Abstract
This study aims to determine the effect of climatic factors (temperature, relative humidity and rainfall) on mosquito abundance and to map mosquito larva breeding sites using GIS application in Eastern Province, Saudi Arabia. The data pertaining to larval and adult mosquito abundance/distribution and climatic factors were collected for the study period of 2014. Bi-variate and multivariate analyses were performed to determine the relationship between mosquito abundance and climatic factors (temperature, relative humidity and rainfall). The utilization of GIS with GPS facilitates to identify and map larva breeding sites in the study area. The result showed strong negative correlation between mosquito abundance and temperature while there appeared a strong positive correlation with relative humidity and moderate positive correlation with rainfall. Low mosquito abundance was observed at high temperatures whereas high and moderate mosquito abundance was observed at high humidity and during rainy months, respectively. In the adult mosquito, the regression model for three climatic factors (temperature, RH and rainfall) and other factors showed a variation of 84.5% of the variance accounted for the climatic factors while the remaining 15.5% were attributed to other factors. In larva, 64.3% of the variance accounted for climatic factors, and the remaining 35.7% attributed to other factors such as the presence of vegetation, waste materials and water reservoirs such as ditches. In comparison, the larva seems influenced by the presence of vegetation, waste material, water reservoirs and ditches and less influenced by climatic factors than the adult mosquito. Development of a risk map by considering the flying distance of the adult mosquito from the studied sites showed three major clusters where a recommendation for management control program was suggested.
Keywords: Mosquito control, Climatic factors, GIS, Eastern Province, Saudi Arabia
1. Introduction
Mosquitoes that belong to the order of Diptera, family Culicidae, are considered as a vector for many diseases such as dengue fever, malaria, yellow fever, Japanese encephalitis, West Nile Virus and filariasis. These mosquito-borne diseases (MBDs) are the results of the interaction of four aspects: the pathogen, the mosquito vector, the human host and the environment (WHO, 2014, Matthews, 2011, Ceccato et al., 2005, Lehane, 2005).
The influence of mosquitoes on human health and well-being is greater than any other insects throughout the world. This is not only due to the diseases they transmit to humans but also the severe nuisance they cause (Gandhi et al., 2013, Pratt et al., 1963). The diseases transmitted by mosquito vectors are among the major contributors to human morbidity and mortality in many tropical and subtropical countries, and also to some extent in temperate zones (WHO, 2014, WHO, 1997).
Each year, over one billion individuals are infected and over one million individuals die from vector-borne diseases with the majority attributed to mosquito-borne diseases (WHO, 2014). The distribution of MBDs is linked to mosquito distribution, as mosquito distribution is mostly dependent upon the spatial distribution of breeding areas, preferred hosts and flight distance (Gimnig et al., 2005).
Many researchers reported the presence of mosquito-borne diseases (MBDs) such as dengue fever, malaria, and rift valley fever (Aziz et al., 2014, Khormi and Kumar, 2013, Alahmed, 2012, Ahmed et al., 2011, Al-Qabati and Al-Afaleq, 2010, El-Gilany et al., 2010, Al-Tawfiq, 2006, Flick and Bouloy, 2005, Fakeeh and Zaki, 2003, Madani et al., 2003, Bashwari et al., 2001) in many parts of Saudi Arabia.
The geographic distribution and abundance of mosquitoes are governed by complex factors. However, climatic factors (temperature, relative humidity and rainfall) are the key determinants of the control over the mosquito vector survival, production, development, abundance and distribution (Bashar and Tuno, 2014, Palaniyandi et al., 2014, Zhou et al., 2007, Reiter, 2001).
The temperature was found to influence the abundance, activity and presence of mosquito in temporary and permanent habitats. It affects the development time from egg to adult mosquito and even the parasite (Byun and Webb, 2012, Costa et al., 2010, Bayoh and Lindsay, 2003, Tun-Lin et al., 2000). Similarly, rainfall influences mosquito distribution either by providing or maintaining more breeding sites. In addition, it also has a negative effect on flushing small breeding sites and decreasing temperature (Byun and Webb, 2012, Ceccato et al., 2005). Variation in relative humidity also affects the number of females laying eggs, the number of eggs laid, feeding frequency and metabolic rate of adult mosquitoes, and survivorship (Ceccato et al., 2005, Costa et al., 2010, Reiter, 2001).
Though the relationship between climatic factors (temperature, humidity and rainfall) and mosquito abundance have been investigated in several studies, (Tian et al., 2015, Alshehri, 2013, Murty et al., 2010, Minakawa et al., 2002), there has been no study examining the effect of climatic factors (temperature, relative humidity and rainfall) on mosquito abundance in the Eastern Province, Saudi Arabia.
Geographic Information System (GIS) is a widely used application in modeling and mapping of public health issues, especially when dealing with mosquito control activity (Rydzanicz et al., 2011). The availability of up-to-date map of mosquito vectors distribution plays a major role in mosquito control programs. GIS together with GPS (Global Positioning System) is used in many mosquito control activities in different countries. The employment of GIS facilitates the identification of the location and size of mosquito larva breeding sites that aids in the selection of types of insecticide to be used in mosquito control measures (Palaniyandi, 2014b, Zou et al., 2006, Mushinzimana et al., 2006).
The objective of this study is to map mosquito larva breeding sites using GIS application and determine the relationship between mosquito abundance and climatic factors (temperature, relative humidity and rainfall) in the Eastern Province, Saudi Arabia.
2. Material and methods
2.1. Study area
The Eastern Province of Saudi Arabia is located along the Arabian Gulf bordered by Northern Province to the north, Kuwait to the northeast and the Sultanate of Oman to the south. It has different geographical features such as sandy soil, coastal lowlands, industrialized areas and farm areas. It is characterized by an arid climate with temperature rising from 15 °C in January to a maximum of about 42 °C in the August–September period. The average annual rainfall ranges from around 100 mm in the north and northeast during winter to less than 10 mm in the Rub al-Khali (Thomas, 2012).
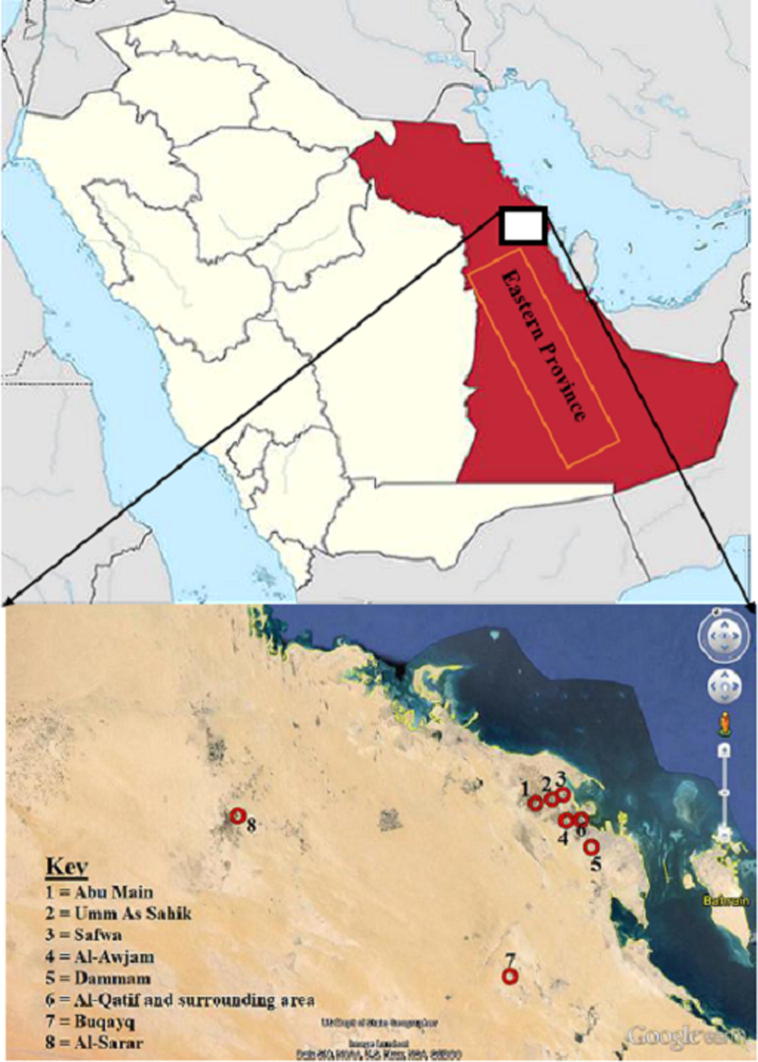
Several environmental factors influence the abundance and distribution of mosquitoes in the area. These factors include temperature, relative humidity and precipitation, the presence of palm gardens/vegetables that hold a large volume of rainwater, widespread salt marshes and irrigation ditches. Poor sanitary sewerage-systems in the areas also cause the accumulation of a large volume of sewage water which serves as a good breeding habitat for mosquitoes in the study area (Alahmed, 2012). 322 larva breeding sites were assessed for the presence of mosquito larvae in eight locations. Those locations include; Abu Main, Umm As Sahik, Safwa, Al-Awjam, Dammam, Al-Qatif and its surrounding area, Buqayq and Al-Sarar. Those sites showed their diverse ecological characteristic and abundance of mosquito species (Fig. 1).
Figure 1.
Locations of the study sites along the Eastern Province, Saudi Arabia.
2.2. Data collection
2.2.1. Field data collection
The spatial information and associated environmental variables were collected using a data collection format. The ‘x’ and ‘y’ coordinates of larva breeding sites including the size of the water body, the presence of terrestrial and immersed vegetation, the presence of fish, algae and waste materials were recorded, and the distance to the nearest house was estimated. A Geographic Positioning System (GPS) [Garmin Model Nuvi 50] was used for recording the coordinates of each breeding site and a hand held camera was used for taking images during field observation.
2.2.2. Secondary data
The larval and adult mosquito data were collected in collaboration with the Ministry of Health branch in Dammam. Data collection was carried out from January to December 2014. The monthly data were collected and compiled to each study site for 2014. A sampling of mosquito larvae was carried out in various breeding habitats by taking 3–5 scoops of water for each sampling location to check for the presence of larvae (Alahmed, 2012). Mosquito larvae were identified using a dissecting microscope. Electric flycatchers and a cow sheet with a spray were used to catch adult mosquitoes from the same sampling locations.
The meteorological data of temperature, relative humidity and precipitation were obtained from the Presidency of Meteorology and Environment (PME) Dammam for the period noted above. Temperature can be defined as the mean average of minimum and maximum temperatures, measured in degree Celsius (°C). Relative humidity (RH), expressed in percentage (%), is the average monthly humidity based on the daily records. Precipitation/rainfall is the amount of rainfall in the month, measured in millimeters.
2.3. Data analysis
Data were analyzed to determine the relationship between mosquito abundance and climatic factors. Bivariate analysis was preformed to determine the relationship between mosquito abundance and each climatic factor (temperature, relative humidity and rainfall). Multivariate regression analysis was performed to identify the overall effect of temperature, relative humidity and rainfall in mosquito abundance. A descriptive analysis was carried out to determine the trends of mosquito abundance and climatic factors. The results of bivariate analysis were expressed in Pearson correlation coefficient.
2.4. Study procedures
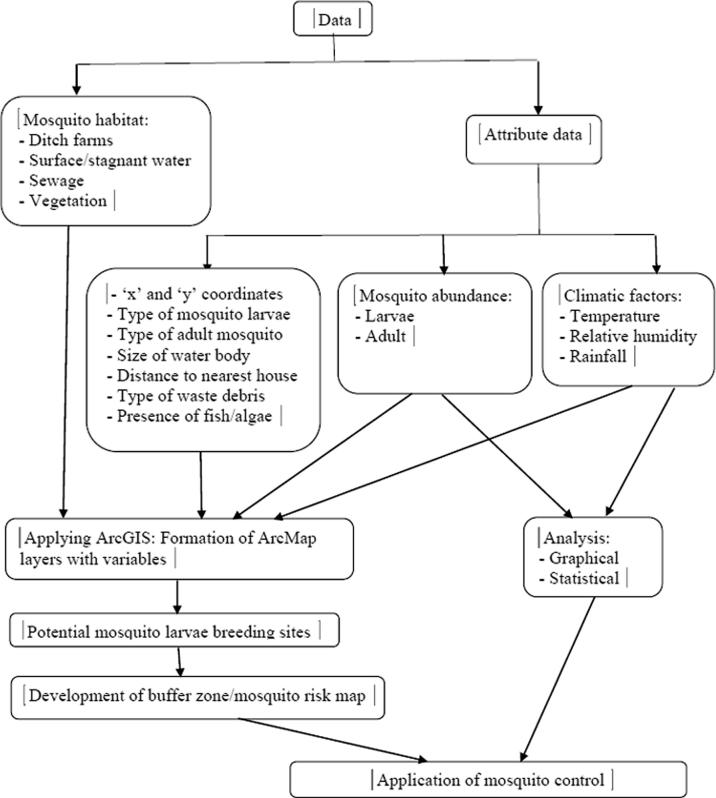
A systematic procedure was carried out to study mosquito distribution and abundance in Eastern Province, Saudi Arabia. To generate the map with layers, converting mosquito larvae habitat into an ArcMap layer map accompanied with variables was performed. The second step was identifying potential hotspots of mosquito larva breeding sites. The final step was determining the buffer zone (buffer distance) to indicate mosquito risk areas from larva breeding sites (Fig. 2).
Figure 2.
Flowchart demonstrating the main steps of the study procedures.
2.5. Mapping mosquito breeding sites
A map of the Kingdom Saudi Arabia showing the governorate boundaries was used as a base map from ESRI to link all thematic data with spatial features in order to construct an accurate database of mosquito breeding sites in the Eastern Province, KSA. In addition, attribute data such as the ‘x’ and ‘y’ coordinates, type of breeding site, the size of water body, vegetation, distance to the nearest house, the presence of fish, algae and debris type were set in the creation of mosquito risk area.
The x and y coordinates recorded using GPS [Garmin Model Nuvi 50] were exported to ArcGIS version 9.3 and then geo-referenced to develop a GIS database. The existing and potential mosquito larva breeding sites were represented by point locations in the GIS map. The GIS map showed the geographical visualization of the mosquito larva breeding sites in order to recognize specific areas with high larval abundance.
The risk map was developed by considering the flying distance of the mosquito. Most adult mosquito species fly within the range of 1–3 km though some species can travel up to 5 km (WHO, 1982) and accordingly a buffer zone of 1 km, 3 km and 5 km was considered as buffer distance or mosquito risk areas.
3. Result and discussion
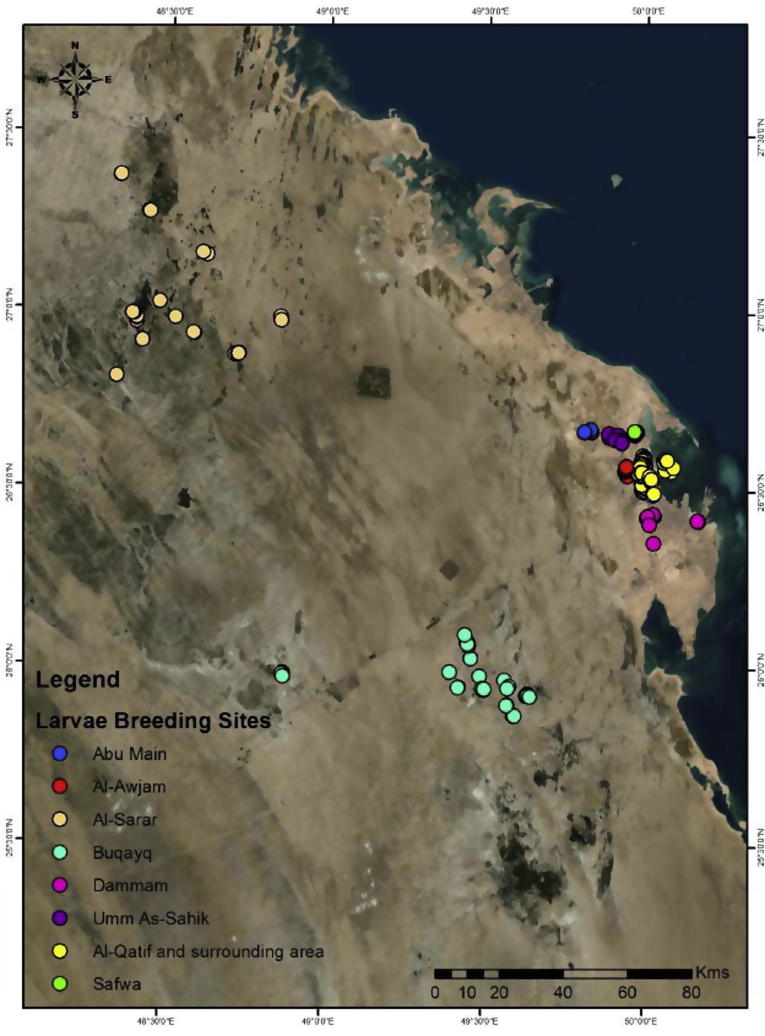
Three hundred twenty-two breeding sites were assessed for the presence of mosquito larvae in eight locations from January to December 2014. Only, 206 sites (64.0%) were found to contain mosquito larvae. Those locations include; Abu Main (5 sites), Umm As Sahik (19 sites), Safwa (10 sites), Al-Awjam (22 sites), Dammam (12 sites), Al-Qatif and its surrounding area (81 sites), Buqayq (32 sites) and Al-Sarar (25 sites). Those sites showed their diverse ecological characteristic and abundance of mosquito species (Fig. 3). A total of 31,041 mosquito larvae and 2036 adult mosquitoes were collected and classified under three genera (Culex, Aedes and Anopheles). The highest number of adults and larvae was observed in Qatif and surrounding area followed by Awjam and Umm As Sahik (Table 1).
Figure 3.
Mosquito breeding locations within each study sites.
Table 1.
Spatial and monthly distribution of larvae and adult mosquito in Eastern Province, Saudi Arabia in 2014.
| Study area | Month |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | ||
| Larval | |||||||||||||
| Abu-Main | 163 | 161 | 137 | 89 | 82 | 74 | 32 | 53 | 75 | 75 | 105 | 121 | 1167 |
| Umm As Sahik | 206 | 207 | 220 | 198 | 208 | 193 | 83 | 248 | 232 | 171 | 179 | 153 | 2298 |
| Safwa | 204 | 188 | 129 | 122 | 137 | 145 | 51 | 83 | 117 | 102 | 141 | 158 | 1577 |
| Al-Awjam | 222 | 271 | 263 | 178 | 192 | 199 | 66 | 181 | 192 | 94 | 140 | 147 | 2145 |
| Dammam | 100 | 120 | 170 | 80 | 110 | 110 | 20 | 100 | 110 | 60 | 60 | 123 | 1163 |
| Al-Qatif and surrounding area | 1027 | 1898 | 2651 | 2082 | 1055 | 1260 | 369 | 1072 | 1015 | 1670 | 2221 | 2806 | 19,126 |
| Buqayq | 243 | 206 | 88 | 111 | 75 | 80 | 50 | 50 | 50 | 50 | 60 | 60 | 1123 |
| Al-Sarar | 297 | 294 | 333 | 275 | 280 | 297 | 0 | 185 | 90 | 141 | 150 | 100 | 2442 |
| Total | 2462 | 3345 | 3991 | 3135 | 2139 | 2358 | 671 | 1972 | 1881 | 2363 | 3056 | 3668 | 31,041 |
| Adult | |||||||||||||
| Abu-Main | 7 | 20 | 21 | 11 | 5 | 4 | 0 | 0 | 0 | 0 | 1 | 59 | 128 |
| Umm As Sahik | 42 | 65 | 35 | 37 | 17 | 18 | 4 | 25 | 16 | 27 | 24 | 62 | 372 |
| Safwa | 3 | 0 | 0 | 0 | 7 | 9 | 0 | 0 | 0 | 0 | 0 | 9 | 28 |
| Al-Awjam | 63 | 58 | 29 | 23 | 11 | 13 | 7 | 32 | 22 | 10 | 22 | 51 | 341 |
| Dammam | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 6 |
| Al-Qatif and surrounding area | 106 | 246 | 295 | 121 | 9 | 0 | 0 | 2 | 0 | 6 | 140 | 236 | 1161 |
| Buqayq | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Al-Sarar | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 221 | 389 | 380 | 192 | 49 | 44 | 11 | 59 | 38 | 43 | 193 | 417 | 2036 |
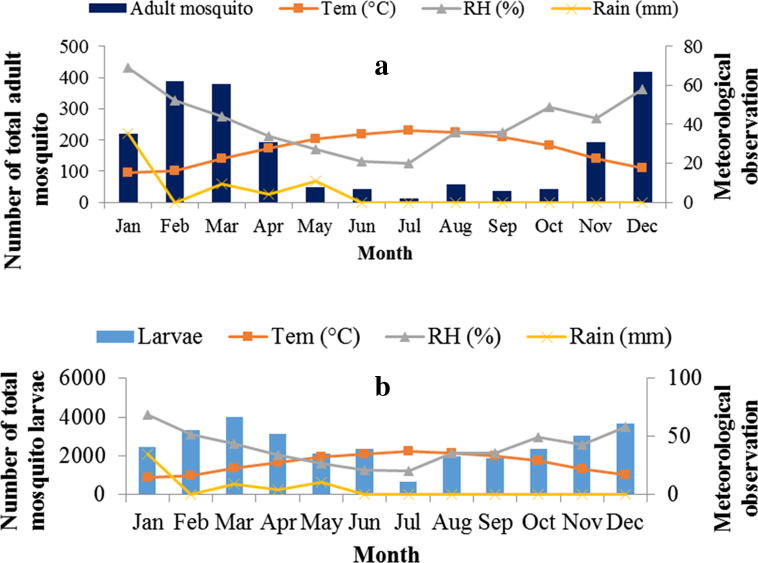
The relationship between larval/adult mosquito abundance and climatic factors of temperature, relative humidity and rainfall is shown (Table 2). It is clear from Fig. 4 that a high number of mosquito larvae were collected during November, December, February, March and April while a high number of adult mosquitoes were observed during November, December, January, February, March and April. These results clearly indicate that temperatures that range from 16.4 °C to 27.7 °C are suitable for the production of larvae and its survival whereas 15–27.7 °C favors the high abundance and spread of the adult mosquito.
Table 2.
Correlation coefficient between climatic factors, larval and adult mosquito abundance in Eastern Province, Saudi Arabia, 2014.
| Pearson correlation | Names of study sites |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AM | US | SA | AW | DM | QS | BU | AS | EP | |
| Adult and climatic factors | |||||||||
| Adult mosquito abundance and temperature | −0.597 | −0.848 | −0.035 | −0.828 | −0.186 | −0.815 | − | − | −0.867 |
| Adult mosquito abundance and RH | 0.467 | 0.757 | −0.056 | 0.825 | 0.048 | 0.563 | – | – | 0.654 |
| Adult mosquito abundance and rain | −0.048 | 0.143 | 0.100 | 0.467 | −0.152 | 0.105 | – | – | 0.147 |
| Larva and climatic factors | |||||||||
| Larva abundance and temperature | −0.941 | −0.075 | −0.810 | −0.441 | −0.373 | −0.627 | −0.682 | −0.350 | −0.733 |
| Larva abundance and relative humidity (RH) | 0.798 | 0.187 | 0.673 | 0.292 | 0.297 | 0.453 | 0.589 | 0.180 | 0.543 |
| Larva abundance and rain | 0.534 | 0.202 | 0.537 | 0.356 | 0.199 | -0.165 | 0.696 | 0.464 | 0.059 |
Note: AM = Abu Main, US = Umm As Sahik, SA = Safwa, AW = Al-Awjam, DM = Dammam, QS = Al-Qatif and its surrounding area, BU = Buqayq, AS = Al-Sarar, EP = Eastern Province.
Figure 4.
Relationship between climatic factors (a) adult mosquito and (b) mosquito larva abundance in Eastern Province, Saudi Arabia, 2014.
In the summer season, the average temperature in the study area became greater than 35 °C, which is unsuitable for both larval and adult mosquito growth. Fig. 4(a and b) shows that limited numbers of larval and adult mosquito were observed in July when the a temperature was 36.9 °C. Overall, a high larval and adult mosquito content were collected at a temperature ranging from 16.4 °C to 27.7 °C and 15 °C to 27.7 °C respectively. Several studies identified temperatures between 20 °C and 29 °C that were favorable for mosquito growth (Tian et al., 2015, Alshehri, 2013, Bayoh and Lindsay, 2003, McMichael et al., 1996).
Temperatures greater than 30 °C decrease mosquito survivorship and abundance (Christiansen-Jucht et al., 2014; Westbrook et al., 2010). Similarly, (Bayoh and Lindsay, 2004) found larval survival decreased with increased temperature. Unlike this study, (Alahmed, 2012, Hopp and Foley, 2001, Tun-Lin et al., 2000) reported that high temperatures speed up mosquito growth and increase mosquito abundance. From this and other studies, it is clear that the effect of temperature on mosquito growth, survival and production is difficult to predict at a specific range (Reiter, 2001).
This study showed that high relative humidity increases the larval and adult mosquito abundance. Average relative humidity that ranges between 44% and 69% was favorable for the increase of mosquito abundance in the study area (Fig. 4a and b). Different studies have indicated that relative humidity influences the survival and activities of the mosquito. It has been reported that the lifespan of the mosquito increases as humidity increases and the high humidity increases mosquito density and abundance (Tian et al., 2015, Bashar and Tuno, 2014, Alshehri, 2013, Alahmed, 2012, Murty et al., 2010, Reiter, 2001).
Costa et al. (2010) found that both the number of female mosquitoes laying eggs and production of eggs (oviposition) are higher at a lower temperature and higher relative humidity, while Hopp and Foley (2001) found that egg production and larva indices increase when both temperature and humidity are high.
This study demonstrated that low humidity was characterized by low larval and adult mosquito abundance. During summer season, a minimum number of larval and adult mosquitoes were collected as relative humidity became low. The increased number of larval and adult mosquitoes was observed during October, November, December, January, February and March when relative humidity is high in all study sites.
In the current study, the influence of precipitation/rainfall was also visible (Fig.4a) on the abundance of the adult mosquito during the rainy months though the average annual rainfall in the Eastern Province was 5 mm. During the rainy months of January, February, March, April and May, more adult mosquito activity was observed compared to the dry seasons. Rainfall influences the number of mosquitoes either positively by providing more/maintaining breeding sites or negatively by flushing out mosquito larvae from small breeding sites (Tian et al., 2015, Bashar and Tuno, 2014, Alshehri, 2013, Byun and Webb, 2012, Murty et al., 2010, Hu et al., 2006, Ceccato et al., 2005, Koenraadt et al., 2004). Overall, a moderate positive correlation between mosquito abundance and rainfall was observed in all study sites.
The statistical analyses performed between adult mosquito abundance and climatic factors are given in Table 2. This statistical analysis was preformed for Abu Main, Umm As-Sahik, Safwa, Al-Awjam, Dammam and Al-Qatif, with the exception of Buqayq and Al Sarar since no adult mosquitoes were collected during field visits. Table 2 clearly shows that mosquito abundance has a negative correlation with temperature. However, a positive correlation was observed with relative humidity with the exception of Safwa site. It is also evident from Table 2 that mosquito abundance has a moderate to low positive correlation with precipitation/rainfall with the exception of Abu Main and Dammam.
The regression model for three climatic factors (temperature, RH and rainfall) explained 84.5% (R2 = 0.845) of the variance in adult mosquito abundance in the Eastern Province. This means that 84.5% of the variance are accounted for the three parameters and the remaining 15.5% attributed to other factors such as the presence of vegetation, waste materials, water reservoirs, ditches, and others.
Mosquito larva abundance has a negative correlation with temperature in general, but a strong negative correlation in Abu Main, Safwa, Al-Qatif and Buqayq. Larva abundance has a positive correlation with relative humidity, with the highest correlation values (0.798, 0.673, and 0.589) in Abu Main, Safwa and Buqayq, respectively. Mosquito larva abundance and precipitation has a moderate to low positive correlation, with the exception of a negative correlation in Al-Qatif. The regression model of the three climatic factors (temperature, RH and rainfall) accounted for 64.3% (R2 = 0.643) of the variance in mosquito larva abundance in the Eastern Province. 64.3% of the variance was explained by the 3 parameters and the remaining 35.7% was attributed to other factors such as the presence of vegetation, waste materials and water reservoirs such as ditches. In a comparison of the two regression models (adults and larvae) and climatic factors, it seems that larvae are more influenced by the presence of vegetation, waste material, water reservoirs and ditches. The presence of floating and terrestrial vegetation, poor environmental sanitation and extensive irrigation activities that create water reservoirs such as ditches are among the major environmental factors for mosquito abundance and their wide distribution in many habitats (Ohta and Kaga, 2014, Alahmed, 2012, Calhoun et al., 2007, Chaikoolvatana et al., 2007).
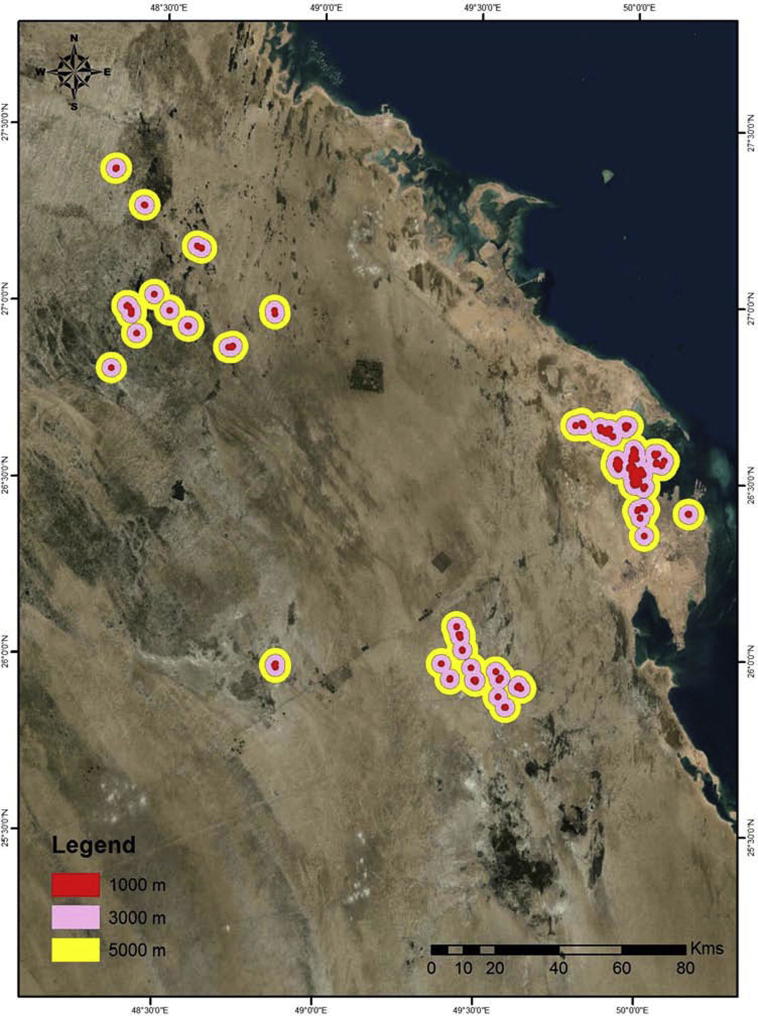
Understanding climatic factors (temperature, relative humidity and rainfall) influencing adult and larvae is the first step to control over mosquito vector distribution and abundance. The other step is utilization of GIS application to improve and advanced control of MBDs and development of a risk map (Palaniyandi et al., 2014, Palaniyandi, 2014a, Palaniyandi, 2014b, Agarwal et al., 2012, Rydzanicz et al., 2011, Chaikoolvatana et al., 2007, Zou et al., 2006). Development of a risk map for the Eastern Province by considering the flying distance of the adult mosquito from the studied sites was performed. Most adult mosquito species fly within the range of 1–3 km, though some species can travel up to 5 km (WHO, 1982). Accordingly, a buffer zone of 1, 3 and 5 km is considered as a buffer distance for mosquito risk areas Fig. 5.
Figure 5.
Buffer distance/mosquito risk area in Eastern Province, Saudi Arabia, 2014.
It seems that the risk areas in our study locations grouped into three clusters. One cluster includes locations 1–6, while the second and the third include location 7 and 8. However, the possibilities of existence of other sites between those locations are possible since this study focused on those three locations. The results show that the buffer distance/mosquito risk area of MBDs is high for locations 1–6, compared to those of 7 and 8. The average distance between all sites at various locations is approximately 0.5 km. Therefore, risk areas for the first cluster will positively overlap comparing to the other two clusters. Based on this finding, it is essential to implement high-risk management control program to focus on sites 1–6 as a first priority, and followed by sites 7 and 8.
4. Conclusion
The results of this study demonstrate a strong negative correlation between mosquito abundance and temperature, while there is a strong and moderate positive correlation between relative humidity and rainfall, respectively. There are variations in the influence of climatic factors on adult mosquitos (84.5%) and larva (64.3%), compared to other factors in adults (15.5%) and larva (37.7%). In addition, GIS was used to identify with detail the potential hotspots of mosquito larva breeding sites, buffer distance/mosquito risk area of MBDs. The study recommends a management control strategy based on current risk area and suggests to focus on specific locations according to their priorities.
Acknowledgements
The authors would like to thank Dr. Alexis Nzila, and Dr. Baqer Al-Ramadan for their constructive comments and support. The authors also would like to thank King Fahd University of Petroleum and Minerals (KFUPM) for the support to conduct the study. The authors are grateful to the Ministry of Health, Dammam Branch and Presidency of Meteorological and Environment, Dammam, for providing required logistic support and meteorological data, for the study.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yasin Jemal, Email: addeymearey@yahoo.com.
Assad A. Al-Thukair, Email: thukair@kfupm.edu.sa.
References
- Agarwal S.A., Sikarwar S.S., Sukumaran D. Application of RS & GIS in risk area assessment for mosquito borne diseases – a case study in a part of Gwalior City (M.P.) Int. J. Adv. Technol. Eng. Res. (IJATER) 2012;2(1):1–4. [Google Scholar]
- Ahmed A.M. Mosquito vectors survey in the AL-Ahsaa district of Eastern Saudi Arabia. J. Insect Sci. 2011;11(176):1–11. doi: 10.1673/031.011.17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmed A.M. Mosquito fauna (Diptera: Culicidae) of the Eastern Region of Saudi Arabia and their seasonal abundance. J. King Saud Univ. Sci. 2012;24(1):55–62. [Google Scholar]
- Al-Qabati A.G., Al-Afaleq A.I. Cross-sectional, longitudinal and prospective epidemiological studies of rift valley fever in Al-Hasa Oasis, Saudi Arabia. J. Anim. Vet. Adv. 2010;9(2):258–265. [Google Scholar]
- Alshehri M.S.A. Dengue fever outburst and its relationship with climatic factors. World Appl. Sci. J. 2013;22(4):506–515. [Google Scholar]
- Al-Tawfiq J.A. Epidemiology of travel-related malaria in a non-malarious area in Saudi Arabia. Saudi Med. J. 2006;27(1):86–89. [PubMed] [Google Scholar]
- Aziz A.T. An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasites Vectors. 2014;7(1):258. doi: 10.1186/1756-3305-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashar K., Tuno N. Seasonal abundance of Anopheles mosquitoes and their association with meteorological factors and malaria incidence in Bangladesh. Parasites Vectors. 2014;7(1):442. doi: 10.1186/1756-3305-7-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashwari L.A. Epidemiological profile of malaria in a university hospital in the eastern region of Saudi Arabia. Saudi Med. J. 2001;22(2):133–138. [PubMed] [Google Scholar]
- Bayoh M.N., Lindsay S.W. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae) Bull. Entomol. Res. 2003;93(5):375–381. doi: 10.1079/ber2003259. [DOI] [PubMed] [Google Scholar]
- Bayoh M.N., Lindsay S.W. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med. Vet. Entomol. 2004;18(2):174–179. doi: 10.1111/j.0269-283X.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- Byun R., Webb C.E. 2012. Guidelines for Mosquito Risk Assessment and Management in Constructed Wetlands. [Google Scholar]
- Calhoun L.M. Combined sewage overflows (CSO) are major urban breeding sites for Culex quinquefasciatus in Atlanta, Georgia. Am. J. Trop. Med. Hyg. 2007;77(3):478–484. [PubMed] [Google Scholar]
- Ceccato P. Application of geographical information systems and remote sensing technologies for assessing and monitoring malaria risk. Parassitologia. 2005;47(1):81–96. [PubMed] [Google Scholar]
- Chaikoolvatana A., Singhasivanon P., Haddawy P. Utilization of a geographical information system for surveillance of Aedes aegypti and dengue haemorrhagic fever in north-eastern Thailand. Dengue Bull. 2007;31:75–82. [Google Scholar]
- Christiansen-Jucht C. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites Vectors. 2014;7(1):489. doi: 10.1186/s13071-014-0489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E.A.P.D.A. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Revista Brasileira de Entomologia. 2010;54(3):488–493. [Google Scholar]
- El-Gilany A.H., Eldeib A., Hammad S. Clinico-epidemiological features of dengue fever in Saudi Arabia. Asian Pac. J. Trop. Med. 2010;3(3):220–223. [Google Scholar]
- Fakeeh M., Zaki A.M. Dengue in Jeddah, Saudi Arabia, 1994–2002. Dengue Bull. 2003;27:13–18. [Google Scholar]
- Flick R., Bouloy M. Rift valley fever virus. Curr. Mol. Med. 2005;5(8):827–834. doi: 10.2174/156652405774962263. [DOI] [PubMed] [Google Scholar]
- Gandhi G. Original research article remote sensing and geographical information system application for mosquito intervention – a case study of Grater Hyderabad. Int. J. Curr. Microbiol. Appl. Sci. 2013;2(12):560–568. [Google Scholar]
- Gimnig J., Hightower A., Hawley W. Application of geographic information systems to the study of the ecology of mosquitoes and mosquito-borne diseases. In: Willem T., Pim M., Robert B., editors. Environmental Change and Malaria Risk: Global and Local Implications. 2005. pp. 27–39. [Google Scholar]
- Hopp M.J., Foley J.A. Global-scale relationships between climate and the dengue fever vector, Aedes aegypti. Clim. Change. 2001;48(2–3):441–463. [Google Scholar]
- Hu W. Rainfall, mosquito density and the transmission of Ross River virus: a time-series forecasting model. Ecol. Modell. 2006;196(3–4):505–514. [Google Scholar]
- Khormi H.M., Kumar L. Using geographic information system and remote sensing to study common mosquito-borne diseases in Saudi Arabia. J. Food Agric. Environ. 2013;11(2):14–17. [Google Scholar]
- Koenraadt C.J.M., Githeko A.K., Takken W. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop. 2004;90(2):141–153. doi: 10.1016/j.actatropica.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lehane M., editor. The Biology of Blood-Sucking in Insects. second ed. Cambridge University Press; 2005. [Google Scholar]
- Madani T.A. Rift valley fever epidemic in Saudi Arabia: epidemiological, clinical and laboratory characteristics. Oxford J. 2003;37:1084–1092. doi: 10.1086/378747. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Matthews G. John Wiley and Sons Ltd; 2011. Integrated Vector Management: Controlling Vectors of Malaria and Other Insect Vector Borne Diseases. [Google Scholar]
- McMichael, A.J. et al., 1996. Climate change and human health. Available at: <http://www.eazphc.tbzmed.ac.ir/research/files/8WHO_EHG_96.7.pdf>.
- Minakawa N. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J. Med. Entomol. 2002;39(6):833–841. doi: 10.1603/0022-2585-39.6.833. [DOI] [PubMed] [Google Scholar]
- Murty U.S., Rao M.S., Arunachalam N. The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. J. Vector Borne Dis. 2010;47(1):26–32. [PubMed] [Google Scholar]
- Mushinzimana E. Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malaria J. 2006;5:13. doi: 10.1186/1475-2875-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Kaga T. Effect of irrigation systems on temporal distribution of malaria vectors in semi-arid regions. Int. J. Biometeorol. 2014;58(3):349–359. doi: 10.1007/s00484-012-0630-y. [DOI] [PubMed] [Google Scholar]
- Palaniyandi M. The environmental aspects of dengue and chikungunya outbreaks in India: GIS for epidemic control. Int. J. Mosq. Res. 2014;1(2):35–40. [Google Scholar]
- Palaniyandi M. Web mapping GIS: GPS under the GIS umbrella for Aedes species dengue and chikungunya vector mosquito surveillance and control. Int. J. Mosq. Res. 2014;1(3):18–25. [Google Scholar]
- Palaniyandi M., Anand P., Maniyosai R. Spatial cognition: a geospatial analysis of vector borne disease transmission and the environment, using remote sensing and GIS. Int. J. Mosq. Res. 2014;1(3):39–54. [Google Scholar]
- Pratt H.D., Barnes R.C., Littig K.S. 1963. Mosquitoes of Public Health Importance and their Control: Training Guide – Insect Control Series. [Google Scholar]
- Reiter P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001;109:141–161. doi: 10.1289/ehp.01109s1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydzanicz K. Implementation of geographic information system (GIS) in an environmental friendly mosquito control programme in irrigation fields in Wroclaw (Poland) J. Eur. Mosq. Control Assoc. 2011;29(January):1–12. [Google Scholar]
- Thomas, J., 2012. Eastern Saudi Arabia: Topography, geography and vegetation. Available at: <http://www.plantdiversityofsaudiarabia.info/Biodiversity-Saudi-Arabia/Vegetation/Eastern%20Province.htm>.
- Tian H.-Y. How environmental conditions impact mosquito ecology and Japanese encephalitis: an eco-epidemiological approach. Environ. Int. 2015;79:17–24. doi: 10.1016/j.envint.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W., Burkot T.R., Kay B.H. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med. Vet. Entomol. 2000;14(1):31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Westbrook C.J. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to Chikungunya virus. Vector Borne Zoonotic Dis. 2010;10(3):241–247. doi: 10.1089/vbz.2009.0035. (Larchmont, N.Y.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 1982. Manual on Environmental Management for Mosquito Control. World Health Organization Offset Publication, No. 66. pp. 284. [PubMed] [Google Scholar]
- WHO . 1997. Vector Control: Methods for use by Individuals and Communities. [Google Scholar]
- WHO . 2014. A Global Brief on Vector-Borne Diseases. pp. 1–56. [Google Scholar]
- Zhou G., Munga S., Minakawa N. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am. J. Trop. Med. Hyg. 2007;77(1):29–35. [PubMed] [Google Scholar]
- Zou L., Miller S.N., Schmidtmann E.T. Mosquito larval habitat mapping using remote sensing and GIS: implications of coalbed methane development and West Nile virus. J. Med. Entomol. 2006;43(5):1034–1041. doi: 10.1603/0022-2585(2006)43[1034:mlhmur]2.0.co;2. [DOI] [PubMed] [Google Scholar]