Abstract
Insulin resistance is a hallmark feature of type-2 diabetes mellitus (T2DM). We determined the homeostatic model assessment insulin resistance (HOMA-IR) and evaluated its association with C-peptide, insulin, fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) in T2DM patients and non-diabetic subjects. This study comprised a total of 47 T2DM patients and 38 non-diabetic controls. Venous blood samples from all the subjects were collected and sera were analyzed for FBG, HbA1c, insulin and C-peptide using an autoanalyzer. HOMA-IR was calculated using the following equation: HOMA-IR = fasting insulin (µU/ml) × fasting glucose (mmol/L)/22.5. There was a significant increase in the levels of FBG and HbA1c in diabetic patients. Although the levels of C-peptide and insulin did not differ significantly between the two groups, a significant increase in HOMA-IR was observed in T2DM patients. Both insulin and C-peptide were significantly correlated with HOMA-IR. In conclusion, C-peptide may serve as a simple and convenient predictor of HOMA-IR.
Keywords: Insulin resistance, Diabetes, C-peptide, Fasting blood glucose, HbA1c, Biomarker
1. Introduction
The pathogenesis of type-2 diabetes mellitus (T2DM) mainly involves abnormalities in insulin secretion and action, the so called insulin resistance (IR) (Weyer et al., 1999). The exact mechanism of IR is multifactorial and has not been precisely established however certain factors such as metabolic syndrome, obesity, dyslipidemia and hyperinsulinemia have been implicated with IR (Weyer et al., 1999, Grundy, 2006, Ma et al., 2012). Over the time, IR and hyperinsulinemia often lead to T2DM or pre-diabetes because the beta cells fail to keep up with the body's increased need for insulin (Martin et al., 1992). It is also probable that chronic inflammation may trigger the onset of IR and subsequently the emergence of T2DM (McNelis and Olefsky, 2014).
Both T2DM and metabolic syndrome are highly prevalent in Saudi Arabia (Alotaibi et al., 2017, Khan et al., 2007, Al-Nozha et al., 2005). Bahijri et al (Bahijri et al., 2010) have suggested that screening of IR in the general population is important for developing strategies to reduce the prevalence of T2DM. Previous studies have shown significant correlations between IR and ghrelin (Al Qarni et al., 2017); adiponectin (Aleidi et al., 2015); leptin (Gulturk et al., 2008); resistin (Al-Harithy and Al-Ghamdi, 2005), C-reactive protein (Alemzadeh and Kichler, 2014), triglycerides (Lee et al., 2011), thyroid hormones (Kumar et al., 2009), vitamin D (Abdelkarem et al., 2016, Maghbooli et al., 2008), and liver enzymes (Esteghamati et al., 2011, Al-Sultan, 2008 Jul). Borai et al. (2011) have shown that HbA1c may serve as a simple and reliable marker for IR in adults with normal glucose tolerance. Vaverková et al. (2017) observed an inverse association between lipoprotein-a and IR as well as metabolic syndrome that precedes an overt T2DM diagnosis.
C-peptide is a byproduct of the insulin synthesis from pro-insulin and roughly indicates the extent of insulin production and release. C-peptide is a biological active compound (Forst et al., 1998) and also serves as an important diagnostic biomarker (Cardellini et al., 2017, Becht et al., 2016, Cabrera de León et al., 2015, Williams et al., 2011). Recently, C-peptide has been suggested as a strong indicator of metabolic syndrome suggesting the importance of this biomolecule in diagnosis of metabolic syndrome (Gonzalez-Mejia et al., 2016). Because both metabolic syndrome and IR are associated with elevated risk for developing T2DM, it would be intriguing to investigate the biomarker potential of C-peptide for screening of IR prone individuals such as pre-diabetics and diabetics.
The homeostasis model assessment insulin resistance (HOMA-IR) developed by Matthews et al. (1985), is a convenient and widely used method for the estimation of IR. In this study, we examined the associations between HOMA-IR and fasting blood glucose (FBG), glycated hemoglobin (HbA1c), insulin and C-peptide in type-2 diabetic patients and healthy controls and evaluated the biomarker potential of C-peptide for screening of IR in diabetic and non-diabetic individuals.
2. Materials and methods
This study comprised a total of 47 T2DM patients and 38 non-diabetic controls who visited the clinics of Prince Sultan Military Medical City, Riyadh, Saudi Arabia. There were 27 males and 20 females in diabetic group and 23 males and 15 females in control group. The average age of the diabetic patients and control subjects were 41.21 ± 4.73 years and 40.79 ± 3.95 years, respectively. The main inclusion criteria for diabetic patients were being a Saudi national and affected with type-2 diabetes. The exclusion criteria included severe chronic illnesses, infections and pregnancy.
Venous blood samples from all the subjects were collected in serum separator tubes after at least 8 h fasting. The sera were analyzed for fasting blood glucose (FBG), glycated hemoglobin (HbA1c), insulin and C-peptide using an autoanalyzer (Roche Modular P-800, Germany). HOMA-IR was calculated using the following equation; fasting insulin (µU/ml) × fasting glucose (mmol/L)/22.5 as reported earlier (Matthews et al., 1985, Wallace et al., 2004).
The data were analyzed by SPSS version 10. Pearson’s correlation test was performed to examine various correlations. Independent samples Student’s t-test (2-tailed) was used to compare the means of different parameters between controls and diabetic patients. P values less than 0.05 were considered as statistically significant.
3. Results
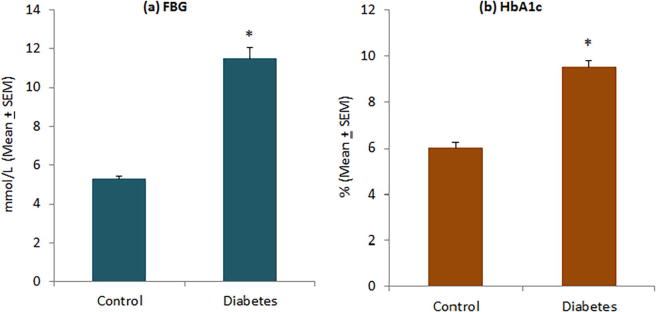
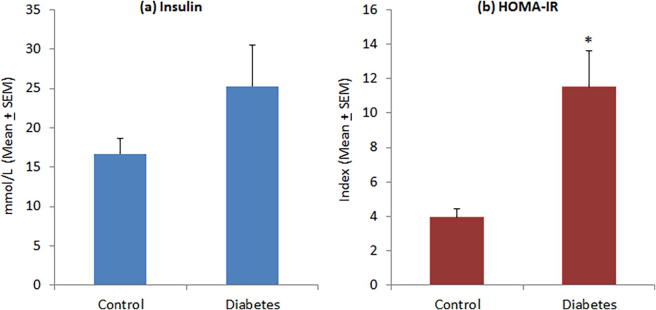
The level of FBG was significantly higher in diabetic patients (11.51 ± 0.55 mmol/L) as compared to controls (5.19 ± 0.13 mmol/L) (Fig. 1a). Diabetic patients had poor glycemic control and their HbA1c levels were significantly higher (9.49 ± 0.31% versus 6.04 ± 0.18%) than normal subjects (Fig. 1b). Although insulin levels were comparatively higher in diabetic patients (25.25 ± 5.39 µU/mL) than controls (16.71 ± 2.02 µU/mL), this difference was not statistically significant (Fig. 2a). Diabetic patients showed a significantly high level of HOMA-IR (11.49 ± 2.10) as compared to normal subjects (3.95 ± 0.46) (Fig. 2b). In diabetic patients, 16 of 47 patients (34.04%) had HOMA-IR > 10 as compared to only 2 of 38 controls (5.26%). There was no significant difference in C-peptide levels between diabetic patients (951.17 ± 100.69 mmol/L) and controls (861.18 ± 63.66 mmol/L) (Fig. 3).
Fig. 1.
Levels of (a) fasting blood glucose and (b) HbA1c in controls and diabetic patients. *P < 0.001 versus control group, t-test.
Fig. 2.
Levels of (a) serum insulin and (b) insulin resistance in controls and diabetic patients. *P < 0.01 versus control group, t-test.
Fig. 3.
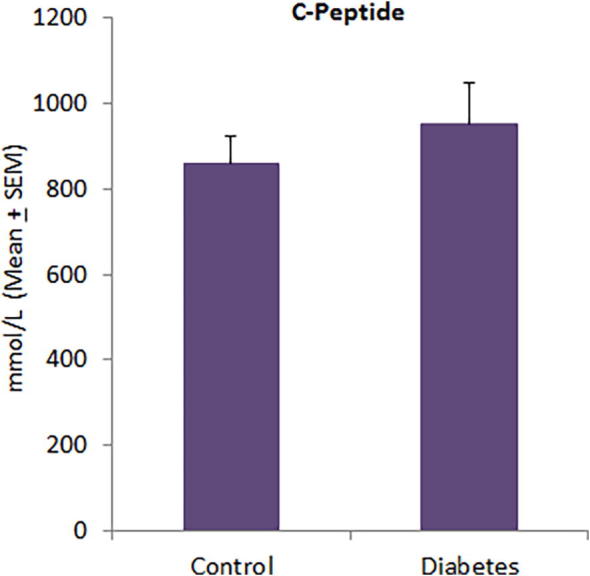
Serum C-peptide levels in controls and diabetic patients.
There were highly significant correlations between HOMA-IR and insulin in both diabetic patients (R = 0.961, P = 0.001) and control subjects (R = 0.975, P = 0.001) (Table 1). C-peptide was also significantly correlated with HOMA-IR in diabetic patients (R = 0.652, P = 0.001) and controls (R = 0.500, P = 0.001). Although HOMA-IR was not correlated with HbA1c in diabetic patients (R = -0.070, P = 0.259), it was significantly correlated with HbA1c in normal subjects (R = 0.355, P = 0.029). Both age and gender were not correlated with HOMA-IR, irrespective of patient or control groups (Table 1).
Table 1.
Correlations between insulin resistance (HOMA-IR) and other variables.
| Variable | Diabetic patients only |
Non-diabetic controls only |
||
|---|---|---|---|---|
| R | P | R | P | |
| Age | −0.140 | 0.347 | −0.019 | 0.909 |
| Gender | 0.202 | 0.173 | 0.007 | 0.966 |
| FBG | −0.041 | 0.784 | 0.244 | 0.140 |
| HbA1c | −0.170 | 0.259 | 0.355 | 0.029* |
| Insulin | 0.961 | 0.001* | 0.975 | 0.001* |
| C-peptide | 0.652 | 0.001* | 0.500 | 0.001* |
Statistically significant.
4. Discussion
Several studies have shown that IR is a strong predictor for the future development of diabetes (Martin et al., 1992, Lillioja et al., 1993). Moreover, current interest in IR and metabolic syndrome is because of their ever-growing incidence and the associated mortality and morbidity due to cardiovascular events, even in non-diabetic subjects (Ford et al., 2002). Thus, a timely identification of IR in non-diabetic population is of a great importance in a community-based strategy to reduce the upcoming prevalence of T2DM, keeping in mind that interventional studies have demonstrated that preservation of b-cell function decreases the transformation of pre-diabetes to diabetes (Kitabchi et al., 2005, Buchanan et al., 2002). It has been suggested that the method of choice for the screening of IR should be suitable for a large-population study, require one blood sample and having a high level of reproducibility and prediction power (Bahijri et al., 2010).
Our results showed significantly high levels of FBG and HbA1c in T2DM patients as compared to control subjects (Fig. 1) indicating a reluctant approach for glycemic control in Saudi individuals (Sherwani et al., 2016, Khan et al., 2014, Ahmad, 2007). We observed that serum insulin levels were comparatively higher in T2DM patients than non-diabetic individuals (Fig. 2). A previous study has shown significantly higher levels of serum insulin in Saudi T2DM patients as compared to healthy controls (Habib et al., 2009). The HOMA-IR was found to be significantly high in T2DM patients as compared to controls (Fig. 2), which is supported by previous studies on Saudi T2DM patients (Al Qarni et al., 2017, Mira et al., 2002). In our study, severe IR (HOMA-IR > 10) was observed in 34.04% of diabetic patients and 5.26% of non-diabetic controls. A recent study on 107 T2DM patients and 101 controls showed the IR frequency of 46.7% in diabetic patients and 5.9% in controls (Al Qarni et al., 2017).
There were no correlations between IR and age, gender or FBG in the subgroups of T2DM patients and non-diabetic controls; however, HbA1c was significantly correlated with IR, only in non-diabetic individuals (Table 1). In a previous study from Saudi Arabia, HOMA-IR values were not associated with age, gender or disease duration Al Qarni et al. (2017). Bahijri et al. (2010) observed that adiposity, but not gender, is a risk factor for IR in healthy adults from Saudi Arabia. A comparative analysis of subjects with normal glucose tolerance (N = 24), impaired fasting glucose (N = 12), impaired glucose tolerance (N = 12), and T2DM (N = 13) showed that HbA1c was more strongly associated with IR in only normal glucose tolerance group (Borai et al., 2011). Al Qarni et al. (2017) observed that HbA1c levels were significantly higher in T2DM subjects with IR than in those without IR; moreover, HbA1c was not correlated with HOMA-IR.
The striking observation of this study was a significant correlation between C-peptide and IR in both T2DM patients and non-diabetic individuals (Table 1) despite C-peptide levels did not differ between these two subgroups (Fig. 3). C-peptide levels were found to be significantly higher in healthy subjects with IR than in those without IR (Al Qarni et al., 2017). High levels of fasting C-peptide pose a risk factor for the development of atherosclerosis in both non-diabetic and diabetic persons (Kim et al., 2011, Patel et al., 2012). Alemzadeh and Kichler (2014) observed that C-peptide levels were significantly higher in patients with metabolic syndrome irrespective of gender difference. Chen et al. (1999) have shown that fasting C-peptide is significantly correlated with several markers of metabolic syndrome in obese adults. Thus, C-peptide may serve as a multi-modal biomarker to identify risky individuals for IR, T2DM, atherosclerosis and metabolic syndrome. It has been recommended that screening for T2DM should begin at the age of 45 years and then be repeated on 3 year intervals; however among the risky individuals, it should begin sooner and be more frequent (Mayfield, 1998). Al Qarni et al. (2017) have suggested the need of a parallel screening for IR as it can be used to trace IR milestones and the progress of the disease with increased age and disease duration.
5. Conclusion
Insulin resistance is highly prevalent in T2DM patients from Saudi Arabia. Both FBG and HbA1c levels do not reflect the intensity of HOMA-IR in T2DM patients. HOMA-IR is also unaffected by age and gender. Serum C-peptide may be used as an appropriate index for detecting IR in community-based surveys. A longer half-life of C-peptide (approximately 5-fold) than insulin (Matthews et al., 1985) also favors its biomarker utility with less fluctuation.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. RGP-009.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelkarem H.M., El-Sherif M.A., Gomaa S.B. Vitamin D status and insulin resistance among young obese Saudi females. Saudi Med. J. 2016;37:561–566. doi: 10.15537/smj.2016.5.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad K.H. Clinical significance of HbA1c as a marker of circulating lipids in male and female type 2 diabetic patients. Acta Diabetol. 2007;44:193–200. doi: 10.1007/s00592-007-0003-x. [DOI] [PubMed] [Google Scholar]
- Al Qarni A.A., Joatar F.E., Das N. Association of plasma ghrelin levels with insulin resistance in Type 2 diabetes mellitus among Saudi subjects. Endocrinol. Metab. (Seoul) 2017;32:230–240. doi: 10.3803/EnM.2017.32.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleidi S., Issa A., Bustanji H., Khalil M., Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm. J. 2015;23:250–256. doi: 10.1016/j.jsps.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemzadeh R., Kichler J. Gender differences in the association of insulin resistance and high-sensitivity c-reactive protein in obese adolescents. J. Diabetes Metab. Disord. 2014;13:35. doi: 10.1186/2251-6581-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harithy R.N., Al-Ghamdi S. Serum resistin, adiposity and insulin resistance in Saudi women with type 2 diabetes mellitus. Ann. Saudi Med. 2005;25:283–287. doi: 10.5144/0256-4947.2005.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nozha M., Al-Khadra A., Arafah M.R., Al-Maatouq M.A., Khalil M.Z., Khan N.B. Metabolic syndrome in Saudi Arabia. Saudi Med. J. 2005;26:1918–1925. [PubMed] [Google Scholar]
- Alotaibi A., Perry L., Gholizadeh L., Al-Ganmi A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: an overview. J. Epidemiol. Glob. Health. 2017;7:211–218. doi: 10.1016/j.jegh.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sultan A.I. Assessment of the relationship of hepatic enzymes with obesity and insulin resistance in adults in Saudi Arabia. Sultan Qaboos Univ. Med. J. 2008 Jul;8(2):185–192. [PMC free article] [PubMed] [Google Scholar]
- Bahijri S.M., Alissa E.M., Akbar D.H., Ghabrah T.M. Estimation of insulin resistance in non-diabetic normotensive Saudi adults by QUICKI, HOMA-IR and modified QUICKI: a comparative study. Ann. Saudi Med. 2010;30:257–264. doi: 10.4103/0256-4947.65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht F.S., Walther K., Martin E., Nauck M.A. Fasting C-peptide and related parameters characterizing insulin secretory capacity for correctly classifying diabetes type and for predicting insulin requirement in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2016;124:148–156. doi: 10.1055/s-0035-1565177. [DOI] [PubMed] [Google Scholar]
- Borai A., Livingstone C., Abdelaal F., Bawazeer A., Keti V., Ferns G. The relationship between glycosylated haemoglobin (HbA1c) and measures of insulin resistance across a range of glucose tolerance. Scand. J. Clin. Lab. Invest. 2011;71:168–172. doi: 10.3109/00365513.2010.547947. [DOI] [PubMed] [Google Scholar]
- Buchanan T.A., Xiang A.H., Peters R.K., Kjos S.L., Marroquin A., Goico J. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- Cabrera de León A., Oliva García J.G., Marcelino Rodríguez I. C-peptide as a risk factor of coronary artery disease in the general population. Diab. Vasc. Dis. Res. 2015;12:199–207. doi: 10.1177/1479164114564900. [DOI] [PubMed] [Google Scholar]
- Cardellini M., Farcomeni A., Ballanti M. C-peptide: A predictor of cardiovascular mortality in subjects with established atherosclerotic disease. Diab. Vasc. Dis. Res. 2017;14:395–399. doi: 10.1177/1479164117710446. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Tsai S.T., Chou P. Correlation of fasting serum C-peptide and insulin with markers of metabolic syndrome-X in a homogenous Chinese population with normal glucose tolerance. Int. J. Cardiol. 1999;68:179–186. doi: 10.1016/s0167-5273(98)00366-0. [DOI] [PubMed] [Google Scholar]
- Esteghamati A., Noshad S., Khalilzadeh O., Khalili M., Zandieh A., Nakhjavani M. Insulin resistance is independently associated with liver aminotransferases in diabetic patients without ultrasound signs of nonalcoholic fatty liver disease. Metab. Syndr. Relat. Disord. 2011;9:111–117. doi: 10.1089/met.2010.0066. [DOI] [PubMed] [Google Scholar]
- Ford E.S., Giles W.H., Dietz W.H. Prevalence of metabolic syndrome among US adults. J. Am. Med. Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Forst T., Kunt T., Pfützner A., Beyer J., Wahren J. New aspects on biological activity of C-peptide in IDDM patients. Exp. Clin. Endocrinol. Diabetes. 1998;106:270–276. doi: 10.1055/s-0029-1212190. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mejia M.E., Porchia L.M., Torres-Rasgado E., Ruiz-Vivanco G., Pulido-Pérez P., Báez-Duarte B.G., Pérez-Fuentes R. C-peptide is a sensitive indicator for the diagnosis of metabolic syndrome in subjects from Central Mexico. Metab. Syndr. Relat. Disord. 2016;14:210–216. doi: 10.1089/met.2015.0067. [DOI] [PubMed] [Google Scholar]
- Grundy S.M. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin. Cornerstone. 2006;8(Suppl. 1):S21–S27. doi: 10.1016/s1098-3597(06)80005-0. [DOI] [PubMed] [Google Scholar]
- Gulturk S., Cetin A., Erdal S. Association of leptin with insulin resistance, body composition, and lipid parameters in postmenopausal women and men in type 2 diabetes mellitus. Saudi Med. J. 2008;29:813–820. [PubMed] [Google Scholar]
- Habib S.S., Aslam M., Shah S.F., Naveed A.K. Lipoprotein (a) is associated with basal insulin levels in patients with type 2 Diabetes Mellitus. Arq. Bras. Cardiol. 2009;93:28–33. doi: 10.1590/s0066-782x2009000700006. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Sobki S.H., Khan S.A. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin. Exp. Med. 2007;7:24–29. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Ola M.S., Alhomida A.S., Sobki S.H., Khan S.A. Evaluation of HbA1c criteria for diagnosis of diabetes mellitus: a retrospective study of 12 785 type 2 Saudi male patients. Endocr. Res. 2014;39:61–65. doi: 10.3109/07435800.2013.828740. [DOI] [PubMed] [Google Scholar]
- Kim S.T., Kim B.J., Lim D.M., SongI G., Jung J.H., Lee K.W., Park K.Y., Cho Y.Z., Lee D.H., Koh G.P. Basal c-peptide level as a surrogate marker of subclinical atherosclerosis in type 2 diabetic patients. Diabetes Metab. J. 2011;35:41–49. doi: 10.4093/dmj.2011.35.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A.E., Temprosa M., Knowler W.C., Kahn S.E., Fowler S.E., Haffner S.M. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H.K., Yadav R.K., Prajapati J., Reddy C.V., Raghunath M., Modi K.D. Association between thyroid hormones, insulin resistance, and metabolic syndrome. Saudi Med. J. 2009;30:907–911. [PubMed] [Google Scholar]
- Lee S.H., Lee B.W., Won H.K., Moon J.H., Kim K.J., Kang E.S., Cha B.S., Lee H.C. Postprandial triglyceride is associated with fasting triglyceride and HOMA-IR in Korean subjects with type 2 diabetes. Diabetes Metab. J. 2011;35:404–410. doi: 10.4093/dmj.2011.35.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Mott D.M., Spraul M., Ferraro R., Foley J.E., Ravussin E. Insulin resistance and insulin secretory dysfunction as precursors of non- insulin- dependent diabetes mellitus: prospective studies of Pima Indians. N. Engl. J. Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- Ma Z.A., Zhao Z., Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp. Diabetes Res. 2012. 2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghbooli Z., Hossein-Nezhad A., Karimi F., Shafaei A.R., Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab. Res. Rev. 2008;24:27–32. doi: 10.1002/dmrr.737. [DOI] [PubMed] [Google Scholar]
- Martin B.C., Warram J.H., Krolewski A.S., Bergman R.N., Soeldner J.S., Kahn C.R. Role of glucose and insulin resistance in development of type II diabetes mellitus: results of a 25- year follow- up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F. Homeostasis model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Rudenski A.S., Burnett M.A., Darling P., Turner R.C. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin. Endocrinol. 1985;23:71–79. doi: 10.1111/j.1365-2265.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Mayfield J. Diagnosis and classification of diabetes mellitus: new criteria. Am. Fam. Phys. 1998;58(1355–62):1369–1370. [PubMed] [Google Scholar]
- McNelis J.C., Olefsky J.M. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36e48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Mira S.A., Akbar D.H., Hashim I.A., Salamah S.H., Zawawi T.H. The insulin resistance syndrome among type II diabetics. Saudi Med. J. 2002;23:1045–1048. [PubMed] [Google Scholar]
- Patel N., Taveira T.H., Choudhary G., Whitlatch H., Wu W.C. Fasting serum C-peptide levels predict cardiovascular and overall death in nondiabetic adults. J. Am. Heart Assoc. 2012;1 doi: 10.1161/JAHA.112.003152. e003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwani S.I., Khan H.A., Ekhzaimy A., Masood A., Sakharkar M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaverková H., Karásek D., Halenka M., Cibíčková L., Kubíčková V. Inverse association of lipoprotein (a) with markers of insulin resistance in dyslipidemic subjects. Physiol. Res. 2017;66(Suppl. 1):S113–S120. doi: 10.33549/physiolres.933583. [DOI] [PubMed] [Google Scholar]
- Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Weyer C., Bogardus C., Mott D.M., Pratley R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.V., Gambhir K.K., Nunlee-Bland G., Abrams C.K., Ganta V., Odonkor W. Significance of plasma C-peptide in obese African American adolescents. J. Natl. Med. Assoc. 2011;103:907–916. doi: 10.1016/s0027-9684(15)30446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]