ABSTRACT
Purpose
This study examined the aftereffects of cognitively demanding acute aerobic exercise on working memory in middle-age individuals.
Methods
In a within-participants design, middle-age males (n = 28) performed a two-back task to assess working memory before, immediately after, and 30 min after the following three interventions: 1) a rest-cognition intervention, in which they performed a cognitive task on a cycle ergometer without exercising; 2) an exercise-cognition intervention, in which they simultaneously exercised on a cycle ergometer and performed a cognitive task; and 3) an exercise-only intervention, in which they only exercised on a cycle ergometer.
Results
The exercise-only intervention resulted in increased hit rate and decreased reaction times and intraindividual variability on correct rejection trials, suggesting that simple aerobic exercise had a beneficial impact on working memory. By contrast, the exercise-cognition intervention resulted in increased intraindividual variability on correct rejection trials, which is suggestive of cognitive fatigue resulting from the additional cognitive demands. Such a decline was not observed even in the rest-cognition condition.
Conclusions
Cognitive fatigue caused by additional cognitive demands during aerobic exercise may cancel beneficial postexercise effects on working memory. Cognitively demanding acute aerobic exercise appears to be less effective than simple aerobic exercise in improving executive function.
Key Words: SINGLE BOUT OF EXERCISE, EXECUTIVE FUNCTION, COGNITIVE FUNCTION, COGNITIVE FATIGUE, MIDDLE AGE
A considerable number of studies published over the last two decades have provided evidence that chronic regular exercise improves cognitive function across the lifespan, and this beneficial effect is disproportionately greater for higher-order cognitive function (i.e., executive function), such as inhibition, working memory, and cognitive flexibility (1,2). More recently, researchers have attempted to shed light on the types of exercise that are more effective for improving cognitive function. Some have found that cognitively demanding chronic exercise (e.g., exergaming) has a greater impact on cognitive function than simple exercise (e.g., running) (3,4). It is possible that this chronic effect is attributable to the increased beneficial effects of acute exercise on cognitive function given by cognitively demanding exercise than simpler exercise because the habitual repetitions of acute exercise result in chronic effects. Several studies have indeed found that cognitively demanding acute exercise had a greater positive impact than simpler exercise on postexercise cognitive performance in children (5,6) and older adults (7). However, other research has suggested that sustained cognitive effort during exercise may not enhance cognitive function. Berman et al. (8) found that working memory performance improved after walking in nature, but not after walking in urban areas. Based on attention restoration theory (9), Berman et al. suggested that walking in nature, which modestly captures bottom-up attention, can provide the opportunity to replenish cognitive abilities. Stated differently, urban environments, which require sustained top-down attention, may induce cognitive fatigue, resulting in inferior working memory performance. Several other studies also found cognitively demanding exercise to be less effective than simpler exercise in enhancing cognitive function in children (10,11) and young adults (12).
Because these acute exercise studies were conducted in a variety of settings, including exergaming (5,7,12), game-based exercise (6), and classrooms (10,11), factors besides cognitive demands that could have influenced postexercise cognitive function, such as motor control demands, social interaction, and enjoyment, were likely to have differed between the exercise conditions. Differences in experimental design (i.e., between-participants vs within-participants) may have also led to different effects on cognitive performance. In acute exercise studies, a within-participants design is generally considered to be more appropriate than a between-participants design in preventing effects of individual differences or other personality attributes. A relatively small number of studies have used a within-participants design to examine the aftereffects of cognitively demanding acute exercise. Studies using a within-participants design showed a smaller improvement in postexercise cognitive performance for cognitively demanding exercise relative to simpler exercise (11,12). Thus, further empirical research is needed to elucidate the aftereffects of cognitively demanding acute exercise on cognitive function in a well-controlled laboratory setting. The present study examined the aftereffects of such exercise on working memory, which is one of the key aspects of executive function.
The majority of acute exercise studies have focused on children, young adults, or older adults (13), and thus little is known about the effects of acute exercise on cognitive function in middle-age individuals. Spartano et al. (14) indicated that midlife aerobic fitness was associated with brain volume nearly 20 yr later, suggesting that aerobic exercise in middle age may be beneficial for healthy brain aging. The secondary purpose of this study was to examine whether middle-age individuals experience effects of aerobic exercise on working memory that are similar to those found in other age groups. Middle-age individuals are in their most productive years, and working memory plays a critical role in supporting them in various aspects of complex everyday cognitive activities (15) including business performance (16). Therefore, an association between aerobic exercise and working memory could encourage middle-age individuals to exercise during working hours.
The present study used a within-participants design in which middle-age participants performed a two-back task, which required updating working memory information before, immediately after, and 30 min after three interventions: 1) a rest-cognition intervention, in which participants performed a cognitive task on a cycle ergometer without exercising; 2) an exercise-cognition intervention, in which participants simultaneously performed a cycling exercise and a cognitive task; and 3) an exercise-only intervention, in which participants only exercised on a cycle ergometer without performing a cognitive task. The exercise-cognition condition and the exercise-only condition were identical in every way except for cognitive demands during the cycling exercise, and the rest-cognition condition served as a rest control condition. Given that a number of studies have shown beneficial effects of acute aerobic exercise on postexercise executive function (13,17), we predicted that working memory performance, as reflected in response accuracy and reaction times (RT), would improve after the interventions in both exercise conditions, but not in the rest-cognition condition. Because of the contradictory findings noted above, we could not make a firm prediction regarding the beneficial effects of acute exercise on working memory in the exercise-cognition versus exercise-only conditions.
METHOD
Participants
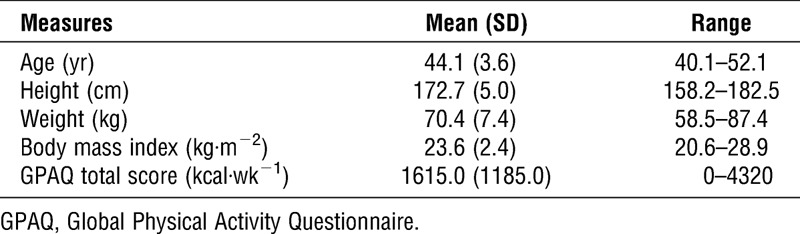
Thirty-six middle-age men were recruited from Shimano Inc., Osaka, Japan. All participants reported being free of neurological disorders, cardiovascular disease, and any medications that influenced central nervous system function, and all were nonsmokers with normal or corrected-to-normal vision. The study was approved by the Ethics Committee on Human Research of Waseda University, and all participants provided written informed consent. Data from eight participants were discarded because they did not complete all experimental conditions (six participants) or performed below chance (i.e., <50%) on the two-back task used to assess working memory (two participants). Thus, data from 28 participants were analyzed. Table 1 presents demographic data for this sample. A sensitivity analysis performed based on this sample with 80% power and alpha of 0.05 demonstrated sufficient sensitivity to detect repeated measures effects exceeding f = 0.247 (assuming correlation between repeated measures ≥0.5) and t-test differences exceeding d = 0.549 (with a two-tailed alpha), as computed using G*Power 3.1.9.2 (18).
TABLE 1.
Mean (SD) values for demographic data.
Laboratory Procedure
On the first day, participants completed the informed consent form, the Physical Activity Readiness Questionnaire (19) to screen for possible medical risks that could be exacerbated by exercise, and the Global Physical Activity Questionnaire (20) to assess their physical activity levels, and had their height and weight measured. They were then given task instructions for the two-back and switch tasks, which were performed in subsequent experimental conditions on different days, and they engaged in practice trials until they reached the criterion of 70% accuracy.
Figure 1 illustrates the experimental protocol. A within-participants repeated-measures design was used. Three experimental conditions were investigated: the rest-cognition condition, the exercise-cognition condition, and the exercise-only condition. Participants performed a two-back task before (pretest), immediately after (post 1), and 30 min after (post 2) each intervention while sitting on a chair. Between post 1 and post 2, they sat on the chair and quietly read a newspaper. Each condition was conducted at the same time on a different day (mean days apart = 10.1 d, SD = 2.8), and the condition order was randomized and counterbalanced across participants. Before the start of each experimental session, participants were fitted with an HR monitor (Garmin Forerunner 735XT, Garmin, Olathe, KS) to measure HR throughout the test.
FIGURE 1.
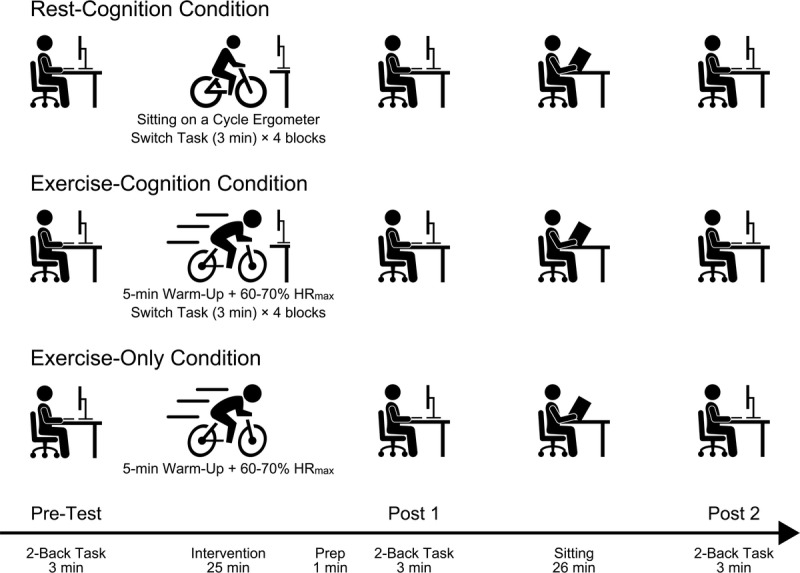
Experimental protocol. Order of experimental conditions was randomized and counterbalanced across participants.
Interventions
The duration of each intervention was 25 min. In the rest-cognition condition, participants sat quietly on a cycle ergometer (BikeFitting BF-PS01/BF-PA01; Shimano, Osaka, Japan) for 5 min and then performed four blocks of a 3-min switch task. They were given 2-min breaks after each block to refocus them on the switch task. In the exercise-cognition condition, a 5-min warm-up was provided, during which the cycling workload was gradually increased, followed by 20 min of cycling exercise at an intensity of 60% to 70% of age-predicted (208 − 0.7 × age) (21) maximum HR (HRmax). Based on a meta-analysis (17), we determined this exercise intensity and duration to be sufficient to detect cognitive benefits after acute aerobic exercise. During the 20-min cycling exercise, participants simultaneously performed four blocks of a 3-min switch task with 2-min breaks after each block (i.e., 3-min exercise with a switch task followed by 2-min exercise without a switch task, repeated four times). Participants were instructed to maintain a cadence between 60 and 70 rpm, and RPE were assessed every 5 min using the 15-point Borg scale (22). The exercise-only condition was identical to the exercise-cognition condition, except that participants did not engage in the switch task during the cycling exercise.
Cognitive Tasks
Stimuli were presented, and responses were recorded using E-Prime 2 software and a Chronos response box (Psychology Software Tools, Inc., Pittsburgh, PA). Working memory performance was assessed with a two-back task as described in the following subsection. During the interventions in both cognition conditions, participants performed an additional cognitive task requiring extensive amounts of top-down cognitive control, a switch task (23), to eliminate practice effects on two-back performance. In both cognitive tasks, stimuli were single-digit numbers, and all stimuli were presented in black on a white background in Courier New font. The viewing distance was approximately 57.3 cm, and each digit subtended 2.3° horizontally and 3.7° vertically. Both accuracy and speed were emphasized in the instructions, and trials with RT shorter than 200 ms and longer than 1500 ms were considered as anticipatory and attentional errors, respectively.
Two-back task
Participants were presented with a continuous sequence of single-digit numbers from 0 to 9, and were instructed to press one of two buttons with their index fingers corresponding to whether the currently presented single-digit matched (right button) or did not match (left) the digit presented two previously in the sequence. Seventy-two stimuli were presented, and the first two trials were discarded, leaving 70 trials to be analyzed. Target stimuli (i.e., matched digits) within the remaining trials were pseudorandomly presented with a probability of 33% (i.e., 23 trials). Each stimulus was presented for 200 ms with a fixed stimulus onset asynchrony of 2500 ms, and thus the task duration was 3 min. Outcome variables for the two-back task included response accuracy and RT on hit trials (i.e., correct identification of matched digits) and correct rejection trials (i.e., correct identification of unmatched digits). We also assessed intraindividual variability (i.e., standard deviation of RT [SDRT]) on hit and correct rejection trials, which is considered as a measure of cognitive fatigue (24).
Switch task
Participants were presented with a continuous sequence of single-digit numbers from 1 to 9, excluding 5, which were enclosed within a solid or dashed square. This task asked participants to press one of two buttons with their index fingers corresponding to whether the digit was lower (left button) or higher (right) than 5 for the digit surrounded by a solid square, or whether the digit was odd (left) or even (right) for the digit surrounded by a dashed square. Seventy-two stimuli were presented, and the first trial was discarded, leaving 71 trials to be analyzed. The task (i.e., low/high or odd/even) changed predictably every two trials (i.e., AABBAA…), resulting in 36 nonswitch and 35 switch trials. All eight digits were pseudorandomly presented with equal probability. Each stimulus was presented for 200 ms with a fixed stimulus onset asynchrony of 2500 ms, and thus the task duration was 3 min. Participants performed four blocks of this 3-min switch task during the interventions in both cognition conditions. Outcome variables for the switch task included response accuracy, RT, and SDRT on nonswitch and switch trials. Data from one participant were lost due to experimenter error, and analyses for the switch task performance were thus conducted for 27 participants.
Statistical Analysis
Data were analyzed using SPSS (SPSS v. 25, Chicago, IL). For the two-back task, response accuracy, RT, and SDRT on hit and correct rejection trials were separately analyzed using a 3 (Condition: rest-cognition, exercise-cognition, exercise-only) × 3 (Time: pretest, post 1, post 2) repeated-measures ANOVA. For the switch task, response accuracy, RT, and SDRT were analyzed using a 2 (Condition: rest-cognition, exercise-cognition) × 2 (Trial: switch, nonswitch) repeated-measures ANOVA. HR was analyzed using a 3 (Condition: rest-cognition, exercise-cognition, exercise-only) × 4 (Time: pretest, during, post 1, post 2) repeated-measures ANOVA. RPE was analyzed using a paired t-test between the exercise-cognition and exercise-only conditions. Analyses with three or more within-participants levels used the Greenhouse–Geisser statistic if the sphericity assumption was violated. Post hoc analyses were conducted using univariate ANOVA and Bonferroni-corrected t-tests. Effect sizes are presented as partial eta-squared and Cohen’s d for ANOVA and t-tests, respectively. All statistical analyses were conducted using a significance level of P = 0.05 before Bonferroni correction.
RESULTS
Intervention
Analysis of HR revealed significant main effects of Condition (F(2, 54) = 129.3, P < 0.001, η2p = 0.83, and Time, F(3, 81) = 499.1, P < 0.001, η2p = 0.95, which were qualified by a Condition–Time interaction (F(6, 162) = 171.6, P < 0.001, η2p = 0.87), as shown in Figure 2. No differences emerged at pretest, (F(2, 54) = 0.5, P = 0.6, η2p = 0.02), whereas significant differences were observed during intervention and at post 1 and post 2 (F(2, 54) ≥ 8.5, P ≤ 0.002, η2p ≥ 0.24). Bonferroni-corrected post hoc t-tests (P < 0.017) indicated that HR was higher for both exercise conditions relative to the rest-cognition condition during intervention and at post 1 and post 2, t’s (27) ≥ 3.0, P’s ≤ 0.006, d’s ≥ 0.56). Although participants had higher HR even at post 2 for both exercise conditions, as expected, mean HR were within 10% of preexercise levels (7.3% and 8.8% for exercise-cognition and exercise-only, respectively), the criterion used in previous studies to examine the aftereffects of acute aerobic exercise on cognition (25,26). More importantly, changes in HR were nearly identical in the two exercise conditions. Analysis of RPE revealed no difference between the exercise-cognition (mean = 11.9, SD = 1.0) and exercise-only (mean = 11.8, SD = 0.9) conditions, t (27) = 1.8, P = 0.2, d = 0.22.
FIGURE 2.
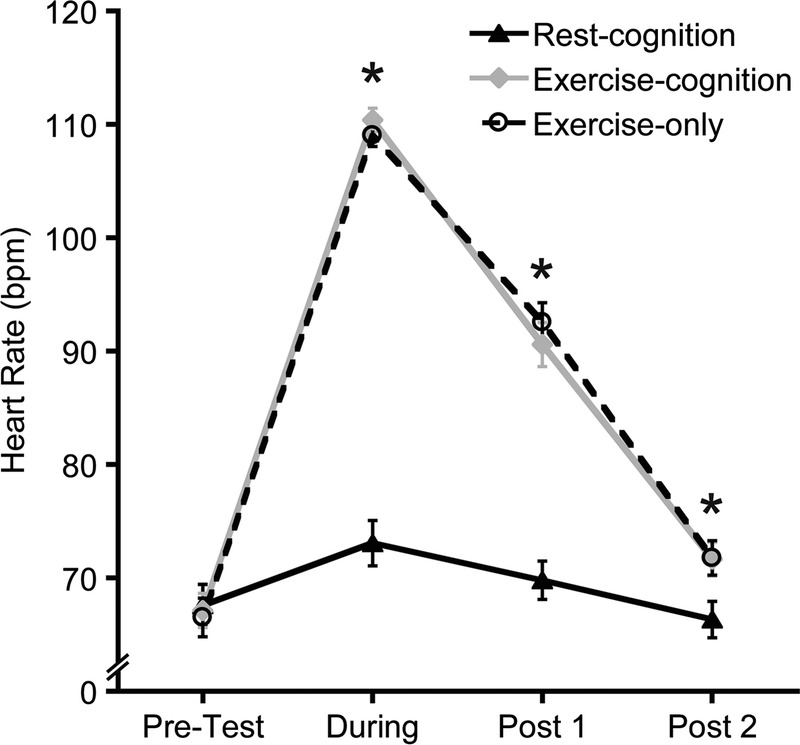
Mean heart rate for each experimental condition. Error bars indicate SE. *P < 0.017 (Bonferroni correction) between rest-cognition condition and both exercise conditions.
Two-Back Task Performance
Analysis of hit accuracy revealed a significant Condition–Time interaction (F(4, 108) = 2.6, P = 0.04, η2p = 0.09), as shown in Figure 3A. No time difference was observed for the rest-cognition and exercise-cognition conditions (F(2, 54) ≤ 1.7, P ≥ 0.2, η2ps ≤ 0.06, whereas a significant time difference emerged in the exercise-only condition (F(2, 54) = 4.2, P = 0.02, η2p = 0.13). Bonferroni-corrected post hoc t-tests (P < 0.017) indicated that hit accuracy increased at post 2 relative to pretest for the exercise-only condition (t (27) = 3.1, P = 0.005, d = 0.58). Analysis of correct rejection accuracy revealed a significant Time main effect (F(2, 54) = 8.8, P = 0.001, η2p = 0.25), which demonstrated decreased correct rejection accuracy at post 1 relative to pretest and post 2 across conditions (t’s (27) ≥ 3.4, P’s ≤ 0.002, d’s ≥ 0.64, as shown in Figure 3B).
FIGURE 3.
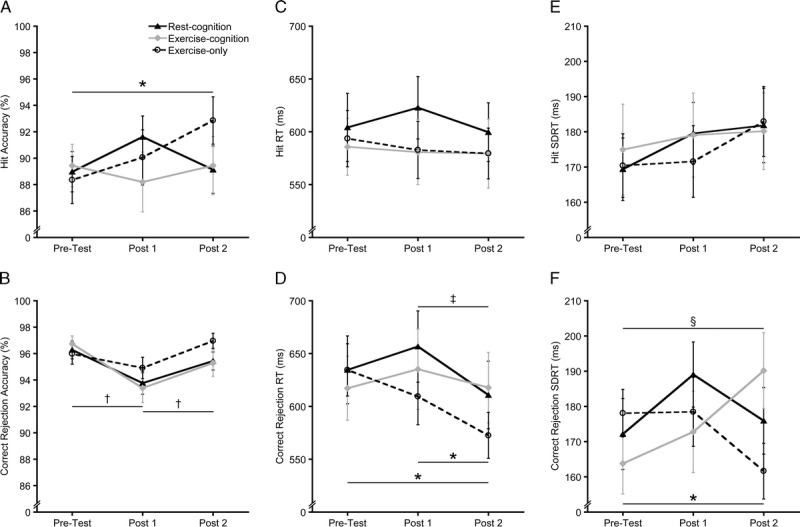
Mean response accuracy (A, B), RT (C, D), and SDRT (E, F) on hit and correct rejection trials during two-back task for each experimental condition. Error bars indicate SE. *P < 0.017 (Bonferroni correction) for exercise-only condition, †P < 0.017 across conditions, ‡P < 0.017 for rest-cognition condition, §P < 0.017 for exercise-cognition condition.
Analysis of hit RT revealed no significant main effects or interaction (P’s ≥ 0.3; Figure 3C). Analysis of correct rejection RT revealed a significant main effect of Time (F(2, 54) = 9.6, P < 0.001, η2p = 0.26), which was qualified by a Condition–Time interaction (F(4, 108) = 3.5, P = 0.01, η2p = 0.11), as shown in Figure 3D. No time difference was found for the exercise-cognition condition (F(2, 54) = 0.8, P = 0.5, η2p = 0.03), but significant time differences were observed for the rest-cognition and exercise-only conditions (F’s(2, 54) ≥ 5.5, P’s ≤ 0.007, η2ps ≥ 0.17). Bonferroni-corrected post hoc t-tests (P < 0.017) indicated that correct rejection RT decreased at post 2 relative to post 1 in the rest-cognition condition (t (27) = 4.1, P < 0.001, d = 0.78) and relative to pretest and post 1 for the exercise-only condition (t’s (27) ≥ 3.2, P’s ≤ 0.004, d’s ≥ 0.60).
Analysis of hit SDRT revealed no significant main effect or interaction (P’s ≥ 0.4; Figure 3E). Analysis of correct rejection SDRT revealed a significant Condition–Time interaction, F(4, 108) = 4.0, P = 0.004, η2p = 0.13, as illustrated in Figure 3F. Significant time differences were observed for the exercise-cognition and exercise-only conditions (F’s(2, 54) ≥ 3.7, P’s ≤ 0.03, η2ps ≥ 0.12), but not for the rest-cognition condition (F(2, 54) = 2.0, P = 0.1, η2p = 0.07). Bonferroni-corrected post hoc t-tests (P < 0.017) indicated that correct rejection SDRT increased at post 2 relative to pretest in the exercise-cognition condition (t (27) = 2.7, P = 0.01, d = 0.52), but decreased at post 2 relative to pretest in the exercise-only condition (t (27) = 2.6, P = 0.016, d = 0.49).
Switch Task Performance
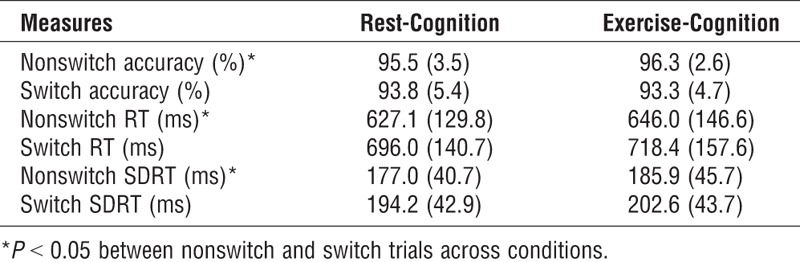
Table 2 presents switch task performance for the rest-cognition and exercise-cognition conditions. Analysis of response accuracy revealed a significant main effect of Trial (F (1, 26) = 21.2, P < 0.001, η2p = 0.45, with higher response accuracy for nonswitch relative to switch trials across conditions. Analysis of RT also revealed a significant main effect of Trial, F (1, 26) = 49.3, P < 0.001, η2p = 0.66, with shorter RT for nonswitch relative to switch trials across conditions. The Trial main effect was also significant in the analysis of SDRT (F (1, 26) = 7.2, P < 0.01, η2p = 0.22), with smaller SDRT for nonswitch relative to switch trials across conditions. No main effects or interactions involving the factor were significant for any switch task performance measures. These results demonstrate the typical switch effect, with no difference in cognitive performance between the rest-cognition and exercise-cognition conditions.
TABLE 2.
Mean (SD) values for switch task performance.
DISCUSSION
In the exercise-only condition, working memory performance improved after moderate aerobic exercise, as indicated by greater hit accuracy and shorter correct rejection RT. This result supports existing evidence that simple aerobic exercise has a beneficial impact on the working memory aspect of executive function (13,17). Furthermore, the positive effects of simple exercise were observed 30 min after, but not immediately following, acute aerobic exercise. This result is consistent with the conclusions of a meta-analysis, which reported that the effects of moderate intensity exercise were more significant after a delay than immediately following the exercise (17). In addition, intraindividual variability on correct rejection trials decreased 30 min after cycling exercise relative to pretest in the exercise-only condition. Intraindividual variability is considered to reflect the ability to monitor and maintain the consistency of task performance during acute cognitive effort (27). Thus, the decreased intraindividual variability following aerobic exercise observed in the exercise-only condition is likely to be indicative of upregulation of executive control to maintain task performance over the entire course of the working memory task.
Contrary to our hypothesis, no such improvement was observed for the exercise-cognition condition. Based on attention restoration theory (8), we suggest that beneficial postexercise effects on working memory may have been nullified because of cognitive fatigue from additional cognitive demands during the cycling exercise. The results of correct rejection SDRT support this assertion. The intraindividual variability on correct rejection trials increased 30 min after cycling exercise relative to pretest for the exercise-cognition condition, which is opposite to the exercise-only condition. Wang et al. (24) examined changes in cognitive performance during a prolonged (3 h) executive function task and found that intraindividual variability increased linearly over time and tended to change more over time than response accuracy and RT. From these findings, they suggested that change in intraindividual variability over time is a key performance measure of cognitive fatigue. Accordingly, the increased intraindividual variability after the present cycling exercise in the exercise-cognition condition is suggestive of cognitive fatigue resulting from the additional cognitive demands during exercise. Thus, the present evidence suggests that cognitively demanding acute aerobic exercise is less effective than simple aerobic exercise in improving postexercise executive function, consistent with prior research using a within-participants design (11,12).
It is notable that two-back performance in the rest-cognition condition after the intervention was mostly sustained relative to pretest, suggesting that the current intermittent switch task did not produce cognitive fatigue independently. Stated differently, the simultaneous combination of the switch task and aerobic exercise (i.e., cognitively demanding exercise) may have resulted in cognitive fatigue. It has been well established that the anterior cingulate cortex (ACC) plays a critical role in executive function (28), and ACC activity is likely to be sensitive to cognitive fatigue. For example, Lorist et al. (29) showed that ACC activity decreased during a prolonged executive function task with concomitant increases in RT and declines in performance adjustments after errors (i.e., disappearance of posterror slowing). This suggests that the reduced task performance caused by cognitive fatigue is partly due to deterioration in ACC functions such as action monitoring, the ability to monitor performance and adjust goal-directed behavior. ACC activity also seems to be associated with perception of effort during cycling exercise (30) and can influence exercise performance. Martin et al. (31) found that cognitive fatigue induced by a prolonged executive function task, which should have resulted in decreased ACC activity, impaired subsequent cycling performance. These findings suggest that ACC activity is important for maintaining both cognitive performance and exercise performance. In the present study, it is likely that the intermittent switch task alone and moderate aerobic exercise alone were not enough to induce fatigue in the ACC, whereas the simultaneous combination of the switch task and aerobic exercise (i.e., cognitively demanding exercise) triggered fatigue in the ACC. Given that the present study did not assess ACC activity, further neuroimaging studies are required to support this assertion.
Although we demonstrated that cognitively demanding acute aerobic exercise was less effective than simple exercise in improving executive function, some caution is warranted in interpreting the present results. First, we should emphasize that because we focused on acute exercise here, the present findings do not mean that cognitively demanding chronic exercise has a smaller effect than simpler exercise on executive function. Several studies have indicated that cognitively demanding chronic exercise is effective in improving cognitive function (3,4). It is possible that a greater amount of load on the brain is required for chronic improvements in cognitive function, as with the overload principle of strength training. Thus, the effects of acute exercise and chronic exercise should be considered separately. Second, because the current switch task, which was used to impose additional cognitive demands during cycling exercise, required extensive amounts of top-down cognitive control (23), cognitive fatigue was likely to have been produced after cognitively demanding exercise. This fatigue in turn may have underlain the observed impaired working memory performance. Further research should examine whether the effects of cognitively demanding exercise on executive function differ depending on the amount of cognitive demand during exercise. Third, as we only examined male participants here, our findings may not generalize to female populations. More importantly, the present study showed that the effects of acute simple aerobic exercise (i.e., the exercise-only intervention) on executive function in middle-age males, who have received little research attention, were similar to those on other age groups, as reported in a meta-analysis (13). Fourth, because the order of conditions was counterbalanced for all 36 participants, and data from eight participants were discarded from the analyses, the presentation order was not perfectly counterbalanced for the remaining 28 participants that were included in the current analyses. However, the order was randomized, and the six possible orders were equally distributed among the 28 participants with four to five participants for each order. Thus, it is reasonable to assume that the order effect was negligible. Lastly, the rest-cognition condition in the present study was not a typical nonexercise control condition. Consequently, this difference might make it difficult to conduct a simple comparison between the current study and past studies. However, we believe that the rest-cognition condition was the appropriate control condition for the present study.
The present study has shown that additional cognitive demands during acute aerobic exercise (i.e., cognitively demanding exercise) can cancel beneficial postexercise effects on working memory. It is well documented that chronic changes in exercise behavior are difficult to sustain (32). Meanwhile, for example, if work efficiency can be improved after a single bout of acute aerobic exercise, people may try to find time to exercise during working hours. Thus, we believe that acute exercise studies are important from a behavioral change perspective. Given that the effects are likely to differ between acute and chronic exercise, future studies are needed to link the relationship between the effects of these types of exercise on cognition.
Acknowledgments
This work was supported by Shimano Inc. This work was also supported in part by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2015–2019 from the Ministry of Education, Culture, Sports, Science, and Technology (S1511017).
K. K. has no conflicts of interest to disclose. K. K. is now at Faculty of Engineering, Information and Systems, University of Tsukuba, Tsukuba, Ibaraki, Japan, and Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan. R. A. is an employee of Shimano Inc. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Erickson KI, Hillman CH, Kramer AF. Physical activity, brain, and cognition. Curr Opin Behav Sci. 2015;4:27–32. [Google Scholar]
- 2.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. [DOI] [PubMed] [Google Scholar]
- 3.Diamond A. Effects of physical exercise on executive functions: going beyond simply moving to moving with thought. Ann Sports Med Res. 2015;2(1):1011. [PMC free article] [PubMed] [Google Scholar]
- 4.Tait JL, Duckham RL, Milte CM, Main LC, Daly RM. Influence of sequential vs. simultaneous dual-task exercise training on cognitive function in older adults. Front Aging Neurosci. 2017;9:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzing V, Heinks T, Eggenberger N, Schmidt M. Acute cognitively engaging exergame-based physical activity enhances executive functions in adolescents. PLoS One. 2016;11(12):e0167501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishihara T, Sugasawa S, Matsuda Y, Mizuno M. The beneficial effects of game-based exercise using age-appropriate tennis lessons on the executive functions of 6–12-year-old children. Neurosci Lett. 2017;642:97–101. [DOI] [PubMed] [Google Scholar]
- 7.Barcelos N, Shah N, Cohen K, et al. Aerobic and cognitive exercise (ACE) pilot study for older adults: executive function improves with cognitive challenge while exergaming. J Int Neuropsychol Soc. 2015;21(10):768–79. [DOI] [PubMed] [Google Scholar]
- 8.Berman MG, Jonides J, Kaplan S. The cognitive benefits of interacting with nature. Psychol Sci. 2008;19(12):1207–12. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan S. The restorative benefits of nature: toward an integrative framework. J Environ Psychol. 1995;15(3):169–82. [Google Scholar]
- 10.Egger F, Conzelmann A, Schmidt M. The effect of acute cognitively engaging physical activity breaks on children’s executive functions: too much of a good thing? Psychol Sport Exerc. 2018;36:178–86. [Google Scholar]
- 11.Gallotta MC, Guidetti L, Franciosi E, Emerenziani GP, Bonavolonta V, Baldari C. Effects of varying type of exertion on children’s attention capacity. Med Sci Sports Exerc. 2012;44(3):550–5. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary KC, Pontifex MB, Scudder MR, Brown ML, Hillman CH. The effects of single bouts of aerobic exercise, exergaming, and videogame play on cognitive control. Clin Neurophysiol. 2011; 122(8):1518–25. [DOI] [PubMed] [Google Scholar]
- 13.Ludyga S, Gerber M, Brand S, Holsboer-Trachsler E, Puhse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology. 2016;53(11):1611–26. [DOI] [PubMed] [Google Scholar]
- 14.Spartano NL, Himali JJ, Beiser AS, et al. Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later. Neurology. 2016;86(14):1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–39. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CE. Cognitive accuracy and intelligent executive function in the brain and in business. Ann N Y Acad Sci. 2007; 1118:122–41. [DOI] [PubMed] [Google Scholar]
- 17.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 19.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci. 1992;17(4):338–45. [PubMed] [Google Scholar]
- 20.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6(6):790–804. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6. [DOI] [PubMed] [Google Scholar]
- 22.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90–3. [PubMed] [Google Scholar]
- 23.Monsell S. Task switching. Trends Cogn Sci. 2003;7(3):134–40. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Ding M, Kluger BM. Change in intraindividual variability over time as a key metric for defining performance-based cognitive fatigability. Brain Cogn. 2014;85:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillman CH, Snook EM, Jerome GJ. Acute cardiovascular exercise and executive control function. Int J Psychophysiol. 2003;48(3):307–14. [DOI] [PubMed] [Google Scholar]
- 26.Kao SC, Westfall DR, Soneson J, Gurd B, Hillman CH. Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology. 2017;54(9):1335–45. [DOI] [PubMed] [Google Scholar]
- 27.Holtzer R, Shuman M, Mahoney JR, Lipton R, Verghese J. Cognitive fatigue defined in the context of attention networks. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18(1):108–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banich MT. Executive function: the search for an integrated account. Curr Dir Psychol Sci. 2009;18(2):89–94. [Google Scholar]
- 29.Lorist MM, Boksem MA, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Brain Res Cogn Brain Res. 2005;24(2):199–205. [DOI] [PubMed] [Google Scholar]
- 30.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol. 2001;90(4):1392–9. [DOI] [PubMed] [Google Scholar]
- 31.Martin K, Staiano W, Menaspa P, et al. Superior inhibitory control and resistance to mental fatigue in professional road cyclists. PLoS One. 2016;11(7):e0159907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster C, Hillsdon M, Thorogood M, Kaur A, Wedatilake T. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005;1:CD003180. [DOI] [PMC free article] [PubMed] [Google Scholar]


