Abstract
On September 7, 2017, three potentially autochthonous cases of chikungunya were notified in the Lazio region. An Outbreak investigation based on established surveillance system data and molecular analysis of viral variant(s) were conducted. Epidemiological analysis suggested the occurrence of 3 main foci of local transmission. The major focus involved 317 cases with epidemiological link with the area of Anzio. The other two foci occurred in Rome (80 cases) and Latina (8 cases). Cumulative incidence in Anzio and Latina were 331.4 and 7.13 per 100,000 residents, respectively. Cumulative incidences ranged from 1.4 to 14.3/100,000 residents in Rome. This is the first report of a chikungunya outbreak involving a densely populated urban area in a western country. The outbreak probably started in Anzio, spread by continuity to neighbouring villages, and then to the metropolitan area of Rome and to the Latina area favoured by the touristic nature of the Anzio area.
Introduction
Chikungunya virus (CHIKV) is an alphavirus associated with a mosquito-borne infection usually causing a systemic self-limiting disease with a wide range of clinical presentations. Chikungunya disease generally includes fever, moderate to severe arthralgia and often maculopapular exanthema. About 15% of CHIKV infections may be asymptomatic [1].
CHIKV was firstly described during an outbreak in southern Tanzania in 1952–53 [2]. Since then the virus was found as one of the main cause of mosquito-borne infection in tropical and subtropical regions where it caused epidemics involving millions of people [3,4]. Following the expansion of the geographical distribution of its mosquito vectors (mainly Aedes aegypti and Aedes albopictus), [5] CHIKV has spread beyond its original tropical locations (Africa and the Indian subcontinent) and, since recently, it has become an emerging issue in temperate regions of Northern Hemisphere. In particular small autochthonous outbreaks occurred, as the consequence of spill over from large ongoing transmission in tropical areas, in continental Europe including Italy in 2007, and in France in 2010, in 2014, and eventually in 201 [6–9].
On September 7, 2017, the Lazio Regional Service for Surveillance and Control of Infectious Diseases (SERESMI) reported to the Italian Ministry of Health (MoH) a cluster of three autochthonous cases of CHIKV infection in Anzio, a coastal town devoted to internal tourism, 1 hour driving from Rome.
Here we report the comprehensive results of the investigations of the largest CHIKV outbreak in a temperate climate with the involvement of a highly populated urban area.
Methods
Study design
Outbreak investigation based on an established surveillance system data and molecular analysis of viral variant(s).
Setting
Lazio is the second most populated Region of Italy (5,898,124 residents). The 48.72% (N = 2,873,486) of Lazio inhabitants live in the Urban area of Rome (which is divided in 15 administrative districts “municipi”; median inhabitants 180,896 IQR 155,201–231,037). All other people live in the remaining 346 municipalities (comuni), mainly towns (median inhabitants 2,619 IQR 1,096–8,094). Anzio (54,311 inhabitants) is a town located 62 Km southern of Rome on the coastline. Due to the proximity to Rome, many residential areas have been developed over the last 40 years to host commuters from Rome that spend the summer in their own beach house.
Since 2015, Lazio Regional Health Authority has implemented an integrated surveillance system for chikungunya, dengue and zika, based on the national plan for surveillance and control of arboviruses transmitted by Aedes mosquito.
Participants and case definition
Following the notification of the autochthonous cluster, the surveillance system was strengthen through the dissemination of a dedicated case definition in order to increase the sensitivity, promptly trace contacts and early identify the affected areas throughout the Region.
All people living in Lazio and matching one of the following definitions:
Suspected case: a person that either A) had a sudden onset of fever and joint pain without history of travel to an endemic country in the 15 days before symptoms’ onset; or B) reporting an epidemiological link with a probable/confirmed case. Epidemiological link was defined as being a relative, living in the same household or being a neighbor (living in radius of 200m from the case household) of a confirmed/probable case;
Person under investigation: any suspected case A) without an available serological and/or molecular test performed or B) with a unique determination of detectable anti-CHIKV IgG;
Probable case: any suspected case that tested positive for anti- CHIKV IgM on a single serum sample;
Confirmed case: any suspected case that: A) tested positive for CHIKV PCR or B) tested positive for anti- CHIKV IgM on a single serum sample confirmed by sero-neutralization or C) seroconverted from negative to positive or D) showed a fourfold increase of Ig titer in two subsequent samples taken at least 2 weeks apart;
Non case: a person that either: A) had undetectable IgM, IgG and PCR if tested ≤10 days since symptoms onset; B) had undetectable IgM and IgG if tested >10 days since symptoms onset.
An imported case was defined as a probable or confirmed case with a history of travel to an endemic country during the 15 days before the onset of symptoms.
Data and sample collection
Any subject responding to the definition of suspected case was notified to SERESMI within 24 hours and samples sent to the Regional Reference Laboratory. In Anzio, a door-to-door active case finding was implemented in the houses and apartments located inside a 200m radius from a probable or confirmed case. Any subject reporting fever and joint pain during the previous 5 months was notified as suspected case and tested. We collected data on patients’ age, sex, place of living, time of symptoms’ onset, history of travel within Italy or abroad during the 15 days before symptoms’ onset. Daily situational reports (epidemic curves, geolocalization) were provided to the MoH and the National Blood Center in order to promptly implement the related control measures.
Virology diagnostics
CHIKV diagnosis was based on the detection of the viral genome by real-time RT-PCR and virus-specific antibodies by serologic tests on serum or plasma samples. For the qualitative detection of CHIKV specific RNA a commercial real-time RT-PCR kit (RealStar Chikungunya RT-PCR Kit 2.0, Altona Diagnostics GmbH, Hamburg, Germany) was used. Serologic tests for the detection of CHIKV specific IgG and IgM were performed using indirect immune fluorescence assay (IFA) (Anti-Chikungunya Virus IIFT Euroimmun AG, Germany). Neutralization assay was performed according to Lindsey HS et al [10].
Molecular investigation
The molecular characterization of E1 was performed for confirmatory purpose on samples collected at various time points (from the first three notified cases, from three cases in mid-September, from two cases at mid-October) [11]. The complete genome sequencing of the isolate obtained from the acute serum sample of a patient living in Anzio, collected on September 11, 2017, was performed. A 11.604 nt long sequence was amplified in 23 overlapping RT-PCR amplicons and Sanger sequenced as described elsewhere (isolate CHIKV/ITA/Lazio-INMI1-2017, GenBank accession number: MG049915) [12]. The phylogenetic tree was built using Maximum-Likelihood method based on the full-length genome sequences of 46 isolates, including the CHIKV/ITA/Lazio-INMI1-2017 sequence, representing the 3 major described CHIKV lineages: ECSA (including the Indian Ocean lineage, n = 29), Asia-Caribbean (n = 12), and West Africa (n = 4). Evolutionary distances were computed using the General Time Reversible model (GTR). The alphavirus O’nyong’nyong was used as an outgroup. Bootstraps were generated using 1,000 replicates. In addition, the CHIKV/ITA/Lazio-INMI1-2017 sequence was compared to the reference sequence S27, the African prototype strain isolated in 1953 [13].
Statistical analysis
Statistical analyses were performed using STATA 13.1. Cumulative incidence, considering the residing population as at risk population, was used as measure of frequency of morbidity. Papulation census from the year 2016 was used (http://demo.istat.it/). Spatial distribution of cases (probable and confirmed) was graphically reported as cumulative incidence per 100,000 residents and plotted on a choropleth map using the 361 administrative area of Lazio (i.e. 346 towns of Lazio and the 15 municipia of Rome).
Ethics statement
According to REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). Informed consent was not obtained from the participants because of the public health emergency during an infectious disease outbreak. All data contained in the manuscript were obtained during the epidemiological investigation as an institutional duty of the Latium Regional Health Authority, in order to identify/contain the ongoing outbreak, to provide recommendations on control measures and to avert complications in infected subjects. The approval of the National Institute for Infectious Diseases Spallanzani’s Institutional Review Board was not required for the same reasons. Patients never underwent individual intervention for the purposes of this study but only according to their needs and clinical judgment. Data have been analyzed anonymously.
Results
Epidemiological investigation
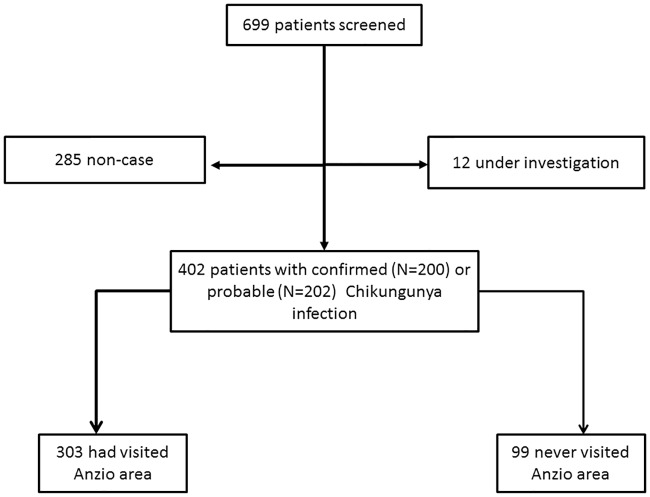
Between January 1, 2017 and January 31, 2018, 823 suspected cases were reported to the regional surveillance system. Of them, 699 (84.9%) responded to the case definition for autochthonous chikungunya suspected cases. Of these, 285 (40.8%) were non cases and 12 (1.7%) remained under investigation. Of the remaining 402 (57.5%) cases, 200 (49.8%) were classified as confirmed cases and 202 (50.2%) as probable cases. Fig 1 reports case definition flowchart.
Fig 1. Case definition flow-chart.
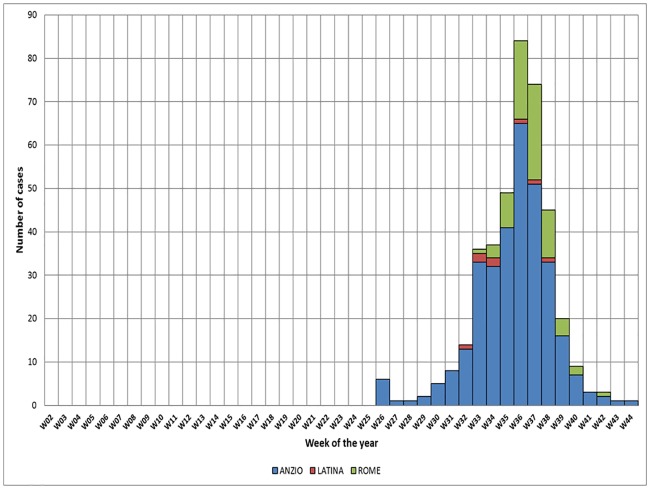
Fig 2 shows the epidemiological curve of cases by possible place of transmission. The first three notified cases had symptoms’ onset on August 5, 11 and 25 and occurred among people living in a residential area, made of single-family detached houses, in Anzio. Following the strengthen of the surveillance system and the door-to-door case finding, the epidemiological investigation suggested the occurrence of three mainfoci of local transmission. The largest focus, involving 317 persons with a direct link with the town of Anzio and its surrounding area, occurred between week 26 and week 42. The earliest symptoms’ onsets were June 26 (2 cases) and 27(2 cases). The other two foci occurred in people who lived in Rome and Latina and had no link with the area of Anzio. In Latina, the second largest urban area of Lazio, 8 cases occurred between week 32 and week 38. The first case was notified on September 12 and earliest reported symptoms’ onset was August 13. The focus of Rome involved 80 cases in the metropolitan area and 2 cases in two towns in the Province between week 33 and week 42. The first case was notified on September 7 and the earliest reported date of symptoms’ onset was August 20. The last symptoms’ onset was November 5 in a case reported from Anzio.
Fig 2. Epidemic curve of autochthonous CHIKV cases by probable place of transmission.
NOTE. Total number of cases reported is 399. Two cases with no epidemiological link with Anzio were residing in two towns in Rome province and one case had not date of symptoms’ onset available. Anzio, cases residing in Anzio or with probable transmission in Anzio; Rome, cases with no epidemiological link with Anzio and with probable transmission in Rome; Latina, cases with no epidemiological link with Anzio and with probable transmission in Latina. Probable place of transmission is defined as the city where the case was continuously residing for the 15 days before the onset of symptoms.
Between January 1, 2017 and January 31, 2018, four imported cases of CHIKV were notified. Only two of them had symptoms’ onset before the notification of the outbreak. One imported case (probable) was notified in January in a traveler returning from Venezuela with symptoms’ onset on January 10 and returning date on January 22. The other case (confirmed) was notified on July 29 with symptoms’ onset on July 27, one day after the return from Cameroon. The molecular characterization of E1 showed a sub-lineage of the ECSA clustering with lineage from Central African Region.
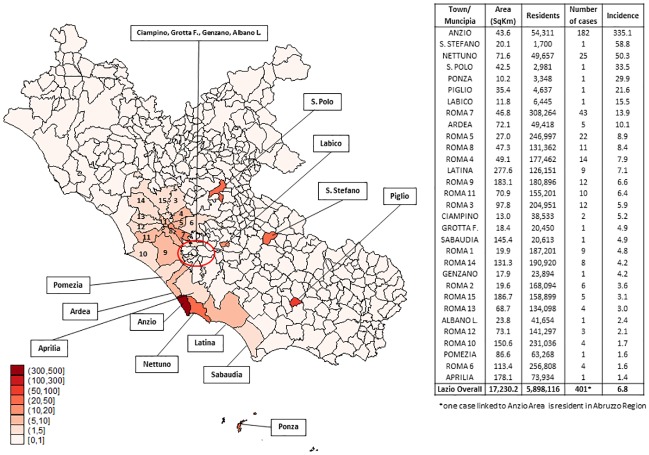
Following the cases in Anzio, incident cases occurred in 16 cities of Lazio and, by the week 33 throughout the 15 districts of Rome. Fig 3 shows the spread of the infection by contiguity across neighboring villages and Rome. Distribution of cumulative incidence (Fig 3) shows higher values in two coastal towns where first cases had occurred (excluding towns were only 1 case occurred): Anzio (N = 182 cases; 335.1 per 100,000 residents) and Nettuno (N = 25 cases; 50.4 per 100,000 residents). The cumulative incidence in Rome shows a variable distribution by municipia ranging from 1.6 to 13.9 per 100,000 residents, in the different districts. Latina reported a cumulative incidence of 7.1 per 100,000 residents. The cumulative incidence in the whole region was 6.8 cases/100,000.
Fig 3. Geographical distribution of cases according to incidence (per 100.000 inhabitants).
NOTE. Incidence has been calculated considering the place of living of the reported cases and not the epidemiological link (history of travel during the 15 days before the symptoms’ onset). *one case linked to Anzio Area is resident in Abruzzo Region.
Clinical presentation
The distribution of demographic and clinical characteristics of the probable or confirmed cases is shown in Table 1. Among the 402 probable/confirmed cases, the infection was equally distributed between gender with a slightly higher proportion among males. Median age was 55 years (IQR 40.0–67.5) and the 56.2% of cases were older than 50 years. The 96·8% (389/402) of cases reported fever, 95.8% (385/402) reported joint pain, 62.9% (253/402) developed a skin rash and 39.6% (159/402) developed arthritis. Sixty-nine suspected cases did not report fever and were tested because close contacts (family/household/neighbor) of a confirmed case. Among these, 13 were confirmed/probable cases and had an epidemiological link with a case in Anzio [8] or in Rome [5] and 56 were classified as non-cases. Thirty-five (9.4%) cases were admitted to hospital. One death was reported in a 77 years old man with underlying cardiovascular disease who was diagnosed with chikungunya infection after an admission for a stroke.
Table 1. Demographic and clinical characteristics of the 402 probable or confirmed cases.
| Demographic and clinical characteristics | Number of cases (%) | |
|---|---|---|
| Age, years | 0–34 | 81 (20.2%) |
| 35–50 | 95 (23.6%) | |
| 51–64 | 99 (24.6%) | |
| >65 | 127 (31.6%) | |
| Sex | Male | 184 (45.8%) |
| Female | 218 (54.2%) | |
| Body temperature | <37.8 °C | 13 (3.2%) |
| > = 37.8 °C | 389 (96.8%) | |
| Hospital admission | No | 367 (90.6%) |
| Yes | 35 (9.4%) | |
| Arthritis | No | 243 (60.4%) |
| Yes | 159 (39.6%) | |
| Headache | No | 196 (48.8%) |
| Yes | 206 (51.2%) | |
| Myalgia | No | 148 (36.8%) |
| Yes | 254 (63.2%) | |
| Retro orbital pain | No | 353 (87.8%) |
| Yes | 49 (12.2%) | |
| Conjuntivitis | No | 342 (85.1%) |
| Yes | 60 (14.9%) | |
| Rash | No | 149 (37.1%) |
| Yes | 253 (62.9%) | |
| Asthenia | No | 91 (22.6%) |
| Yes | 311 (77.4%) | |
| Arthralgia | No | 17 (4.2%) |
| Yes | 385 (95.8%) | |
Molecular characterization
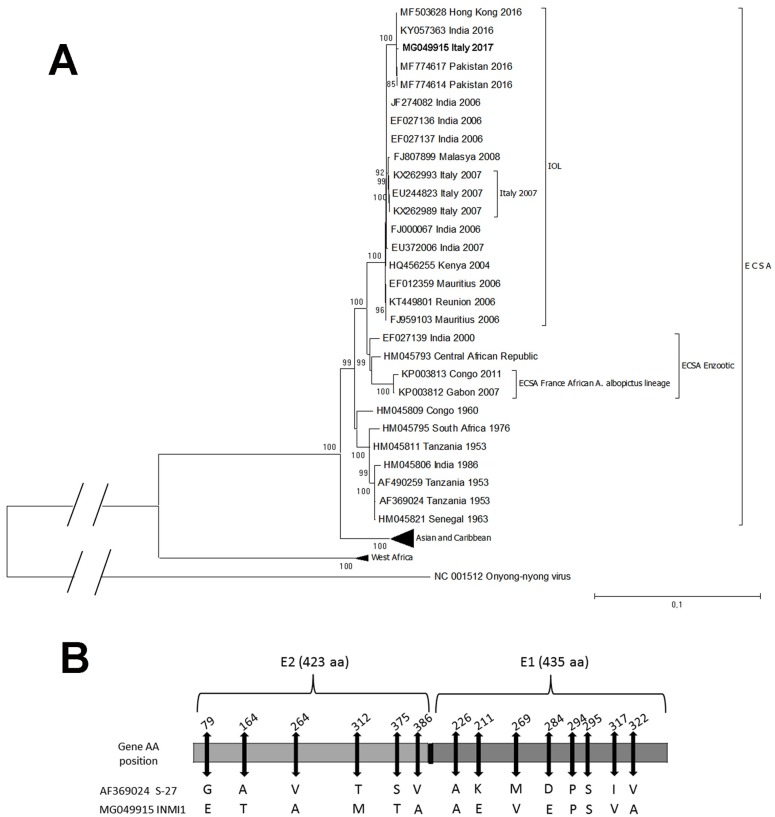
Preliminary molecular characterization, based on partial sequence of the E1, indicated that the sequences from samples collected at different time points of the outbreak were all identical, and belonged to the Indian Ocean subclade (IOL) of the ECSA lineage, as observed for the sequences from the 2007 outbreak in Italy. In Fig 4, phylogenetic tree of the complete genome sequence of the isolate CHIKV/ITA/Lazio-INMI1-2017 is shown in the context of sequences representing the 3 major described CHIKV lineages: ECSA, Asia and Caribbean, and West Africa. As can be seen, CHIKV/ITA/Lazio-INMI1-2017 is located on a branch of the IOL sublineage that is distinct from that comprising of the 2007 Italian autochthonous sequences, and clusters with recent strains originating from Pakistan and with an isolate obtained in China, from a traveler returning from India. Considering E1 and E2 polymorphisms at sites considered relevant for vector fitness, CHIKV/ITA/Lazio-INMI1-2017 lacks the adaptive mutation A226V in E1, and, more generally, shows the genetic signature of strains using Ae. aegypti as preferential vector (Fig 4B) [12].
Fig 4. Phylogenetic tree of the complete genome sequence (A) and polymorphisms in E1 and E2 envelope glycoproteins (B).
A) Phylogenetic tree of the complete genome sequence of the isolate CHIKV/ITA/Lazio-INMI1-2017 obtained from the current outbreak, Lazio region, Italy. The Maximum-likelihood phylogenetic tree was built with the complete genome sequence of the isolate CHIKV/ITA/Lazio-INMI1-2017 (outlined in bold characters) in the context of whole genome sequences representing the 3 major described CHIKV lineages: ECSA (including the Indian Ocean lineage), Asia and Caribbean, and West Africa. These sequences are indicated with their accession number, geographic origin and year of sampling. Asian and Caribbean and West Africa sequences are collapsed to increase the clarity of the figure. Bootstraps were generated using 1,000 replicates; only those >80 are shown. The bar represents genetic distance (substitutions per nucleotide position). B) Polymorphisms in E1 and E2 envelope glycoproteins of CHIKV/ITA/Lazio-INMI1-2017, at positions considered relevant for vector adaptation.
Discussion
This is the first report of a CHIKV outbreak involving a highly populated urban area in a western country. Based on the epidemic curve and on the incidence map, the outbreak may have started in the area of Anzio and spread by continuity to the neighboring villages and then to the Urban area of Rome and to the city of Latina favored by the touristic nature of the Anzio area. Anzio is one of the most popular location for internal tourism of people living in Rome who spend summer time in their second houses on the coast commuting to Rome on daily basis. The touristic nature of Anzio could explain also secondary outbreak of CHIKV in September and October 2017, in Guardavalle Marina, a small village of 2,346 inhabitants in the province of Catanzaro (CZ), Calabria region (Southern Italy) [14].
The cumulative incidence was higher in coastal and rural areas compared to that reported in Rome as also predicted by transmission models based on mosquitoes abundance and biting rate [15]. The cumulative incidence in Anzio and Rome area is lower than the incidence in the 2007 outbreak in Castiglione di Cervia (5.4%) and in Castiglione di Ravenna (2.5%) and the incidence in tropical areas [16–18] but higher than the incidence observed during the 2017 event in France in Le Cannet-des-Maures (9 cases out of 4.500 inhabitants) [9].
The median age of the patients was 55 years (IQR 40.0–67.5). Similar age profile was reported in the 2007 outbreak in Italy (median 60 years) [6] and in 2017 outbreak in France (range 33–77 years) [9]. The increasing prevalence rate of CHIKV infection with increasing age is consistent with other outbreaks, which report lower seroprevalence in children in Italy, in Bagan Panchor, Malaysia, in Mayotte, Indian Ocean and in Managua, Nicaragua [19–22]. Age is a proxy-factor for specific behaviors that cause higher exposure to Ae. albopictus bites (i.e., staying outdoors during daytime) or less tendency toward individual protection (i.e., use of insect repellents) in elder people. Elder people are also more likely to show symptoms and to access to the health structures than young people are [22].
Fever and joint pain, usually localized in both the arms and legs, is reported in 90% of patients [23]. Skin rash is usually reported in a variable proportion of cases (between 20% and 80%). The distribution of symptoms is similar to the one reported during the previous outbreak in Italy in 2007 [6] and during the 2017 outbreak in France [9]. The relative frequencies of fever, joint pain and rash were higher than those reported from the Pakistan 2016 outbreak (caused by the same clade), where fever and joint pain occurred in 85·9% and 88·4%, respectively, and rash occurred in only 29.1% of cases [24]. The case fatality rate was 2.5 per 1,000 clinical cases, lower than the one reported in the 2007 outbreak in Italy (0.5%) but consistent with those reported from la Reunion (1 death per 1,000 clinical cases) [6,25].
As reported by Carletti et al [12], the phylogenetic analysis shows that the virus involved in the current Lazio outbreak belongs to the East, Central, and South Africa (ECSA) clade, and clusters within the IOL. The sequences from the current outbreak are placed in a separate branch of the phylogenetic tree compared with the isolates from the 2007 Italian outbreak. Isolates from recent outbreaks in Pakistan and India are placed in the same branch, suggesting a more recent origin of the new epidemic strain. Given the molecular characterization of the strain and its phylogenetic characteristics, a possible introduction of the virus from the ongoing epidemic in Pakistan by travelers could be hypothesized [26]. This hypothesis is also supported by the seasonal synchronicity between Italy and India or Pakistan, as it happened also during the outbreak in Italy in 2007 [27]. The concurrent 2017 French outbreak showed a sub-lineage of the ECSA clustering with lineage from Central African Region and carrying the A226V mutation [9]. This excludes a possible link between the two outbreaks. Finally, the epidemiological and molecular characteristics of the imported cases prior to the outbreak rule out these cases as possible source of introduction. Of note, the characterization of E1 performed at various time points during the outbreak indicated a virtually complete identity of the virus sequences along the outbreak suggesting no additional introductions during the outbreak.
E1 sequences lack the A226V substitution associated with increased viral fitness in Ae. Albopictus [11]. The virus was isolated also from mosquitoes in Anzio area and showed the same clade and mutational patterns [28] of the human strain.
Ae. albopictus is disseminated through at least nine regions in Italy: Veneto, Lombardy, Emilia-Romagna, Liguria, Tuscany, Lazio, Piedmont, Campania, Sardinia [29] and Calabria [30].
The absence of the A226V may have had implication for transmission efficiency in the present outbreak, given that Ae. Albopictus is the unique competent vector circulating in Italy. This could explain the lower cumulative incidence compared with other urban outbreaks although the role of different background vector density or climate-dependent vector behavior cannot be excluded. The 2017 was characterized by an exceptional dry summer season. Despite the low cumulative incidence, this outbreak showed a very long duration (from June to November). Fig 4B shows a greater number of mutations that, considering E1 and E2 polymorphisms at sites relevant for vector fitness, provide the genetic signature of strains that use Ae. aegypti as preferential vector. The role of these mutations in the virus’ ability to better replicate and disseminate in Ae. Albopictus is still unclear. A comparison of the vector competence for the 2007 and 2017 Italian strains showed similar vector competence for both strains suggesting A226V could not be the sole responsible for ability of CHIKV to replicate in Ae. Albopictus [31]. Moreover, as already demonstrated for dengue and yellow fever [32], the genetic background of the autochtonous Ae. Albopictus could facilitate the competence for the virus despite the presence of the A226V mutation and should be further evaluated.
Vector control activities have been carried out by using insecticides for adult mosquitoes knock-down (space spraying with pyrethrines) and residual insecticides for resting mosquitoes (etofenprox based products) applied on vegetation. Preventive blood safety measures were also introduced in the different areas following the spread of the infection. Given the large and populated area of Rome and the consequences of a interruption of blood donations on the regional blood supply, a risk-benefit evaluation based on daily epidemiological data was performed [33].
The reappearance of CHIKV in Italy and the involvement of urban settings could have been influenced by several factors. The globalization of travel together with the global spread of CHIKV could facilitate the importation of the infection, as was the case in the 2007 outbreak [6]. The adaptation of the CHIKV to specific vector species though genome mutation is a key factor in the CKIKV spread. The influence of climate change on the vector’s climatic suitability put the Mediterranean region at high risk for CHIKV dissemination [34, 35].
The date of symptoms’ onset of first reported case was August 5, 2017 and the earliest symptoms’ onset was June 26. The surveillance system was able to detect the first autochthonous case only after the peak of the outbreak as it was in 2007 [6]. Early identification of autochthonous cases of chikungunya infection is the main challenge for the passive surveillance system in place. In Italy, the activity of the vector mosquitoes is mainly restricted to the summer season (usually between June and October) when outbreaks could be triggered by the arrival of imported cases from endemic areas and may result in secondary cases. This highlights the importance of an integrated surveillance system that should promptly identify autochthonous transmission. The integration of the passive surveillance with different surveillance tools (such as laboratory-based surveillance, syndromic surveillance, novel data stream) [36] combined with entomological surveillance should facilitate the detection, response and control of arboviruses spreading, including CHIKV.
Finally, information and training for medical professionals might be useful to favor early diagnosis and reporting of exotic viral diseases such as chikungunya.
Supporting information
This a minimum anomyzed data set is available. 0, Absent; 1, Present; F, Female; M, Male.
(XLSX)
Acknowledgments
Chikungunya Lazio Outbreak Group (in alphabetical order): Rosa Francesca Alicata, Alessandro Agresta, Silvia Aquilani, Giuseppina Bartolomei, Licia Bordi, Carlo Cerocchi, Francesca Colavita, Angela Corpolongo, Maurizio D’Amato, Claudio De Liberato, Stefano De Luca, Virginia Di Bari, Giuseppe Di Luzio, Enrico Di Rosa, Giorgio Esterini, Federica Ferraro, Roberto Giammattei, Marco Iannetta, Vincenzo Labriola, Eleonora Lalle, Mariarosaria Loffredo, Adele Magliano, Donatella Mandolini, Fabrizio Magrelli, Andrea Mariano, Pier Angela Napoli, Alessio Pendenza, Alberto Perra, Alberto Piccoli, Raffaella Pisapia, Patricia Porcelli, Pasquale Rombolà, Amilcare Ruta, Monica Sanè Schepisi, Sara Simeoni, Donatella Vincenti.
The authors would like also to acknowledge the contributions of all staff of Local Public Health Units who managed the report of single cases and performed preliminary epidemiologic investigation of cases.
Data Availability
A minimum anonymized dataset has been uploaded as additional file.
Funding Statement
This intervention and the study were funded by Directorate of Health and Social Welfare, Latium Region, the Local Public Health Units, Latium Region and Ricerca Corrente – Italian Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brouard C, Bernillon P, Quatresous I, Pillonel J, Assal A, De Valk H, et al. Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion 2008; 48: 1333–41. 10.1111/j.1537-2995.2008.01646.x [DOI] [PubMed] [Google Scholar]
- 2.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines 2012; 11: 1087–101. 10.1586/erv.12.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chretien JP, Anyamba A, Bedno SA, Breiman RF, Sang R, Sergon K, et al. Drought-associated chikungunya emergence along coastal East Africa. Am J Trop Med Hyg 2007; 76: 405–7. [PubMed] [Google Scholar]
- 5.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae.albopictus. Elife. 2015. June 30;4:e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007. December 1;370:1840–6. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- 7.Grandadam M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux AB, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17:910–3. 10.3201/eid1705.101873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveillance. 2015;20:21108 [DOI] [PubMed] [Google Scholar]
- 9.Calba C, Guerbois-Galla M, Franke F, Jeannin C, Auzet-Caillaud M, Grard G, et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22:17–00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsey HS, Calisher CH, Mathews JH. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol. 1976;4:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bordi L, Carletti F, Lalle E, Colavita F, Meschi S, Di Caro A, et al. Molecular Characterization of Autochthonous Chikungunya Cluster in Lazio Region, Italy. Emerg Infect Dis. 2018. January 15;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carletti F, Marsella P, Colavita F, Meschi S, Lalle E, Bordi L, et al. Full-Length Genome Sequence of a Chikungunya Virus Isolate from the 2017 Autochthonous Outbreak, Lazio Region, Italy. Genome Announc. 2017. December 7;5(49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AH, Morita K, Parquet Md Mdel C, Hasebe F, Mathenge EG, Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83:3075–84. 10.1099/0022-1317-83-12-3075 [DOI] [PubMed] [Google Scholar]
- 14.ECDC Rapid Risk Assessment. Clusters of autochthonous chikungunya cases in Italy First update 9 October 2017 https://ecdc.europa.eu/sites/portal/files/documents/RRA-chikungunya-Italy-update-9-Oct-2017.pdf
- 15.Manica M, Guzzetta G, Poletti P, Filipponi F, Solimini A, Caputo B, et al. Transmission dynamics of the ongoing chikungunya outbreak in Central Italy: from coastal areas to the metropolitan city of Rome, summer 2017. Euro Surveill. 2017;22(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry M, Francis L, Asin V, Polson-Edwards K, Olowokure B. Chikungunya virus outbreak in Sint Maarten, 2013–2014. Rev Panam Salud Publica. 2017;41:e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastula DM, Hancock WT, Bel M, Biggs H, Marfel M, Lanciotti R, et al. Chikungunya virus disease outbreak in Yap State, Federated States of Micronesia. PLoS Negl Trop Dis. 2017. March1;11:e0005410 10.1371/journal.pntd.0005410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balmaseda A, Gordon A, Gresh L, Ojeda S, Saborio S, Tellez Y, et al. Clinical Attack Rate of Chikungunya in a Cohort of Nicaraguan Children. Am J Trop Med Hyg. 2016;94:397–9. 10.4269/ajtmh.15-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro ML, Gagliotti C, Silvi G, Angelini R, Sambri V, Rezza G, et al. Chikungunya virus in North-Eastern Italy: a seroprevalence survey. Am J Trop Med Hyg. 2010;82:508–11. 10.4269/ajtmh.2010.09-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayu SM, Lai LR, Chan YF, Hatim A, Hairi NN, Ayob A, et al. Seroprevalence survey of Chikungunya virus in Bagan Panchor, Malaysia. Am J Trop Med Hyg. 2010;83:1245–8. 10.4269/ajtmh.2010.10-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, et al. Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PLoS One. 2008;3:e3066 10.1371/journal.pone.0003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuan G, Ramirez S, Gresh L, Ojeda S, Melendez M, Sanchez N, et al. Seroprevalence of Anti-Chikungunya Virus Antibodies in Children and Adults in Managua, Nicaragua, After the First Chikungunya Epidemic, 2014–2015. PLoS Negl Trop Dis. 2016;10:e0004773 10.1371/journal.pntd.0004773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–9 10.1056/NEJMra1406035 [DOI] [PubMed] [Google Scholar]
- 24.Naqvi S, Bashir S, Rupareliya C, Shams A, Giyanwani PR, Ali Z, et al. Clinical Spectrum of Chikungunya in Pakistan. Cureus. 2017;9:e1430 10.7759/cureus.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks—the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–71. 10.1056/NEJMp078013 [DOI] [PubMed] [Google Scholar]
- 26.Aamir UB, Badar N, Salman M, Ahmed M, Alam MM. Outbreaks of chikungunya in Pakistan. Lancet Infect Dis. 2017;17:483 10.1016/S1473-3099(17)30191-3 [DOI] [PubMed] [Google Scholar]
- 27.Charrel RN, de Lamballerie X. Chikungunya virus in north-eastern Italy: a consequence of seasonal synchronicity. Euro Surveill. 2008;13(1). [PubMed] [Google Scholar]
- 28.Venturi G, Di Luca M, Fortuna C, Remoli ME, Riccardo F, Severini F, et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill. 2017;22(39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romi R. History and updating on the spread of Aedes albopictus in Italy. Parassitologia 1995; 37: 99–103 [PubMed] [Google Scholar]
- 30.Bonacci T, Mazzei A, Hristova VK, Ayaz Ahmad M. Monitoring of Aedes albopictus (Diptera, Cilicidae) in Calabria, Southern Italy." International Journal of Scientific & Engineering Research 2015; 6:1186–89. [Google Scholar]
- 31.Fortuna C, Toma L, Remoli ME, Amendola A, Severini F, Boccolini D, et al. Vector competence of Aedes albopictus for the Indian Ocean lineage (IOL) Chikungunya viruses of the 2007 and 2017 outbreaks in Italy: a comparison between strains with and without the E1:A226V mutation. Euro Surveill. 2018;23(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lourenço de Oliveira R, Vazeille M, de Filippis AM, Failloux AB. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am J Trop Med Hyg. 2003;69:105–14 [PubMed] [Google Scholar]
- 33.Marano G, Pupella S, Pati I, Masiello F, Franchini M, Vaglio S, et al. Ten years since the last Chikungunya virus outbreak in Italy: history repeats itself. Blood Transfus. 2017;15:489–490. 10.2450/2017.0215-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer D, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, Beierkuhnlein C, Semenza JC. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int J Health Geogr. 201312:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ECDC (2008)a. Meeting Report, Stockholm, 29–30 March 2007, Environmental Change and Infectious Disease Workshop. European Centre for Disease Prevention and Control, Stockholm, May 2008.
- 36.Vairo F, Di Pietrantonj C, Pasqualini C, Mammone A, Lanini S, Nicastri E, ey al. The Surveillance of Chikungunya Virus in a Temperate Climate: Challenges and Possible Solutions from the Experience of Lazio Region, Italy. Viruses. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This a minimum anomyzed data set is available. 0, Absent; 1, Present; F, Female; M, Male.
(XLSX)
Data Availability Statement
A minimum anonymized dataset has been uploaded as additional file.