Abstract
Background
The real-world data for the effectiveness and safety of sofosbuvir/ledipasvir (SOF/LDV) with or without ribavirin (RBV) in patients with hepatitis C virus genotype 1 (HCV-1) infection remain limited in Taiwan.
Methods
A total of 273 chronic HCV-1 patients receiving 8, 12, or 24 weeks of SOF/LDV with or without RBV were enrolled. The sustained virologic response rate at week 12 off-therapy (SVR12) by evaluable population (EP) and per-protocol population (PP) were assessed for effectiveness. The treatment discontinuation rate due to adverse events (AEs) and serious AE rate were assessed for safety. Baseline patient characteristics and on-treatment HCV viral kinetics associated with SVR12 were analyzed.
Results
The SVR12 rates by EP and PP analyses were 96.7% (95% confidence interval [CI]: 93.9%-98.3%) and 97.5% (95% CI: 94.8%-98.8%), respectively. The rates of treatment discontinuation due to AE and serious AE were 0.4% and 4.4%, respectively. Seven patients with true virologic failure were relapsers. In 2 patients who were lost-to follow-up, one expired at treatment week 3 due to pneumonia which was considered not related to treatment, and one declined follow-up at off-therapy week 4. The SVR12 rates were comparable in terms of baseline patient characteristics and viral decline at week 4 of treatment.
Conclusions
SOF/LDV with or without RBV for 8–24 weeks is well tolerated and achieves a high SVR12 rate in patients with HCV-1 infection in Taiwan.
Introduction
Hepatitis C virus (HCV) infection is a challenging health problem which affects approximately 71.1 million people worldwide [1]. Over a period of 20–30 years, about 20% of chronic HCV-infected patients will evolve to cirrhosis which may progress to hepatic decompensation and hepatocellular carcinoma (HCC) [2,3]. Apart from the liver-related morbidity and mortality, HCV infection may also induce extra-hepatic manifestations which adversely affects the patients’ health outcome and quality of life [4]. On the other hand, the prognosis is improved once patients achieve sustained virologic response (SVR) following anti-HCV agents [5–8]. Currently, HCV genotype 1 (HCV-1) infection is predominant around the world [9]. Compared to patients with non-HCV-1 infection, those with HCV-1 infection have an increased risk of cirrhosis and HCC [10,11]. Therefore, an effective and safe HCV treatment strategy, particularly for patients with HCV-1infection, is mandatory.
The introduction of interferon (IFN)-free direct acting antiviral agents (DAAs) has revolutionized the care of HCV infection. Sofosbuvir (SOF) is a pyrimidine nucleotide analogue that inhibits the HCV non-structural protein 5B (NS5B) ribonucleic acid (RNA)-dependent RNA polymerase. After intra-hepatic metabolism, the active uridine triphosphate form is incorporated to HCV RNA by NS5B polymerase and acts as the chain terminator [12]. Clinically, SOF is administered once-daily with pangenotypic potency, excellent tolerability, high genetic barriers to drug resistance, and few potential drug-drug interactions (DDIs). Currently, SOF can be used with ledipasvir (LDV) as a formula of fixed-dose combination which is active against HCV-1, 4, 5 or 6 infection. The efficacy and safety of SOF/LDV with or without RBV for 8–24 weeks for ordinary HCV-1 patients are excellent in phase III trials [13–16]. Furthermore, the therapeutic profiles remain excellent among patients with human immunodeficiency virus (HIV) coinfection, decompensated cirrhosis, or organ transplantation [17–21]. Therefore, SOF/LDV-based regimens for HCV-1 patients are appealing to most health care providers.
Regarding to the real-world effectiveness and safety of SOF/LDV with or without RBV for HCV-1 patients, data from Western and Eastern countries showed that the SVR rates ranged from 92%-98% and most patients tolerated the treatment well [22–26]. On the basis of these encouraging results, we aimed to evaluate the real-world performance of SOF/LDV with or without RBV for HCV-1 patients in Taiwan.
Materials and methods
Patients
Between April 2015 and August 2017, HCV-1 infected patients who received SOF/LDV for 8, 12 or 24 weeks with or without ribavirin (RBV) were retrospectively enrolled at the National Taiwan University Hospital (NTUH) and NTUH Yun-Lin Branch. All patients were aged ≥ 20 years and had chronic HCV infection, defined as detectable HCV antibody (anti-HCV; Abbott HCV EIA 2.0, Abbott Laboratories, Abbott Park, Illinois, USA) and quantifiable serum HCV RNA (Cobas TaqMan HCV Test v2.0, Roche Diagnostics GmbH, Mannheim, Germany, lower limit of detection [LLOD]: 15 IU/mL) for ≥ 6 months. Patients who had non-HCV-1 infection, had prior DAA exposure, had active HCC, had estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2, received treatment regimens outside the guideline recommendation, or refused to provide written informed consent were excluded from the study [27–29]. The study was approved by the NTUH Research Ethics Committee (201205058RIC) and was conducted in accordance with the principles of Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. All patients provided written informed consent before the study.
Study design
Baseline patient demographics, hemogram, serum biochemical data (albumin, total bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], creatinine, eGFR, as calculated by modification of diet in renal disease (MDRD) equation, anti-HCV, hepatitis B virus (HBV) surface antigen (Abbott Architect HBsAg qualitative assay, Abbott Laboratories, Abbott Park, Illinois, USA), HCV RNA, HCV genotype (Abbott RealTime HCV Genotype II, Abbott Laboratories, Abbott Park, Illinois, USA) and anti-HIV (Abbott Architect HIV Ag/Ab Combo, Abbott Laboratories, Abbott Park, Illinois, USA) were collected [30]. The status of cirrhosis was determined by liver biopsy, clinical signs of portal hypertension, imaging studies, AST-to-platelet ratio index (ARPI) with a cutoff value of > 2.0 or liver stiffness measurement (LSM, FibroScan, Echosens, Paris, France) with a cutoff value of > 12.5 kPa when appropriate [31,32]. The stage of cirrhosis was graded by Child-Pugh score. Baseline serum HBV DNA (Cobas AmpliPrep/Cobas Taqman HBV test v.2.0, Roche Diagnostics GmbH, Mannheim, Germany, LLOD: 20 IU/mL) or HIV RNA (Cobas AmpliPrep/Cobas Taqman HIV-1 test v.2.0, Roche Diagnostics GmbH, Mannheim, Germany, LLOD: 20 copies/mL) level was determined for patients with HBV or HIV coinfection [33].
Patients received fixed-dose combination of SOF/LDV (400mg/90mg, Harvoni, Gilead Sciences, Carrigtohill, Co. Cork, Ireland) 1 tablet per day for 8, 12 or 24 weeks. Treatment-naïve, non-cirrhotic patients with baseline HCV RNA level < 6,000,000 IU/mL can receive 8 or 12 weeks of SOF/LDV treatment. Patients with compensated cirrhosis (Child-Pugh A) can receive 12 weeks of SOF/LDV with or without weight-based RBV (Robatrol, 200 mg capsule, Genovate Biotechnology Co. Ltd., Hsinchu, Taiwan; 1,200 mg per day if the body weight ≥ 75 kg; 1,000 mg per day if the body weight < 75 kg) or 24 weeks of SOF/LDV at the discretion of the physicians. Patients who had decompensated cirrhosis (Child-Pugh B or C) or had undergone liver transplantation can receive 12 weeks of SOF/LDV with weight-based RBV or 24 weeks of SOF/LDV. For patients with baseline eGFR between 30–50 mL/min/1.73m2, the RBV was adjusted to 200 mg/400 mg per day at alternative dosage.
Effectiveness
Patients received on-treatment serum HCV RNA monitoring at week 4 and at the end of treatment (EOT). Furthermore, they received off-therapy serum HCV RNA testing at week 12 to assess SVR12. Patients were considered failure to achieve SVR12 if they lacked SVR12 data. We adopted two different endpoints for effectiveness: the evaluable population (EP) which assessed the SVR12 for patients who received at least one dosage of treatment, and the per-protocol population (PP) which assessed the SVR12 by excluding non-SVR12 patients due to non-virologic failure.
Safety
The rate of treatment completion was assessed for all patients. The reasons for patients who prematurely discontinued treatment or were lost-to follow-up were assessed through the chart review. The on-treatment constitutional and laboratory adverse events (AEs), and serious AEs were also evaluated. In patients who were seropositive for HBsAg, serum HBV DNA levels were evaluated after the initiation of DAA treatment. HBV reactivation was defined as the presence of HBV DNA level ≥ LLOD in patients with baseline HBV DNA level < LLOD, or increase of HBV DNA level > 1 log10 IU/mL in patients with baseline HBV DNA level ≥ LLOD [34]. HBV-associated hepatitis was defined as HBV reactivation and hepatitis flare presenting with ALT increase ≥ 3 times baseline and > 100 U/L [35].
Statistical analysis
All analyses were performed using Statistical Program for Social Sciences (SPSS Statistics Version 23.0, IBM Corp., Armonk, New York, USA). The baseline characteristics were shown in median (range) and numbers (percentages) when appropriate. The rates of antiviral response were shown in numbers (percentages) with 95% confidence interval (CI) and the AE rates were shown in numbers (percentages). The stratified analysis of SVR12 by EP analysis for baseline characteristics and week 4 viral decline were assessed and shown in percentages with 95% CI.
Results
Patient characteristics
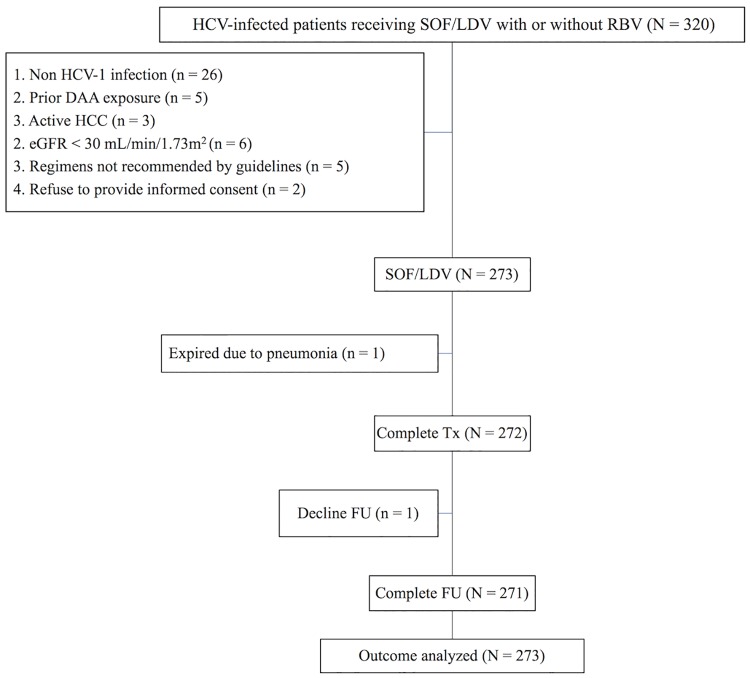
Of 320 HCV-infected patients receiving SOF/LDV with or without RBV, 47 were excluded from the study because of non-HCV-1 infection, prior DAA exposure, active HCC, eGFR < 30 mL/min/1.73m2, receiving antiviral regimens not recommended by guidelines, or refusal to provide informed consent. The remaining 273 patients were eligible for the analysis (Fig 1). Table 1 shows the baseline patient characteristics. One hundred twenty-seven (46.5%) patients were male and 182 (66.7%) were treatment-naïve. Nine (3.3%) and 13 (4.8%) patients had HBV and HIV coinfection, respectively. Twenty-six (9.5%) and 13 (4.8%) patients underwent liver and renal transplantation, respectively. Among the 88 (32.2%) patients receiving RBV, all received treatment for 12 weeks, including 25 who underwent liver transplantation (one with decompensated cirrhosis), 25 with decompensated cirrhosis (one who underwent liver transplantation), and 39 with compensated cirrhosis (37 with prior IFN-based treatment). All patients who underwent renal transplantation were treatment-naïve and received SOF/LDV for 12 weeks. One decompensated cirrhotic, one treatment-naïve compensated cirrhotic, and one liver transplantation patients received SOF/LDV for 24 weeks. With regard to HCV subgenotype distribution, 21 (7.7%), 242 (88.6%) and 10 (3.7) patients had HCV-1a, HCV-1b, and unsubtypable HCV-1 infections. One hundred thirty-eight (50.5%) patients were non-cirrhotic. Among the 135 cirrhotic patients, 109 (80.7) and 26 (19.3%) of them had compensated and decompensated cirrhosis, respectively. Seven (77.8%) and 12 (92.3%) patients with HBV or HIV coinfection had baseline undetectable serum HBV DNA or HIV RNA.
Fig 1. Study flow.
Table 1. Baseline patient characteristics.
| Characteristics* | Patient (N = 273) |
|---|---|
| Age, year, median (range) | 64 (29–86) |
| Age ≥ 60 years | 188 (68.9) |
| Male | 127 (46.5) |
| Treatment-naive | 182 (66.7) |
| HBV coinfection | 9 (3.3) |
| HIV coinfection | 13 (4.8) |
| Prior history of HCC | 57 (20.9) |
| Liver transplantation | 26 (9.5) |
| Renal transplantation | 13 (4.8) |
| Treatment duration, week | |
| 8 weeks | 5 (1.8) |
| 12 weeks | 265 (97.1) |
| 24 weeks | 3 (1.1) |
| RBV usage | 88 (32.2) |
| Hemoglobin, g/dL, median (range) | 13.5 (7.7–17.6) |
| White cell count, 109 cells/L, median (range) | 4.9 (1.9–12.5) |
| Platelet count, 109 cells/L, median (range) | 136 (33–433) |
| Albumin, g/dL, median (range) | 4.1 (2.1–5.0) |
| Total bilirubin, mg/dL, median (range) | 0.8 (0.3–8.8) |
| AST, ULN, median (range) | 1.7 (0.3–8.5) |
| ALT, ULN, median (range) | 1.7 (0.3–10.6) |
| Creatinine, mg/dL, median (range) | 0.8 (0.4–2.1) |
| eGFR, mL/min/1.73m2, median (range) † | 84 (32–186) |
| eGFR < 60 mL/min/1.73m2 † | 52 (19.0) |
| HCV RNA, log10 IU/mL, median (range) | 6.17 (2.85–7.69) |
| HCV RNA > 6,000,000 IU/mL | 48 (17.6) |
| HCV genotype | |
| 1a | 21 (7.7) |
| 1b | 242 (88.6) |
| 1‡ | 10 (3.7) |
| Cirrhosis | |
| Absent | 138 (50.5) |
| Present | 135 (49.5) |
| Child-Pugh A | 109 (80.7) |
| Child-Pugh B and C | 26 (19.3) |
HBV: hepatitis B virus; HIV: human immunodeficiency virus; HCC: hepatocellular carcinoma; RBV: ribavirin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ULN: upper limit of normal; eGFR: estimated glomerular filtration rate.
* Values are numbers (percentages) unless otherwise indicated.
† eGFR was calculated by MDRD equation.
‡ Failed subtyping for major genotyping.
Effectiveness
Of the 272 patients with available HCV RNA data at week 4 of treatment, 218 (80.2%) of them had undetectable serum HCV RNA. All 272 (100%) patients with available HCV RNA data had undetectable HCV RNA at EOT. One Child-Pugh C cirrhotic patient receiving SOF/LDV with RBV died at treatment week 3 did not receive HCV RNA testing at week 4 and EOT. The overall SVR12 rates were 96.7% (264 of 273 patients; 95% CI: 93.9%-98.3%) by EP analysis, and 97.5% (264 of 271 patients; 95% CI: 94.8%-98.8%) by PP analysis (Table 2).
Table 2. Virologic responses.
| HCV RNA < LLOD | Patient (N = 273) | |
|---|---|---|
| n/N (%) | 95% CI | |
| During treatment | ||
| Week 4* | 218/272 (80.2) | 75.0–84.5 |
| EOT* | 272/272 (100) | 98.6–100 |
| After treatment | ||
| SVR12 (EP)† | 264/273 (96.7) | 93.9–98.3 |
| SVR12 (PP)‡ | 264/271 (97.5) | 94.8–98.8 |
| Reason for non-SVR12, n | ||
| Relapse | 7 | |
| Lost to follow-up | 2 | |
| During treatment | 1 | |
| After treatment | 1 | |
LLOD: lower limit of detection; EOT: end-of-treatment; EP: evaluate population; PP: per-protocol population; CI: confidence interval.
* One patient who expired at treatment week 3 did not have week 4 and EOT HCV RNA data.
† Patients who received at least one dosage of treatment were included in the analysis.
‡ Patients with non-virologic failure were excluded from the analysis.
Among patients who failed to achieved SVR12, 7 (2.6%) were relapsers and 2 (0.7%) were lost to follow-up. Among the relapser, 5 (71.4%) were female, 6 (85.7%) were treatment-naïve, 4 (57.1%) had cirrhosis and all received SOF/LDV with or without RBV for 12 weeks. In 2 patients who were lost to follow-up, one died of pneumonia at treatment week 3, and the other one declined follow-up at post-treatment week 4 (S1 Table).
Stratified analysis of patient characteristics predictive of SVR12
Table 3 shows the stratified SVR12 rates of SOF/LDV with or without RBV by baseline characteristics and week 4 treatment response. The SVR12 rates were comparable with regard to age at a cut-off value of 60 years, sex, prior IFN exposure, HBV or HIV coinfection, liver or renal transplantation, scheduled treatment duration, use of RBV, eGFR at a cut-off value of 60 mL/min/1.73m2, baseline HCV viral load at a cut-off value of 6,000,000 IU/mL, HCV genotype, cirrhosis and week 4 viral decline. The SVR12 rates for patients with HCV-1a, HCV-1b, and unsubtypable HCV-1 infections were 90.5% (95% CI: 71.1%-97.4%), 97.9% (95% CI: 95.3%-99.1%), and 80.0% (95% CI: 49.0%-94.3%), respectively. Among the cirrhotic patients, the SVR12 rates for those with compensated and decompensated cirrhosis were 97.3% (95% CI: 92.2%-99.1%) and 88.5% (95% CI: 71.0%-96.0%), respectively.
Table 3. SVR12 according to baseline patient characteristics and on-treatment HCV viral decline.
| Characteristics | Patient (N = 273) | ||
|---|---|---|---|
| Patient No. | SVR12 (%) | 95% CI | |
| Age, years | |||
| < 60 | 85 | 94.1 | 87.0–97.5 |
| ≥ 60 | 188 | 97.9 | 94.7–99.2 |
| Sex | |||
| Female | 146 | 96.6 | 92.2–98.5 |
| Male | 127 | 96.9 | 92.2–98.8 |
| Treatment experience | |||
| Naïve | 182 | 96.7 | 93.0–98.5 |
| Experienced | 91 | 96.7 | 90.8–98.9 |
| HBV coinfection | |||
| Absent | 264 | 96.6 | 93.7–98.2 |
| Present | 9 | 100 | 70.1–100 |
| HIV coinfection | |||
| Absent | 260 | 96.5 | 93.6–98.2 |
| Present | 13 | 100 | 77.2–100 |
| Prior HCC history | |||
| Absent | 216 | 96.8 | 93.5–98.4 |
| Present | 57 | 96.5 | 88.1–99.0 |
| Liver transplantation | |||
| No | 247 | 96.4 | 93.2–98.1 |
| Yes | 26 | 100 | 87.1–100 |
| Renal transplantation | |||
| No | 260 | 96.5 | 93.6–98.2 |
| Yes | 13 | 100 | 77.2–100 |
| Treatment duration, week | |||
| 8 | 5 | 100 | 56.6–100 |
| 12 | 265 | 96.6 | 93.7–98.2 |
| 24 | 3 | 100 | 43.9–100 |
| RBV usage | |||
| No | 185 | 97.3 | 93.8–98.8 |
| Yes | 88 | 95.5 | 88.9–98.2 |
| eGFR, mL/min/1.73m2 | |||
| < 60 | 52 | 98.1 | 89.9–99.7 |
| ≥ 60 | 221 | 96.4 | 93.0–98.2 |
| HCV RNA, IU/mL | |||
| < 6,000,000 | 225 | 96.4 | 93.1–98.2 |
| ≥ 6,000,000 | 48 | 97.9 | 89.1–99.6 |
| HCV genotype | |||
| 1a | 21 | 90.5 | 71.1–97.4 |
| 1b | 242 | 97.9 | 95.3–99.1 |
| 1 | 10 | 80.0 | 49.0–94.3 |
| Cirrhosis | |||
| Absent | 138 | 97.8 | 93.8–99.3 |
| Present | 135 | 95.6 | 90.7–98.0 |
| Child-Pugh A | 109 | 97.3 | 92.2–99.1 |
| Child-Pugh B and C | 26 | 88.5 | 71.0–96.0 |
| Week 4 HCV RNA < LLOD* | |||
| No | 54 | 94.4 | 84.9–98.1 |
| Yes | 218 | 97.7 | 94.8–99.0 |
NA: not assessed.
* One patient who expired at treatment week 3 did not have week 4 HCV RNA data.
In patients who were treatment-naïve, non-cirrhotic and had baseline HCV RNA level < 6,000,000 IU/mL, the SVR12 rates were 100% (95% CI: 56.6%-100%) and 98.7% (95% CI: 92.8%-99.8%) for patients receiving 8 and 12 weeks of SOF/LDV. The SVR12 rates of SOF/LDV for 12 weeks in compensated cirrhotic patients who were treatment-naïve and treatment-experienced were 96.5% (95% CI: 88.1%-99.0%) and 100% (95% CI: 61.0%-100%), respectively. Furthermore, the SVR12 rates of SOF/LDV with RBV for 12 weeks in compensated cirrhotic patients who were treatment-naïve and treatment-experienced were 100% (95% CI: 61.0%-100%) and 97.4% (95% CI: 86.8%-99.6%), respectively. In patients with decompensated cirrhosis, the SVR12 rates of SOF/LDV with RBV for 12 weeks and SOF/LDV for 24 weeks were 88% (95% CI: 70.0%-95.8%) and 100% (95% CI: 20.7%-100%), respectively (Table 4).
Table 4. SVR12 in patients of specific interest.
| Patients of specific interest* | Patient No. | SVR12 (%) | 95% CI |
|---|---|---|---|
| Treatment-naïve, non-cirrhotic, & baseline HCV RNA level < 6,000,000 IU/mL | |||
| SOF/LDV, 8 weeks | 5 | 100 | 56.6–100 |
| SOF/LDV, 12 weeks | 75 | 98.7 | 92.8–99.8 |
| Treatment-naïve, Child-Pugh A cirrhotic | |||
| SOF/LDV, 12 weeks | 57 | 96.5 | 88.1–99.0 |
| SOF/LDV with RBV, 12 weeks | 6 | 100 | 61.0–100 |
| SOF/LDV, 24 weeks | 1 | 100 | 20.7–5100 |
| Treatment-experienced, Child-Pugh A cirrhotic | |||
| SOF/LDV, 12 weeks | 6 | 100 | 61.0–100 |
| SOF/LDV with RBV, 12 weeks | 39 | 97.4 | 86.8–99.6 |
| Child-Pugh B or C cirrhotic | |||
| SOF/LDV with RBV, 12 weeks | 25 | 88 | 70.0–95.8 |
| SOF/LDV, 24 weeks | 1 | 100 | 20.7–100 |
* Patients receiving liver or renal transplantation were not included in the analysis.
Safety
Two hundred seventy-two (99.6%) patients completed the scheduled treatment. One treatment-naïve Child-Pugh C cirrhotic patients receiving SOF/LDV with RBV died at treatment week 3 due to pneumonia, which was not related to treatment. Twelve (4.4%) patients experienced on-treatment serious AEs, and none were considered related to DAA treatment. The rates and severity of hematological and hepatic AEs were generally low and mild in grade. The common AEs with event rates ≥ 10% included fatigue (27.1%), headache (20.5%), nausea (17.9%) and insomnia (13.9%) (Table 5). The rates for serious AE, fatigue, hemoglobin level < 10 g/dL, and elevated total bilirubin level were higher in patients with decompensated cirrhosis than those with no cirrhosis and with compensated cirrhosis. Among the 9 patients with HBV coinfection, 2 (22.2%) experienced HBV reactivation after treatment, but none had HBV-associated hepatitis that needed anti-HBV treatment.
Table 5. Safety summary.
| Variable, n (%) | All patient (N = 273) | No cirrhosis (n = 138) | Child-Pugh A cirrhosis (n = 109) | Child-Pugh B/C cirrhosis (n = 26) |
|---|---|---|---|---|
| Serious adverse event | 12 (4.4) | 1 (0.7) | 5 (4.6) | 6 (23.1) |
| Pneumonia | 1 (0.4) | 0 (0) | 0 (0) | 1 (3.8) |
| Spontaneous bacterial peritonitis | 3 (1.1) | 0 (0) | 0 (0) | 3 (11.5) |
| Variceal bleeding | 2 (0.7) | 0 (0) | 1 (0.9) | 1 (3.8) |
| Hepatocellular carcinoma | 4 (1.5) | 0 (0) | 3 (2.8) | 1 (3.8) |
| Herpes zoster | 1 (0.4) | 0 (0) | 1 (0.9) | 0 (0) |
| Duodenal ulcer bleeding | 1 (0.4) | 1 (0.7) | 0 (0) | 0 (0) |
| Discontinuation due to adverse event* | 1 (0.4) | 0 (0) | 0 (0) | 1 (3.8) |
| Death* | 1 (0.4) | 0 (0) | 0 (0) | 1 (3.8) |
| Adverse event in ≥ 10% of patients | ||||
| Fatigue | 74 (27.1) | 30 (21.7) | 32 (29.4) | 12 (46.2) |
| Headache | 56 (20.5) | 28 (20.3) | 22 (20.2) | 6 (23.1) |
| Nausea | 49 (17.9) | 22 (15.9) | 21 (19.3) | 6 (23.1) |
| Insomnia | 38 (13.9) | 18 (13.0) | 15 (13.8) | 5 (19.2) |
| Laboratory adverse event | ||||
| Hemoglobin | ||||
| 8.0–10.0 g/dL | 21 (7.7) | 1 (0.7) | 14 (12.8) | 6 (23.1) |
| < 8.0 g/dL | 4 (1.5) | 0 (0) | 3 (2.8) | 1 (3.8) |
| White blood cell count | ||||
| 2.0–3.0 x 109 cells/L | 7 (2.6) | 0 (0) | 5 (4.6) | 2 (7.7) |
| < 2.0 x 109 cells/L | 2 (0.7) | 0 (0) | 1 (0.9) | 1 (3.8) |
| Platelet count | ||||
| 50–75 x 109 cells/L | 46 (16.8) | 3 (2.2) | 24 (22.0) | 19 (73.1) |
| < 50 x 109 cells/L | 10 (3.7) | 0 (0) | 4 (3.7) | 6 (19.2) |
| Total bilirubin | ||||
| 1.5–3.0 x ULN | 21 (7.7) | 2 (1.4) | 11 (10.1) | 8 (30.8) |
| > 3.0 x ULN | 9 (3.3) | 0 (0) | 4 (3.7) | 5 (19.2) |
| ALT | ||||
| 3–5 x ULN | 6 (2.2) | 3 (2.2) | 2 (1.8) | 1 (3.8) |
| > 5x ULN | 2 (0.7) | 1 (0.7) | 1 (0.9) | 0 (0) |
| eGFR | ||||
| 15–30 mL/min/1.73m2 | 3 (1.1) | 0 (0) | 2 (1.8) | 1 (3.8) |
| < 15 mL/min/1.73m2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
* One patient expired due to pneumonia at treatment week 3, which was considered not related to DAA treatment.
Discussion
Compared to protease inhibitor (PI)-containing HCV DAA regimens for HCV-1 infection, the PI-free SOF/LDV regimen has lower pill burden, fewer potential drug-drug interactions (DDIs), and can be applied to decompensated cirrhotic patients [36–38]. Therefore, treatment by SOF/LDV with or without RBV is appealing to most health care providers in the management of HCV-1 infection.
Our real-world study which enrolled a heterogeneous group of HCV-1 patients showed that the SVR12 rates by EP and PP analyses in patients receiving SOF/LDV with or without RBV for 8–24 weeks were excellent (96.7% and 97.5%, respectively) and were comparable to the response rates in clinical trials and real-world studies [13,14,15,17–20,23–26]. Furthermore, 99.6% of our patients completed the scheduled treatment and 4.4% of them experienced on-treatment serious AEs, which were also comparable to the pooled safety analysis for patients receiving SOF/LDV with or without RBV for 8–24 weeks in ION studies [16]. Only one decompensated cirrhotic patient prematurely discontinued treatment due to pneumonia, which was considered not related to SOF/LDV. The rates of common constitutional AEs, including fatigue, headache, nausea, and insomnia were also in line with the ION reports [16]. However, patients with decompensated cirrhosis tended to have higher risks of serious AE, fatigue, anemia and hyperbilirubinemia than those with no cirrhosis and with compensated cirrhosis, implying that the treating physicians should be alert to the clinical presentations in patients with decompensated cirrhosis to secure the safety profiles [18,19]. Based on the excellent safety and effectiveness in our study, applying SOF/LDV with or without RBV may serve as an ideal regimen for HCV-1 infection.
In terms of patient characteristics, our study showed that the SVR12 rates were similar regardless of age, sex, prior treatment experience, HBV or HIV coinfection, prior HCC history, HCV viral load, eGFR level or week 4 viral decline [17,39–41]. The SVR12 rate in compensated cirrhotic patients was also comparable to non-cirrhotic patients [13,14]. Furthermore, the SVR12 rate in decompensated cirrhotic patients was 88.5% (95% CI: 71.0%-96%) and was comparable to the reports in SOLAR-1 and SOLAR-2 studies [18,19]. Patients with decompensated cirrhosis had lower SVR12 rate than patients with no cirrhosis or with compensated cirrhosis, probably due to lower drug delivery, altered drug metabolism, and impaired immune response in these patients [42–44]. Applying velpatasvir (VEL), which exhibits a higher genetic barrier to N55A resistance associated substitutions (RASs) than LDV, in combination with SOF and RBV for 12 weeks, or treating patients following liver transplantation, may improve the clinical outcome in decompensated cirrhotic patients [18,19,32,45]. Although there were no statistical differences, the SVR12 rate in patients with HCV-1a infection were numerically lower than that with HCV-1b infection, which may be reasoned by the greater loss of response rates for HCV-1a patients receiving SOF/LDV than for HCV-1b patients in the presence of NS5A RASs [46].
Among our HCV-1 patients receiving liver transplantation, 25 patients were treated by SOF/LDV with RBV for 12 weeks and one were treated by SOF/LDV for 24 weeks. All of them achieved SVR12, implying that the effectiveness of SOF/LDV-based therapies remained excellent in this special population [18,19]. In contrast, 13 HCV-1 patients receiving renal transplantation were treated by SOF/LDV for 12 weeks and all achieved SVR12, implying that RBV-free SOF/LDV regimen can be applied to patients receiving non-liver solid organ transplantation [20,21,47].
In ION-3 and real-world studies, treatment-naïve, non-cirrhotic HCV-1 patients with baseline HCV RNA < 6,000,000 IU/mL can receive SOF/LDV for 8 weeks without compromising the treatment responses [15,48]. All 5 (100%) patients and 74 of 75 (98.7%) patients who met such criteria achieved SVR12 by 8 and 12 weeks of SOF/LDV, respectively. In treatment-naïve, compensated cirrhotic HCV-1 patients, our study showed that adding RBV to SOF/LDV for 12 week or extending SOF/LDV treatment to 24 weeks did not benefit the SVR12 rates, compared to SOF/LDV for 12 weeks [49]. In contrast to Western studies, our data were in line with Asian reports indicating that there was no benefit to improve the SVR12 rate by adding RBV to SOF/LDV for 12 weeks in treatment-experienced, compensated cirrhotic HCV-1 patients [50,51]. Further studies are needed to explore the potential mechanisms for such discrepancies.
Among the 9 patients with HBV coinfection, the risks of HBV reactivation and the HBV-related hepatitis after DAA treatment were 22.2% and 0%, which were comparable to the report in a meta-analysis enrolling 242 HBV-coinfected patients [52]. Although there were no apparent clinical events related to HBV reactivation in our study, watchful surveillance of HBV activity is still needed to detect and treat potential complications related to HBV reactivation at the earliest stage.
Although we confirmed that SOF/LDV with or without RBV had excellent safety and effectiveness for HCV-1 patients in Taiwan, several limitations existed in our study. First, the numbers of patients receiving SOF/LDV for 8 or 24 weeks were small and more data are needed to confirm the overall performance in patients of specific interests. Second, HCV-6 patients may potentially be misclassified to unsubtypable HCV-1 patients by Abbott RealTime HCV Genotype II testing, which might affect the SVR12 rate in our study [53]. Third, we did not evaluate the effects baseline NS5A RASs on the treatment responses in our patients, particularly for HCV-1a patients.
In summary, SOF/LDV with or without RBV for 8–24 weeks is well tolerated and achieves a high SVR12 rate in HCV-1 infection, which may improve the care of such patients in Taiwan.
Supporting information
(DOCX)
Acknowledgments
The authors thank Hui-Ju Lin and Pin-Chin Huang for clinical data management; the 7th Core Lab of National Taiwan University Hospital and the 1st Common Laboratory of National Taiwan University Hospital, Yun-Lin Branch for instrumental and technical support.
Data Availability
All relevant data are within the manuscript and its Supporting Information file.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2: 161–176. 10.1016/S2468-1253(16)30181-9 [DOI] [PubMed] [Google Scholar]
- 2.Kao JH. Hepatitis C virus infection in Taiwan: Past, present, and future. J Formos Med Assoc. 2016;115: 65–66. 10.1016/j.jfma.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 3.Liu CH, Kao JH. Nanomedicines in the treatment of hepatitis C virus infection in Asian patients: optimizing use of peginterferon alfa. Int J Nanomedicine. 2014;9: 2051–2067. 10.2147/IJN.S41822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, et al. ; R.E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206: 469–477. 10.1093/infdis/jis385 [DOI] [PubMed] [Google Scholar]
- 5.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308: 2584–2593. 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 6.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158: 329–337. 10.7326/0003-4819-158-5-201303050-00005 [DOI] [PubMed] [Google Scholar]
- 7.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64: 495–503. 10.1136/gutjnl-2014-308163 [DOI] [PubMed] [Google Scholar]
- 8.Mahale P, Engels EA, Li R, Torres HA, Hwang LY, Brown EL, et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67: 553–561. 10.1136/gutjnl-2017-313983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61: 77–87. 10.1002/hep.27259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol. 2013;85: 1724–1733. 10.1002/jmv.23661 [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28: 4587–4593. 10.1200/JCO.2010.29.1500 [DOI] [PubMed] [Google Scholar]
- 12.Keating GM. Sofosbuvir: a review of its use in patients with chronic hepatitis C. Drugs. 2014;74: 1127–1146. 10.1007/s40265-014-0247-z [DOI] [PubMed] [Google Scholar]
- 13.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. ; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370: 1889–1898. 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 14.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. ; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370: 1483–1493. 10.1056/NEJMoa1316366 [DOI] [PubMed] [Google Scholar]
- 15.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. ; ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879–1888. 10.1056/NEJMoa1402355 [DOI] [PubMed] [Google Scholar]
- 16.Alqahtani SA, Afdhal N, Zeuzem S, Gordon SC, Mangia A, Kwo P, et al. Safety and tolerability of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: Analysis of phase III ION trials. Hepatology. 2015;62: 25–30. 10.1002/hep.27890 [DOI] [PubMed] [Google Scholar]
- 17.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. ; ION-4 Investigators. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373: 705–713. 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, et al. ; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149: 649–659. 10.1053/j.gastro.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. ; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16: 685–697. 10.1016/S1473-3099(16)00052-9 [DOI] [PubMed] [Google Scholar]
- 20.Colombo M, Aghemo A, Liu H, Zhang J, Dvory-Sobol H, Hyland R, et al. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med. 2017;166: 109–117. 10.7326/M16-1205 [DOI] [PubMed] [Google Scholar]
- 21.Liu CH, Chen YS, Wang SS, Liu CJ, Su TH, Yang HC, et al. Sofosbuvir-based interferon-free direct acting antiviral regimens for heart transplant recipients with chronic hepatitis C virus infection. Clin Infect Dis. 2018;66: 289–292. 10.1093/cid/cix787 [DOI] [PubMed] [Google Scholar]
- 22.Terrault NA, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros PJ, Frazier LM, et al. ; HCV-TARGET Study Group. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151: 1131–1140.e5. 10.1053/j.gastro.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151: 457–471.e5. 10.1053/j.gastro.2016.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64: 405–414. 10.1002/hep.28625 [DOI] [PubMed] [Google Scholar]
- 25.Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, et al. ; Spanish Group for the Study of the Use of Direct-acting Drugs Hepatitis C Collaborating Group. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol. 2017;66: 1138–1148. 10.1016/j.jhep.2017.01.028 [DOI] [PubMed] [Google Scholar]
- 26.Ji F, Wei B, Yeo YH, Ogawa E, Zou B, Stave CD, et al. Systematic review with meta-analysis: effectiveness and tolerability of interferon-free direct-acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment Pharmacol Ther. 2018;47: 550–562. 10.1111/apt.14507 [DOI] [PubMed] [Google Scholar]
- 27.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62: 932–954. 10.1002/hep.27950 [DOI] [PubMed] [Google Scholar]
- 28.European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60: 392–420. 10.1016/j.jhep.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10: 702–726. 10.1007/s12072-016-9717-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CH, Liang CC, Liu CJ, Lin CL, Su TH, Yang HC, et al. Comparison of Abbott RealTime HCV Genotype II with Versant Line Probe Assay 2.0 for hepatitis C virus genotyping. J Clin Microbiol. 2015;53: 1754–1757. 10.1128/JCM.03548-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CH, Liu CJ, Hong CM, Su TH, Yang HC, Chen KM, et al. A noninvasive diagnosis of hepatic fibrosis by BioFibroScore in chronic hepatitis C patients. J Gastroenterol Hepatol. 2018;33: 291–297. 10.1111/jgh.13834 [DOI] [PubMed] [Google Scholar]
- 32.Liu CH, Sun HY, Liu CJ, Sheng WH, Hsieh SM, Lo YC, et al. Generic velpatasvir plus sofosbuvir for hepatitis C virus infection in patients with or without human immunodeficiency virus coinfection. Aliment Pharmacol Ther. 2018;47: 1690–1698. 10.1111/apt.14647 [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Sheng WH, Sun HY, Hsieh SM, Lo YC, Liu CJ, et al. Peginterferon plus ribavirin for HIV-infected patients with treatment-naïve acute or chronic HCV infection in Taiwan: a prospective cohort study. Sci Rep. 2015;5: 17410 10.1038/srep17410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu CH, Liu CJ, Su TH, Fang YJ, Yang HC, Chen PJ, et al. Hepatitis B virus reactivation in patients receiving interferon-free direct-acting antiviral agents for chronic hepatitis C virus infection. Open Forum Infect Dis. 2017;4: ofx028 10.1093/ofid/ofx028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67: 1560–1599. 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu CH, Liu CJ, Su TH, Yang HC, Hong CM, Tseng TC, et al. Real-world effectiveness and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with or without ribavirin for patients with chronic hepatitis C virus genotype 1b infection in Taiwan. J Gastroenterol Hepatol. 2018;33: 710–717. 10.1111/jgh.13912 [DOI] [PubMed] [Google Scholar]
- 37.Liu CH, Yu ML, Peng CY, Hsieh TY, Huang YH, Su WW, et al. Comorbidities, concomitant medications, and potential drug-drug interactions with interferon-free direct-acting antiviral agents in hepatitis C patients in Taiwan. Aliment Pharmacol Ther. 2018;48: 1290–1300. 10.1111/apt.15011 [DOI] [PubMed] [Google Scholar]
- 38.Liu CH, Huang YJ, Yang SS, Chang CH, Yang SS, Sun HY, et al. Generic sofosbuvir-based interferon-free direct acting antiviral agents for patients with chronic hepatitis C virus infection: a real-world multicenter observational study. Sci Rep. 2018;8: 13699 10.1038/s41598-018-32060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, et al. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology. 2018;154: 989–997. 10.1053/j.gastro.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 40.Maasoumy B, Vermehren J, Welker MW, Bremer B, Perner D, Höner Zu Siederdissen C, et al. Clinical value of on-treatment HCV RNA levels during different sofosbuvir-based antiviral regimens. J Hepatol. 2016;65: 473–482. 10.1016/j.jhep.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 41.Fourati S, Guedj J, Chevaliez S, Nguyen THT, Roudot-Thoraval F, Ruiz I, et al. Viral kinetics analysis and virological characterization of treatment failures in patients with chronic hepatitis C treated with sofosbuvir and an NS5A inhibitor. Aliment Pharmacol Ther. 2018;47: 665–673. 10.1111/apt.14478 [DOI] [PubMed] [Google Scholar]
- 42.Al Marzooqi SH, Feld JJ. Sorting out cirrhosis: mechanisms of non-response to hepatitis C therapy. Liver Int. 2015;35: 1923–1933. 10.1111/liv.12861 [DOI] [PubMed] [Google Scholar]
- 43.Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful interferon-free therapy of chronic hepatitis C virus infection normalizes natural killer cell function. Gastroenterology. 2015;149: 190–200.e2. 10.1053/j.gastro.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serti E, Park H, Keane M, O’Keefe AC, Rivera E, Liang TJ, et al. Rapid decrease in hepatitis C viremia by direct acting antivirals improves the natural killer cell response to IFNα. Gut. 2017;66: 724–735. 10.1136/gutjnl-2015-310033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373: 2618–2628. 10.1056/NEJMoa1512614 [DOI] [PubMed] [Google Scholar]
- 46.Zeuzem S, Mizokami M, Pianko S, Mangia A, Han KH, Martin R, et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: prevalence and effect on treatment outcome. J Hepatol. 2017;66: 910–918. 10.1016/j.jhep.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 47.D’Ambrosio R, Aghemo A, Rossetti V, Carrinola R, Colombo M. Sofosbuvir-based regimens for the treatment of hepatitis C virus in patients who underwent lung transplant: case series and review of the literature. Liver Int. 2016;36: 1585–1589. 10.1111/liv.13203 [DOI] [PubMed] [Google Scholar]
- 48.Kowdley KV, Sundaram V, Jeon CY, Qureshi K, Latt NL, Sahota A, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65: 1094–1103. 10.1002/hep.29005 [DOI] [PubMed] [Google Scholar]
- 49.Reddy KR, Bourlière M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology. 2015;62: 79–86. 10.1002/hep.27826 [DOI] [PubMed] [Google Scholar]
- 50.Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15: 645–653. 10.1016/S1473-3099(15)70099-X [DOI] [PubMed] [Google Scholar]
- 51.Chuang WL, Chien RN, Peng CY, Chang TT, Lo GH, Sheen IS, et al. Ledipasvir/sofosbuvir fixed-dose combination tablet in Taiwanese patients with chronic genotype 1 hepatitis C virus. J Gastroenterol Hepatol. 2016;31: 1323–1329. 10.1111/jgh.13305 [DOI] [PubMed] [Google Scholar]
- 52.Mücke MM, Backus LI, Mücke VT, Coppola N, Preda CM, Yeh ML, et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3: 172–180. 10.1016/S2468-1253(18)30002-5 [DOI] [PubMed] [Google Scholar]
- 53.Mallory MA, Lucic D, Ebbert MT, Cloherty GA, Toolsie D, Hillyard DR. Evaluation of the Abbott RealTime HCV genotype II plus RUO (PLUS) assay with reference to core and NS5B sequencing. J Clin Virol. 2017;90: 26–31. 10.1016/j.jcv.2017.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information file.