Abstract
Inhibitors of the epidermal growth factor receptor (EGFR) are important treatment options for non–small cell lung cancer (NSCLC) patients with activating EGFR mutations. Erlotinib, gefitinib, afatinib, and osimertinib are approved for use in NSCLC patients, and several other agents are in clinical development. The objectives of this article are to review the pharmacokinetic and known drug interaction data for EGFR tyrosine kinase inhibitors (TKIs) available for use in NSCLC patients, as well as adverse events (AEs) commonly observed with EGFR-TKI treatment, and to discuss relevant management strategies. The importance of this information for patient care is explored from the perspective of advanced practitioners. Pharmacokinetic, drug-interaction, and safety data are included for EGFR inhibitors approved for NSCLC (erlotinib, gefitinib, afatinib, and osimertinib). Relevant dose modifications and AE management strategies are also reviewed. The interdisciplinary health-care team plays an essential role in patient education, care planning, and medication administration. As such, it is essential that advanced practitioners understand the safety profiles and the potential for drug interactions with EGFR TKIs to ensure patients achieve the maximum benefit from these agents.
The identification of activating mutations in the epidermal growth factor receptor (EGFR) has expanded treatment options for non–small cell lung cancer (NSCLC), where the presence of these mutations can sensitize tumors to EGFR inhibitors (Rosell et al., 2010). For patients whose tumors have sensitizing EGFR mutations, EGFR tyrosine kinase inhibitors (TKIs) are important components of the NSCLC treatment landscape. Four EGFR TKIs are approved by the US Food and Drug Administration (FDA) for use in NSCLC patients (erlotinib [Tarceva], gefitinib [Iressa], afatinib [Gilotrif], and osimertinib [Tagrisso]), and several others are in development. A thorough understanding of the safety profiles and drug interactions of EGFR TKIs is critical for advanced practitioners, who have a key role in educating patients on their safe and effective use. Here, we review relevant pharmacokinetic (PK) data and known drug interactions for each of the FDA-approved EGFR TKIs. We also summarize the most common EGFR-TKI-associated adverse events (AEs) and discuss management strategies, highlighting the role of advanced practitioners in safely managing EGFR-TKI use to ensure maximum patient benefit.
APPROVED EGFR TKIS
Erlotinib
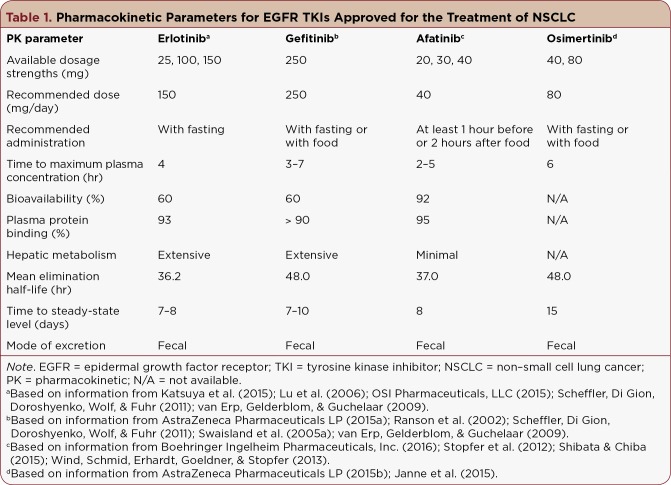
Erlotinib is an oral, reversible inhibitor of wild-type and mutant EGFR (Figure 1) indicated for the first-line treatment of metastatic NSCLC harboring deletion 19 (del19) or exon 21 (L858R) substitution EGFR mutations (OSI Pharmaceuticals, LLC, 2015). Erlotinib is also indicated for the treatment of locally advanced NSCLC after chemotherapy failure and for maintenance treatment of locally advanced or metastatic NSCLC that has not progressed after 4 cycles of platinum-based therapy (OSI Pharmaceuticals, LLC, 2015). The recommended erlotinib dose is 150 mg/day on an empty stomach, as PK studies have demonstrated that bioavailability is increased with food (Katsuya et al., 2015; OSI Pharmaceuticals, LLC, 2015). Additional PK analyses (Table 1) have shown that erlotinib is ~60% bioavailable, has a long half-life (> 36 hours), and is metabolized primarily by cytochrome P450 (CYP) enzymes, particularly CYP3A4, in the liver (Lu et al., 2006; OSI Pharmaceuticals, LLC, 2015).
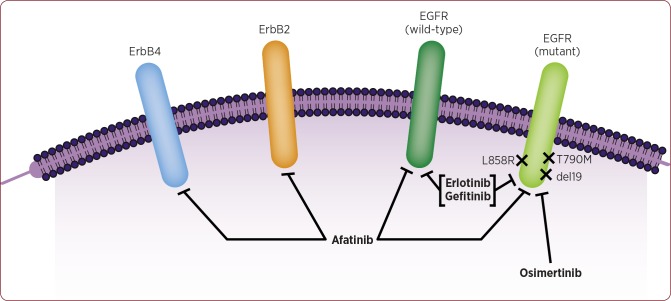
Figure 1.
Mechanisms of action of approved EGFR TKIs for NSCLC. Erlotinib and gefitinib are reversible EGFR inhibitors that bind to both wild-type and mutant EGFR, including L858R and del19 forms. In contrast, afatinib irreversibly binds to wild-type and mutant EGFR, as well as to the ErbB family members ErbB2 and ErbB4. The recently approved, mutant-specific, EGFR inhibitor osimertinib binds preferentially to mutant forms of the receptor, particularly T790M. EGFR = epidermal growth factor receptor; TKI = tyrosine kinase inhibitor; NSCLC = non–small cell lung cancer; L858R = exon 21; del19 = deletion 19.
Table 1.
Pharmacokinetic Parameters for EGFR TKIs Approved for the Treatment of NSCLC
Generally, no significant effects on PK were observed with age, gender, or weight differences (Lu et al., 2006; OSI Pharmaceuticals, LLC, 2015), although one study (N = 55) demonstrated lower erlotinib exposure in African-American NSCLC patients (Phelps et al., 2014). Patients with mild or moderate hepatic impairment had similar PK as patients with normal liver function; thus, erlotinib dose modifications are not recommended for impaired hepatic function, but patients should be monitored closely (O’Bryant et al., 2012). Hepatotoxicity can occur with erlotinib, and patients with baseline hepatic impairment have increased risk. Periodic liver testing should be performed, and erlotinib should be withheld for total bilirubin levels greater than three times the upper limit of normal or transaminases greater than five times the upper limit of normal. No studies have been conducted in patients with renal failure, although a case study reported that erlotinib was tolerated in three NSCLC patients with chronic renal failure (Gridelli, Maione, Galetta, & Rossi, 2007). Accordingly, there are no dose modifications recommended for these patients (OSI Pharmaceuticals, LLC, 2015).
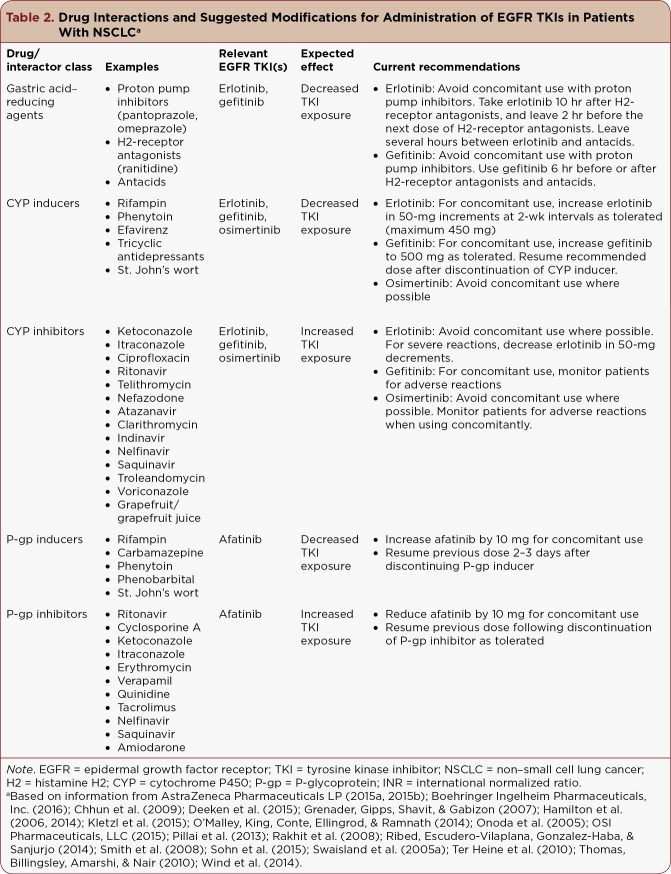
Erlotinib exposure may be affected by concomitant use of other drugs (Table 2). Drugs that decrease acid can decrease erlotinib exposure. Patients should avoid use of proton pump inhibitors, such as pantoprazole and omeprazole, while taking erlotinib due to potential effects on erlotinib concentration (Kletzl et al., 2015; OSI Pharmaceuticals, LLC, 2015; Ter Heine et al., 2010). When histamine H2-receptor antagonists (H2 antagonists), such as ranitidine, are used concomitantly, erlotinib should be given 10 hours after the H2 antagonist and ≥ 2 hours before the next antagonist dose (Kletzl et al., 2015; OSI Pharmaceuticals, LLC, 2015). Antacids should be administered several hours before or after erlotinib (Kletzl et al., 2015; OSI Pharmaceuticals, LLC, 2015). It is important that these recommendations are followed, as decreased erlotinib efficacy was reported in NSCLC patients using gastric acid–suppressing medications (Chu et al., 2015). Alternate means of managing gastroesophageal reflux disease include sucralfate and limiting gastric irritants and foods high in acidity.
Table 2.
Table 2. Drug Interactions and Suggested Modifications for Administration of EGFR TKIs in Patients With NSCLCa
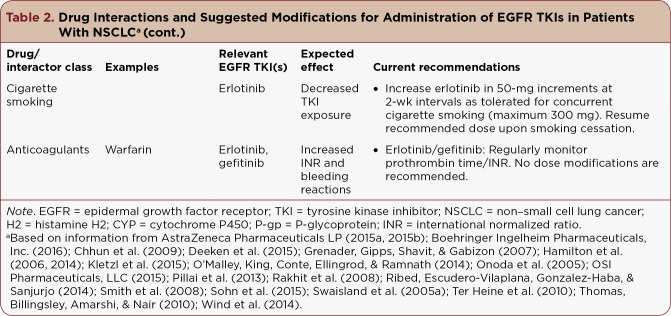
Table 2.
Table 2. Drug Interactions and Suggested Modifications for Administration of EGFR TKIs in Patients With NSCLCa (cont.)
Due to its extensive metabolism by CYP3A4, erlotinib may be affected by other drugs that enhance or inhibit CYP activity or undergo CYP-mediated metabolism (Table 2). Advanced practitioners should be aware if patients are taking CYP inhibitors, including certain antifungal, antibiotic, and antiretroviral medications, as these agents can increase erlotinib exposure (Deeken et al., 2015; Pillai et al., 2013; Rakhit et al., 2008). Additionally, grapefruit or grapefruit juice are CYP inhibitors and may increase erlotinib plasma concentrations (Smith et al., 2008). Likewise, patients should avoid concomitant use of erlotinib and CYP inducers, such as rifampin, phenytoin, and St. John’s wort, as their use may affect drug exposure (Deeken et al., 2015; Grenader, Gipps, Shavit, & Gabizon, 2007; Hamilton et al., 2014; Pillai et al., 2013). Use of erlotinib with dexamethasone, also a CYP inducer, resulted in a 0.6-fold decrease in erlotinib exposure and may require dose escalation up to the maximum of 450 mg daily (Deeken et al., 2015). For situations where concomitant use of erlotinib with CYP interactors is necessary, erlotinib dose should be decreased (CYP inhibitors) or increased (CYP inducers) in 50-mg decrements/increments at 2-week intervals as tolerated (Table 2).
Drug interactions may also occur between erlotinib and drugs cleared by uridine diphosphate–glucuronosyltransferases, organic cation transporters (OCTs), and organic anion transporters (OATs), potentially affecting exposure to drugs that are substrates of these transporters (e.g., metformin, hydroxyurea, methotrexate, certain statins; Johnston, Rawling, Chan, Zhou, & Murray, 2014; Liu, Ramirez, House, & Ratain, 2010; Minematsu & Giacomini, 2011).
Erlotinib exposure also can be decreased by cigarette smoking (Hamilton et al., 2006; O’Malley, King, Conte, Ellingrod, & Ramnath, 2014; Sohn et al., 2015). Current smokers, defined as smoking a minimum of 10 cigarettes per day for at least 1 year, required double the dose (300 mg) of erlotinib as nonsmokers (150 mg) to achieve therapeutic concentrations of the drug (Hamilton et al., 2006). Median survival times for patients receiving erlotinib are longest for those who never smoked compared with former smokers and current smokers (O’Malley et al., 2014). Erlotinib dose increases of 50 mg are advised at 2-week intervals to a maximum of 300 mg daily for concurrent cigarette smoking (OSI Pharmaceuticals, LLC, 2015). The interdisciplinary team of health-care providers can be instrumental in smoking cessation education; once cigarette smoking has ceased, the recommended erlotinib dose (100 or 150 mg daily) can be immediately resumed (Table 2).
Adverse interactions between erlotinib and coumarin-based anticoagulants, such as warfarin, have been observed, including increased international normalized ratios (INRs) and bleeding reactions (Thomas, Billingsley, Amarshi, & Nair, 2010). Patients using warfarin should be monitored closely for changes in INR while using erlotinib (Table 2).
Gefitinib
Gefitinib is another reversible inhibitor of wild-type and mutant EGFR (Figure 1) indicated for the first-line treatment of patients with metastatic NSCLC whose tumors have del19 or L858R EGFR mutations (AstraZeneca Pharmaceuticals LP, 2015a). Gefitinib should be taken orally at a dose of 250 mg/day with or without food (AstraZeneca Pharmaceuticals LP, 2015a). The PK profile of gefitinib is similar to that of erlotinib, but gefitinib has a longer half-life of 48 hours (Ranson et al., 2002; Table 1); gefitinib availability is not significantly influenced by food (Swaisland et al., 2005a). Patient age, weight, gender, or ethnicity do not have any apparent effect on gefitinib PK (AstraZeneca Pharmaceuticals LP, 2015a; Li et al., 2006). Gefitinib PK were shown to be impacted by hepatic impairment due to cirrhosis but not liver metastases (Horak et al., 2011), and patients with moderate to severe hepatic impairment should be monitored for an increased risk of adverse reactions (AstraZeneca Pharmaceuticals LP, 2015a). Dose modifications are recommended based on results of periodic liver testing: for grade 2 or higher transaminase elevations, gefitinib should be withheld, and for severe hepatic impairment, treatment should be discontinued. No studies have been conducted to evaluate gefitinib PK in patients with renal impairment.
Due to their similar structure and PK profile, gefitinib and erlotinib share similar drug-interaction potential. Like erlotinib, gefitinib metabolism occurs mainly through CYP enzymes in the liver, including CYP3A4 and CYP2D6 (Li, Zhao, He, Hidalgo, & Baker, 2007). CYP inhibitors (e.g., itraconazole) may increase gefitinib plasma levels, and patients taking gefitinib with CYP inhibitors should be monitored closely (Swaisland et al., 2005b). For concomitant use with strong CYP inducers, such as phenytoin and rifampin, which can decrease gefitinib levels (Chhun et al., 2009; Swaisland et al., 2005b), gefitinib daily dose can be increased from 250 mg to 500 mg. Patients should resume the recommended dose of 250 mg after discontinuation of the CYP inducer (AstraZeneca Pharmaceuticals LP, 2015a). Gefitinib can inhibit the activity of drug transporters, such as OCTs (metformin, oxaliplatin) and OATs (montelukast, certain statins, sulfated estrogens, thyroxine), potentially altering the exposure of coadministered medications that are substrates for these transporters (Johnston et al., 2014; Minematsu & Giacomini, 2011).
Like erlotinib, acid-reducing medications may decrease gefitinib exposure. Gefitinib should not be used with proton pump inhibitors and, when concomitant use is necessary, dosing of gefitinib and the proton pump inhibitor should be spaced by 12 hours (AstraZeneca Pharmaceuticals LP, 2015a). When treatment with antacids or H2 antagonists is required, gefitinib should be taken 6 hours before or after the acid-reducing agent (AstraZeneca Pharmaceuticals LP, 2015a). Similar to erlotinib, gefitinib use has been linked to adverse reactions, including hemorrhage and INR elevations, in patients using warfarin or other coumarin derivatives (Onoda et al., 2005; Ribed, Escudero-Vilaplana, Gonzalez-Haba, & Sanjurjo, 2014), and therefore these patients should be monitored closely for potential reactions.
Afatinib
Afatinib is an oral, irreversible ErbB family blocker that targets mutant and wild-type EGFR/ErbB1, human epidermal growth factor receptor (HER) 2/ErbB2, and HER4/ErbB4, which results in the inhibition of HER3/ErbB3 phosphorylation (Li et al., 2008; Solca et al., 2012; Figure 1). It is currently indicated for the first-line treatment of patients with metastatic NSCLC whose tumors harbor del19 or L858R EGFR mutations and for metastatic squamous NSCLC progressing after platinum therapy (Boehringer Ingelheim Pharmaceuticals, 2016). The recommended afatinib dose is 40 mg once daily, taken ≥ 1 hour before or 2 hours after a meal (Boehringer Ingelheim Pharmaceuticals, 2016).
The PK profile of afatinib is presented in Table 1 (Wind, Schmid, Erhardt, Goeldner, & Stopfer, 2013). Weight, age, gender, race, and mild or moderate hepatic impairment do not have a significant effect on afatinib PK (Freiwald et al., 2014; Schnell et al., 2014). Severe renal impairment has been shown to increase afatinib exposure; thus, according to the US prescribing information, these patients should receive a starting dose of 30 mg afatinib (Boehringer Ingelheim Pharmaceuticals, 2016). Afatinib is minimally metabolized by hepatic enzymes (Shibata & Chiba, 2015; Stopfer et al., 2012); therefore, CYP-interacting agents are unlikely to impact afatinib PK. No dose modifications are recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment (Boehringer Ingelheim Pharmaceuticals, 2016). No studies have examined the effects of afatinib on patients with severe hepatic impairment (Child-Pugh C), although these patients should be closely monitored and have their dose adjusted for tolerability (Boehringer Ingelheim Pharmaceuticals, 2016). If periodic liver testing shows worsening of liver function, gefitinib should be withheld; for those who develop severe hepatic impairment, treatment should be discontinued.
Afatinib is a substrate and inhibitor of the P-glycoprotein (P-gp) drug transporter, and inhibitors and enhancers of P-gp activity may impact afatinib exposure. One study demonstrated that concomitant administration of afatinib with ritonavir, a P-gp inhibitor, increased afatinib exposure, although this increase was minimized by spacing out the medications by 6 hours (Wind et al., 2014). When concomitant use of afatinib with P-gp inhibitors is required, the afatinib dose should be reduced by 10 mg, as tolerated (Boehringer Ingelheim Pharmaceuticals, 2016; Table 2). Additionally, coadministration of afatinib with the P-gp inducer rifampin decreased afatinib plasma concentrations (Wind et al., 2014). Patients taking afatinib with a P-gp inducer should increase their afatinib dose by 10 mg (Boehringer Ingelheim Pharmaceuticals, 2016; Table 2). There is also evidence for an inhibitory effect of afatinib on OATs, although this effect is less potent than that observed with erlotinib or gefitinib (Johnston et al., 2014).
Osimertinib
Osimertinib was approved for metastatic NSCLC patients whose tumors harbor the T790M EGFR mutation following progression on EGFR-TKI therapy in 2015 (AstraZeneca Pharmaceuticals LP, 2015b; Kuiper et al., 2014). Osimertinib inhibits mutant forms of EGFR, including T790M, del19, and L858R, with minimal inhibition of wild-type EGFR protein (Cross et al., 2014; Figure 1). The recommended osimertinib dose is 80 mg once daily, taken orally with or without food (AstraZeneca Pharmaceuticals LP, 2015b).
There are limited PK and drug interaction data available for osimertinib, as its approval was recent and studies are ongoing. Pharmacokinetic data suggest a long half-life and relatively longer time to maximum plasma concentration compared with other approved EGFR TKIs (AstraZeneca Pharmaceuticals LP, 2015b; Jänne et al., 2015; Table 1). Based on its metabolism by CYP3A enzymes, osimertinib exposure may be impacted by CYP enzyme inducers or inhibitors, although extensive studies have not yet been performed (AstraZeneca Pharmaceuticals LP, 2015b). Patients taking osimertinib should avoid concomitant use of strong CYP inhibitors or inducers, and patients should be closely monitored when coadministration is necessary (AstraZeneca Pharmaceuticals LP, 2015b; Table 2).
SAFETY CONSIDERATIONS WITH EGFR-TKI THERAPY
EGFR TKIs have been associated with specific AEs, including diarrhea, mucositis, rash, and paronychia (Melosky & Hirsh, 2014). Because the incidence of these AEs can be high with EGFR-TKI use, dose optimization (where applicable) can mitigate their effects. Advanced practitioners can help anticipate and effectively manage these AEs so that patients can remain on therapy and maximize the clinical benefits of EGFR TKIs.
Diarrhea
Diarrhea is one of the most commonly observed AEs with EGFR TKIs (Melosky & Hirsh, 2014) and is graded from 1 to 5 based on frequency and severity (U.S. Department of Health and Human Services, National Institutes of Health, & National Cancer Institute, 2010). Grade 1 is defined as an increase of less than 4 stools per day over baseline, grade 2 as 4 to 6 stools per day over baseline, grade 3 as more than 7 stools per day over baseline or incontinence, grade 4 includes life-threatening consequences, and grade 5 is death (Cancer.net, 2017). In clinical trials supporting the approval of EGFR TKIs, incidence of any grade diarrhea ranged from 30.8% with gefitinib to 95.2% with afatinib (Douillard et al., 2014; Jänne et al., 2015; Rosell et al., 2012; Sequist et al., 2013). The incidence of grade ≥ 3 diarrhea was ≤ 5% for erlotinib, gefitinib, and osimertinib and ranged from 5.4% to 14.4% with afatinib when starting at 40 mg (Douillard et al., 2014; Jänne et al., 2015; Rosell et al., 2012; Sequist et al., 2013; Wu et al., 2014). Management strategies for diarrhea include dietary modifications such as avoiding foods that are difficult to digest (e.g., broccoli, cabbage) and following the bananas, rice, applesauce, and toast (BRAT) diet until symptoms begin to resolve (Melosky & Hirsh, 2014). Loperamide can also be used to treat diarrhea; 4 mg should be given after the first episode of diarrhea, and 2 mg can be administered every 2 to 4 hours until diarrhea stops, typically not exceeding 16 mg/day for prescription use (Drugs.com, 2016; Hirsh, 2011; Melosky & Hirsh, 2014; Sipples, Andan, & Eaby-Sandy, 2015). For more severe diarrhea, diphenoxylate and atropine or octreotide can be considered. The EGFR TKI should be discontinued until severe diarrhea resolves, at which point the EGFR TKI can often be reintroduced (Hirsh, 2011; Melosky & Hirsh, 2014).
Rash
Dermatologic AEs, such as rash and acne, are also commonly observed with EGFR TKIs, and patient education should be provided at the initiation of therapy (Figure 2). Reported rates for these AEs in clinical trials were between 40.0% and 89.1% for rash of any grade and up to 16.2% for grade ≥ 3 rash (Douillard et al., 2014; Jänne et al., 2015; Rosell et al., 2012; Sequist et al., 2013). Rashes typically present in stages, with early erythema and edema observed in weeks 1 to 2 of treatment. In week 2, papulopustular eruptions can occur in skin with a high density of sebaceous glands, followed by crusting of the skin in week 4 (Lacouture et al., 2011). Over the next 4 to 6 weeks, patients can experience dry skin and erythema (Lacouture & Melosky, 2007). Based on data from randomized controlled trials, preventive therapy is recommended using 1% hydrocortisone, alcohol-free emollient creams, sunscreen, and doxycycline 100 mg twice daily (Hirsh, 2011; Lacouture et al., 2011; Melosky & Hirsh, 2014). Additional treatments for EGFR-TKI-associated rash include medium- to high-potency topical corticosteroids and isotretinoin at doses lower than those used for acne (Lacouture et al., 2011). EGFR-TKI treatment should be discontinued for severe reactions and can often be reintroduced at a modified dose upon resolution of the rash (Hirsh, 2011; Melosky & Hirsh, 2014).
Figure 2.
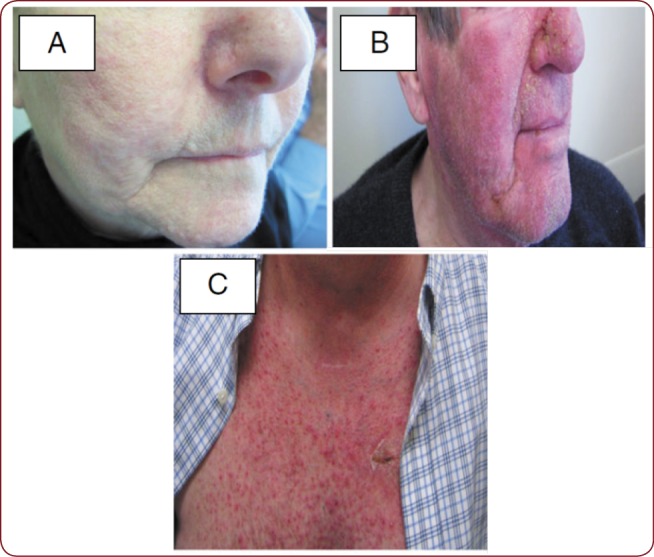
Mild papulopustular (acneiform) rash (A), and papulopustular (acneiform) rash (B and C).
Stomatitis/Mucositis
Stomatitis (inflammation of the mouth) and mucositis (inflammation of the gastrointestinal tract) have been observed with EGFR-TKIs (Melosky & Hirsh, 2014; Soria et al., 2015). In the LUX-Lung 3 trial, stomatitis/mucositis was seen in 72.1% of patients receiving afatinib, with 8.7% experiencing grade ≥ 3 stomatitis/mucositis (Sequist et al., 2013). Grades 1 and 2 mucosal inflammation were also reported in 12% of patients receiving osimertinib, 18% receiving erlotinib (1% grade ≥ 3), and 7% receiving gefitinib (0.3% grade ≥ 3) (AstraZeneca Pharmaceuticals LP, 2015a; Jänne et al., 2015; OSI Pharmaceuticals, LLC, 2015).
Suggested management strategies for stomatitis/mucositis include maintaining good oral hygiene with alcohol-free products, triamcinolone acetonide, clobetasol, erythromycin, viscous lidocaine, and magic mouthwash (Melosky & Hirsh, 2014; Sipples et al., 2015). Analgesics may also be temporarily required for pain management associated with stomatitis/mucositis (Harris, 2006). As with other EGFR-TKI-associated AEs, it is suggested that treatment with the EGFR TKI be paused until severe cases of stomatitis/mucositis are resolved or improved. Following improvement of the AE, the EGFR TKI can be reintroduced at a lower dose (Melosky & Hirsh, 2014).
Paronychia
Another AE seen more frequently with afatinib and osimertinib is paronychia, an infection of the tissue where the skin meets the nail, which can often be tender and painful. Paronychia was reported in 56.8% of patients receiving afatinib, with grade ≥ 3 paronychia observed in 11.4% (Sequist et al., 2013). Paronychia was reported in 17% of patients taking osimertinib, with less than 0.5% of cases reported as grade ≥ 3 (Jänne et al., 2015). Recommended strategies for managing paronychia include the use of topical clobetasol or antibiotics/antiseptics, vinegar soaks, silver nitrate, and nail avulsion for severe cases (Melosky & Hirsh, 2014; Sipples et al., 2015).
Dose-Modification Protocols for Adverse Events
Dose modifications are recommended for patients taking EGFR TKIs who experience grade ≥ 3 AEs, particularly diarrhea and rash. These modifications generally involve withholding the TKI until AE improvement, at which time the TKI can be reintroduced at a lower dose (AstraZeneca Pharmaceuticals LP, 2015a, 2015b; OSI Pharmaceuticals, LLC, 2015). A dose-optimization scheme has been recommended for patients taking afatinib who experience drug-related grade 3 and certain prolonged grade 2 AEs (diarrhea persisting 2 or more consecutive days despite antidiarrheal medication, cutaneous reactions lasting more than 7 days or that is intolerable, and renal impairment; Sequist et al., 2013; Sipples et al., 2015; Yang et al., 2016). Patients should discontinue afatinib temporarily, and appropriate supportive care measures should be implemented. Upon AE improvement, afatinib can be reintroduced at a dose 10 mg lower than the previous dose. Importantly, progression-free survival was similar for patients who dose-reduced on afatinib vs. those who did not reduce their dose, suggesting that dose modification can allow patients to manage AEs without compromising afatinib efficacy (Yang et al., 2016).
DISCUSSION AND HEALTH-CARE TEAM PERSPECTIVES
EGFR TKIs are a key component of the NSCLC treatment landscape, and familiarity with these agents is critical for achieving maximum treatment benefits for patients. Health-care team members are responsible for understanding the drug profile of medications they are prescribing or administering, are key informants to patients, and are crucial to ensuring patient safety and adherence. Comprehensive assessment of patients taking EGFR TKIs helps to identify and quantify adverse reactions and contributes to increased medication compliance. Furthermore, advanced practitioners are pivotal to the interdisciplinary team, help to quantify the impact of cancer treatments on patients’ quality of life, and modify treatment care plans as necessary to meet the needs of their patients and their treatment outcomes.
The health-care team provides patient education about EGFR TKIs and can encourage open communication with patients about concurrent medication use and onset of side effects. Providing education can help keep patients out of the hospital due to uncontrolled AEs and can reduce health-care spending by preventing avoidable hospitalizations (Brooks et al., 2014; Newcomer, 2014). Effective counseling and education empowers the patient to deal with EGFR-TKI-associated side effects, and advanced practitioners are an important resource to patients and families on how to manage and mitigate EGFR-TKI-associated toxicities. Continued understanding of the anticipated drug-interaction and side-effect profiles of targeted therapies is the key to keeping patients on the proper doses of these medications to maximize benefit.
Acknowledgment
The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Lauren Fink, PhD, of MedErgy, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Footnotes
Ms. Kucharczuk has no conflicts of interest to disclose. Dr. Ganetsky has served on a speakers bureau for Amgen and advisory boards for Astellas and Genentech. Dr. Vozniak has served on an advisory board for Eisai and is an employee of Merck & Co., Inc. (affiliation change after article submission).
References
- 1.AstraZeneca Pharmaceuticals LP. Iressa (gefitinib) tablets for oral use package insert. . 2015a Retrieved from http://www.azpicentral.com/pi.html?product=iressa&country=us&popup=no.
- 2.AstraZeneca Pharmaceuticals LP. Tagrisso (osimertinib) tablet for oral use package insert. 2015b Retrieved from http://www.azpicentral.com/pi.html?product=tagrisso&country=us&popup=no.
- 3.Boehringer Ingelheim Pharmaceuticals, Inc. Gilotrif (afatinib) package insert. 2016 Retrieved from http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Gilotrif/Gilotrif.pdf?DMW_FORMAT=pdf.
- 4.Brooks Gabriel A, Abrams Thomas A, Meyerhardt Jeffrey A, Enzinger Peter C, Sommer Karen, Dalby Carole K, Uno Hajime, Jacobson Joseph O, Fuchs Charles S, Schrag Deborah. Identification of potentially avoidable hospitalizations in patients with GI cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:496–503. doi: 10.1200/JCO.2013.52.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer.net. Diarrhea. 2017 Retrieved from http://www.cancer.net/navigating-cancer-care/side-effects/diarrhea.
- 6.Chhun Stephanie, Verstuyft Celine, Rizzo-Padoin Nathalie, Simoneau Guy, Becquemont Laurent, Peretti Ilana, Swaisland Alan, Wortelboer Robert, Bergmann Jean Francois, Mouly Stephane. Gefitinib-phenytoin interaction is not correlated with the C-erythromycin breath test in healthy male volunteers. British journal of clinical pharmacology. 2009;68:226–237. doi: 10.1111/j.1365-2125.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu Michael P, Ghosh Sunita, Chambers Carole R, Basappa Naveen, Butts Charles A, Chu Quincy, Fenton David, Joy Anil A, Sangha Randeep, Smylie Michael, Sawyer Michael B. Gastric Acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clinical lung cancer. 2015;16:33–39. doi: 10.1016/j.cllc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Cross Darren A E, Ashton Susan E, Ghiorghiu Serban, Eberlein Cath, Nebhan Caroline A, Spitzler Paula J, Orme Jonathon P, Finlay M Raymond V, Ward Richard A, Mellor Martine J, Hughes Gareth, Rahi Amar, Jacobs Vivien N, Red Brewer Monica, Ichihara Eiki, Sun Jing, Jin Hailing, Ballard Peter, Al-Kadhimi Katherine, Rowlinson Rachel, Klinowska Teresa, Richmond Graham H P, Cantarini Mireille, Kim Dong-Wan, Ranson Malcolm R, Pao William. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer discovery. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeken John F, Beumer Jan H, Anders Nicole M, Wanjiku Teresia, Rusnak Milan, Rudek Michelle A. Preclinical assessment of the interactions between the antiretroviral drugs, ritonavir and efavirenz, and the tyrosine kinase inhibitor erlotinib. Cancer chemotherapy and pharmacology. 2015;76:813–819. doi: 10.1007/s00280-015-2856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douillard J-Y, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, Milenkova T. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. British journal of cancer. 2014;110:55–62. doi: 10.1038/bjc.2013.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drugs.com. Loperamide (loperamide hydrochloride) package insert. . 2016 Retrieved from https://www.drugs.com/pro/loperamide.html.
- 12.Freiwald Matthias, Schmid Ulrike, Fleury Angele, Wind Sven, Stopfer Peter, Staab Alexander. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer chemotherapy and pharmacology. 2014;73:759–770. doi: 10.1007/s00280-014-2403-2. [DOI] [PubMed] [Google Scholar]
- 13.Grenader Tal, Gipps Maya, Shavit Linda, Gabizon Alberto. Significant drug interaction: phenytoin toxicity due to erlotinib. Lung cancer (Amsterdam, Netherlands) 2007;57:404–406. doi: 10.1016/j.lungcan.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Gridelli Cesare, Maione Paolo, Galetta Domenico, Rossi Antonio. Safety profile of erlotinib in patients with advanced non-small cell lung cancer with chronic renal failure. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007;2:96–98. doi: 10.1097/JTO.0b013e31802bffb0. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton Marta, Wolf Julie L, Drolet Daniel W, Fettner Scott H, Rakhit Ashok K, Witt Karsten, Lum Bert L. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer chemotherapy and pharmacology. 2014;73:613–621. doi: 10.1007/s00280-014-2390-3. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton Marta, Wolf Julie L, Rusk Jason, Beard Shannon E, Clark Gary M, Witt Karsten, Cagnoni Pablo J. Effects of smoking on the pharmacokinetics of erlotinib. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 17.Harris Debra J. Cancer treatment-induced mucositis pain: strategies for assessment and management. Therapeutics and clinical risk management. 2006;2:251–258. doi: 10.2147/tcrm.2006.2.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh V. Managing treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Current oncology (Toronto, Ont.) 2011;18:126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horak Jiri, White Jeff, Harris Adrian L, Verrill Mark, Carmichael James, Holt Alison, Cantarini Mireille, Macpherson Merran, Swaisland Alan, Swaisland Helen, Twelves Chris. The effect of different etiologies of hepatic impairment on the pharmacokinetics of gefitinib. Cancer chemotherapy and pharmacology. 2011;68:1485–1495. doi: 10.1007/s00280-011-1611-2. [DOI] [PubMed] [Google Scholar]
- 20.Jänne Pasi A, Yang James Chih-Hsin, Kim Dong-Wan, Planchard David, Ohe Yuichiro, Ramalingam Suresh S, Ahn Myung-Ju, Kim Sang-We, Su Wu-Chou, Horn Leora, Haggstrom Daniel, Felip Enriqueta, Kim Joo-Hang, Frewer Paul, Cantarini Mireille, Brown Kathryn H, Dickinson Paul A, Ghiorghiu Serban, Ranson Malcolm. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. The New England journal of medicine. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 21.Johnston Rosie A, Rawling Tristan, Chan Ting, Zhou Fanfan, Murray Michael. Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug metabolism and disposition: the biological fate of chemicals. 2014;42:1851–1857. doi: 10.1124/dmd.114.059097. [DOI] [PubMed] [Google Scholar]
- 22.Katsuya Yuki, Fujiwara Yutaka, Sunami Kuniko, Utsumi Hirofumi, Goto Yasushi, Kanda Shintaro, Horinouchi Hidehito, Nokihara Hiroshi, Yamamoto Noboru, Takashima Yuki, Osawa Satoko, Ohe Yuichiro, Tamura Tomohide, Hamada Akinobu. Comparison of the pharmacokinetics of erlotinib administered in complete fasting and 2 h after a meal in patients with lung cancer. Cancer chemotherapy and pharmacology. 2015;76:125–132. doi: 10.1007/s00280-015-2778-8. [DOI] [PubMed] [Google Scholar]
- 23.Kletzl Heidemarie, Giraudon Mylene, Ducray Patricia Sanwald, Abt Markus, Hamilton Marta, Lum Bert L. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anti-cancer drugs. 2015;26:565–572. doi: 10.1097/CAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper J L, Heideman D A M, Thunnissen E, Paul M A, van Wijk A W, Postmus P E, Smit E F. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung cancer (Amsterdam, Netherlands) 2014;85:19–24. doi: 10.1016/j.lungcan.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Lacouture Mario E, Anadkat Milan J, Bensadoun René-Jean, Bryce Jane, Chan Alexandre, Epstein Joel B, Eaby-Sandy Beth, Murphy Barbara A. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1079–1095. doi: 10.1007/s00520-011-1197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacouture M E, Melosky B L. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: A dermatology-oncology perspective. Skin Therapy Letter. 2007;12(6):1–5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17762902. [PubMed] [Google Scholar]
- 27.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac L R, Padera R F, Shapiro G I, Baum A, Himmelsbach F, Rettig W J, Meyerson M, Solca F, Greulich H, Wong K-K. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Jing, Karlsson Mats O, Brahmer Julie, Spitz Avery, Zhao Ming, Hidalgo Manuel, Baker Sharyn D. CYP3A phenotyping approach to predict systemic exposure to EGFR tyrosine kinase inhibitors. Journal of the National Cancer Institute. 2006;98:1714–1723. doi: 10.1093/jnci/djj466. [DOI] [PubMed] [Google Scholar]
- 29.Li Jing, Zhao Ming, He Ping, Hidalgo Manuel, Baker Sharyn D. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:3731–3737. doi: 10.1158/1078-0432.CCR-07-0088. [DOI] [PubMed] [Google Scholar]
- 30.Liu Yong, Ramírez Jacqueline, House Larry, Ratain Mark J. Comparison of the drug-drug interactions potential of erlotinib and gefitinib via inhibition of UDP-glucuronosyltransferases. Drug metabolism and disposition: the biological fate of chemicals. 2010;38:32–39. doi: 10.1124/dmd.109.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Jian-Feng, Eppler Steve M, Wolf Julie, Hamilton Marta, Rakhit Ashok, Bruno Rene, Lum Bert L. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clinical pharmacology and therapeutics. 2006;80:136–145. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Frontiers in Oncology. 2014;4:238. doi: 10.3389/fonc.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minematsu Tsuyoshi, Giacomini Kathleen M. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Molecular cancer therapeutics. 2011;10:531–539. doi: 10.1158/1535-7163.MCT-10-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newcomer Lee N. Innovative payment models and measurement for cancer therapy. Journal of oncology practice. 2014;10:187–189. doi: 10.1200/JOP.2014.001378. [DOI] [PubMed] [Google Scholar]
- 35.O’Bryant Cindy L, Haluska Paul, Rosen Lee, Ramanathan Ramesh K, Venugopal Balaji, Leong Stephen, Boinpally Ramesh, Franke Amy, Witt Karsten, Evans Jeffry, Belani Chandra, Gail Eckhardt S, Ramalingam Suresh. An open-label study to describe pharmacokinetic parameters of erlotinib in patients with advanced solid tumors with adequate and moderately impaired hepatic function. Cancer chemotherapy and pharmacology. 2012;69:605–612. doi: 10.1007/s00280-011-1733-6. [DOI] [PubMed] [Google Scholar]
- 36.O’Malley Meaghan, King Amanda N, Conte Marisa, Ellingrod Vicki L, Ramnath Nithya. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:917–926. doi: 10.1097/JTO.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 37.Onoda Sayaka, Mitsufuji Hisashi, Yanase Nobuo, Ryuge Shinichiro, Kato Emi, Wada Mayumi, Ishii Kaori, Hagiri Shintaro, Yamamoto Michiko, Yokoba Masanori, Yanaihara Tomoko, Kuboto Masaru, Takada Nobukazu, Katagiri Masato, Abe Tadashi, Tanaka Naohiko, Kobayashi Hirosuke, Masuda Noriyuki. Drug interaction between gefitinib and warfarin. Japanese journal of clinical oncology. 2005;35:478–482. doi: 10.1093/jjco/hyi122. [DOI] [PubMed] [Google Scholar]
- 38.OSI Pharmaceuticals, LLC. Tarceva (erlotinib) tablets for oral use package insert. 2015 Retrieved from http://www.gene.com/gene/products/information/pdf/tarceva-prescribing.pdf.
- 39.Phelps M A, Stinchcombe T E, Blachly J S, Zhao W, Schaaf L J, Starrett S L, Wei L, Poi M, Wang D, Papp A, Aimiuwu J, Gao Y, Li J, Otterson G A, Hicks W J, Socinski M A, Villalona-Calero M A. Erlotinib in African Americans with advanced non-small cell lung cancer: a prospective randomized study with genetic and pharmacokinetic analyses. Clinical pharmacology and therapeutics. 2014;96:182–191. doi: 10.1038/clpt.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillai Venkateswaran C, Venkataramanan Raman, Parise Robert A, Christner Susan M, Gramignoli Roberto, Strom Stephen C, Rudek Michelle A, Beumer Jan H. Ritonavir and efavirenz significantly alter the metabolism of erlotinib--an observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:1843–1851. doi: 10.1124/dmd.113.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakhit Ashok, Pantze Michael P, Fettner Scott, Jones Hannah M, Charoin Jean-Eric, Riek Myriam, Lum Bert L, Hamilton Marta. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. European journal of clinical pharmacology. 2008;64:31–41. doi: 10.1007/s00228-007-0396-z. [DOI] [PubMed] [Google Scholar]
- 42.Ranson Malcolm, Hammond Lisa A, Ferry David, Kris Mark, Tullo Andrew, Murray Philip I, Miller Vince, Averbuch Steve, Ochs Judy, Morris Charles, Feyereislova Andrea, Swaisland Helen, Rowinsky Eric K. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 43.Ribed A, Escudero-Vilaplana V, Gonzalez-Haba E, Sanjurjo M. Increased INR after gefitinib and acenocoumarol co-administration. European review for medical and pharmacological sciences. 2014;18:1720–1722. [PubMed] [Google Scholar]
- 44.Rosell Rafael, Carcereny Enric, Gervais Radj, Vergnenegre Alain, Massuti Bartomeu, Felip Enriqueta, Palmero Ramon, Garcia-Gomez Ramon, Pallares Cinta, Sanchez Jose Miguel, Porta Rut, Cobo Manuel, Garrido Pilar, Longo Flavia, Moran Teresa, Insa Amelia, De Marinis Filippo, Corre Romain, Bover Isabel, Illiano Alfonso, Dansin Eric, de Castro Javier, Milella Michele, Reguart Noemi, Altavilla Giuseppe, Jimenez Ulpiano, Provencio Mariano, Moreno Miguel Angel, Terrasa Josefa, Muñoz-Langa Jose, Valdivia Javier, Isla Dolores, Domine Manuel, Molinier Olivier, Mazieres Julien, Baize Nathalie, Garcia-Campelo Rosario, Robinet Gilles, Rodriguez-Abreu Delvys, Lopez-Vivanco Guillermo, Gebbia Vittorio, Ferrera-Delgado Lioba, Bombaron Pierre, Bernabe Reyes, Bearz Alessandra, Artal Angel, Cortesi Enrico, Rolfo Christian, Sanchez-Ronco Maria, Drozdowskyj Ana, Queralt Cristina, de Aguirre Itziar, Ramirez Jose Luis, Sanchez Jose Javier, Molina Miguel Angel, Taron Miquel, Paz-Ares Luis. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet. Oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 45.Rosell Rafael, Morán Teresa, Carcereny Enric, Quiroga Vanessa, Molina Miguel Angel, Costa Carlota, Benlloch Susana, Tarón Miquel. Non-small-cell lung cancer harbouring mutations in the EGFR kinase domain. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010;12:75–80. doi: 10.1007/S12094-010-0473-0. [DOI] [PubMed] [Google Scholar]
- 46.Scheffler Matthias, Di Gion Paola, Doroshyenko Oxana, Wolf Jürgen, Fuhr Uwe. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on 4-anilinoquinazolines. Clinical pharmacokinetics. 2011;50:371–403. doi: 10.2165/11587020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Schnell David, Buschke Susanne, Fuchs Holger, Gansser Dietmar, Goeldner Rainer-Georg, Uttenreuther-Fischer Martina, Stopfer Peter, Wind Sven, Petersen-Sylla Marc, Halabi Atef, Koenen Rüdiger. Pharmacokinetics of afatinib in subjects with mild or moderate hepatic impairment. Cancer chemotherapy and pharmacology. 2014;74:267–275. doi: 10.1007/s00280-014-2484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequist Lecia V, Yang James Chih-Hsin, Yamamoto Nobuyuki, O'Byrne Kenneth, Hirsh Vera, Mok Tony, Geater Sarayut Lucien, Orlov Sergey, Tsai Chun-Ming, Boyer Michael, Su Wu-Chou, Bennouna Jaafar, Kato Terufumi, Gorbunova Vera, Lee Ki Hyeong, Shah Riyaz, Massey Dan, Zazulina Victoria, Shahidi Mehdi, Schuler Martin. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 49.Shibata Yoshihiro, Chiba Masato. The role of extrahepatic metabolism in the pharmacokinetics of the targeted covalent inhibitors afatinib, ibrutinib, and neratinib. Drug metabolism and disposition: the biological fate of chemicals. 2015;43:375–384. doi: 10.1124/dmd.114.061424. [DOI] [PubMed] [Google Scholar]
- 50.Sipples R, Andan C, Eaby-Sandy B. Practical management of adverse events associated with afatinib. Oncology Nursing Forum. 2015;42(2) [Google Scholar]
- 51.Smith N F, Baker S D, Gonzalez F J, Harris J W, Figg W D, Sparreboom A. Modulation of erlotinib pharmacokinetics in mice by a novel cytochrome P450 3A4 inhibitor, BAS 100. British journal of cancer. 2008;98:1630–1632. doi: 10.1038/sj.bjc.6604353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sohn Hyun Soon, Kim Hyunah, Song Im-Sook, Lim Eunjeong, Kwon Mihwa, Ha Ji-Hye, Kwon Jin-Won. Evidence supporting the need for considering the effects of smoking on drug disposition and effectiveness in medication practices: a systematic narrative review. International journal of clinical pharmacology and therapeutics. 2015;53:621–634. doi: 10.5414/CP202260. [DOI] [PubMed] [Google Scholar]
- 53.Solca Flavio, Dahl Goeran, Zoephel Andreas, Bader Gerd, Sanderson Michael, Klein Christian, Kraemer Oliver, Himmelsbach Frank, Haaksma Eric, Adolf Guenther R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. The Journal of pharmacology and experimental therapeutics. 2012;343:342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 54.Soria Jean-Charles, Felip Enriqueta, Cobo Manuel, Lu Shun, Syrigos Konstantinos, Lee Ki Hyeong, Göker Erdem, Georgoulias Vassilis, Li Wei, Isla Dolores, Guclu Salih Z, Morabito Alessandro, Min Young J, Ardizzoni Andrea, Gadgeel Shirish M, Wang Bushi, Chand Vikram K, Goss Glenwood D. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. The Lancet. Oncology. 2015;16:897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 55.Stopfer Peter, Marzin Kristell, Narjes Hans, Gansser Dietmar, Shahidi Mehdi, Uttereuther-Fischer Martina, Ebner Thomas. Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer chemotherapy and pharmacology. 2012;69:1051–1061. doi: 10.1007/s00280-011-1803-9. [DOI] [PubMed] [Google Scholar]
- 56.Swaisland Helen C, Ranson Malcolm, Smith Robert P, Leadbetter Joanna, Laight Alison, McKillop David, Wild Martin J. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clinical pharmacokinetics. 2005;44:1067–1081. doi: 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- 57.Swaisland Helen C, Smith Robert P, Laight Alison, Kerr David J, Ranson Malcolm, Wilder-Smith Clive H, Duvauchelle Thierry. Single-dose clinical pharmacokinetic studies of gefitinib. Clinical pharmacokinetics. 2005;44:1165–1177. doi: 10.2165/00003088-200544110-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ter Heine Rob, Fanggiday James C, Lankheet Nienke A G, Beijnen Jos H, Van Der Westerlaken Monique M L, Staaks Gerald H A, Malingré Mirte M. Erlotinib and pantoprazole: a relevant interaction or not? British journal of clinical pharmacology. 2010;70:908–911. doi: 10.1111/j.1365-2125.2010.03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas Kelly S, Billingsley Amanda, Amarshi Naseem, Nair Balagopalan A. Elevated international normalized ratio associated with concomitant warfarin and erlotinib. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2010;67:1426–1429. doi: 10.2146/ajhp090202. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Department of Health and Human Services, National Institutes of Health, & National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. 2010 Retrieved from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 61.van Erp Nielka P, Gelderblom Hans, Guchelaar Henk-Jan. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer treatment reviews. 2009;35:692–706. doi: 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Wind Sven, Giessmann Thomas, Jungnik Arvid, Brand Tobias, Marzin Kristell, Bertulis Julia, Hocke Julia, Gansser Dietmar, Stopfer Peter. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clinical drug investigation. 2014;34:173–182. doi: 10.1007/s40261-013-0161-2. [DOI] [PubMed] [Google Scholar]
- 63.Wind Sven, Schmid Marion, Erhardt Julia, Goeldner Rainer-Georg, Stopfer Peter. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clinical pharmacokinetics. 2013;52:1101–1109. doi: 10.1007/s40262-013-0091-4. [DOI] [PubMed] [Google Scholar]
- 64.Wu Yi-Long, Zhou Caicun, Hu Cheng-Ping, Feng Jifeng, Lu Shun, Huang Yunchao, Li Wei, Hou Mei, Shi Jian Hua, Lee Kye Young, Xu Chong-Rui, Massey Dan, Kim Miyoung, Shi Yang, Geater Sarayut L. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. The Lancet. Oncology. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 65.Yang J C-H, Sequist L V, Zhou C, Schuler M, Geater S L, Mok T, Hu C-P, Yamamoto N, Feng J, O'Byrne K, Lu S, Hirsh V, Huang Y, Sebastian M, Okamoto I, Dickgreber N, Shah R, Märten A, Massey D, Wind S, Wu Y-L. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27:2103–2110. doi: 10.1093/annonc/mdw322. [DOI] [PubMed] [Google Scholar]