Abstract
Background
Cancer patients when treated with different chemotherapeutic drugs often develop mild to severe sight threatening diseases during or after chemotherapy. The mechanism involved in the pathogenesis of ocular toxicities is poorly understood. Oxidative stress, inflammation and MMPs (angiogenic factor) are involved in the progression of chemotherapy related ocular disorders.
Materials and methods
The concentration of oxidative stress markers such as MDA, NO and levels of different antioxidant molecules such as SOD, CAT, GSH, GPx, GPr, VIT A, VIT E and VIT C present in the serum of chemotherapy treated patients (n = 50) and in normal persons (n = 20) were estimated by the direct spectrophotometric method while the concentration of TNF-α and MMP-9 activity were determined using human TNF-α and MMP-9 ELISA kits.
Results
The concentration of SOD and CAT (0.356 ± 0.05 μg/dl and 1.26 ± 0.01 μmol/mol of protein) was significantly lower as compared to that (1.09 ± 0.03 μg/dl and 3.99 ± 0.04 μmol/mol of protein) in controls. The levels of GPx (0.06 ± 0.01 mmol/dl) in the cancer patients were much lower than those in the controls (0.78 ± 0.06 mmol/dl). Lower level of GSH (0.96 ± 0.003 μg/dl) in serum of the diseased group was observed as compared to healthy group (7.26 ± 1.40 μg/dl). The level of Vit A, Vit C and Vit E was lower in systemic circulation of cancer patients (109.99 ± 6.35 μg/ml, 1.26 ± 0.36 μg/ml and 1.29 ± 0.191 μg/ml) as compared to control subjects (166.35 ± 14.26 μg/ml, 3.25 ± 0.099 μg/ml and 6.354 ± 2.26 μg/ml) respectively. The concentration of nitric oxide was significantly higher in the cancer patients (45.26 ± 6.35 ng/ml) than that in the normal subjects (16.35 ± 3.26 ng/ml). The higher concentration of MDA (8.65 ± 3.26 nmol/ml) was observed in the patients than normal ones (1.254 ± 0.065 nmol/ml). The quantity of TNF-α was significantly higher in chemotherapy treated patients (32.68 ± 4.33 pg/ml) as compared to the control group (20.979 ± 1.98 pg/ml). Significantly higher concentration of MMP-9 (40.26 ± 3.26 ng/ml) was observed in the cancer patients than the controls (7.256 ± 1.95 ng/ml).
Conclusion
Lower levels of antioxidant enzymes and non-enzymatic small molecules and higher levels of oxidative stress and inflammatory clinical parameters such as NO, MDA, TNF-α and MMP-9 may be involved in the pathogenesis of systemic chemotherapy related ocular complications such as cataract, glaucoma, blepharitis, retinitis pigmentosa, macular degeneration, pterygium and retinal degeneration.
Abbreviations: NO, nitric oxide; MDA, malondialdehyde; TNF, tumor necrosis factor; IL, interleukin; MMPs, matrix metalloproteinases; HIV, human immunodeficiency virus; ROS, reactive oxygen species
Keywords: Oxidative stress, Cancer, Chemotherapeutic drugs, TNF-α, MMPs, Ocular complications
1. Introduction
Cancer arises due to the abnormal cells behavior, such as it goes under uncontrolled cell differentiation and rapid cell profilation. It can move away from its origin to other parts of body via blood and lymphatic system (Klein, 2008, Cree, 2011). Normal cells become cancerous, when a wide array of mutations is accumulated in the cell system or DNA repair system is unable to overwhelm these potential mutations. In cancerous cells, generally proto-onco genes, tumor suppressor genes and genes that are part of DNA repair machinery are normally mutated (Douglas and Weinberg, 2011). Most often changes in tumor suppressor genes like P53 and Ras are associated with the development of different malignant cancers (Karnoub and Robert, 2008). The major carcinogenic factors that may contribute to the progression of cancer are increased consumption of tobacco, alcohol, rich calorie food, hypertension, viral and bacterial infections, toxic environmental pollutants and loss of physical activity (Ananad et al., 2008), thus a change in life style may also be considered as an important factor responsible for reducing the risk of cancer development.
Nowadays, chemotherapy is considered as the most promising, effective and frequently used therapy for the treatment of different types of life threatening cancers, all over the world. It has been suggested that most of the chemotherapeutic drugs kill the cancerous cells by producing reactive oxygen species (ROS) as a part of their mechanism of action but now it is believed that it also destroys the normal cells (Maiti, 2012). So, these ROS producing chemotherapeutic drugs increase oxidative stress in normal cells that are associated with a diverse array of toxicities. Chemotherapy related ocular toxicities that are from mild ocular changes to severe sight threatening complications are frequently observed by ophthalmologists, and if these are not treated in time they can cause irreversible blindness. Human eye is equipped with an efficient antioxidant defense system containing superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase and non-enzymatic antioxidants such as vitamin C, vitamin E, vitamin D and glutathione to neutralize the reactive oxygen species and a well maintained system of ocular barriers prevents the entry of toxic agents (Čejková et al., 2004, Cunha-Vaz, 2009). One of the possibilities is that drugs cross ocular barriers and may overwhelm the natural antioxidant defense mechanism. Inflammation and oxidative stress are associated with increase in vascular permeability of blood retinal barrier for the passage of drugs that are unable to penetrate in normal situation (Kaur et al., 2008). Chemotherapeutic drugs are metabolized in liver and excreted by kidney and in case of renal or hepatic dysfunction, their concentration increases in the systemic circulation (Superfin et al., 2007). Lipophilic drugs mostly bind to melanin, which acts as a ROS neutralizer. Their prolong binding may produce drug induced toxicity in different melanin containing ocular tissues (Hu et al., 2008).
In eyes, oxidative stress is associated with the progression of various ocular pathologies such as macular degeneration, cataract, glaucoma, optic neuropathy and diabetic retinopathy (Choi et al., 2009). Oxidative stress enhances the production of pro-inflammatory cytokines by activating MAPK signaling pathways, stimulating transcription factors such as NF-KB and AP-1 which in turn activate the expression of different pro-inflammatory cytokine genes such as TNF-α, IL-1, IL-6 and IL-10 (Luo et al., 2004, Bala and Haldar, 2013). Higher level of TNF-α is also involved in the up-regulation of matrix metallo-proteinases (MMPs) by activating nuclear transcription factors, AP-1 and NF-kB in keratinocytes. The aims and objectives of the present study were to assess the diagnostic factors associated with anti-oxidative status and expression of matrix metallo-proteinases in patients with ocular disorders induced by cancer therapy.
2. Materials and methods
The study was conducted at the Institute of Molecular Biology and Biotechnology (IMBB), The University of Lahore. Fifty patients (n = 50) with glaucoma, macular degeneration, optic neuropathies, vascular retinopathies, uveitis, retinitis pigmentosa, pterygium and cataract when they were treated with traditional chemotherapeutic drugs and compared them with a control group comprising of twenty (n = 20) healthy persons.
2.1. Inclusion criteria
Patients with glaucoma, macular degeneration, optic neuropathies, vascular retinopathies, uveitis, retinitis pigmentosa, pterygium and cataract were substituted in the study.
2.2. Exclusion criteria
Patients with Diabetes, HIV and any other congenital diseases were excluded from the study.
2.3. Blood separation
Five mL of blood was collected from the affected and healthy individuals were taken and stored at −20 °C after centrifugation for 15 min at 4000 rpm for further biochemical analysis. This study was approved by local Ethics Committee of the University of Lahore (UOL).
2.4. Biochemical analysis
Determination of enzymatic antioxidants like SOD, catalase, GPx and GPr in the serum of cancer patients were determined following the methods described by Kakkar et al. (1984), (Aebi, 1974, Wendel, 1980, David and Richard, 1983) respectively, non-enzymatic antioxidants like GSH, vitamin A, C and E were appraised following Moron et al. (1979), (Bayfield and Cole, 1980, Chinoy et al., 1976, Rosenberg, 1992), while stress parameters like NO, MDA, TNF-α and MMP-9 were assessed by the Griess reagent, Ohkawa et al. (1979), human TNF-alpha ELISA kit (Diaclone, France) and human Matrix Metalloprotinase-9 (MMP-9) ELISA kit (GSCIENCE, USA).
2.5. Demographic data
All patients had ocular morbidity in at least one of their eyes. Leading cause of the disorder remained presbyopia and effect of the disease increases in age above 35. Other causes of OM affected lens and conjunctiva. No Association could be seen in between educational attainment, employment and OM. Demographic data in patients with ocular morbidity receiving chemotherapy has been depicted in Table 1.
Table 1.
Demographic data in patients with ocular morbidity receiving chemotherapy.
| Ocular morbidity | Chemotherapeutic agent | (n = 100) | Chemotherapeutic exposure | Route of administration |
|---|---|---|---|---|
| Cataract | 5-Fluorouuracil Cisplatin |
16 | 3 | Intravenous |
| Glaucoma | Methotrexate Carmustine |
15 | 4 | Intravenous |
| Blepharitis | Interferon Tamoxifen |
02 | 3 | Intravenous |
| Retinitis Pigmentosa | Tamoxifen | 31 | 7 | Intravenous |
| Macular Degeneration | Cisplatin | 18 | 5 | Intravenous |
| Retinal Degeneration | Carmustine | 16 | 2 | Intravenous |
| Corneal Opacity | Cytosine Arabinoside 5-Fluorouuracil |
02 | 4 | Intravenous |
Table showing different type of ocular morbidity along with their thareuptic approach with different chemotherapeutic agents. Diagnosed cases of ocular morbidity patients such as cataract were administered 5-Fluorouuracil Cisplatin, Glaucoma were given Methotrexate carmustine, Blepharitis were provided with Interferon Tamoxifen, Retinitis Pigmentosa were treated with Tamoxifen, and Macular degradation were give Cisplatin intravenously. While patients of Retinal Degradation and Corneal Opacity were treated with Carmustine and Cytosine Arabinoside 5-Fluorouuracil respectively by I.V line.
2.6. Statistical analysis
The study design was a prospective case control and the data for all stress parameters in the serum of cancer patients were statistically analyzed using independent t-test SPSS version 16. All results were expressed as mean ± standard deviation. The results were considered statistically significant if their P-value was less than 0.05.
3. Results
The cancer patients diagnosed with different types of cancer were treated with traditional chemotherapeutic drugs such as alkylating agents, anti-metabolites, taxanes, anthracycline etc. These patients were with different types of ocular disorders during or after treatment as cytotoxic effects of drugs. Increased levels of oxidative stress markers or decreased levels of enzymatic or non-enzymatic antioxidants in the serum of patients with different ocular disorders indicate increasing oxidative stress. The concentration of SOD (0.356 ± 0.05 μg/dl) was significantly lower as compared to that (1.09 ± 0.03 μg/dl) in the controls as shown in Table 2. The level of catalase in the cancer patients (1.26 ± 0.01 μmol/mol of protein) was significantly (P = 0.015) lower as observed in the normal group (3.99 ± 0.04 μmol/mol of protein). The levels of GPx (0.06 ± 0.01 mmol/dl) in the cancer patients much lower than those in the controls (0.78 ± 0.06 mmol/dl). The levels of GPr were non-significantly lower in the patients (1.26 ± 0.002 μmol/ml) than in the control group (1.35 ± 0.035 μmol/ml) (Table 2). Their lower levels were associated with increasing oxidative stress parameters in the diseased persons. Human ocular tissues are equipped with non-enzymatic antioxidants, helping in scavenging reactive oxygen species, but in diseased condition perhaps due to inadequate antioxidants they are unable to neutralize the increasing concentration of free radicals. Lower level of GSH (0.96 ± 0.003 μg/dl) in the serum of the diseased group was observed that shows the impaired levels of thiol group which is important in maintaining glutathione redox homeostasis under normal conditions. The level of vitamin A was lower in the systemic circulation of the cancer patients (109.99 ± 6.35 μg/ml) than in the control subjects (166.35 ± 14.26 μg/ml) (Table 2). The low concentration of vitamin A was found to be associated with increasing oxidative stress in the patients. Significantly (P = 0.036) lower levels of vitamin C (1.26 ± 0.36 μg/ml) were observed in the patients as compared that in the healthy subjects (3.25 ± 0.099 μg/ml). The activity of vitamin E was significantly (P = 0.015) lower in the patients receiving chemotherapeutic drugs (1.29 ± 0.191 μg/ml) than in the healthy ones (6.354 ± 2.26 μg/ml). The concentration of nitric oxide was significantly higher in the cancer patients (45.26 ± 6.35 ng/ml) than that in the normal persons (16.35 ± 3.26 ng/ml). The concentration of nitric oxide increased due to increased expression of iNOS, and mRNA that was associated with increasing concentration of inflammatory cytokines by activating the transcription factor NF-κβ. It is also an important mediator of increasing oxidative stress by upregulating reactive oxygen and nitrogen species contributing to progression of a wide array of mild to severe sight threatening disorders. The higher concentration of MDA (8.65 ± 3.26 nmol/ml), the indicator of lipid peroxidation was observed in the patients than that in the normal ones (1.254 ± 0.065 nmol/ml). TNF-α, a pro-inflammatory cytokine and important mediator of inflammation, usually elevates in chronic inflammatory eye diseases. It is a multipotent molecule in diseased conditions, associated with upregulation of MMP-9, mRNA of iNOS, and has a role in ROS production. The level of TNF-α was significantly (P = 0.043) higher in the chemotherapy treated patients (32.68 ± 4.33 pg/ml) than in the controls (20.979 ± 1.98 pg/ml). In the current study, significantly higher concentration of MMP-9 (40.26 ± 3.26 ng/ml) was observed in the cancer patients than that in the control subjects (7.256 ± 1.95 ng/ml). MMP-9 is an angiogenic factor that may also increases the concentration of inflammatory cytokines which in turn enhances the oxidative stress by producing reactive oxygen species. MMP-9 is considered as a stress stimulus and its chronic levelsare associated with increased pathogenesis of different vascular sight threatening diseases and if not treated in time it may cause irreversible blindness.
Table 2.
Levels of different variables in the serum of cancer patients undergone chemotherapy and healthy persons.
| Variables | Control | Patients | P < 0.05 |
|---|---|---|---|
| Vit. A (µg/ml) | 166.35 ± 14.26 | 109.99 ± 6.35 | 0.0325 |
| Vit. E (µg/ml) | 6.354 ± 2.26 | 1.29 ± 0.191 | 0.015 |
| Vit. C (µg/ml) | 3.25 ± 0.099 | 1.26 ± 0.356 | 0.036 |
| MDA (nmol/ml) | 1.254 ± 0.065 | 8.65 ± 3.26 | 0.011 |
| GPx (mmol/dl) | 0.780 ± 0.056 | 0.065 ± 0.0065 | 0.0015 |
| GRr(µmol/ml) | 1.35 ± 0.035 | 1.265 ± 0.0016 | 0.065 |
| GSH (µg/dl) | 7.26 + 1.40 | 0.956 ± 0.0025 | 0.014 |
| SOD (µg/dl) | 1.09 ± 0.025 | 0.356 ± 0.0458 | 0.0035 |
| CAT (µmol/mol of protein) | 3.995 ± 0.35 | 1.26 ± 0.011 | 0.015 |
| NO (ng/ml) | 16.35 ± 3.26 | 45.26 ± 6.35 | 0.0329 |
| TNF-α (pg/ml) | 20.979 ± 1.98 | 32.68 ± 4.33 | 0.043 |
| MMP9 (ng/ml) | 7.256 ± 1.95 | 40.26 ± 3.26 | 0.003 |
All data were statistically analyzed as mean ± SD. Results are significant at P ≤ 0.05.
4. Discussion
The present study shows that the lower concentrations of antioxidants and higher levels of oxidative stress markers i.e. nitric oxide (NO) and malondialdehyde (MDA), and inflammatory markers (TNF-α and MMP-9) were observed in the serum of chemotherapy receiving cancer patients. All of thesemay develop ocular diseases by activating processes of neo-vascularization. Systemic anticancer drugs produce reactive oxygen species (ROS) that activate multiple stress related signaling pathways including inflammation and activation of Ca-dependent MMPs that are involved in the pathophysiology of different eye diseases such as cataract, pterygium, macular degeneration, glaucoma, retinitis pigmentosa, uveitis and proliferative retinopathy (Chen et al., 2007).
It has been suggested that chemotherapeutic treatment increases the oxidative stress by enhancing the production of ROS/RNS or by decreasing thelevels of antioxidant molecules in multiple organs which may cause organ related toxicity (Yan et al., 2015). It has been also shown that the levels of oxidants/antioxidants were disturbed in the serum of the patients undergoing these chemotheraputic drugs (bleomycin, vinblastine, adriamycin and dacarbasine) (Thanon, 2007). High doses of cyclophosphamide, vincristine and adriamycin in combination resulted in increased concentration of NO and MDA while reduced the level of total antioxidant capacity in serum (Crohns et al., 2009). The current study has also shown theup-regulation of stress markers and downregulation of antioxidant parameters in the serum of the patients receiving chemotherapy. It has been reported that oxidative stress may be a major factor in inducing chemotherapy related mild anemia, fatigue, loss of hairs, vomiting, nousea, mouth sores and diareheato severe organ toxicities such as cisplatin induced ototoxicity, neuropathy and renal failure, doxorubicin-paclitaxel induced cardiomyopathy and taxan induced neuropathy asobserved in different studies (Pinches et al., 2012, Bhatnager et al., 2014).
TNF-alpha (TNF-α), amacrophage/monocyte derived multi-functional pro-inflammatory cytokine and an important mediator in ROS generation is shown to increase the concentration of TNF-α by activating NF-Kβ which in turn upregulates the expression of ROS and iNOS through activating c-JunN-terminal kinase (JNK) and NF-Kβ (Chandel and Schumacke et al., 2001, Lee et al., 2014), also shown in Fig. 1. So, ROS can activate pro-inflammatory cytokine genes through the activation of transcription factor NF-Kβ and is associated with pathogenesis of different eye diseases. TNF-α and ROS upregulate the concentration of MMP-9 through phsphorylation of mitogen activated protein kinases (MAPK) which in turn activate the NF-KB (Ranaivo et al., 2012). ROS can activatethe expression of MMP-9, an angiogenic factor through the activation of transcription factor AP-1 and can cause retinal degeneration by destroying retinal ganglion and retinal pigment epithelial cells. It has been suggested that elevated levels of phosphosylated JNK, P38 MAPK, ERK1/2 were associated with upregulated levels of IL-1β, TNF-α and MMP-9 in corneal, conjunctival epitheliumand in tear fluid, as represented in Fig. 1. Upregulated levels of MMPs (Cree, 2011, Cunha-Vaz, 2009, Kaur et al., 2008, Luo et al., 2004, Bala and Haldar, 2013). and downregulated levels of TIMP1 and TIMP2 were detected in both cancer and cataract patients (Määttä et al., 2006). It has been demonstrated that the higher quantity of MMP-9 was examined in both posterior and anterior segments of eye after experimentally induced retinal reperfusion injury but the levels of TIMP 1 and 2 remained unchanged (Zhang et al., 2002). Thus, use of TIMP 1 & 2 or MMP-9 inhibitors can prevent corneal injuries. So, a vicious cycle of ROS induced oxidative stress, inflammation and MMP-9 were involved in the development of various ocular diseases.
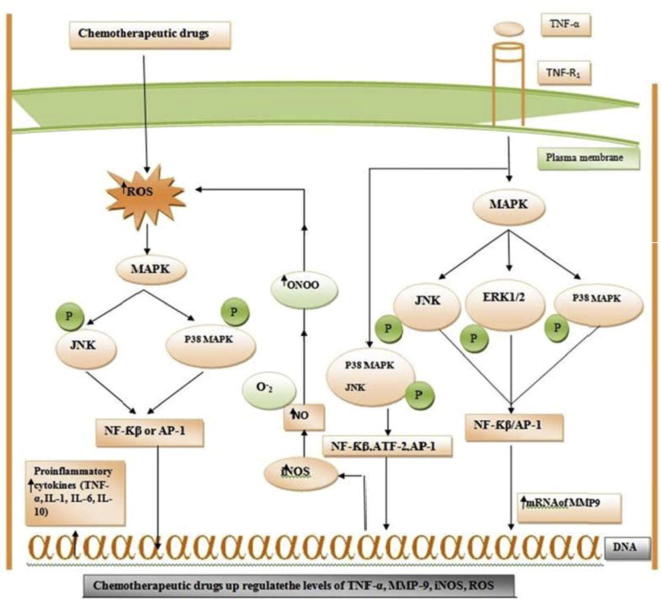
Fig. 1.
Chemotheraputic drugs when enter in living cell, it can up-regulate the levels of reactive oxygen species (ROS), which in turn phosphorylate different stress related MAPKs. These MAP kinases up-regulate the levels of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6 and IL-10. Similarly, TNF-α when bind to its receptor TNFR1 it may up regulate both mRNA of MMP9 and inflammatory iNOS through phosphorylating different MAPKs, JNK, ERK1/2 and P38MAPK through activating nuclear transcription factors that are NF-Kβ, AP-1 and ATF-2. Inducible nitric oxide synthase increases concentration of nitric oxide to chronic level under pathological condition. Nitric oxide will react with super oxide anion and form peroxynitrite ONOO, which in turn increase the oxidative stress associated toxicity in multiple organs. Abbreviation: MMP9: matrix metalloproteinase 9, TNF-α: tumor necrosis factor-alpha, ROS: reactive oxygen species, iNOS: inducible nitric oxide synthase, O−2: superoxide anion, P38MAPK: P38 mitogen activated protein kinase, JNK: c-Jun N- terminal kinases, ERK1/2: extracellular signal-regulated kinases, AP-1: activator protein 1. ATF-2: activating transcription factor-2.
5. Conclusion
The present study concludes that chemotherapy may increase the oxidative stress by elevating the concentration of TNF-alpha and MMP-9 that may develop different ocular complications which should be treated in time to avoid irreversible blindness. Regular examination should be done during and after the process of therapy. Moreover, the use of antioxidants, anti-TNFα inhibitors, inhibitors of MMP-9 and anti-VEGF may be useful in the development of novel therapeutic approches that are relevant for the management of cancer therapy inducing ocular complications. But further studies and clinical trials are required to investigate the exact mechanism involved in the pathogenesis and progression of chemotherapy associated ocular adverse effects.
Conflict of interest
There is no conflict of interest by any author.
Acknowledgement
This project was supported by the NSTIP strategic technologies program in the Kingdom of Saudi Arabia – Project No. (12-MED3078-03). The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. 3rd ed. Academic Press; New York: 1974. Methods in Enzymatic Analysis; pp. 674–684. [Google Scholar]
- Ananad P., Ajaikumar B.K., Chitra S., Kuzhuvelil B.H., Sheeja T.T., Oiki S.L., Bharat B.A. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala A., Haldar P.K. Free radical biology in cellular inflammation related to rheumatoid arthritis. OA Arthritis. 2013;1(2):15. [Google Scholar]
- Bayfield, R.F., Cole, E.R., 1980. Colorimetric determination of vitamin A with trichloroacetic acid. In: McCormick, D.B,. Wright, L.D. (Eds.) Methods in Enzymology, Part F. Vitamins and Coenzymes. Academic Press, New York, 67, 189–195. [DOI] [PubMed]
- Bhatnager B., Gilmore S., Goloubeva O., Pelser C., Medeiros M., Chumsri S., Tkaczuk K., Edelman M., Bao T. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springer Plus. 2014;3:366. doi: 10.1186/2193-1801-3-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čejková J., Típek S., Crkovská J., Ardan T., Pláteník J., Čejka Cand, Midelfart A. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol. Res. 2004;53:1–10. [PubMed] [Google Scholar]
- Chandel, S.N., Schumacke, T.P., Arch, H.R., 2001. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 276, 42728–42736. [DOI] [PubMed]
- Chen, Y., Jungsuwadee, P., Vore, M., Butterfield, D.A., St, Clair. D.K., 2007. Collateral damage in cancer chemotherapy: Oxidative stress in non-targeted tissues. Mol Interv. 7(3), 147–156. [DOI] [PubMed]
- Chinoy J.J., Singh Y.D., Gurumurthi K. The role of ascorbic acid in growth, differentiation and metabolism of plants. J. Plant. Physiol. 1976;22:122. [Google Scholar]
- Choi S., Kim T., Kim S.K., Kim B., Cho H., Lee K.H., Cho Hand, Kim K.E. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type-2. Am. J. Pathol. 2009;175(1):248–261. doi: 10.2353/ajpath.2009.081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree I.A. Cancer biology Methods. Mol Biol. 2011;731:1–11. doi: 10.1007/978-1-61779-080-5_1. [DOI] [PubMed] [Google Scholar]
- Crohns, M., Liippo, K,, Erhola, M., Kankaananta, H., Moikinen, E., Alho, H., Kellokumpu, Lehtinen. P., 2009. Concurrent decline of several antioxidants and markers of oxidative stress during combination chemotherapy for small lung cancer. Clin. Biochem. 42, 1236–1245. [DOI] [PubMed]
- Cunha-Vaz J. The blood–retinal barrier in retinal disease. Eur. Ophthalmic Rev. 2009;3:105–108. [Google Scholar]
- David, M., Richard, J.S., 1983. In: Bergmeyer, J., Grab, M., (Eds), Methods of Enzymatic Analysis, Verlag Chemie Wenhein Deer Field. Beach Floride, 358.
- Douglas H., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hu D.N., Simon D.J., Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008;84(3):639–644. doi: 10.1111/j.1751-1097.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984;21:131–132. [PubMed] [Google Scholar]
- Karnoub E.A., Robert A.W. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell. Biol. 2008;8:275–283. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Foulds W.S., Ling E.A. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res. 2008;27(6):622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Klein C. Canc. Biol. Methods. Sci. 2008;321:785–787. [Google Scholar]
- Lee H.Y., Su B.S., Huang C.C., Sheu M.H., Tsai C.J., Lin H.C., Wang J.Y., Wang J.B. N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through induction of cell death, ROS related signailing and cytokine expression. PLoS ONE. 2014;9(9):108317. doi: 10.1371/journal.pone.0108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L., De, Quan. L., Amish, D., William, F., Rosa, M.C., Stephen, C.P., 2004. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. O.phthalmol. Vis. Sci. 45(12), 4293–4301. [DOI] [PubMed]
- Määttä M., Tervahartiala T., Vesti E., Airaksinen J., Sorsa T. Levels and activation of matrix metalloproteinases in aqueous humor are elevated in uveitis-related secondary glaucoma. J, Glaucoma. 2006;15(3):229–237. doi: 10.1097/01.ijg.0000212229.57922.72. [DOI] [PubMed] [Google Scholar]
- Maiti K.A. Elevate the ROS level to kill cancer cells during chemotherapy. Chemotherapy. 2012;1(5):1000–1119. [Google Scholar]
- Moron, M.S., Depierre, J., Mannervik, B., 1979. Levels of glutathione, glutathione reductase and glutathione -S- transferase activities in rat lung and liver. Biochim. Biophys. Acta 58, 267–278. [DOI] [PubMed]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pinches M.D., Betts C.J., Bickerton S.J., Beattie L., Burdett L.D., Thomas H.T., Derbyshire N.A., Moores M. Evaluation of novel urinary renal biomarkers with a cisplatin model of kidney injury: effects of collection period. Toxicol. Pathol. 2012;40(3):534–540. doi: 10.1177/0192623311432437. [DOI] [PubMed] [Google Scholar]
- Ranaivo R.H., Hodge N.J., Choi N., Wainwright S.M. Albumin induces upregulation of matrix metalloprotinases-9 in astrocytes via MAPK and ROS-dependent pathway. J. Neuroinflammation. 2012;9:68. doi: 10.1186/1742-2094-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H.R. Inter Science Publishers Inc.; New York: 1992. Chemistry and Physiology of Vitamins; pp. 452–453. [Google Scholar]
- Superfin D., Iannucci A.A., Davies M.A. Commentary: Oncologic drugs in patients with organ dysfunction: a summary. The Oncologist. 2007;12(9):1070–1083. doi: 10.1634/theoncologist.12-9-1070. [DOI] [PubMed] [Google Scholar]
- Thanon J.A.I. Oxidative stress and immunoglobulin level in patients with hodgkin’s lymphoma. MJBU. 2007;25(2):23–27. [Google Scholar]
- Wendel, A., 1980. Glutathione Peroxidases. In: Enzymatic Basis of Detoxification. Academic Press. New York. p. 333–353.
- Yan, X.H., Guo Xiang Yang, Jiao Fu Yong, Liu Xuan, Liu Yong. Activation of large-conductance Ca2+-activated K+ channels inhibits glutamate-induced oxidative stress through attenuating ER stress and mitochondrial dysfunction. Neurochem Int 2015;90:28–35. [DOI] [PubMed]
- Zhang X., Sakamoto T., Hata Y., Kubota T., Hisatomi T., Murata T., Ishibashi T., Inomata H. Expression of matrix metalloproteinases and their inhibitors in experimental retinal ischemia-reperfusion injury in rats. Exp. Eye Res. 2002;74(5):577–584. doi: 10.1006/exer.2001.1152. [DOI] [PubMed] [Google Scholar]