders (Delta HbA1 at 6 months <0) | Non-responders (Delta HbA1c at 6 months ≥0) | P | ||
|---|---|---|---|---|
|
| ||||
| n | 110 | 45 | --- | |
| Age (year-old) | 60.5 (51.0, 66.4) | 60.4 (52.3, 68.6) | .553 | |
| Gender (male) | 71 (64.5) | 28 (62.2) | .785 | |
| Hypertension | 71 (64.5) | 31 (68.9) | .605 | |
| Dyslipidemia | 79 (71.8) | 36 (80.0) | .291 | |
|
| ||||
| Switched DPP4i | Sitagliptin | 38 (34.5) | 15 (33.3) | .485 |
| Vildagliptin | 36 (32.7) | 15 (33.3) | ||
| Saxagliptin | 16 (14.5) | 3 (6.7) | ||
| Linagliptin | 19 (17.3) | 12 (26.7) | ||
|
| ||||
| Insulin | 40 (36.4) | 16 (35.6) | .924 | |
| Sulfonylurea | 80 (72.7) | 32 (71.1) | .281 | |
| Metformin | 92 (83.6) | 37 (82.2) | .831 | |
| Pioglitazone | 14 (12.7) | 6 (13.3) | .919 | |
| Cancer history | 10 (9.1) | 3 (6.7) | .621 | |
| MACE history | 5 (4.5) | 3 (6.7) | .588 | |
|
| ||||
| HbA1c (%) | Baseline | 9.2 (8.3, 10.0) | 8.0 (7.6, 9.0) | <.001 |
| at 3 months | 8.2 (7.6, 9.1) | 8.3 (7.8, 9.2) | .547 | |
| at 6 months | 7.9 (7.4, 8.6) | 8.5 (7.9, 9.4) | <.001 | |
|
| ||||
| P value of HbA1c in the same group | Baseline vs 3 months | <.001 | .006 | --- |
| Baseline vs 6 months | <.001 | <.001 | ||
| 3 months vs 6 months | <.001 | .086 | ||
|
| ||||
| Body weight (kg) | Baseline | 73.0 (65.8, 84.0) | 71.0 (65.0, 77.5) | .143 |
| at 3 months | 72.0 (65.0, 81.8) | 70.0 (64.5, 75.0) | .125 | |
| at 6 months | 72.3 (64.3, 82.9) | 69.8 (62.8, 74.3) | .168 | |
|
| ||||
| P value of body weight in the same group | Baseline vs 3 months | <.001 | .002 | --- |
| Baseline vs 6 months | <.001 | <.001 | ||
| 3 months vs 6 months | .282 | .012 | ||
|
| ||||
| Body mass index (kg/m2) | Baseline | 27.5 (25.0, 30.6) | 26.2 (24.0, 28.6) | .054 |
| at 3 months | 27.0 (24.3, 30.2) | 25.9 (23.9, 28.2) | .185 | |
| at 6 months | 26.7 (24.3, 30.2) | 25.6 (23.1, 28.4) | .088 | |
|
| ||||
| P value of body mass index in the same group | Baseline vs 3 months | <.001 | .003 | --- |
| Baseline vs 6 months | <.001 | <.001 | ||
| 3 months vs 6 months | .087 | .016 | ||
|
| ||||
| Baseline ACR (mg/g) | 25.5 (8.9, 95.7) | 34.9 (8.8, 144.8) | .379 | |
| Baseline creatinine (mg/dL) | 0.80 (0.67, 0.98) | 0.84 (0.71, 1.09) | .238 | |
| Creatinine at 3 months | 0.82 (0.69, 0.99) | 0.88 (0.70, 1.20) | .165 | |
| Creatinine at 6 months | 0.85 (0.68, 1.01) | 0.88 (0.71, 1.09) | .688 | |
Values are number (percentage) or median and interquartile ranges. DPP4i: Dipeptidyl peptidase-4 inhibitors; MACE: major adverse cardiovascular events; HbA1: glycated hemoglobin; ACR: urinary albumin creatinine ratio; Delta HbA1c at 6 months was defined as the difference in HbA1c level at 6 months minus its level at baseline.
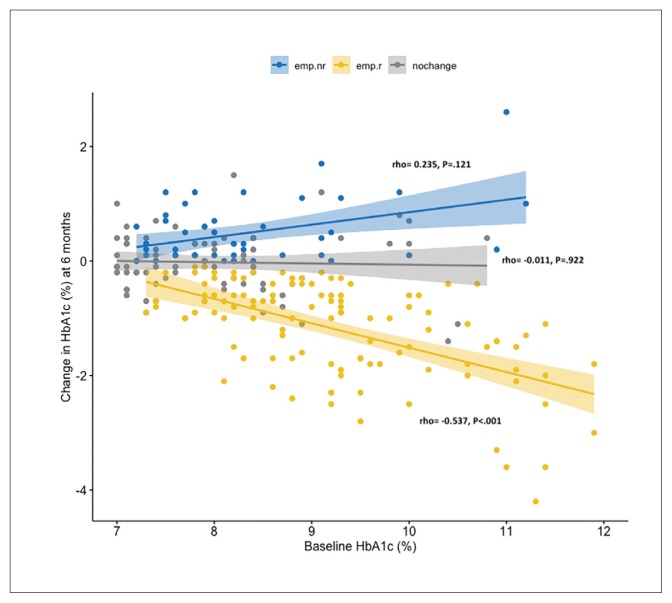
We analyzed correlations between the levels of baseline HbA1c and delta HbA1c at 6 months in the patients in the three study groups (Figure 1). A significant negative correlation was found between the level of baseline HbA1c and the change in HbA1c at 6 months in the 110 responders in the switched-to-empagliflozin group (rho=-0.537, P<.001). The negative correlation remained significant after adjusting for BMI, age, gender, and creatinine (rho=-0.577, P<.001). There were no significant correlations between the level of baseline HbA1c and the change in HbA1c at 6 months in the 81 patients in the remained-on-DPP4i group (rho=-0.011, P=.922) or the 45 non-responders in the switched-to-empagliflozin group (rho=0.235, P=.121).
Figure 1.
Correlations between baseline glycated hemoglobin (HbA1c) and change in HbA1c at 6 months. The patients who did not adjust their antidiabetic agents except for switching their dipeptidyl peptidase-4 inhibitor (DPP4i) to empagliflozin were grouped as responders (emp.r) and non-responders (emp.nr) according to the change in HbA1c at 6 months. The remaining patients continued taking DPP4i (nochange). The scatterplots and regression line (95% CI) are stratified by the three groups. The correlations were analyzed using Spearman’s correlation. The responders in the switched-to-empagliflozin had a significant negative correlation (rho=-0.537, P<.001), which remained significant after adjusting for BMI, age, gender, and serum creatinine (rho=-0.577, P<.001).
DISCUSSION
Although studies comparing the effects of SGLT2is and DPP4is on metabolic parameters in diabetic patients naïve to both categories of drugs have been reported, 6–8 no studies have reported the metabolic impact of replacing a DPP4i with an SGLT2i in patients with type 2 diabetes. Our results revealed that the median HbA1c and BMI of the patients in the switched-to-empagliflozin group were significantly reduced as early as 3 months after switching from a DPP4i to empagliflozin. In addition, the higher the baseline value of HbA1c, the better the improvement in HbA1c after the switch in these patients. One previous study hypothesized that the effectiveness of SGLT2is in reducing HbA1c in subjects with a higher baseline HbA1c could be related to an increased fraction of filtered glucose excreted by SGLT2is during hyperglycemia, resulting in upregulation of sodium-glucose cotransporter 2 protein in the kidneys.8,9 To the best of our knowledge, this is the first study to evaluate glycemic and BW changes in type 2 diabetic patients who switched from a DPP4i to empagliflozin while still taking other anti-diabetic agents.
In this study, 29% of the non-responders in the switched-to-empagliflozin group had an HbA1c level equal to or higher than their baseline value, although they had a significantly reduced BMI at 3 and 6 months after replacement. For non-responders with clinical indications for an SGLT2i, but with no improvement in HbA1c, other treatment modalities such as insulin injection and/or adding-on other oral anti-diabetic agents can be considered when there are clinically relevant indications such as an HbA1c level >9.0% or excessive weight loss.
It is not clear why the HbA1c level of 29% of the patients in the switched-to-empagliflozin group did not improve. The baseline BMI of the patients in the switched-to-empagliflozin group was higher than that in the remained-on-DPP4i group. Furthermore, the baseline BMI level was relatively higher in the responders than in the non-responders in the switched-to-empagliflozin group. Studies focusing on Asian subjects have reported a significant negative correlation between BMI and the HbA1c-lowering effect in patients treated with DPP4is.10 Therefore, the patients in our switched-to-empagliflozin group, especially the responders, may be considered to be non-responders to DPP4is. Whether differences in the serum concentration of empagliflozin resulted in the different responsiveness is not clear as we did not measure the serum concentration of empagliflozin. Genetic variations in the sodium-glucose transporter gene (SGLT2) and differences in the expression of sodium-glucose cotransporter 2 protein in the kidneys reportedly can affect regulation of glucose homeostasis.11,12 However, further studies are needed to investigate whether these factors are related to different responses in patients who switch to empagliflozin.
The weight reduction benefits of SGLT2is are reported to be related to the amount of caloric loss in the form of urinary glucose excretion.10 In our study, the BW and BMI of the responders in the switched-to-empagliflozin group significantly decreased, and this may have been related to the beneficial effect of SGLT2is on weight reduction. However, the decreases in BW and BMI in the non-responders in the switched-to-empagliflozin group may have been due to both the effect of empagliflozin and worsened blood glucose control after switching to empagliflozin. This 6-month panel study was too short to conclude any cardiovascular benefits. There were no cases of mortality or cardiovascular morbidity in this study. In addition, there were no obvious differences in blood pressure and lipid levels between the responders and non-responders in the switched-to-empagliflozin group. Although the blood glucose responses after replacement may have differed between patients, most benefited from weight reduction after the switch to empagliflozin.
The limitations of this study are the retrospective design, the short observation period, the small number of patients, and lack of evaluation of quality of life, side effects, or the cost. Also, we lacked data on SGLT2is other than empagliflozin. Confirmation of the changes in glycemic levels and BW in patients with both type 2 diabetes and a history of major adverse cardiovascular events may need more clinical evidence. Although all of the patients in this study were counselled by the same team of diabetes educators, the effects of different lifestyle modifications on metabolic parameters may also be a limitation of this retrospective study. However, this study still demonstrated the metabolic impact of switching from a DPP4i to a well-known SGLT2i, and provided clinical experience for diabetic patients who have to make a decision between a SGLT2i and DPP4i. Further large-scale trials are warranted to study the long-term effects of replacing a DPP4i with a SGLT2i. Our data showed an improvement in metabolic parameters including BW, BMI, and/or HbA1c in 71% of our patients with type 2 diabetes whose DPP4i was replaced with the SGLT2i empagliflozin.
Footnotes
Funding: None.
CONFLICT OF INTEREST: No any relationship or support from the manufacturer of empagliflozin or other agents.
REFERENCES
- 1.Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes. JAMA. 2018;319:1580–91. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, Eynatten MV, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S73–85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–93. doi: 10.2337/dc14-2364. [DOI] [PubMed] [Google Scholar]
- 6.Muhammad AG. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes? Diabetes Care. 2015;38:373–5. doi: 10.2337/dc14-2517. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–83. doi: 10.2337/dc14-1142. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes. 2013;62:3324–8. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucoselowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 11.Enigk U, Breitfeld J, Schleinitz D, Dietrich K, Halbritter J, Fischer-Rosinsky A, et al. Role of genetic variation in the human sodium–glucose cotransporter 2 gene (SGLT2) in glucose homeostasis. Pharmacogenomics. 2011;12:1119–26. doi: 10.2217/pgs.11.69. [DOI] [PubMed] [Google Scholar]
- 12.Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, et al. SGLT2 expression is increased in human diabetic nephropathy: SGLT2 inhibition decreases renal lipid accumulation, inflammation and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292:5335–48. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]