Abstract
Agarwood (Oudh), is often used by people in the Kingdom of Saudi Arabia. The Oudh has been mentioned in the Hadith and is traditionally used for its aroma (perfuming smell) and potential medicinal applications. The aim of the study was to isolate mycotoxigenic fungi that grow on agarwood and the factors and storage conditions that enhance their growth potential. In addition to the detection of associated mycotoxins like: Aflatoxin B1 (AFB1) and ochratoxin A (OTA) from agarwood. Agarwood samples were collected from local markets of Jeddah governorate, Kingdom of Saudi Arabia. Standard dilution plate method was used for the isolation of fungi. Isolated fungi were identified based on morphological characteristics and confirmed using molecular biology techniques. AFB1 and OTA were detected by High Performance Liquid Chromatography (HLPC). The results indicated that the most commonly isolated fungal genera were in the following descending order: Aspergillus, Penicillium, Fusarium and Rhizopus. Among Aspergillus genera, A. flavus and A. ochraceus were detected based on their morphology and confirmed by PCR using specific primers. It was also noted that AFB1 was released by 15.3 and 55.0% of A. flavus and A. parasiticus isolates respectively with levels reaching up to 14.60 µg/L. The moisture content in the samples ranged from 3% to 10% affected fungal growth. AFB1 was detected in 22 out of 50 of the samples. The maximum level of AFB1 (50.7 µg/kg) was detected in samples with higher moisture content (12%) stored at a temperature of 32 °C. Aspergillus fungi were found to be the most predominant fungal genera found on agarwood. Moisture content (9–10%) and storage temperature (32 °C) stimulated fungal growth and their ability to produce mycotoxins. For this reason, storage conditions at the marketing place should be adequate in order not to provide a conducive environment for fungal growth which is associated with the mycotoxin production. In order to prevent fungal growth and mycotoxin production, it would be recommended to store agarwood at temperatures not exceeding 25 °C and moisture content of up to a maximum of 5–6%.
Keywords: Agarwood (Oudh), 18S rDNA, Fungi, Mycotoxins, Aflatoxin B1, Agriculture commodities, Ochratoxin A
1. Introduction
Agarwood (Known in Arabic as Oudh) is a dark resinous wood product of relatively high economic value originated from the trunk of some Aquilaria species tree (Thymelaeaceae) (Sangareswari@Nagajothi et al., 2016). It is formed as a defence reaction of trees either physically or chemically when they are exposed to biotic and abiotic stresses. Agarwood essential oil is used as perfumes in Western cities, whereas agarwood smoke and oil are customarily used as perfumes in the Middle East (Sangareswari@Nagajothi et al., 2016). Agarwood incense, when burned, produces a pleasant aroma, which can be used as a general perfume or as an element in important religious occasions and its essential oil is the most important ingredient in high-end perfumes because of its unique fragrance (Zhang et al., 2012).
The occurrence of toxigenic mycoflora and mycotoxins in medicinal and herbal plants has been confirmed and the two main genera that produce mycotoxins are Aspergillus and Penicillium (Rodriguez-Amaya and Sabino, 2002). Several environmental factors influence fungal proliferation and mycotoxin production, whereas temperature, moisture, and relative humidity are considered the most severe (Bhat and Vasanthi, 2003).
Some negative effects caused by the use of contaminated herbal drugs on human have been reported (Vartika and Shanta, 2005).
Mycotoxins such as aflatoxins (AFs) and ochratoxin A (OTA), the secondary metabolites produced by Aspergillus spp. are of great concern and are an emerging issue in many countries especially in humid tropics (FAO, 1991). The International Agency for Research on Cancer categorized aflatoxins as class1 carcinogens, as they are highly poisonous, toxic substances (IARC, 1993). Thus, direct exposure to aflatoxin-contaminated commodities may impose a great risk to the consumer. There are many factors involved in mycotoxin infectivities such as biological factors, harvesting, storage and processing conditions and climate change which is the most important factor (Arijit et al., 2013).
Modern molecular techniques are rapid, specific and sensitive, their application is being increasingly used for microbial species identification. Molecular techniques have been used to ascertain the aflatoxigenicity of A. flavus and A. niger fungi in food and feed (Toma and Abdulla, 2013).
The agarwood shelf life will be expanded if not exposed to fungal infestation. Up till now, no reports are available for the microbiological quality of agarwood. Therefore, the present study was designed to shed the light on the safety of agarwood (Oudh) through isolation of mycotoxigenic fungi as well as detection of AFB1 and OTA contaminants in agarwood samples collected from local markets of Jeddah governorate, Kingdome of Saudi Arabia. On the other hand, the effect of environmental conditions on the level of AFB1 in agarwood samples was studied.
2. Methodology
2.1. Agarwood samples
Fifty different agarwood samples were randomly purchased from local markets in Jeddah Governorate, Kingdom of Saudi Arabia. Each sample was placed in a dry sealed sterile container and kept in a refrigerator at 5 ± 2 °C until used.
2.2. Fungal isolation
Standard dilution plate: Ten grams of each agarwood sample were transferred into 250 mL screw-capped medicinal bottle containing 90 mL of sterile distilled water and prepared as described by (Toma and Abdulla, 2013).
Appropriate tenfold serial dilution was prepared and 1 mL of suitable dilutions of the resulting samples suspensions was used to inoculate a set of three Petri dishes each containing 15 mL Potato Dextrose Agar (PDA) or Rose Bengal Agar (RBA) medium (Laboratories Conda, Madrid, Spain). Plates were then incubated for 7 days at 28 ± 2 °C. The developing fungal colonies were counted and the average numbers per gram dry sample were determined and expressed as Colony Forming Units (CFU) of the sample (Sahab et al., 2014).
2.3. Identification of the fungal genera
Fungal isolates were sub-cultured for purification and then identified on the basis of their colony morphology and spore characteristics (Rajankar et al., 2007).
2.4. Molecular identification of fungi
Genomic DNA was extracted from pure mycelial cultures of the fungal isolate; grown on PDA using Extract-N-Amp Plant PCR Kit (Sigma–Aldrich Co., USA) following the manufacturer’s instructions. The crude lysate (freshly prepared) was subjected to18S rDNA PCR partial amplification using the protocol of Gene Jet genomic DNA purification kit. Identification of the fungal isolate was performed based on molecular genetic analysis using the internal transcribed spacer region (ITS). Partial sequences of the isolates 18S rDNA were obtained using a strategy based on Boekhout et al., 1994. A divergent 5′ domain of the gene was amplified using primers forward (5′-AGAGTTTGATCCTGGCTCAG) and reverse (5′-GGTTACCTTGTTACGACTT). DNA amplification involved the 25 cycles. Amplified products were isolated with a silica matrix (Geneclean II Kit; Bio 101). Sequencing results were individually inputted online into the nucleotide BLAST program (BLASTN 2.2.29) through the NCBI database (http://blast.ncbi.nlm.nih.gov/) to identify the isolates (Altschul et al., 1997).
2.5. Ability of isolated fungi to produce mycotoxins
Each fungal isolate (A. flavus, A. parasiticus, A. ochraceus and A. niger) was inoculated into 250 mL Erlenmeyer flasks containing 50 mL Potato dextrose broth (PDB), and incubated at 28 ± 2 °C for 7 days. AFB1 and OTA were extracted according to following methods.
2.6. Mycotoxin standards
Aflatoxin B1 and ochratoxin A standards were purchased from Sigma–Aldrich Chemical Co. St. Louis, MO, USA.
2.7. Analyses of aflatoxin B1
Agarwood samples (25 g) or cultures were mixed with 125 mL methanol/water (70/30 v/v) and homogenated at high speed for 1 min. The extract was filtered using Whatman No. 4 and collected in a vessel. Fifteen milliliters of the filtrate was diluted with 30 mL of purified water and filtered through a glass microfiber filter. Ten mL of filtrate was passed through an immunoaffinity columns, AflaTest®, (VICAM, Watertown, MA, USA) followed by 5 mL of purified water. The extract was eluted with 1.0 mL methanol HPLC grade (BDH Laboratory supplies, Pool, England, UK) at a rate of 1–2 drops/s and then evaporated to dryness under a stream of nitrogen and analyzed using HPLC.
2.8. Analysis of ochratoxin A
Agarwood samples (10 g) or cultures were mixed in a blender for 1 min with 72 mL of acetonitrile/water (60/40) and filtered. The acetonitrile was evaporated under nitrogen, 30 mL of water was added and the extract was partitioned with 30 mL of chloroform which then evaporated under nitrogen. OTA was analyzed using HPLC.
2.9. HPLC system
HPLC system consisting of a Waters Binary pump Model 1525, a Rheodyne manual injector, a 2475 multi-wavelength fluorescence detector (Waters, Milford, MA, USA). Column type and size: C18, 250 × 4.5 mm I.D., 5-micron particle size. The mobile phase acetonitrile/water/methanol (1/6/3 v/v/v) was used for the separation of AFB1 at ambient temperature at a flow rate of 1.0 mL/min. Mobile phase acetonitrile/water/acetic acid (99/99/2 v/v/v) was used for the separation of OTA at a flow rate of 1.0 mL/min.
2.10. Determination of moisture content
Ten grams of each sample were dried in an oven at 100 °C for two hours to determine their moisture content on the basis of weight loss using the following formula:
where MC = moisture content; IW = initial weight; FW = final weight (Essono et al., 2007).
2.11. The effect of storage conditions on AFB1 production
Twenty-five grams of agarwood were washed by sterilized water for three minutes and then soaked in another 40 mL overnight in Erlenmeyer flasks and the moisture content was adjusted individually to 6%, 8%, and 10% before the start of the experiment with sterilized water. The flasks were inoculated with one mL of A. flavus spore suspension (1mL of 1.0 × 105) and then the flasks were incubated at three different temperatures (25, 28 and 32 ± 2 °C) for 21 days. AFB1 were estimated after 7, 14 and 21 days.
3. Results
3.1. Isolation and morphological identification
Analysis of different fungal species isolated from agarwood samples for morphological and cultural characteristics showed that there was variation in the colony colour, margins, and texture and colony reverse colours. The fungal analysis of the agarwood samples showed that 601 and 633 of the fungal isolates were counted on PDA and RBA media respectively. The isolated species belonged to four different genera; namely, Aspergillus spp., Fusarium, Penicillium, and Rhizopus. However, the most common genus was Aspergillus spp. as shown in Figure 1.
Figure 1.
Occurrence of isolated fungi on Potato dextrose agar (PDA) and Rose Bengal agar (RBA) media.
Among Aspergillus genus isolated from agarwood samples on RBA media; A. flavus, A. niger, A. ochraceus and A. parasiticus. Both A. flavus and A. parasiticus were detected in 78.0 and 60.0% of the samples respectively while A. niger and A. ochraceus were detected in 72.0 and 58.0% of the samples respectively. It was also noticed that A. carbonaris was not detected on RBA media, whereas it was detected on PDA media.
The percentage of occurrence of fungi in agarwood samples was calculated to the total number of fungal isolates and to the total Aspergillus species. Aspergillus niger recorded the highest count (26.95% and 35.06%) of the total fungal isolates and total Aspergillus species respectively on PDA media, followed by A. flavus (22.29% and 29%), A. parasiticus (15.54% and 20.34%), A. ochraceus (7.48% and 9.74%) and A. carbonaris (4.49% and 5.84%) in descending order. Using RBA, the results were as follows in a descending order: Aspergillus niger recorded the highest count (25.27% and 29.30%) of the total fungal isolates and total Aspergillus species respectively, followed by A. flavus (23.53% and 27.28%), A. ochraceus (18.79% and 21.79%), A. parasiticus (18.64% and 21.61%), and A. carbonaris was not detected at all.
3.2. Molecular identification of Aspergillus flavus and Aspergillus ochraceus isolates
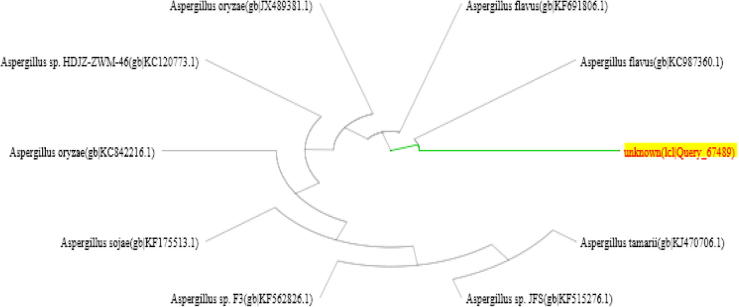
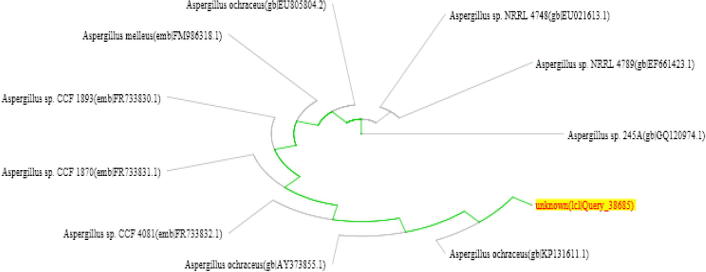
The molecular identification of the fungal isolates was performed by partial 18S rDNA sequencing. The apparent size of the PCR amplicon was 682 bp, and 550 bp for Aspergillus flavus and Aspergillus ochraceus respectively. The obtained 18S rDNA nucleotide sequence was compared with available 18S ribosomal sequences in the NCBI database using BLASTN. The fungal isolates were found to be closely related to Aspergillus flavus strain GHBF09 (Accession No. KC987360.1) and to Aspergillus ochraceus strain IHEM 18887 (Accession No. KP131611.1) (Tables 1 and 2, respectively). As a result, phylogenetic trees were mapped using the neighbor joining method (Figures 2 and 3, respectively).
Table 1.
Sequence producing significant alignments for the Aspergillus flavus isolate.
| Description | Max. score | Total score | Query cover | E value | Ident. | Accession # |
|---|---|---|---|---|---|---|
| Aspergillus flavus strain GHBF09 18S ribosomal RNA gene, partial sequence | 1260 | 1260 | 100% | 0.0 | 100% | KC987360.1 |
| Aspergillus flavus isolate EFB2.3A 18S ribosomal RNA gene, partial sequence | 1229 | 1229 | 100% | 0.0 | 99% | KF691806.1 |
| Aspergillus tamarii strain ZJUT ZQ013 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KJ470706.1 |
| Aspergillus sp. JFS 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KF515276.1 |
| Aspergillus sp. F3 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KF562826.1 |
| Aspergillus sojae strain JPDA1 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KF175513.1 |
| Aspergillus oryzae 16S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KC842216.1 |
| Aspergillus sp. HDJZ-ZWM-46 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | KC120773.1 |
| Aspergillus oryzae strain Yz12 18S ribosomal RNA gene, partial sequence | 1223 | 1223 | 100% | 0.0 | 99% | JX489381.1 |
Table 2.
Sequence producing significant alignments for the Aspergillus ochraceus isolate.
| Description | Max. score | Total score | Query cover | E value | Ident. | Accession # |
|---|---|---|---|---|---|---|
| Aspergillus ochraceus strain IHEM 18887 isolate ISHAM-ITS_ID MITS290 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 942 | 942 | 100% | 0.0 | 100% | KP131611.1 |
| Aspergillus ochraceus strain SRRC 65 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 942 | 942 | 100% | 0.0 | 100% | AY373855.1 |
| Aspergillus pallidofulvus 18S rRNA gene (partial), ITS1, 5.8S rRNA gene, ITS2 and 28S rRNA gene (partial), culture collection CCF<CZE>:4081 | 942 | 942 | 100% | 0.0 | 100% | FR733832.1 |
| Aspergillus pallidofulvus 18S rRNA gene (partial), ITS1, 5.8S rRNA gene, ITS2 and 28S rRNA gene (partial), culture collection CCF<CZE>:1870 | 942 | 942 | 100% | 0.0 | 100% | FR733831.1 |
| Aspergillus pallidofulvus 18S rRNA gene (partial), ITS1, 5.8S rRNA gene, ITS2 and 28S rRNA gene (partial), culture collection CCF<CZE>:1893 | 942 | 942 | 100% | 0.0 | 100% | FR733830.1 |
| Aspergillus melleus 18S rRNA gene (partial), ITS1, 5.8S rRNA gene, ITS2 and 28S rRNA gene (partial), strain CECT 2092 | 942 | 942 | 100% | 0.0 | 100% | FM986318.1 |
| Aspergillus ochraceus strain AS III 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 942 | 942 | 100% | 0.0 | 100% | EU805804.2 |
| Aspergillus sp. NRRL 4789 internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 942 | 942 | 100% | 0.0 | 100% | EF661423.1 |
| Aspergillus sp. NRRL 4748 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 942 | 942 | 100% | 0.0 | 100% | EU021613.1 |
| Aspergillus sp. 245A 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence | 931 | 931 | 100% | 0.0 | 99% | GQ120974.1 |
Figure 2.
Phylogenetic tree based on partial 18S rDNA sequences of Aspergillus flavus.
Figure 3.
Phylogenetic tree based on partial 18S rDNA sequences of Aspergillus ochraceus.
3.3. Ability of isolated fungi to produce mycotoxins
Regarding the capability of Aspergillus species isolated from different agarwood samples to produce mycotoxins (AFB1, OTA) was estimated after incubation for 14 days at 28°. Data revealed that about 15.4% and 55.0% of A. flavus, and A. parasiticus isolates respectively were able to produce AFB1 with a range from 1.6 to 12.4 and from 3.4 to 7.9 µg/l respectively. However, the ability of A. ochraceus and A. niger to produce OTA revealed that 25.0 and 18.2% of both isolates were able to produce OTA.
3.4. The effect of moisture content on fungal count
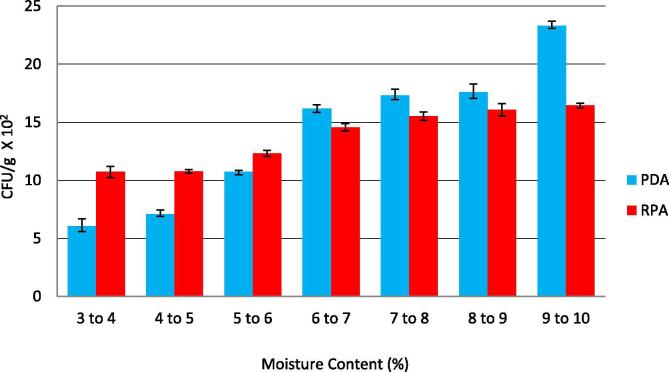
On studying the effect of different moisture content on the fungal count, results showed that the lowest counts of fungi (6.0 × 102 and 10.71 × 102 CFU/g) were detected in the samples with 3–4% moisture content. The fungal counts increased with the increase of the moisture content (Figure 4). The fungal counts in samples with 8–9% moisture recorded 17.58 × 102 and 16.06 × 102 CFU/g on PDA and RBA media respectively. Results also revealed that samples with moisture content 6–7% showed high total fungal count (15.2 × 103 CFU/g), which might be due to the presence of high content of oil in the ground samples.
Figure 4.
Fungal counts of agarwood samples with different moisture content. Results are mean ± SD of three replicates.
3.5. The effect of moisture contents on mycotoxin production
Data showed that contaminated samples with AFB1 ranged had a moisture content between 27.0% and 45.0% for the samples with 3–6% and 6–8% moisture contents respectively. On the other hand, the percentage of contaminated samples with OTA had a moisture content ranging between 18.0 to 55.0%. The levels of AFB1 for the samples with different moisture contents varied recording 7.76 to 11.60 µg/kg for lower and moderate moisture contents respectively. The maximum concentrations of AFB1 and OTA reached 12.40 and 6.30 µg/kg respectively for the samples with higher moisture content.
3.6. The effect of storage conditions on AFB1 production
The current results showed that the biosynthesis of AFB1 increased as a result of increasing moisture and temperature during storage period. The detected levels of AFB1 for the samples with 4.0% moisture content and stored at 25 ranged between 5.8 and 7.5 µg/kg respectively during the incubation period. The maximum level of AFB1 recorded 50.7 µg/kg after 21 days at 32 ± 2 °C for samples with 12.0% moisture.
4. Discussion
To our knowledge, this study is considered the first report on the mycotoxigenic fungi as contaminants of agarwood. Approximately 633 isolates were counted from the tested samples which are in good harmony with those recorded by Singh et al. (2008) who isolated a total of 858 fungal species from the raw materials of different medicinal plants. Mandeel (2005) isolated a total of 665 fungal isolates, representing 14 species from seventeen imported raw spice samples obtained from retail outlets. Similar results were reported by Nagajothi et al. (2016) who revealed that 17 isolates were collected from agarwood in India that was identified based on the morphological and molecular studies, whereas Turjaman et al. (2016) reported that over than 20 fungal species have been identified in the aromatic agarwood. The percentage of Aspergillus infection of agarwood samples reached 90 and 96% on PDA and RBA media respectively and was considered the most common isolated genus. Similar results were reported by Bokhari (2007) who found that Aspergillus was the most common genus in the different spices tested collected from Saudi Arabia. Singh et al. (2008) reported that the most dominant genus causing infection to most of the raw materials was found to be the genus Aspergillus. Bugno et al. (2006) investigated medicinal plants for the fungal contamination and found that 89.9% of the isolates belonged to Aspergillus and Penicillium genera. In recent studies Nagajothi et al., 2016, Turjaman et al., 2016 revealed that the agarwood was naturally infected with Aspergillus, Fusarium and Penicillium among other fungal species.
Among the Aspergillus genus, Aspergillus flavus, A. ochraceus, A. parasiticus and A. niger, were the most commonly isolated fungi. Wang et al. (2013) reported that more than 93% of different Chinese herbal medicines were contaminated with different species of fungi. The presence of high percentages of mycoflora particularly isolates belonging to Aspergillus is important because these species are known to produce mycotoxins and cause mycotoxicosis to human and animals (Bennett and Klich, 2003).These findings are in good harmony with those recorded by Moorthy et al., 2010, Kong et al., 2014.
The classification and identification of Aspergillus have been based on phenotypic characters but in the last decades was strongly influenced by molecular and chemotaxonomic characterization (Hathout, et al., 2015), (Abdel-Hadi et al., 2010). Various molecular approaches have been used previously for rapid detection of Aspergillus from environmental and clinical samples (Henry et al., 2000). Automated molecular techniques are presently under commercial development for identification of fungal pathogens (Loeffler et al., 2002).
Our results revealed that some of the identified Aspergillus fungi were able to produce mycotoxins (AFB1, OTA). This may be due to the presence of A. flavus in the powdered samples which initially colonizes the substrate and produce the AFs (Singh et al., 2008, Mandeel, 2005, Dereje et al., 2009). The potential risk of A. niger in stored agarwood should also be considered, according to previous reports (Noonimabc et al., 2009) which recorded that the occasional isolates of A. niger can produce OTA, fumonisin B2, sterigmatocystin which exhibits acute toxic, carcinogenic, mutagenic, teratogenic, immunotoxic or oestrogenic effects in animals and humans (Bennett and Klich, 2003, Frisvad et al., 2005, Zinedine et al., 2006). The results of this investigation revealed that several toxigenic fungal metabolites could be present in the same samples since about 12 and 8% of the samples with low moisture content were contaminated with AFB1 and OTA respectively. Moreover, the same isolate can produce different mycotoxins as mentioned above. Similar results were reported by Tassaneeyakul et al. (2004) who found that 18% of herbal samples were contaminated with a detectable amount of the total AFs.
Our results revealed that the moisture content in agarwood samples ranged between 3.2% and 9.8%, which was considered lower than that of Moorthy et al. (2010) who reported that moisture content ranged from 6.31% to 12.99% in all spices samples and herbal drugs. These results indicated the potential ability of fungi for growth if the moisture content arises during processing, transportation, and inadequate storage conditions. Many researchers found the high temperature is considered a major factor influencing fungal growth and consequently aflatoxin contamination (Kaaya and Kyamuhangire, 2006). Alborch et al. (2011) added that moisture content and temperature are the two key environmental factors that influence the growth of fungi. Nonetheless, few countries have effectively established regulations as for Aflatoxins (AFs) in the medicinal plant. For instance, the maximum tolerable limit for AFs allowed in the European Union (EU) member States has been set at 5 µg/kg for AFB1 (EC, 2006). AFB1 and OTA levels in our results are in safe limits and in good harmony with those recorded by Moorthy et al. (2010). However, the results showed that these levels were lower than those recorded for AFB1 (69.28 μg/kg) for some dried spices and herbs in Qatar State (Abdulkadar et al., 2004) and for OTA (33.0 μg/kg) as recorded by Karan et al. (2005). Moreover, the current results showed that the high production of AFB1 occurred under the high temperature, high moisture, and long term storage. From this overview, we noticed that the lower temperature and initial moisture influenced not only the rate of fungal spoilage but also the production of mycotoxins. It was also noticed that high moisture content and temperature is optimal for mycotoxin biosynthesis in agarwood (Akbar et al., 2016). Hygiene and sanitation from harvest to storage are key factors in eliminating sources of infection and reducing levels of contamination. So it is necessary to establish the Hazards Analyses Critical Control Point (HACCP) during storage and importing agarwood from its origin country to consumers.
5. Conclusion
This study concluded that Aspergillus sp. contaminated agarwood samples during marketing and under storage. The ability of isolated mycotoxigenic Aspergillus sp. to produce AFB1 and OTA was based on different storage conditions. The AFB1 and OTA production levels in our results are in safe limits but alarming and raising a question mark for the safety of agarwood. Moisture content and temperature are considered the two main environmental factors that influenced fungal growth and consequently mycotoxin production.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. 74/130/1433. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Acknowledgments
Conflict of interest
The authors declare no conflicts of interests.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Hadi A., Carter D., Magan N. Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B1 production during storage of peanuts under different water activity levels. J. Appl. Microbiol. 2010;109:1914–1922. doi: 10.1111/j.1365-2672.2010.04820.x. [DOI] [PubMed] [Google Scholar]
- Abdulkadar A.H.W., Al-Ali A.A., Al-Kildi A.M., Al-Jedah J.H. Mycotoxins in food products available in Qatar. Food Cont. 2004;15:543–548. [Google Scholar]
- Akbar A., Medina A., Magan N. Impact of interacting climate change factors on growth and ochratoxin A production by Aspergillus section Circumdati and Nigri species on coffee. World Mycotoxin J. 2016;9:863–874. [Google Scholar]
- Alborch L., Bragulat M.R., Abarca M.L., Cabañes F.J. Effect of water activity, temperature and incubation time on growth and ochratoxin A production by Aspergillus niger and Aspergillus carbonarius on maize kernels. Int. J. Food Microbiol. 2011;147:53–57. doi: 10.1016/j.ijfoodmicro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl. Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arijit D., Sourav B., Muthusamy P., Jayaraman A. Molecular identification of Aspergillus flavus GHBF09 involved in aflatoxin B1 production in rice straw. Ann. Biol. Res. 2013;4:102–110. [Google Scholar]
- Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, R.V., Vasanthi, S., 2003. Mycotoxin food safety risks in developing countries. Food Safety in Food Security and Food Trade. 2020 Vision for Food, Agriculture and the Environment, Focus 10.
- Boekhout T., Kurtzman C.P., O’Donnell K., Smith M.T. Phylogeny of the yeast genera Hanseniaspora (anamorph Kloeckera), Dekkera (anamorph Brettanomyces), and Eeniella as inferred from partial 26s ribosomal DNA nucleotide sequences. Int. J. Syst. Bacteriol. 1994;44:781–786. doi: 10.1099/00207713-44-4-781. [DOI] [PubMed] [Google Scholar]
- Bokhari F.M. Spices mycobiota and mycotoxins available in Saudi Arabia and their abilities to inhibit growth of some toxigenic fungi. Mycobiology. 2007;35:47–53. doi: 10.4489/MYCO.2007.35.2.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno A., Adriana A.B.A., Tatiana C.P., Terezinha A.P., Myrna S. Occurrence of toxigenic fungi in herbal drugs. Braz. J. Microbiol. 2006;37:47–51. [Google Scholar]
- Dereje T., Asefa A., Ragnhild O., Gjerde B., Maan S., Sidhu C., Solveig D., Cathrine F., Kure D., Truls E., Ida S. Molds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009;128:435. doi: 10.1016/j.ijfoodmicro.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Essono G., Ayodele M., Akoa A., Foko J., Olembo S., Gock J. Aspergillus species on cassava chips in storage in rural areas of southern Cameroon: their relationship with storage duration, moisture content and processing methods. Afr. J. Microbiol. 2007;1:1–8. [Google Scholar]
- FAO, Food and Agriculture Organization 1991. Food for the Future.
- Frisvad C.J., Skouboe P., Samson A.R. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin, and 3-O-methylsterigmatocystin, Aspergillus rumbelli sp. nov. Syst. Appl. Microbiol. 2005;28:442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Henry T., Iwen P.C., Hinrichs S.H. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 2000;38:1510–1515. doi: 10.1128/jcm.38.4.1510-1515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC, International Agency for Research on Cancer 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In: IARC monographs on the evaluation of carcinogenic risks to humans (pp. 245-395). IARC, Lyon, France, vol. 56.
- Kaaya A.N., Kyamuhangire W. The effect of storage time and agro-ecological zone on mold incidence and aflatoxin contamination of maize from traders in Uganda. Int. J. Food Microbiol. 2006;110:217–223. doi: 10.1016/j.ijfoodmicro.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Karan D., Vukojević J., Milićević D., Ljajević-Grbić M., Janković V. prisustvaplesniimikotoksina u pojedinimzačinimakoji se koriste u industriji mesa. Tehnologija Mesa. 2005;46:306–310. [Google Scholar]
- Kong W., Wei R., Logrieco A.F., Wei J., Wen J., Xiao X., Yang Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chem. 2014;146:320–326. doi: 10.1016/j.foodchem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Loeffler J., Schmidt K., Hebart H., Schumacher U., Einsele H. Automated Extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA pure LC system. J. Clin. Microbiol. 2002;40:2240–2243. doi: 10.1128/JCM.40.6.2240-2243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeel Q.A. Fungal contamination of some imported spices. Mycopathologia. 2005;159:291–298. doi: 10.1007/s11046-004-5496-z. [DOI] [PubMed] [Google Scholar]
- Moorthy K., Prasanna N., Thajuddin S., Arjunan T., Gnanendra S., Hussain Z. Occurrence of mycopopulation in spices and herbal drugs. Int. J. Biodiversity. 2010;1:6–14. [Google Scholar]
- Nagajothi M., Parthiban K.T., Kanna S.U., Karthiba L., Saravanakumar D. Fungal microbes associated with Agarwood formation. Am. J. Plant Sci. 2016;7:1445–1452. [Google Scholar]
- Noonimabc P., Mahakarnchanakulb W., Nielsend K.F., Frisvadd J.C., Samsona R.A. Fumonisin B2 production by Aspergillus niger in Thai coffee beans. Food Addit. Contam. 2009;26:94–100. doi: 10.1080/02652030802366090. [DOI] [PubMed] [Google Scholar]
- Rajankar P.N., Tambekar D.H., Wate S.R. Study of phosphate solubilization efficiencies of fungi and bacteria isolated from saline belt of purna river basin. Res. J. Agric. Biol. Sci. 2007;3:701–703. [Google Scholar]
- Rodriguez-Amaya D.B., Sabino M. Mycotoxin research in Brazil: the last decade in review. Braz. J. Microbiol. 2002;33:1–11. [Google Scholar]
- Sahab A.F., Aly S.E., Hathout A.S., Sabry B.A. Application of some plant essential oils to control Fusarium isolates associated with freshly harvested maize in Egypt. J. Essent. Oil Bear. Plants. 2014;17:1146–1155. [Google Scholar]
- Sangareswari@Nagajothi M., Thangamuthu Parthiban K., Umesh Kanna S., Karthiba L., Saravanakumar D. Fungal microbes associated with agarwood formation. Am. J. Plant Sci. 2016;7:1445–1452. [Google Scholar]
- Singh P., Srivastava B., Kumar A., Dubey N.K. Fungal contamination of raw materials of some herbal drugs and recommendation of Cinnamomum camphora oil as herbal fungi toxicant. Microb. Ecol. 2008;56:555–560. doi: 10.1007/s00248-008-9375-x. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W., Razzazi-Fazeli E., Porasuphatana S., Bohm J. Contamination of aflatoxins in herbal medicinal products in Thailand. Mycopathologia. 2004;158:239–244. doi: 10.1023/b:myco.0000041892.26907.b4. [DOI] [PubMed] [Google Scholar]
- Toma F.M., Abdulla N.Q.F. Isolation and identification of fungi from spices and medicinal plants. Res. J. Environ. Earth Sci. 2013;5:131–138. [Google Scholar]
- Turjaman, M., A. Hidayat, E. Santoso, 2016. Development of Agarwood induction technology using endophytic fungi. Agar, Part of the series Tropical Forestry, pp. 57–71.
- Vartika, R., M. Shanta, 2005. Toxic Contaminants in Herbal Drugs. Environment News 11.
- Wang W.L., Xu H., Chen H.Z., Zheng R.S., Tan J., Zhan R.T., Chen W.W. Separation and molecular identification of fungal contamination on surface of 15 Chinese herbal medicines. Zhongguo Zhong Yao ZaZhi. 2013;38:191–204. [PubMed] [Google Scholar]
- Zhang X.L., Liu Y.Y., Wei J.H., Yang Y., Zhang Z., Huang J.Q., Chen H.Q., Liu Y.J. Production of high-quality agarwood in Aquilaria sinensis trees via whole-tree agarwood-induction technology. Chinese Chem. Lett. 2012;23:727–730. [Google Scholar]
- Zinedine A., Brera C., Elakhdari S., Catano C., Debegnach F., Angelini S., De Santis B., Faid M., Benlemlih M., Minardi V., Miraglia M. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Cont. 2006;17:868–874. [Google Scholar]