Abstract
This study was conducted to examine the role of arbuscular mycorrhiza fungi (AMF) in alleviating the adverse effects of drought stress on damask rose (Rosa damascena Mill.) plants. Four levels of drought stress (100, 75, 50, and 25% FC) were examined on mycorrhizal and non-mycorrhizal plants in pots filled with sterilized soil. Our results showed that increasing drought stress level decreased all growth parameters, nutrient contents, gas exchange parameters, and water relations indicators. Under different levels of drought stress, mycorrhizal colonization significantly increased all studied parameters. Pn, gs, and E of the mycorrhizal plants was higher than those of non-mycorrhizal plants under different levels of drought stress. The increase in those rates was proportional the level of the mycorrhizal colonization in the roots of these plants. Majority of growth, nutrition, water status and photosynthetic parameters had a great dependency on the mycorrhizal colonization under all levels of drought stress. The results obtained in this study provide a clear evidence that AMF colonization can enhance growth, flower quality and adaptation of rose plants under different drought stress levels, particularly at high level of drought stress via improving their water relations and photosynthetic status. It could be concluded that colonization with AMF could help plants to tolerate the harmful effects caused by drought stress in arid and semi-arid regions.
Keywords: AMF, Gas exchange, Glomus, Pigments content, Rosa damascena Mill., Drought stress
1. Introduction
Drought stress is negatively affect the plant production all over the world, especially in arid and semi-arid regions like Saudi Arabia, and is expected to increase with climate changes such as increasing of global temperature and increasing soil drought (EEA, 2011). Soil drought is a widespread problem in the World, which restricts plant growth and biomass production, particularly in arid and semi-arid regions (Asrar et al., 2012).
Damask rose (Rosa damascena Mill.) belongs to Rosacea family. It is a vigorous, arching, deciduous shrub with grey-green leaves, divided into five leaflets (rarely seven), ovate to elliptic leaflets. The flowers are famous for their fine fragrance, and are commercially harvested for aromatic rose oil to be used in perfumery and to make rose water. The flower petals are considered safe for human consumptions. They could be used directly to flavor food and drinking water, and to make tea. Damask rose is reported to be grown in Bulgaria, Turkey, Spain, France, India, Syria, Morocco, Tunisia, and Saudi Arabia (Chevallier, 1996). These roses are mainly cultivated depending on rain water. Therefore, they are exposed to several drought periods affecting their growth and productivity.
Plants of 95% of plant families is known to form a mutualistic relationship with the arbuscular mycorrhizal fungi (Trappe, 1987). The fungus associates with the plant roots in such relationship enhances the plant ability to adsorb water and nutrition via increasing the absorbing area through its large surface area of mycelium (Selosse et al., 2006). The plant, in turn, provides the fungus directly with needed carbohydrates including glucose and sucrose (Harrison, 2005). Many studies indicate that the symbiotic relationship between plants and the arbuscular mycorrhizal fungi (AMF) is a key factor helping plants to tolerate and/or resist drought stress (e.g. Abdel-Fattah et al., 2014a, Abdel-Fattah et al., 2014b, Asrar et al., 2012, Ruiz-Lozano and Aroca, 2010). There is evidence that AMF improve drought tolerance of rose plants (Augé et al., 1986a, Augé et al., 1986b). Therefore, the aim of this study was to examine the role of AMF in alleviating the harmful effects of drought stress on the damask rose plants.
2. Materials and methods
2.1. Inoculum preparation
The mycorrhizal inoculum was prepared in an open-culture of sudangrass (Sorghum halepense L.) plants. The wet sieving and decanting technique (Gerdemann and Nicolson, 1963) was followed to isolate the AMF used in this study from different sites cultivated with rose plants subjected to drought stress in Taif region, Saudi Arabia. The spores of AMF were cultured for 6 months on sudangrass plants cultivated in pots containing sandy soil (autoclaved 3 times, 121 °C, 30 min, 1.5 air pressure for three separate consequent time) in an environmentally controlled greenhouse (25/16 °C day/night, relative humidity of 60–65%, 16/8 light/dark photoperiod with light intensity of 700 µmol m−2 s−1). The inoculum used was consisted of 20 g of the sudangrass rhizosphere containing roughly 950 mycorrhizal spores and about 0.5 g of colonized roots.The inoculum average infection level was 78.5%. The mycorrhizal inoculums were placed at 3 cm depth of the rose cutting media upon planting.
2.2. Growth conditions
The experiment was conducted in the greenhouse with temperature 25/16 °C day/night, relative humidity of 60–65%, 16/8 light/dark photoperiod with light intensity of 700 µmol m−2 s−1 (measured using LI-1500 data logger, LI-COR® Biosciences, Lincoln, NE, USA). Damask rose plants were obtained from a commercial farm in Taif region, Saudi Arabia. In spring (March 2012), cuttings were prepared as basal 10 cm long cuttings and planted in 15 cm diameter pots containing autoclaved perlite for rooting, after being treated with IBA (500 ppm) to stimulate root formation. Then, rooted homogeneous cuttings were replanted in 30 cm diameter pots containing autoclaved (121 °C, 20 min, 1.5 air pressure for three separate consequent time) soil mixture consisting of sand and clay (v:v, 1:1). The soil used in the study had the following characteristics: organic matter (0.43%), pH (soil solution) 7.61, nitrogen (25.0 mg kg−1), phosphorus (7.02 mg kg−1), magnesium (85 mg kg−1); potassium (52 mg kg−1); and electrical conductivity “EC” of 0.35 dS m−1. These pots were randomly divided into two groups (mycorrhizal and non-mycorrhizal treatments). For mycorrhizal colonization, each mycorrhizal-designated pot received 20 g of the prepared inoculum. Each group divided into four sub-groups where each sub-group received one of the determined four different drought stress treatments; namely, 100% of field capacity, FC, (Control), 75% of FC, 50% of FC and 25% of FC.
The method described by (Khan et al., 2008) was used to determine the soil FC. Separate experiments were conducted to find out weights of water present in 1 kg soil at 100, 75, 50 and 25% FC levels. Completely randomized design was used to range the pots in the green house under the following controlled conditions: 680-μmol m−2 s−1 light intensity, 16-h photoperiod, 25/20 °C day/night temperatures and 70–75%, relative humidity.
For the different four treatments, water was supplied daily to maintain the water content of the pots at the determined level of field capacity using the weight as indicator. The pots were weighed every day and the loss in water based on weight was added to maintain the soil water content at the desired level according to the different treatments. Drought stress was applied directly after transplanting the cuttings. Harvesting (five plants from each treatment) was carried out at two stages; vegetative and flowering. The flowers, roots and shoots of each plants were separated and the different studied parameters was measured.
2.3. Parameters studied
2.3.1. Flowering parameters
At full flowering stage, five plants of each treatment were harvested to measure flower fresh weight (FW) and dry weight (DW). The FW was measured immediately after harvesting, while the DW was measured after oven drying the plant flowers and roots separately at 80 °C for 48 h. Number and diameter of flowers per plant were recorded. Moreover, the number of leaves and leaf area of each plant were measured. LI-3000C Portable Leaf Area Meter (LI-COR® Biosciences, Lincoln, NE, USA) was used to measure plant leaf area.
2.3.2. Gas exchange
LI-6400XT portable photosynthetic system (LI-COR® Biosciences, Lincoln, NE, USA) was used to measure the net photosynthetic rate (Pn), transpiration rate (E), and stomatal conductance (gs) both at vegetative growth and flowering stages. The first third upper leaf of the plants were used for gas exchange measurements. Gas exchange measurements were made under saturated light conditions with intensity of 1000-μmol m−1 s−1. The measurement temperature was 25 °C and CO2 level adjusted to 400 μmol mol−1 air with five replicates for each treatment.
2.3.3. Leaf water status
The forth full leaf from the apices of the rose plant was used to measure the water content (WC), relative water content (RWC), and water saturation deficit (WSD) according to the method described by (Ünyayar et al., 2004).
Electrolyte leakage (EL) was measured as described by Wang et al. (2011). The leaves were cut into strips and soaked in 10 ml of distilled water. The leaf sample solution was put on a shaker (120 rpm) at room temperature for 2 h, and then the first conductivity of the solution (EC1) was measured with a conductivity meter (Waterproof pH/EC system, Hanna Instruments, France). After that, the solution was boiled for 10 min to reach the complete leakage and cooled down before the second conductivity (EC2) was measured. The relative EL was calculated using following formula (Wang et al., 2011):
Leaf water potential (Ψleaf) was measured using a PSYPRO Water Potential System (WESCOR INC, Logan, UT, USA) with five replicates for each treatment. An attached leaf of the plant was fitted into the leaf hygrometer. The cycle was as follows: 15 s cooling time, 4 s plateau delay, 5 s read average seconds, and 25 s measurement period.
2.3.4. Mineral analysis
Oven-dried shoot and root samples of five randomly chosen plants from each treatment were grounded and sieved using a sieve with diameter of 0.5 mm. For the estimation of the macronutrients (P, K, Mg and Ca), five grams of the grounded shoots and roots were chemically digested using a mixture of nitric acid (HNO3), sulfuric acid (H2SO4) and hydrochloric acid (HCl 60%) with a ratio of 10:1:4 v:v:v. The vanadate-molybdate colorimetric method described by (Jackson, 1973) was used to analyze the total phosphorus (P). Potassium (K) was assayed using flame photometer (Corning 400, UK). The atomic absorption system (Perkin Elmer, Model 2380, USA) was used to determine the content of calcium (Ca) and magnesium (Mg) in plant material. The total nitrogen (N) was measured using the Micro-Kjeldahl method described by (Nelson and Sommers, 1973).
2.3.5. Proline content
Estimation of proline was done according to the method of (Bates et al., 1973). The absorbance was read by a spectrophotometer at 520 nm and calculated as µmoles g–1 FW against standard proline (Sigma–Aldrich Chemie, Germany) using the following equation:
2.3.6. Pigment contents
The total chlorophyll was extracted and assayed according to the method of (Porra, 2002). The crude extraction supernatant was used for absorbance reading at 646.6, 663.6 and 470 nm with a spectrophotometer. The chlorophyll and carotenoids contents were calculated according to the following equations:
2.3.7. Levels of the mycorrhizal colonization
To calculate the mycorrhizal colonization levels, the root system of the plants was washed thoroughly immediately after harvest using tap water to clean the roots from soil particles. The washed roots were, then, cut into 0.5–1.0 cm fragments and thoroughly mixed. The root fragments were cleared in 10% potassium hydroxide (KOH) solution. Then, the cleared root fragments were stained following the method described by (Phillips and Hayman, 1970) using 0.05% trypan blue in lactophenol. Stained root pieces were examined under a compound microscope at 40× magnification. Intensity of mycorrhizal infection (M%), Rtate of the colonization (F%), and arbuscules development (A%) in the rose infected roots was calculated using the Mycocalc software following the method described by (Trouvelot et al., 1986).
The mycorrhizal dependency (MD) is known as the percentage of increase in plant growth as a result of mycorrhizal colonization. MD was calculated for each studied parameter using the formula of (Menge et al., 1978) as follow:
where M is the parameter value of the mycorrhizal plants and NM is the parameter value of the non-mycorrhizal plants.
2.4. Statistical analysis
The experiment was conducted using a 2 × 4 factorial arranged in a completely randomized design with two arbuscular mycorrhizal treatments [inoculated (M) and non-inoculated plants (NM)] and four levels of drought stress treatments [100% FC, 75% FC, 50% FC and 25% FC]. Five replicates was used for each treatment. Each replicate consists of 10 experimental units (pots), one plant per pot. Thus, the total number of plants in this study was 400 plants. Data were subjected to statistical analysis using two-factor analysis of variance (ANOVA). Means were separated by the least significant difference test (LSD, P ≤ 0.05) method using the SPSS 22.0 software. Correlation was measured using the Pearson’s coefficient. All of the measurements were performed five times for each treatment, and the mean was reported.
3. Results
3.1. Flower yield
Flower FW and DW, flower number and flower diameter of drought stressed M and NM rose plants were significantly lower than those of control ones (Table 1); however, the reductions in flower yield parameters due to drought stress were more pronounced in NM than in M rose plants. On the other hand, the M plants had higher flower number and diameter than the NM plants regardless of the water treatments.
Table 1.
Flower yield of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed levels.
| Treatments |
Flower fresh weight (g plant−1) |
Flower dry weight (g plant−1) |
Number of flowers (flowers plant−1) | Flower diameter (cm) | |
|---|---|---|---|---|---|
| Irrigation (% FC) | AMF status | ||||
| 100 | −AMF | 253.49c | 113.38c | 8.33b | 8.83c |
| +AMF | 350.58a | 174.01a | 12.67a | 12.83a | |
| 75 | −AMF | 216.30de | 93.82d | 7.67bc | 7.17de |
| +AMF | 298.83b | 165.72a | 11.00a | 11.50b | |
| 50 | −AMF | 194.79e | 89.09d | 6.33c | 6.17e |
| +AMF | 229.90cd | 152.45b | 7.67bc | 8.17cd | |
| 25 | −AMF | 0.00g | 0.00f | 0.00e | 0.00f |
| +AMF | 146.62f | 75.89e | 3.33d | 6.37e | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
3.2. Gas exchange
Pn, E and gs of leaves of rose plants were significantly decreased as the drought stress increased both at the vegetative growth and the flowering stages (Table 2). Those effects were more remarked at vegetative growth stage. Moreover, the gas exchange parameters of M plants were significantly higher than those of the NM plants. Such simulation in Pn was linked to the level of the mycorrhizal colonization in each treatment. The differences in Pn and E were not significant between the M and NM plants grown at the highest level of drought stress, both at vegetative and flowering stages.
Table 2.
Leaf net photosynthetic rate (Pn), transpiration rate (E) and stomatal conductance (gs) of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed levels during vegetative growth and flowering stages.
| Treatments |
Vegetative growth stage |
Flowering stage |
|||||
|---|---|---|---|---|---|---|---|
| Irrigation (% FC) | AMF status |
Pn (µmol m−2 s−1) |
E (mmol m−2 s−1) |
gs (mol m−2 s−1) |
Pn (µmol m−2 s−1) |
E (mmol m−2 s−1) |
gs (mol m−2 s−1) |
| 100 | −AMF | 2.58cd | 0.51d | 0.02d | 3.39b | 0.64b | 0.03bcd |
| +AMF | 3.58b | 0.46d | 0.02d | 3.13b | 1.26a | 0.01d | |
| 75 | −AMF | 2.99bc | 1.19cd | 0.05cd | 3.35b | 1.25a | 0.05abc |
| +AMF | 5.73a | 3.83a | 0.22a | 6.00a | 1.58a | 0.02cd | |
| 50 | −AMF | 2.67c | 0.86cd | 0.03cd | 2.16b | 0.73b | 0.02cd |
| +AMF | 2.70c | 2.78b | 0.15b | 2.96b | 1.38a | 0.07ab | |
| 25 | −AMF | 1.91de | 1.18cd | 0.07cd | 2.00b | 1.45a | 0.06abc |
| +AMF | 1.29e | 1.37c | 0.1bc | 1.80b | 1.56a | 0.08a | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
3.3. Leaf water status
Water content, relative water content, and water saturation deficit were highly affected by the AMF inoculation and application of drought stress (Table 3). The water content and relative water content in M and NM plants were markedly decreased by drought stress. Moreover, M plants had significantly higher RWC than NM plants had, regardless of water treatments. Yet, plants grown under higher levels of drought stress exhibited more benefits from colonization with mycorrhizal fungi.
Table 3.
Water status of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed conditions.
| Treatments |
Water content (%) | Relative water content (%) | Water saturation deficit (%) | Water potential (MPa) | |
|---|---|---|---|---|---|
| Irrigation (% FC) | AMF status | ||||
| 100 | −AMF | 73.67a | 82.68a | 17.32d | −2.01a |
| +AMF | 78.19a | 86.83a | 13.17d | −2.34a | |
| 75 | −AMF | 68.16b | 72.22b | 27.78c | −1.79c |
| +AMF | 74.06a | 75.97b | 24.03c | −2.25a | |
| 50 | −AMF | 54.57d | 64.40c | 35.60b | −1.38e |
| +AMF | 63.22c | 74.65b | 25.35c | −1.70cd | |
| 25 | −AMF | 46.18e | 55.85d | 44.15a | −1.45e |
| +AMF | 55.67d | 63.23c | 36.77b | −1.54de | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
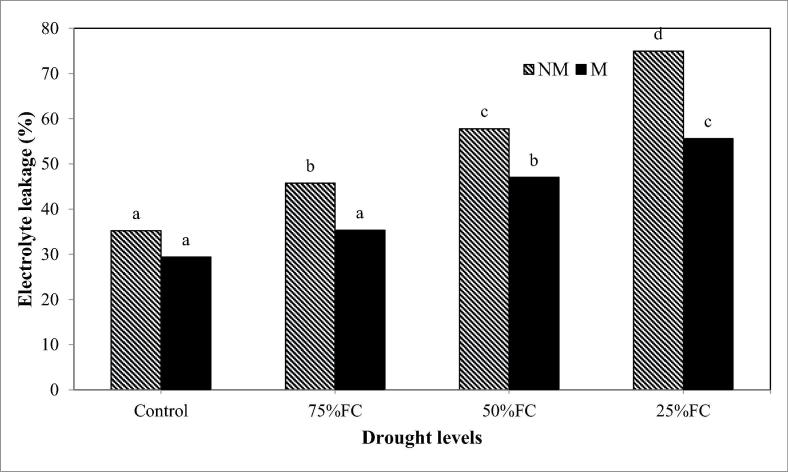
Drought stress markedly increased the electrolyte leakage of rose plants comparing to those of non-stressed plants (Fig. 1). This outcome was increased as drought stress increased. The higher the level of drought stress was, the more EL in the leaves of rose plants was. However, inoculation with AMF has markedly helped to reduce the EL in the leaves of M rose plants as compared to NM plants under both control and drought conditions.
Fig. 1.
The beneficial roles of inoculation with AMF on electrolyte leakage of damask rose plants grown under different levels of drought stress.
Leaf water potential was significantly less negative in M plants than in NM plants, regardless the level of the drought stress. It was also less negative at low levels of drought stress (Table 3).
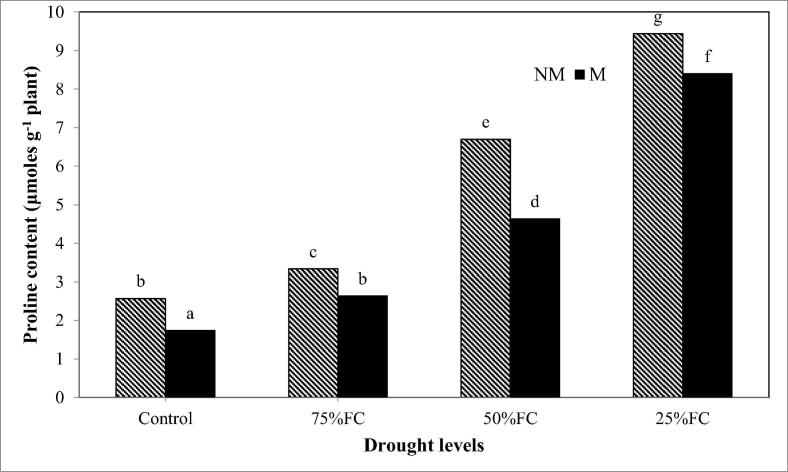
3.4. Proline content
The proline contents of the M and M rose leaves were increased by increasing the drought stress level (Fig. 2). However, M plants had lower proline content in leaves than in leaves of the NM plants, regardless of the water treatments. This difference was more remarked under different drought stress conditions.
Fig. 2.
The function of inoculation with AMF on proline content of damask rose plants grown under different levels of drought stresses.
3.5. Nutrient contents
In general, N, P, K, Mg, and Ca contents in shoots and roots of M and NM rose plants decrease as the drought stress level increases. Reduction in leaf nutrient content because of the drought stress was more evident in NM than in M plants. Furthermore, inoculation with AMF increased the contents of different nutrients both in shoot and root of M rose plants conparing to NM plants, especially at the higher levels of drought stress (Table 4). Among different drought stress treatments, there was a great association between AMF colonization and increment of the nutrients content.
Table 4.
Concentrations (%) of N, P, K, Ca, and Mg and proline content in leaves of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed conditions.
| Treatments |
Macronutrients (%) |
|||||
|---|---|---|---|---|---|---|
| Irrigation (% FC) |
AMF status | N | P | K | Ca | Mg |
| 100 | −AMF | 2.19de | 0.111cd | 1.61bc | 1.28f | 0.213bc |
| +AMF | 2.83ab | 0.151a | 1.90a | 1.52d | 0.273a | |
| 75 | −AMF | 2.43c | 0.107de | 1.70ab | 1.62c | 0.223bc |
| +AMF | 2.99a | 0.140ab | 1.96a | 1.92a | 0.233b | |
| 50 | −AMF | 2.40cd | 0.093ef | 1.40cd | 1.43e | 0.207c |
| +AMF | 2.85ab | 0.126bc | 1.72ab | 1.81b | 0.217bc | |
| 25 | −AMF | 2.14e | 0.077f | 1.29d | 1.28f | 0.183d |
| +AMF | 2.75b | 0.107de | 1.62bc | 1.58cd | 0.202cd | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
3.6. Photosynthetic pigments
Drought stress significantly decreased the content of chlorophyll a, chlorophyll b, and carotenoids contents in leaves of M and NM rose plants (Table 5). The total chlorophyll content in leaves of M plants was significantly higher than that in leaves of NM plants, regardless of the water treatment.
Table 5.
Pigments content of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed conditions.
| Treatments |
Chl a (mg g−1 plant) |
Chl b (mg g−1 plant) |
Total chlorophyll (mg g−1 plant) |
Carotenoids (mg g−1 plant) |
|
|---|---|---|---|---|---|
| Irrigation (% FC) | AMF status | ||||
| 100 | −AMF | 944.33b | 715.33b | 1659.66c | 203.33c |
| +AMF | 1043.33a | 803.67a | 1847.00a | 313.00a | |
| 75 | −AMF | 827.33c | 723.33b | 1550.66d | 207.67c |
| +AMF | 953.33b | 800.67a | 1754.00b | 273.33b | |
| 50 | −AMF | 840.33c | 608.33d | 1448.66e | 180.67d |
| +AMF | 957.00b | 660.67c | 1617.66c | 206.00c | |
| 25 | −AMF | 626.00e | 410.33f | 1036.33g | 134.67e |
| +AMF | 727.00d | 524.33e | 1251.33f | 195.67cd | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
3.7. Mycorrhizal colonization levels
The results revealed that the frequency of the mycorrhizal colonization (F%) and intensity of the mycorrhizal colonization (M%) were generally significantly decreased in rose root tissues as the level of the drought stress raised (Table 6). However, no significant differences were observed in the rate of arbuscular frequency (A%) among M plants grown under the different levels of drought stress. No mycorrhizal colonization was detected in the NM rose plants.
Table 6.
Frequency of mycorrhizal colonization (F), intensity of mycorrhizal colonization (M) and arbuscular frequency (A) of mycorrhizal (+AMF) and non-mycorrhizal (−AMF) damask rose plants grown under different irrigation-stressed conditions.
| Treatments |
Mycorrhizal colonization levels (%) |
|||
|---|---|---|---|---|
| Irrigation (% FC) | AMF status | F | M | A |
| 100 | −AMF | 0.0 | 0.0 | 0.0 |
| +AMF | 83.67a | 83.58a | 12.62a | |
| 75 | −AMF | 0.0 | 0.0 | 0.0 |
| +AMF | 73.66b | 43.31b | 11.66ab | |
| 50 | −AMF | 0.0 | 0.0 | 0.0 |
| +AMF | 63.33c | 35.26c | 10.90b | |
| 25 | −AMF | 0.0 | 0.0 | 0.0 |
| +AMF | 54.17d | 33.10d | 10.32b | |
Values in each column followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
3.8. Mycorrhizal dependency
Results of the mycorrhizal dependency (MD) on improving plant growth, flower yield, chlorophyll and some of nutrients of damask rose plants grown under well-watered conditions (100% FC) or high drought stress conditions (25% FC) are shown in Table 7. Mycorrhizal dependency values of the rose plants in responding to AMF inoculation were significantly higher under drought stress than under control conditions among all calculated parameters (P ≤ 0.05). Under drought stress, the improved growth of damask rose plants depended highly on the presence of the arbuscular mycorrhizal fungi, indicating that AMF colonization can mitigate the damaging effects of the drought stress on growth of rose plants.
Table 7.
Mycorrhizal dependency (MD)* of several parameters of damask rose plants grown under different irrigation-stressed conditions.
| Parameters | 100% FC | 25% FC |
|---|---|---|
| No. of leaves | 35.50b | 75.35a |
| Water potential | 6.58b | 16.24a |
| Total pigments | 11.34b | 20.75a |
| N | 15.22b | 28.32a |
| P | 16.01b | 38.69a |
| K | 17.81b | 25.98a |
Values in each row followed by the same letters are not significantly different at P ≤ 0.05.
FC: Field capacity.
MD = [(M − NM)/NM] × 100, where M is parameter value of mycorrhizal plants and NM is parameter value of non-mycorrhizal plants.
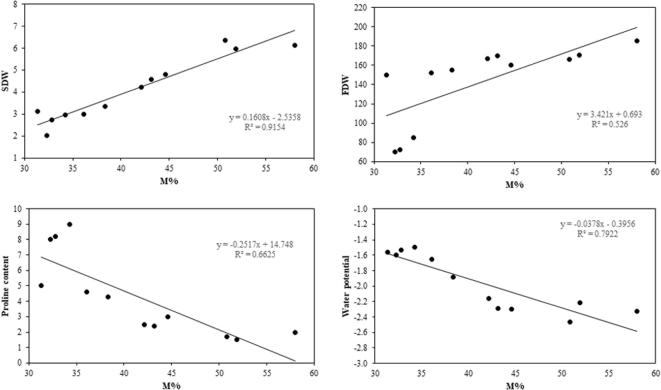
The correlation between M% of the mycorrhizal fungus and other several parameters of the M damask rose plants was studied. The results of the correlation analysis are shown in Fig. 3. There was a positive relationship between M% and shoot dry and flower dry weights. There was, also, a negative relationship between M% and both proline content and water potential in damask rose plants.
Fig. 3.
Correlation analysis between intensity of mycorrhizal colonization. (M%) and shoot dry weight (SDW), fruit dry weight (FDW), proline content and water potential.
4. Discussion
Drought stress is a worldwide problem, which reduces plant growth, flower yield and other physiological processes of most field and ornamental economical crops. Since soil usually first begin to dry, the shoot growth can be inhibited before any leaf dehydration occurs through the root-to-shoot monohydraulic signaling mechanisms (Asrar et al., 2014, Davies et al., 1994). In the present study, the rate of growth in response to mycorrhizal colonization was more pronounced at higher levels of drought stress. Such increases in growth parameters of rose plants because of the mycorrhizal colonization were directly related proportionally to the respective level of the mycorrhizal colonization. The enhanced growth effects of the M plants are often related to the improvement of P and other nutrients (N, K, Ca Mg) acquisitions, as the availability of soil P is reduced by soil drying (Augé, 2001). Moreover, it has been stated that the main mechanism for enhancing drought tolerance in the M plants was the improvement of P nutrition (Giri et al., 2007).
Nitrogen, potassium, magnesium and calcium contents were significantly higher in leaves of M than those in leaves of the NM rose plants regardless of drought stress level. This finding supports the previous studies who mentioned that arbuscular mycorrhizal fungi absorb more N, P, Ca and Mg than NM plants during drought stress (Al-Karaki and Al-Raddad, 1997, Asrar and Elhindi, 2011). In this regard, (Augé, 2001) stated, also, that the positive effects of AMF relies mainly on the uptake and transport of water and on an improved uptake of nutrients, especially of available soil P and other immobile mineral nutrients, resulting in hydration of plant tissues, a sustainable physiology and a clear promotion of growth. Furthermore, the protection of the M plants against drought stress was related to the mycorrhizal induction of leaf conductance (Abdel-Fattah et al., 2014a, Abdel-Fattah et al., 2014b). Potassium plays a key role in plant drought stress and its cationic solute, which is responsible for stomatal movement because of changes in the bulk leaf status (Ruiz-Lozano et al., 1995). In this study, the role of AMF to protect rose plants from the harmful effects of drought stress and K content in plants were positively related.
The dependency on the mycorrhiza was increased with increasing the drought stress. In addition, the strong positive correlation between M% and dry weights of plants’ shoot, and flowers suggests that the growth of damask rose plants are significantly affected by AMF colonization. The enhanced growth effects of M plants are often related to the AMF symbiosis, which could help plants to cope with the adverse effects of drought stress directly or indirectly through enhancing plant functionality both above- and/or below-ground (Rapparini and Peñuelas, 2014).
In this study, drought stress often caused reductions in flower parameters of the M and NM damask rose plants comparing to control plants. Plants grown under controlled or stressed conditions and colonized with AMF had greater flower yield, better flower number and diameter than those NM plants had. This is in agreement with the previous findings on other ornamental plants (Asrar et al., 2012, Augé et al., 1987, Levy and Krikun, 1980) in which M plants had better flower yield than the NM plants had. Such increases in flower yield parameters were related directly to the level of the AMF colonization at different drought stress levels.
The contents of the photosynthetic pigments in leaves of the M and NM damask rose plants were reduced as the level of the drought stress increased. In spite of this, all treatments of damask rose plants colonized by the AMF had greater photosynthetic contents comparing to these of the NM plants. Drought stress may lead to oxidative stress on plants via production of the reactive oxygen species (ROS), such as the singlet oxygen, superoxides, hydrogen peroxide, and hydroxyl radicals (Rapparini and Peñuelas, 2014). Carotenoids is a part of the plant’s antioxidant defense system (Mittler, 2002). The improvement of the stress tolerance mechanism by AMF symbiosis is often related to the amelioration of the antioxidant activities in plants (Baslam and Goicoechea, 2012). The higher levels of carotenoids contents in M plants observed in this study represent an evident for mycorrhizal role in alleviating adverse effects of drought stress on rose plants. Furthermore, the overall reduction in chlorophyll content with drought stress may be due to the reduction in Mg and K concentrations (Augé, 2001). In the present study, the contents of those elements are usually found to be higher in M than in NM plants.
Under drought stress conditions, plants accumulate some small molecules including organic solutes like soluble sugars and proline (Farooq et al., 2009). The results showed that NM plants accumulated more proline than the M plants and this accumulation was increased with increasing the drought stress levels. This suggests that AMF colonization enhanced the host plant’s drought stress tolerance and thus; plants were less stressed than the NM plants (Tang et al., 2009, Wu and Xia, 2006). Moreover, the correlation analysis showed a strong negative relationship between M% and proline content in damask rose plants. These results approved that mycorrhizal fungi could alleviate the adverse effects of drought on plants.
It is evident from the present study that Pn, gs, and E were significantly higher in leaves of M than in leaves of NM rose plants grown under different levels of drought stress. There was a strong relation between the increase in these processes and the levels of AMF colonization. These data are in agreement with (Augé et al., 2008) and (Abdel-Fattah et al., 2013) who observed an increase in E and gs in M plants grown under drought conditions. A higher transpiration rate in leaves of M plants would be consistent with the higher rates of stomatal conductance that often accompany the mycorrhizal symbiosis, and are supposed to be necessary to supply carbon needs for the fungal symbiont (Augé, 2001). A significant increase in P content, through its transport by hyphae has also been noticed as well as the AMF symbiosis affects the stomatal behavior (Ruiz-Lozano and Azcón, 1995). Moreover, the M rose plants in this study showed more shoot and root dry matter than the NM plants. This might partially explain why M plants had higher gas exchange parameters than the NM plants. The ability of AMF to increase leaf number, leaf area and root density had been reported by many earlier investigations (Abdel-Fattah et al., 2013, Al-Karaki and Al-Raddad, 1997). In this study, the mycorrhizal plants had higher contents of K than NM plants had. Potassium is a key factor in plant’s drought stress tolerance and it is responsible of the stomatal movement to cope with changes in leaf water status as a whole (Ruiz-Lozano et al., 1995).
Water status parameters in leaves of the M and NM rose plants were highly reduced by increasing the level of drought stress. However, all M rose plants had significantly higher water content than of the NM plants had. The AMF absorb mineral nutrients and water, which benefits the host plants comparing to the NM plants. Similar results have been reported by (Allen, 1982) who stated that AMF could affect water uptake ability and water use efficiency in host plants. In this experiment, the low water potential of M plants under control conditions (100% FC) indicates that AMF symbiosis adapted well to drought conditions. Moreover, it could be indicated that water potential of M plants under drought stress levels, especially at high levels; did not decrease significantly. The water content was higher in M plants, signifying that AMF association had increased water uptake. The low electrolyte leakage values indicate more root hydraulic conductance in M plants. Thus, mycorrhization might increase plant’s water uptake ability via increasing the effectiveness of the root hydraulic conductivity.
In this study, reductions in AMF colonization levels with increasing levels of drought stress were observed. This is in consistent with previous studies of (Al-Karaki and Al-Raddad, 1997, Asrar et al., 2012). In contrast, other studies indicated that the percentage of roots colonized by AMF is not affected by drought stress (Bryle and Duniway, 1997, Morte et al., 2000). It has been also observed in this study that the beneficial effects of AMF on flowering growth of rose plants are more pronounced in higher levels of drought stress (Table 1). These positive effects may be due to the improvement of phosphorus and nitrogen nutrition (Smith et al., 2011), the enhancement of gas exchange parameters (Augé, 2001) and to the increase of root density (Bryle and Duniway, 1997).
The results obtained here concluded that drought stress had harmful effect on growth, flower yield, nutrition and water status of rose plants particularly at high level. Mycorrhizal rose plants had higher growth and flower yield than those in NM plants. Therefore, the results suggested that the benefits of AMF colonization on rose plants grown under drought stress conditions were due to the improvement of water relations, nutrients content, gas exchange status and photosynthetic pigments. Finally, the results showed that benefits of AMF colonization could protect plant against the deleterious effects of drought stress.
5. Conclusions
In many arid and semiarid regions, like in Saudi Arabia, drought stress affects the physiological and biochemical processes of the plants, resulting in altering growth, yield, water relations and metabolic pathways. Reduction in plant growth under drought stress is due to the osmotic stress resulted from decreasing of soil water potential and nutrient uptake. The present investigation showed that AMF inoculation significantly alleviated the harmful effects of drought stress on damask rose plants (Rosa damascena Mill.) grown under different soil drought stress through enhancing growth and flower yield, increasing P, N, K and Mg contents, improving water relation parameters, stimulating gas exchange status, and increasing proline content. These benefits in response to the mycorrhizal inoculation are generally accelerated when the level of the drought stress increases in soil. These studies conclude that AMF colonization can mitigate the deleterious effects of drought stress on growth and flower yield of rose plants.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Saudi Arabia for funding this work through research group no. (RGP-1438-053).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Fattah G.M., Asrar A.A., Al-Amri S.M., Abdel-Salam E.M. Influence of arbuscular mycorrhiza and phosphorus fertilization on the gas exchange, growth and phosphatase activity of soybean (Glycine max L.) plants. Photosynthetica. 2014;52:581–588. [Google Scholar]
- Abdel-Fattah G.M., Asrar A.A., Al-Amri S.M., Abdel-Salam E.M. Influence of arbuscular mycorrhiza and phosphorus fertilization on the gas exchange, growth and phosphatase activity of soybean (Glycine max L.) plants grown in a sandy loam soil. J. Food Agric. Environ. 2014;12:150–165. [Google Scholar]
- Abdel-Fattah G.M., Ibrahim A.H., Al-Amri S.M., Shoker A.E. Synergistic effect of arbuscular mycorrhizal fungi and spermine on amelioration of salinity stress of wheat (Triticum aestivum L. cv. Gimiza) Aust. J. Crop Sci. 2013;7:1525–1532. [Google Scholar]
- Al-Karaki G.N., Al-Raddad A. Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza. 1997;7:83–88. [Google Scholar]
- Allen M.F. Influence of vesicular-arbuscular mycorrhizae on water movement through Bouteloua gralics (H.B.K.) Lag Ex Steud. New Phytol. 1982;91:191–196. [Google Scholar]
- Asrar A.-W.A., Elhindi K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011;18:93–98. doi: 10.1016/j.sjbs.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrar A.A., Abdel-Fattah G.M., Elhindi K.M. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica. 2012;50:305–316. [Google Scholar]
- Asrar A.A., Abdel-Fattah G.M., Elhindi K.M., Abdel-Salam E.M. The impact of arbuscular mycorrhizal fungi in improving growth, flower yield and tolerance of kalanchoe (Kalanchoe blossfeldiana Poelin) plants grown in NaCl-stress conditions. J. Food Agric. Environ. 2014;12:105–112. [Google Scholar]
- Augé R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Augé R.M., Schekel K.A., Wample R.L. Leaf water and carbohydrate status of VA mycorrhizal rose exposed to drought stress. Plant Soil. 1987;99:291–302. [Google Scholar]
- Augé R.M., Schekel K.A., Wample R.L. Greater leaf conductance of well-watered VA mycorrhizal rose plants is not related tp phosphorus nutrition. New Phytol. 1986;103:107–116. [Google Scholar]
- Augé R.M., Schekel K.A., Wample R.L. Osmotic adjustment in leaves of VA mycorrhizal and nonmycorrhizal rose plants in response to drought stress. Plant Physiol. 1986 doi: 10.1104/pp.82.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé R.M., Toler H.D., Sams C.E., Nasim G. Hydraulic conductance and water potential gradients in squash leaves showing mycorrhiza-induced increases in stomatal conductance. Mycorrhiza. 2008;18:115–121. doi: 10.1007/s00572-008-0162-9. [DOI] [PubMed] [Google Scholar]
- Baslam M., Goicoechea N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza. 2012;22:347–359. doi: 10.1007/s00572-011-0408-9. [DOI] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bryle D.R., Duniway J.M. Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol. 1997;136:581–590. doi: 10.1046/j.1469-8137.1997.00780.x. [DOI] [PubMed] [Google Scholar]
- Chevallier A. A DK Publishing book; DK Pub: 1996. The Encyclopedia of Medicinal Plants. [Google Scholar]
- Davies W.J., Tardieu F., Trejo C.L. How do chemical signals work in plants that grow in drying soil? Plant Physiol. 1994 doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EEA, E.E.A., 2011. Global and European temperature (CSI 012/CLIM 001), Copenhagen [WWW Document].
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. [Google Scholar]
- Gerdemann J.W., Nicolson T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963;46:235–244. [Google Scholar]
- Giri B., Kapoor R., Mukerji K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007;54:753–760. doi: 10.1007/s00248-007-9239-9. [DOI] [PubMed] [Google Scholar]
- Harrison M.J. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005;59:19–42. doi: 10.1146/annurev.micro.58.030603.123749. [DOI] [PubMed] [Google Scholar]
- Jackson M.L. Prentice Hall Inc.; New Delhi, India: 1973. Soil Chemical Analysis. [Google Scholar]
- Khan I.A., Ayub N., Mirza S.N., Nizami S.M., Azam M. Yield and water use efficiency (WUE) of Cenchrus ciliaris as influenced by vesicular arbuscular mycorrhizae (VAM) Pakistan J. Bot. 2008;40:931–937. [Google Scholar]
- Levy Y., Krikun J. Effect of vesicular-arbuscular mycorrhiza on Citrus jambhiri water relations. New Phytol. 1980;85:25–31. [Google Scholar]
- Menge J.A., Johnson E.L.V., Platt R.G. Mycorrhizal dependency of several citrus cultivars under three nutrient regimes. New Phytol. 1978;81:553–559. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Morte A., Lovisolo C., Schubert A. Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense-Terfezia claveryi. Mycorrhiza. 2000;10:115–119. [Google Scholar]
- Nelson D.W., Sommers L.E. Determination of total nitrogen in plant material1. Agron. J. 1973;65:109–112. [Google Scholar]
- Phillips J.M., Hayman D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Brit Mycol Soc Trans. 1970 [Google Scholar]
- Porra R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002;73:149–156. doi: 10.1023/A:1020470224740. [DOI] [PubMed] [Google Scholar]
- Rapparini F., Peñuelas J. Mycorrhizal Fungi to Alleviate Drought Stress on Plant Growth. In: Miransari M., editor. Use of Microbes for the Alleviation of Soil Stresses. Vol. 1. New York; NY: 2014. pp. 21–42. (Springer). [Google Scholar]
- Ruiz-Lozano J.M., Aroca R. Modulation of Aquaporin Genes by the Arbuscular Mycorrhizal Symbiosis in Relation to Osmotic Stress Tolerance. In: Seckbach J., Grube M., editors. Symbioses and Stress: Joint Ventures in Biology. Springer; Netherlands, Dordrecht: 2010. pp. 357–374. [Google Scholar]
- Ruiz-Lozano J.M., Azcón R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995;95:472–478. [Google Scholar]
- Ruiz-Lozano J.M., Azcon R., Gomez M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995 doi: 10.1128/aem.61.2.456-460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M.-A., Richard F., He X., Simard S.W. Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol. 2006;21:621–628. doi: 10.1016/j.tree.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Smith S.E., Jakobsen I., Grønlund M., Smith F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Chen H., Huang J.C., Tian Z.Q. AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biol. Biochem. 2009;41:936–940. [Google Scholar]
- Trappe J. Phylogenetic and ecologic aspects of mycotrophy in the angiosperms from an evolutionary standpoint. In: Safir G.R., editor. Ecophysiology of VA Mycorrhizal Plants. CRC Press; Florida, FL: 1987. pp. 2–25. [Google Scholar]
- Trouvelot, A., Kough, J.L., Gianinazzi-Pearson, V., 1986. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson, V., Gianinazzi, S. (Eds.), Physiological and genetical aspects of mycorrhizae = Aspects physiologiques et genetiques des mycorhizes: proceedings of the 1st European Symposium on Mycorrhizae, Dijon, 1–5 July 1985. Institut National de la Recherche Agronomique Press, Paris.
- Ünyayar S., Keleş Y., Ünal E. Proline and ABA levels in two sunflower genotypes subjected to water stress. Bulg. J. Plant Physiol. 2004;30:37–47. [Google Scholar]
- Wang J., Sun P.-P., Chen C.-L., Wang Y., Fu X.-Z., Liu J.-H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011;62:2899–2914. doi: 10.1093/jxb/erq463. [DOI] [PubMed] [Google Scholar]
- Wu Q.-S., Xia R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006;163:417–425. doi: 10.1016/j.jplph.2005.04.024. [DOI] [PubMed] [Google Scholar]