Abstract
The present study was aimed to evaluate the influence of flaxseed oil on renal toxicity induced by thioacetamide in male rats. The animals were distributed into four groups. Rats of the first group were served as control. Rats of the second group were exposed to thioacetamide. Rats of the third group were treated with flaxseed oil and thioacetamide. Rats of the fourth group were treated with flaxseed oil. Significant increases of blood creatinine and uric acid were observed in TAA-treated rats after three weeks. In thioacetamide group, the levels of serum creatinine, blood urea nitrogen and uric acid were significantly elevated after six weeks. Histopathologically, the renal sections from thioacetamide-treated rats showed severe alterations in the structure of renal corpuscles including a degeneration of glomeruli and Bowman’s capsules. Administration of flaxseed oil protects the observed biochemical and histopathological alterations induced by thioacetamide exposure. Hence, the results of this study suggest that flaxseed oil protects against thioacetamide-induced renal injury and the protective influence of flaxseed oil may be attributed to its antioxidant role.
Keywords: Thioacetamide, Renal toxicity, Flaxseed oil, Rats
1. Introduction
The kidney is a vital organ to implement important roles such as an excretion of toxic substances and metabolites, and homeostasis maintenance (Ferguson et al., 2008). The kidney is a major target organ for toxic factors (Kim and Moon, 2012). Globally, renal (kidney) dysfunctions are considered as a major health problem. (Schieppati and Remuzzi, 2005, Nasri, 2014). Chronic renal dysfunctions can lead to increased risk of cardiac disease and costs of healthcare (McLaughlin et al., 2001, Vlagopoulos and Sarnak, 2005, Marcen, 2006). Moreover, they increase the percentage of deaths yearly (Schieppati and Remuzzi, 2005). The increased prevalence of chronic diseases, including chronic renal dysfunctions and diseases is attributed to the aging population and advanced life expectancy (Nasri, 2014). Acute renal injury is related to different causes such as chemical toxicity, burns, shock, sepsis, trauma and severe diarrhea, at any age (Bicalho et al., 2015).
Thioacetamide (TAA) is a classic liver toxic chemical used for the induction of liver fibrosis and cirrhosis (Nozu et al., 1992). Exposure of TAA causes liver cirrhosis and hepatic neoplasms at medium and long terms respectively in experimental animals (Becker, 1983, Muller et al., 1988). TAA induced apoptosis in the rat liver based on histochemical observations (Ledda-Columbano et al., 1991). Additionally, TAA can stimulate the hepatic DNA synthesis and mitosis for liver regeneration (Mangipudy et al., 1995). TAA is a hepatotoxin frequently used in experimental investigations because its effectiveness to induce liver disorder in experimental animals (Reddy et al., 2004, Wang et al., 2004). Moreover, many studies showed that TAA caused liver fibrosis and injuries of kidney, brain, lung, stomach, intestine and spleen in experimental animals (Al-Bader et al., 2000, Liu et al., 2000, Caballero et al., 2001, Latha et al., 2003, Al-Attar, 2011, Al-Attar, 2012, Kadir et al., 2013, Zargar, 2014, Al-Attar and Shawush, 2015, Al-Attar et al., 2016, Al-Attar et al., 2017, Hsin et al., 2016).
The plant kingdom contains a large number of medical herbs that are used in many crude forms such as syrups, tea, powders and liniments (Solecki, 1975). The traditional treatment of different illnesses is successful using herbal constituents. Globally, most populations depend on medical herbs for the treatment of different diseases (Mamun et al., 2003). Flaxseed oil is one of the famous plant oils which is extracted from the seeds of the plant Linium usitatissimum. However, the seeds of Linium usitatissimum are used as edible oil in different countries. Many nutritional, physiological, biochemical and pharmacological researches focused on the effects of flaxseed extracts due to the health properties of flaxseed constituents (Abdel-Moneim et al., 2010, Akpolat et al., 2011, Khan et al., 2012, Rizwan et al., 2014, Jangale et al., 2016, Yari et al., 2016). The aim of the present study is to investigate the influence of flaxseed oil against TAA-induced renal injury in male rats.
2. Materials and methods
2.1. Animals
Male rats (Rattus norvegicus), weighing 91.5–129.8 g were utilized in the present study. Rats were procured from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. Rats were allowed to adapt to the laboratory conditions for one week before the initiation of experimentation. Throughout the period of adaptation and experimental durations, rats were housed using plastic cages in a room at 20 ± 1 °C with 65% of humidity and 12:12 h light–dark cycle. Rats were fed chow diet and tap water ad libitum. The adaptation and experimental treatments were carried out according to the ethical guidelines of the Animal Care and Use Committee of King Abdulaziz University.
2.2. Experimental protocol
A total of sixty rats were distributed into four groups, fifteen of rats each. Rats of group 1 were served as controls and were intraperitoneally injected with saline solution (0.9% NaCl), twice weekly. Rats of group 2 were given 250 mg/kg body weight of TAA (Sigma–Aldrich Corp., St. Louis, MO, USA) by intraperitoneal injection, twice weekly. Rats of group 3 were orally supplemented with flaxseed oil (Sigma–Aldrich Corp., St. Louis, MO, USA) at a dose of 2 g/kg body weight/day. Moreover, they were intraperitoneally injected with TAA at the same dose given to group 2. Rats of group 4 were intraperitoneally injected with saline solution (0.9% NaCl), twice weekly and were orally supplemented with flaxseed oil at a dose of 2 g/kg body weight/day.
2.3. Blood serum analysis
After three and six weeks, rats were fasted for 12 h and then anesthetized using diethyl ether. Blood samples were collected from orbital venous plexus in serum separator tubes. After centrifugation process (2500 rpm for 15 min), the blood sera were collected and kept at 4 °C. Creatinine value was estimated according to the method of Larsen (1971). Blood urea nitrogen (BUN) level was measured using the method of Patton and Crouch (1977). The method of Young (1990) was used to determine the level of uric acid.
2.4. Histopathological examination
After blood sampling at three and six weeks, rats were dissected and kidney tissues were collected and fixed in 10% buffered formaldehyde. Kidney tissues were dehydrated with different ethanol solutions and embedded in paraffin, then cut into 4 μ thick sections. These sections were stained with hematoxylin and eosin and examined using a photomicroscope.
2.5. Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS for windows, version 12.0). The data were expressed as mean ± standard deviation (S.D.) and values were analyzed using a two-way analysis of variance (ANOVA) to evaluate the differences between the mean values of experimental groups. The results were regarded statistically significant at P was less than 0.05.
3. Results
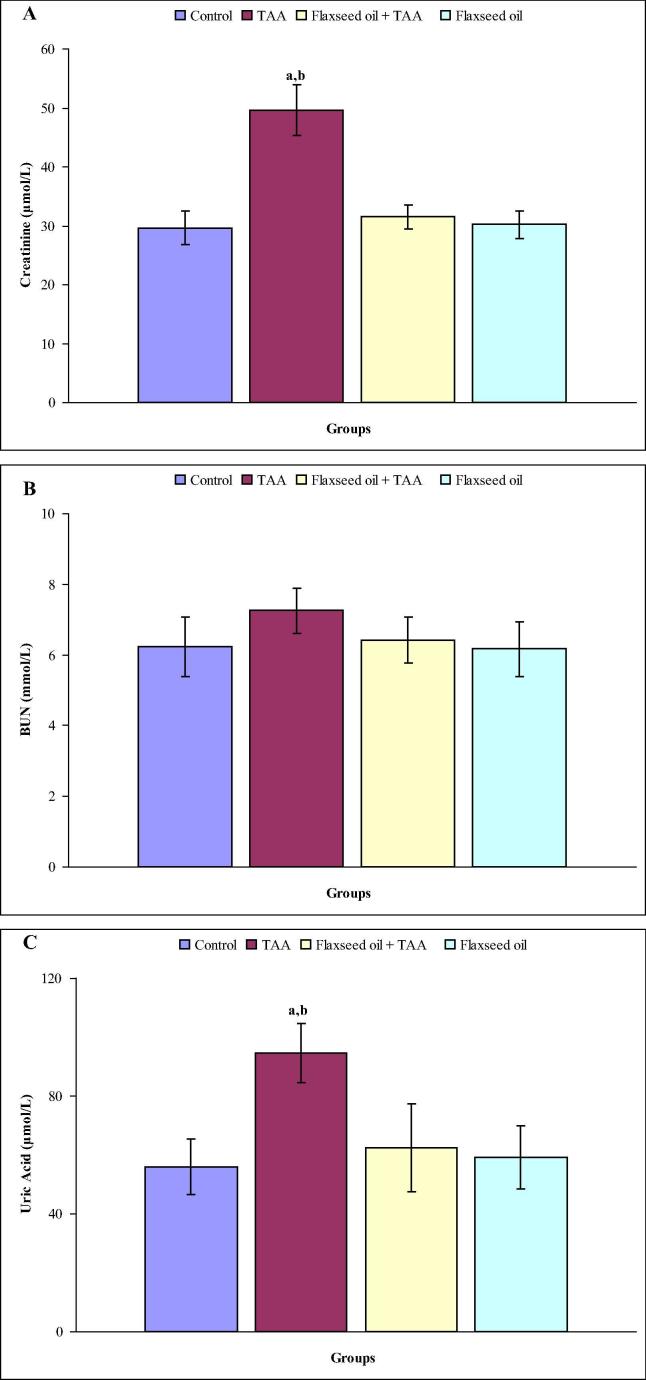
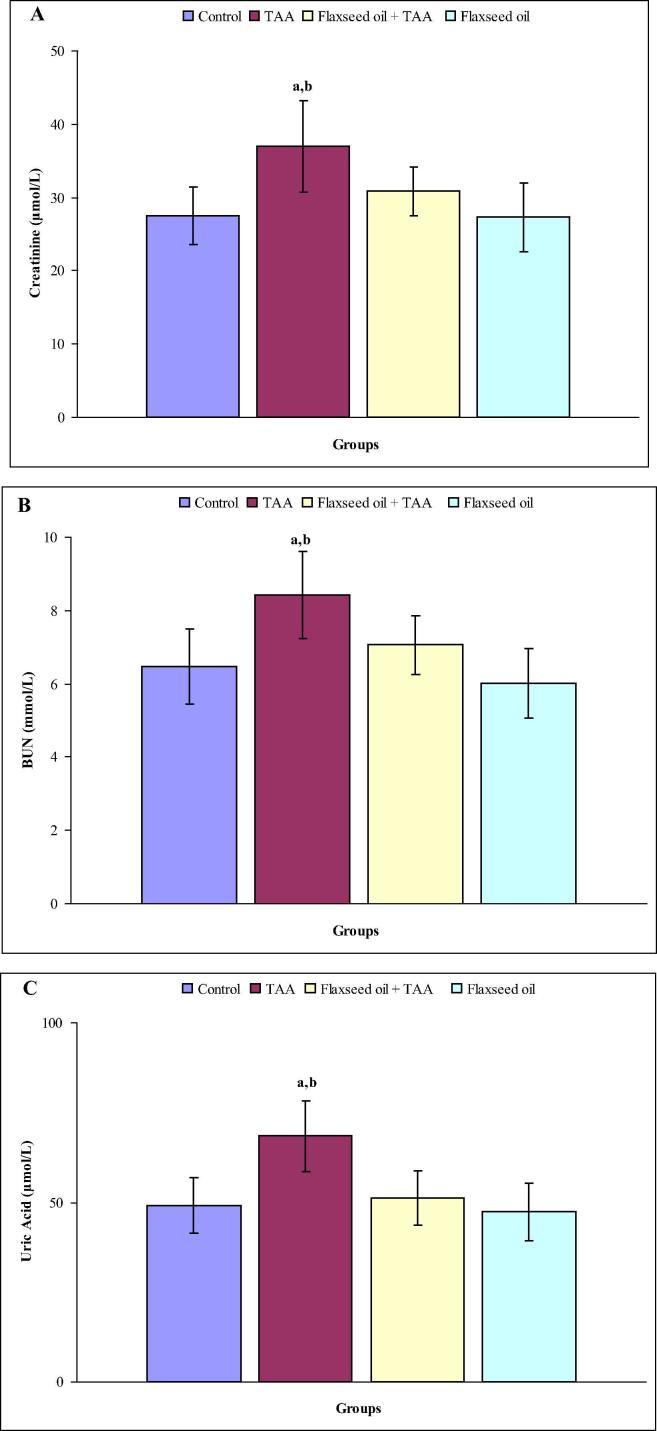
Fig. 1(A–C) shows the levels of serum creatinine, BUN and uric acid in all experimental groups after three weeks. Remarkable elevations in the levels of serum creatinine (67.2%) and uric acid (68.8%) were observed as compared with control rats. On the other hand, insignificant alterations of serum creatinine and BUN levels were noted in flaxseed oil plus TAA and flaxseed oil treated rats as compared with control rats. Moreover, no statistically significant differences were observed in the level of serum BUN in rats treated with TAA, flaxseed oil plus TAA and flaxseed oil as compared with control group. Fig. 2(A–C) shows the levels of serum creatinine, BUN and uric acid in all experimental groups after six weeks. In the TAA group, the levels of serum creatinine (34.5%), BUN (30.1%) and uric acid (39.3%) were significantly elevated as compared with the control group, while these parameters were statistically unchanged in flaxseed oil plus TAA and flaxseed oil treated rats compared with control rats.
Figure 1.
(A–C) The levels of serum creatinine (A), BUN (B) and uric acid in control, TAA, flaxseed oil plus TAA and flaxseed oil treated rats after three weeks. aindicates a significant difference between control and treated groups. bindicates a significant difference between rats treated with TAA and flaxseed oil plus TAA and flaxseed oil.
Figure 2.
(A–C) The levels of serum creatinine (A), BUN (B) and uric acid in control, TAA, flaxseed oil plus TAA and flaxseed oil treated rats after six weeks. aindicates a significant difference between control and treated groups. bindicates a significant difference between rats treated with TAA and flaxseed oil plus TAA and flaxseed oil.
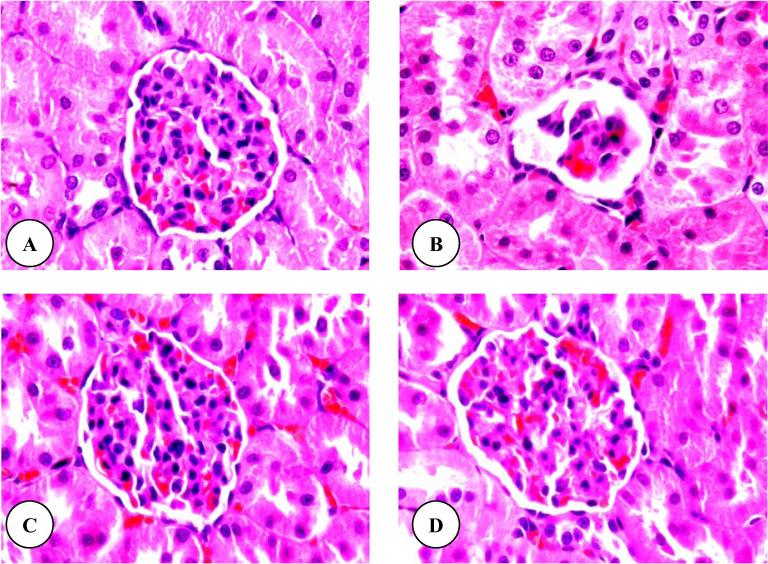
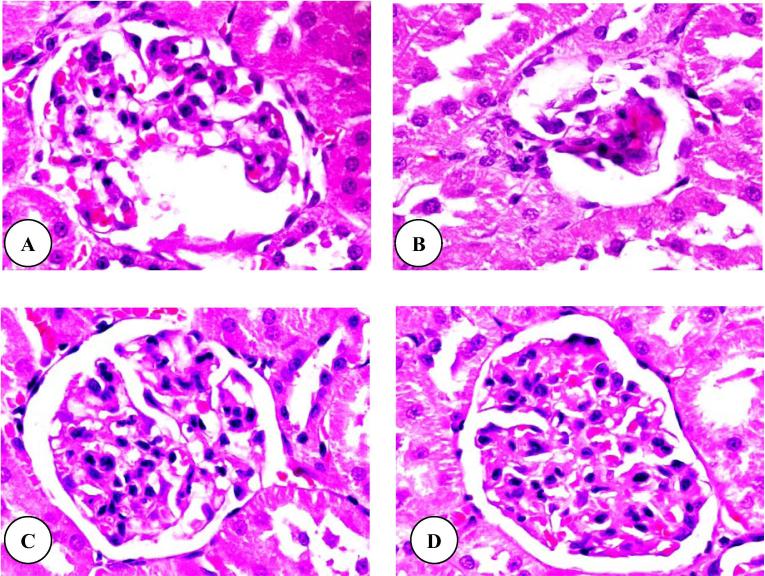
Histopathologically, normal structures of renal cortex and medulla were observed in control rats. Fig.3A shows the normal structure of renal (Malpighian) corpuscle. After three weeks, similar observations were noted in rats subjected to flaxseed oil plus TAA (Fig.3C) and flaxseed oil (Fig.3D). In rats treated with only TAA, there were several alterations in the structure of many renal corpuscles including a degeneration of glomeruli and Bowman’s capsules (Fig.3B). After six weeks of TAA treatment (group 2), the treated rats revealed advanced renal architecture destruction including increases of the degeneration and necrosis of glomeruli and Bowman’s capsules (Fig.4A and B). Moreover, the histopathological examination of flaxseed oil plus TAA (Fig.4C) and flaxseed oil (Fig.4D) treated rats showed normal renal corpuscle structures.
Figure 3.
(A–D) Renal corpuscle micrographs of control (A), TAA (B), flaxseed oil plus TAA (C) and flaxseed oil (D) treated rats after three weeks. Original magnification (×1000).
Figure 4.
(A–D) Renal corpuscle micrographs of TAA (A and B), flaxseed oil plus TAA (C) and flaxseed oil (D) treated rats after six weeks. Original magnification (×1000).
4. Discussion
As seen in the present study, the levels of serum creatinine, BUN and uric acid were statistically increased in rats treated with only TAA. Moreover, the present findings were confirmed by the histopathological alterations of renal corpuscles. It is known that the increase of creatinine, BUN and uric acid in serum indicates renal injury. Moreover, the disturbance of kidney functions led to the decline of creatinine excretion and increase its level in blood which accompanied with renal disorder (Hayes, 1994). Furthermore, the main waste products of protein metabolism are creatinine, BUN and uric acid which must be excreted by kidney, so the increase of these parameters in blood is considered as a major marker of renal injury (Panda, 1999). TAA is considered as a nephrotoxic chemical factor due to its heaped up and fast elimination injury through free radical pathway (Dashti et al., 1997, Silva, 2004, Begum et al., 2011). Damage of different tissues by TAA is due to its metabolites (sulfoxide and sulfone) distribution via different organs such as blood, kidney, liver, adrenals (Edward and Baker, 1974). Additionally, the extensive metabolic process of TAA led to produce acetate which appear in urine within 24 h (Spira and Raw, 2000, Kadir et al., 2011). The main toxic effect of TAA is for specific cells such as liver and kidney cells, and cortical thymocytes. Experimental investigations showed that the injury of kidney by toxin is attributed to the inhibition of glomerular filtration rate as a result of occlusion and back leak of renal filtrate and reactive oxygen species (Edward and Baker, 1974, Leena and Balaraman, 2005).
In the study of Kadir et al. (2013), they showed that the levels of blood urea and creatinine were increased in TAA treated rats as a result of the nephrotoxicity. Histopathological evaluation of renal tissues for the TAA treated group revealed severe histopathological changes. Additionally, they reported that the renal histopathological evaluation showed severe alterations which are confirmed by tubular epithelial cell necrosis, diffuse tubular swelling, glomerular congestion and inflammatory cell infiltration. Al-Attar et al. (2017) demonstrated that TAA intoxication caused renal dysfunction in male mice. The renal dysfunction was confirmed by the increases of serum creatinine, BUN and uric acid levels, and histopathological alterations of renal corpuscles. Furthermore, they showed that TAA caused more changes in cortex than medulla.
The present results showed that the pretreatment of rats with flaxseed oil improved the biochemical and histopathological alterations induced by TAA exposure. This indicated the effectiveness of flaxseed oil in prevention of TAA nephrotoxicity. The possible mechanism of the studied flaxseed oil as renal protective factor may be attributed to its antioxidant effect which inhibits and blocks the influence of TAA. The antioxidants occurring in food play many functions to block and reduce the oxidation processes which led to the prevention of oxidative stress. Many types of antioxidant factors extracted from herbal sources were extensively studied due to their antioxidative properties. (Rice-Evans et al., 1996, Kähkönen et al., 1999, Dragland et al., 2003, Yoo et al., 2008, Sharma et al., 2016). However, it is very important to increase the antioxidants in our food as preventive agents against oxidative stress and many diseases (Rubiolo et al., 2008). Moreover, antioxidants are used as food additives to block the oxidative activities of lipids (Wasowicz et al., 2004, El Bedawey et al., 2010, Erukainure et al., 2012).
Khan et al. (2012) examined whether intake of fish oil/flaxseed oil would have protective effect against sodium nitroprusside-induced nephrotoxicity in male rats. In sodium nitroprusside treated rats, the levels of serum creatinine and BUN were evoked and the levels of lactate dehydrogenase and glucose-6-phosphate dehydrogenase were also increased. The levels of malate dehydrogenase, glucose-6-phosphatase, fructose-1,6-bisphosphatase and malic enzyme were statistically decreased. The levels of enzymes of brush membrane (alkaline phosphatase, γ-glutamyl transpeptidase and leucine amino peptidase) were declined. Moreover, the levels of catalase and glutathione peroxidase were decreased with an elevation of lipid peroxidation. These results indicate that sodium nitroprusside caused renal injury. Supplementation of fish oil and flaxseed inhibited the alterations induced by sodium nitroprusside exposure. They suggested that omega-3 polyunsaturated fatty acids-enriched fish oil and flaxseed attenuated the nephrotoxicity and oxidative damage induced by sodium nitroprusside intoxication.
In addition, Rizwan et al. (2014) investigated the influence of flaxseed oil on renal injury induced by sodium arsenate. The nephrotoxicity of sodium arsenate was detected by increases of serum creatinine and BUN values. Renal enzymes levels of brush border membrane were statistically decreased. Moreover, lipid peroxidation and total sulfhydryl groups were changed. The levels of some enzymes and antioxidant defense system were decreased due to sodium arsenate exposure. Additionally, they demonstrated that sodium arsenate caused severe renal histopathological injury. Flaxseed oil treatment attenuated the alterations of these measured parameters and the renal histological structures induced by sodium arsenate exposure. Based on the present findings, it can be concluded that this study shows that flaxseed oil produces significant anti-nephrotoxicity influence in TAA treated rats. Further studies are needed to know the mechanism action of flaxseed oil against the nephrotoxicity induced by TAA and other toxic factors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Moneim A.E., Dkhil M.A., Al-Quraishy S. The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol. Trace Elem. Res. 2010;143:457–467. doi: 10.1007/s12011-010-8882-z. [DOI] [PubMed] [Google Scholar]
- Akpolat M., Kanter M., Topcu-Tarladacalisir Y., Aydogdu N. Protective effect of flaxseed oil on renal injury in hyperlipidaemic rats: the effect of flaxseed oil on hyperlipidaemia. Phytother. Res. 2011;25:796–802. doi: 10.1002/ptr.3334. [DOI] [PubMed] [Google Scholar]
- Al-Attar A.M. Hepatoprotective influence of vitamin C on thioacetamide-induced liver cirrhosis in Wistar male rats. J. Pharmacol. Toxicol. 2011;6:218–233. [Google Scholar]
- Al-Attar A.M. Attenuating effect of Ginkgo biloba leaves extract on liver fibrosis induced by thioacetamide in mice. J. Biomed. Biotechnol. 2012;2012:1–9. doi: 10.1155/2012/761450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Shawush N.A. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J. Biol. Sci. 2015;22:157–163. doi: 10.1016/j.sjbs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Alrobai A.A., Almalki D.A. Effect of Olea oleaster and Juniperus procera leaves extracts on thioacetamide induced hepatic cirrhosis in male albino mice. Saudi J. Biol. Sci. 2016;23:363–371. doi: 10.1016/j.sjbs.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Alrobai A.A., Almalki D.A. Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi J. Biol. Sci. 2017;24:15–22. doi: 10.1016/j.sjbs.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader A., Mathew T.C., Koursheed M., Asfar S., al-Sayer Dashti H., Dashti H.M. Thioacetamide toxicity and the spleen: histological and biochemical analysis. Anat. Histol. Embryol. 2000;29:3–8. doi: 10.1046/j.1439-0264.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Becker F.F. Thioacetamide hepatocarcinogenesis. J. Natl Cancer Inst. 1983;71:553–558. [PubMed] [Google Scholar]
- Begum Q., Noori S., Mahboob T. Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pak. J. Biochem. Mol. Biol. 2011;44:21–26. [Google Scholar]
- Bicalho M.D., Soares D.B., Botoni F.A., Reis A.M.M., Martins M.A.P. Drug-induced nephrotoxicity and dose adjustment recommendations: agreement among four drug information sources. Int. J. Environ. Res. Public Health. 2015;12:11227–11240. doi: 10.3390/ijerph120911227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero M.E., Berlanga J., Ramirez D., Lopez-Saura P., Gozalez R., Floyd D.N., Marchbank T., Playford R.J. Epidermal growth factor reduces multiorgan failure induced by thioacetamide. Gut. 2001;48:34–40. doi: 10.1136/gut.48.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti H.M., Mathew T.C., Jadaon M.M., Ashkanani E. Zinc and liver cirrhosis: biochemical and histopathologic assessment. Nutrition. 1997;13:206–212. doi: 10.1016/s0899-9007(96)00403-0. [DOI] [PubMed] [Google Scholar]
- Dragland S., Senoo H., Wake K., Holte K., Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003;133:1286–1290. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- Edward A., Baker E.A.S. Nonhepatic thioacetamide injury. The morphologic features of proximal renal tubular injury. Am. J. Pathol. 1974;74:576–590. [PMC free article] [PubMed] [Google Scholar]
- El Bedawey A.A., Mansour E.H., Zaky M.S., Hassan A.A. Characteristics of antioxidant isolated from some plant sources. Food Nutr. Sci. 2010;2010(1):5–12. [Google Scholar]
- Erukainure O.L., Oke O.V., Owolabi F.O., Kayode F.O., Umanhonlen E.E., Aliyu M. Chemical properties of Monodora myristica and its protective potential against free radicals in vitro. Oxid. Antioxid. Med. Sci. 2012;1:127–132. [Google Scholar]
- Ferguson M.A., Vaidya V.S., Bonventre J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.W. third ed. Raven Press; New York, NY, USA: 1994. Principles and Methods of Toxicology. [Google Scholar]
- Hsin I.F., Lee J.Y., Huo T.I., Lee F.Y., Huang H.C., Hsu S.J., Wang S.S., Ho H.L., Lin H.C., Lee S.D. 2’-Hydroxyflavanone ameliorates mesenteric angiogenesis and portal-systemic collaterals in rats with liver fibrosis. J. Gastroenterol. Hepatol. 2016;31:1045–1051. doi: 10.1111/jgh.13197. [DOI] [PubMed] [Google Scholar]
- Jangale N.M., Devarshi P.P., Bansode S.B., Kulkarni M.J., Harsulkar A.M. Dietary flaxseed oil and fish oil ameliorates renal oxidative stress, protein glycation, and inflammation in streptozotocin-nicotinamide-induced diabetic rats. J. Physiol. Biochem. 2016;7:327–336. doi: 10.1007/s13105-016-0482-8. [DOI] [PubMed] [Google Scholar]
- Kadir F.A., Kassim N.M., Abdulla M.A., Yehye W.A. Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement Altern. Med. 2013;13:294. doi: 10.1186/1472-6882-13-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir F.A., Othman F., Abdulla M.A., Hussan F., Hassandarvish P. Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J. Pharmacol. 2011;13:64–68. doi: 10.4103/0253-7613.75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:362–3954. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Khan M.W., Priyamvada S., Khan S.A., Khan S., Naqshbandi A., Yusufi A.N.K. Protective effect of ω-3 polyunsaturated fatty acids (PUFAs) on sodium nitroprusside-induced nephrotoxicity and oxidative damage in rat kidney. Hum. Exp. Toxicol. 2012;31:1035–1049. doi: 10.1177/0960327112444475. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. 2012;20:268–272. doi: 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 1971;41:209–217. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- Latha M.S., Mirabel R.P., Pushpalatha P.K. Thioacetamide toxicity and the lung: histological analysis. Indian J. Physiol. Pharmacol. 2003;47:476–478. [PubMed] [Google Scholar]
- Ledda-Columbano G.M., Coni P., Curto M., Giacomini L., Faa G., Oliverio S., Piacentini M., Columbano A. Induction of two different modes of cell death, apoptosis and necrosis, in rat liver after a single dose of thioacetamide. Am. J. Pathol. 1991;139:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Leena P., Balaraman R. Effect of green tea extract on cisplatin induced oxidative damage on kidney and testes of rats. Ars. Pharm. 2005;46:5–18. [Google Scholar]
- Liu, L., Han, D., Ren, D., 2000. Effect of intestinal endotoxemia induced by thioacetamide. Chung-HuaKan Tsang Ping Tsa Chin. 8, 174–179. [PubMed]
- Mamun M.M., Billah M.M., Ashek M.A., Ahasan M.M., Hossain M.J., Sultana T. Evaluation of diuretic activity of Ipomoea aquatica (kalmisak) in mice model study. J. Med. Sci. 2003;3:395–400. [Google Scholar]
- Mangipudy R.S., Chanda S., Mehendale H.M. Tissue repair response as a function of dose in thioacetamide hepatotoxicity. Environ. Health Perspect. 1995;103:260–267. doi: 10.1289/ehp.95103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcen R. Cardiovascular risk factors in renal transplantation-Current controversies. Nephrol. Dial. Transplant. 2006;21:3–8. doi: 10.1093/ndt/gfl298. [DOI] [PubMed] [Google Scholar]
- McLaughlin K., Manns B., Culleton B., Donaldson C., Taub K. An economic evaluation of early versus late referral of patients with progressive renal insufficiency. Am. J. Kidney Dis. 2001;38:1122–1128. doi: 10.1053/ajkd.2001.28619. [DOI] [PubMed] [Google Scholar]
- Muller A., Machnik F., Zimmermann T., Schubert H. Thioacetamide-induced cirrhosis-like liver lesions in rats–usefulness and reliability of this animal model. Exp. Pathol. 1988;34:229–236. doi: 10.1016/s0232-1513(88)80155-5. [DOI] [PubMed] [Google Scholar]
- Nasri H. World kidney day. Chronic kidney disease and aging: a global health alert. Iran. J. Public Health. 2014;43:126–127. [PMC free article] [PubMed] [Google Scholar]
- Nozu F., Takeyama N., Tanaka T. Changes of hepatic fatty acid metabolism produced by chronic thioacetamide administration in rats. Hepatology. 1992;15:1099–1106. doi: 10.1002/hep.1840150621. [DOI] [PubMed] [Google Scholar]
- Panda N.C. Textbook of biochemistry and human biology. second ed. Prentise hall; India: 1999. Kidney; pp. 290–296. [Google Scholar]
- Patton G.J., Crouch S.R. Determination of urea (urease modified Berthelot reaction) Anal. Chem. 1977;49:464–469. [Google Scholar]
- Reddy P.V., Murthy Ch.R., Reddanna P. Fulminant hepatic failure induced oxidative stress in nonsynaptic mitochondria of cerebral cortex in rats. Neurosci. Lett. 2004;368:15–20. doi: 10.1016/j.neulet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure- antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Rizwan S., Naqshbandi A., Farooqui Z., Khan A.A., Khan F. Protective effect of dietary flaxseed oil on arsenic-induced nephrotoxicity and oxidative damage in rat kidney. Food Chem. Toxicol. 2014;68:99–107. doi: 10.1016/j.fct.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Rubiolo J.A., Mithieux G., Vega F.A. Resveratrol protects primary rat hepatocytes against oxidative stress damage. Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Schieppati A., Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int. 2005;98:S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- Sharma C.K., Sharma M., Sharma V. Therapeutic potential of the medicinal plant Aegle Marmelos (Linn.) Correa: Insight. J. Environ. Pathol. Toxicol. Oncol. 2016;3:1–10. doi: 10.1615/JEnvironPatholToxicolOncol.2015014485. [DOI] [PubMed] [Google Scholar]
- Silva F.G. Chemical-induced nephropathy: a review of the renal tubulointerstitial lesions in humans. Toxicol. Pathol. 2004;32:71–84. doi: 10.1080/01926230490457530. [DOI] [PubMed] [Google Scholar]
- Solecki R. Shanidar IV, a neanderthal flower burial in northern Iraq. Science. 1975;190:880–881. [Google Scholar]
- Spira B., Raw I. The effect of thioacetamide on the activity and expression of cytosolic rat liver glutathione-S-transferase. Mol. Cell. Biochem. 2000;211:103–110. doi: 10.1023/a:1007114801362. [DOI] [PubMed] [Google Scholar]
- Vlagopoulos P.T., Sarnak M.J. Traditional and nontraditional cardiovascular risk factors in chronic kidney disease. Med. Clin. North Am. 2005;89:587–611. doi: 10.1016/j.mcna.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Jawan B., Lee T.H., Hung K.S., Chou W.Y., Lu C.N., Liu J.K., Chen Y.J. Single injection of naked plasmid encoding alpha-melanocyte-stimulating hormone protects against thioacetamide-induced acute liver failure in mice. Biochem. Biophys. Res. Commun. 2004;322:153–161. doi: 10.1016/j.bbrc.2004.07.091. [DOI] [PubMed] [Google Scholar]
- Wasowicz E., Gramza A., Hêś M., Jeleوń H.H., Korczak J., Matecka M., Mildner-Szkudlarz S., Rudzińska M., Samotyja U., Zawirska-Wojtasiak R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004;13(54):87–100. [Google Scholar]
- Yari Z., Rahimlou M., Eslamparast T., Ebrahimi-Daryani N., Poustchi H., Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open labeled, controlled study. Int. J. Food Sci. Nutr. 2016;67:461–469. doi: 10.3109/09637486.2016.1161011. [DOI] [PubMed] [Google Scholar]
- Yoo K.M., Lee C.H., Lee H., Moon B., Lee C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106:929–936. [Google Scholar]
- Young, D.S., 1990. Effects of Drugs on Clinical Laboratory Test. third ed., 3, pp.19–25.
- Zargar S. Protective effect of Trigonella foenum-graecum on thioacetamide induced hepatotoxicity in rats. Saudi J. Biol. Sci. 2014;21:139–145. doi: 10.1016/j.sjbs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]