Abstract
Aims
Rising antimicrobial resistance (AMR) is a global health crisis. India has among the highest resistance rates and antibiotic consumption internationally. Extensive use of fixed‐dose combination (FDC) antibiotics and of unapproved formulations are claimed contributory factors but there has been no systematic examination of formulations or volumes sold. The aim of the present study was to investigate the regulatory approval status and sales volumes of systemic antibiotics marketed in India.
Methods
This was an ecological study using regulatory records in India, the UK and the US to determine the approval status in each country of systemic antibiotic FDCs and single‐drug formulations (SDFs) sold in India. Pharmatrac® sales data were used to determine the formulations and volumes sold (2007–2012), branded‐product numbers and manufacturers.
Results
Of 118 systemic antibiotic FDC formulations sold in India, 43 (36%) were approved but 75 (64%) had no record of regulatory approval; four (3%) formulations were approved in the UK and/or US. Almost half of formulations (58/118; 49%) comprised dual antimicrobials, most unapproved in India (43/58; 74%), and many were pharmacologically problematic. In contrast, 80/86 (93%) SDFs were approved in India and over two‐thirds in the UK and/or US. Total antibiotic sales increased by 26%, from 2056 million units (2007–08) to 2583 million units (2011–12). FDC sales rose by 38% vs. 20% for SDFs. By 2011–12, FDCs comprised one‐third of sales (872 million units). Over one‐third of FDCs sold (300.26 million units; 34.5%) were of unapproved formulations. Multinational companies manufactured unapproved formulations and accounted for 19% of all FDC and SDF sales annually.
Conclusions
Sales in India of antibiotic FDCs, including unapproved formulations, are rising. In the context of increasing AMR rates nationally and globally, unapproved antibiotic FDCs undermine India's national AMR strategy and should be banned from sale.
Keywords: antibiotics, antimicrobial resistance, fixed‐dose combination (FDC) formulations, regulatory approval, unapproved formulations
What is Already Known about this Subject
India has among the highest rates globally of antimicrobial resistance and of antibiotic consumption.
Prescription medicines must have central regulatory approval before they can be sold.
Indian government reports claim extensive use of unapproved fixed‐dose combination (FDC) formulations of antibiotics.
What this Study Adds
There were 118 antibiotic FDC formulations on the market in India (2007–2012) compared with four in the UK and/or US.
Only 43 of the 118 antibiotic FDC formulations (36%) had central regulatory approval; 75 (64%) were unapproved, even though the sale of unapproved new drugs is illegal in India.
Multinational companies (MNCs) manufactured 53 of the 118 FDC formulations; only 33 (62%) of these were Central Drugs Standard Control Organization‐approved and four were Medicines and Healthcare Products Regulatory Agency/European Medicines Agency‐ and /or Food and Drug Administration‐approved formulations.
Total antibiotic sales volumes increased by 26%, from 2056 million units (2007–08) to 2583 million units (2011–12); FDC sales rose by 38% vs. 20% for single‐drug formulations (SDFs).
By 2011–12, FDCs comprised one‐third of systemic antibiotic sales (872 million units) but over one‐third of FDCs sold (300 million units; 34.5%) were of unapproved formulations.
MNCs accounted for almost 20% of FDC and SDF sales annually.
Introduction
Antimicrobial resistance (AMR) is an acknowledged global crisis but antibiotic consumption is rising, despite access for many being poor 1, 2, 3, 4. In 2015, the World Health Assembly endorsed a five‐point AMR global action plan 5, and the World Health Organization (WHO) has urged its adoption. For decades, the WHO has promoted rational medicines use and universal access through its Model List of Essential Medicines, which acts as a guide for individual national lists of essential medicines (NLEMs) 6. The WHO classifies antibiotics in ascending order of priority: important, highly important, critically important and highest priority critically important 7. Highest priority critically important antibiotics are those for which loss of efficacy due to resistance would have a major impact on human health owing to high numbers of people affected by infections for which they are the sole treatment, or one of few effective treatments. They include third/fourth‐generation cephalosporins, fluoroquinolones, glycopeptides and macrolides.
During 2000–2010, antibiotic sales across 71 countries rose by 36% 8. Five countries (Brazil, China, India, Russia, South Africa) accounted for 76% of the increase. Consumption per capita was highest in India, a major producer of antibiotics with a fragile health system and among the highest rates globally of AMR 2, 8, 9. Parliamentary investigations have highlighted failures of its drug regulatory system, including approvals of ‘irrational’ fixed‐dose combination (FDC) formulations of antibiotics and marketing of FDCs unapproved by the national regulator, the Central Drugs Standard Control Organization (CDSCO), despite the sale or supply of unapproved new drugs being unlawful in India 10, 11, 12, 13, 14, 15, 16. The availability of FDCs ‘not approved anywhere in the world’ has been criticized and a recommendation made that such formulations ‘may not be cleared for use in India unless there is a specific disease or disorder prevalent in India, or a very specific reason backed by scientific evidence and irrefutable data applicable specifically to India, that justifies the approval of a particular FDC.’ 10 However, no systematic examination has been conducted of antibiotic FDCs available in India or of their approval status.
FDCs
FDCs are formulations comprising two or more drugs combined in a fixed ratio of doses and available in a single dosage form. Some antibiotic FDCs comprise an antibiotic plus non‐antibiotic drug – e.g. amoxicillin (a beta‐lactam antibiotic) plus clavulanic acid (an inactivation inhibitor) 17. Others include dual antimicrobials – e.g. trimethoprim plus sulfamethoxazole 18. Dual antimicrobial FDCs are appropriate in well‐defined situations where a particular combination is of proven efficacy, doses are stable throughout treatment and the drugs have compatible pharmacological characteristics. Dual antimicrobial FDCs are to be distinguished from the concomitant use of two antibiotics as single‐drug formulations (SDFs). While infection treatment guidelines recommend two antibiotics in some situations, the antibiotics and their doses vary depending on epidemiological, host and drug factors, and they are prescribed as SDFs 19, 20, 21, 22, 23, 24.
Evaluating drug consumption in India
The AMR global action plan 5 urges improved surveillance of antimicrobial use, but for many countries, including India, this is challenging. Studies using antibiotic sales data as a proxy for use have demonstrated rising sales of individual antibiotics and pharmacological groups but have not reported the formulations sold or their approval status 1, 8, 24, 25. We therefore analysed systemic antibiotic sales in India during a 5‐year period, 2007–2012, according to formulation (FDC or SDF) and approval in India and two other countries, the UK and US. We determined if formulations were listed in the NLEM‐India, if they included highest priority critically important antibiotics 7 and if they were manufactured by multinational pharmaceutical companies (MNCs) as well as by Indian companies.
Aim
To examine the systemic antibiotic formulations sold in India in the context of their regulation and governance for licensing and to determine if these formulations were approved in the UK and/or the US.
Objectives
To identify the FDC and single drug formulations on the Indian market, determine their regulatory approval status in India, the UK and the US and their NLEM‐India listing.
To examine 12‐monthly sales trends (2007–2012), identify the top‐selling antibiotic formulations (2011–12), and determine sales of WHO‐designated ‘highest‐priority‐critically‐important’ antibiotics categorised in each case according to approval.
To determine the numbers of branded products on the market and manufacture by multi124 national companies (MNCs).
Methods
This was an ecological study quantifying systemic antibiotic sales in India during 2007–2012 and including a cross‐sectional analysis of the individual formulations sold in 2011–12.
Data sources
Drug sales data
These were obtained from PharmaTrac®, a commercial database of Indian pharmaceutical sales 26. The data comprised monthly sales audits collected through multiple routes (some 5000 pharmaceutical companies, 18 000 distributers/stockists, 32 000 sub‐stockists, 500 000 retailers and hospitals, and dispensing doctors in 23 regions of India). The audits captured 35% of national sales and were projected to estimate total national sales. The data included formulation composition, generic and branded product names, manufacturers, sales volumes and value, and date of market launch. Sales volumes were reported monthly in units (a strip of 10 tablets/capsules or one bottle (liquid formulations) for oral drugs or one injection for parenteral drugs). Formulations were coded according to therapy type and treatment group. For example, the FDC ceftriaxone plus vancomycin (J1D42) was an anti‐infective in the cephalosporin group of systemic antibacterial agents.
Approvals in India
The CDSCO website published approvals for FDCs from 1961–2014 and for SDFs from 1971–2011 27, 28, 29. Listed chronologically, they included formulation content, indication and approval date. For the purposes of the present study, we assumed that CDSCO records were complete. State drug authority records of licences granted for drug manufacture/distribution/sale were unavailable.
Approvals in the UK and USA
The Medicines and Healthcare Products Regulatory Agency (MHRA) and/or European Medicines Agency (EMA) and Food and Drug Administration (FDA) websites were used to determine the approval status in the UK and US, respectively, of antibiotic formulations sold in India 30, 31, 32.
NLEM‐India
The 2011 list was used to determine the antibiotics recommended for use in India by a core committee of experts from the Ministry of Health and Family Welfare during the period of the cross‐sectional analysis of sales, 2011–2012 6.
Data Extraction
We examined PharmaTrac® data for the period October 2007 to November 2012. Systemic antibiotic formulations were identified independently by two people (P.M., A.K.). Information was extracted in duplicate onto Excel® spreadsheets. Discrepancies were resolved by consensus. We excluded antituberculosis and primary antifungal or antiviral formulations. We categorized systemic antibiotics according to FDC and SDFs. We subdivided FDCs into formulations comprising: (i) dual antimicrobials – e.g. two antibiotics or one antibiotic plus an antiprotozoal or antiviral agent; or (ii) one antibiotic plus other agents – e.g. amoxicillin plus clavulanic acid (a beta‐lactamase inhibitor). Although they have some antimicrobial activity, we did not consider beta‐lactamase inhibitors as antibiotics in their own right because they are not used as antibiotic monotherapy, so we did not count these combinations as dual antimicrobials. We counted the numbers of brand‐named products arising from each formulation, and the numbers of manufacturers, and noted whether the manufacturer was Indian or an MNC. We summed monthly sales volumes (in millions of units) to examine sales during five 12‐month periods from October 2007 to November 2012, where one unit was a strip of 10 oral tablets or capsules, one injection vial or one bottle of oral medicine.
Formulations were further categorized by CDSCO, MHRA/EMA and FDA approval status, and by NLEM‐India listing. For approvals, we focused on the first approval granted. A formulation was deemed ‘approved’ if ever recorded in the regulators' lists of approved medicines, irrespective of dose amount or modified release variations, and ‘unapproved’ if it was not included. We adhered to Strengthening the Reporting of Observational Studies in Epidemiology ‐ Anti‐Microbial Studies (STROBE‐AMS) guidelines for reporting 33.
Results
FDCs and SDFs marketed in India
There were 132 systemic antibiotic FDC formulations listed in PharmaTrac; 118/132 FDCs specified the full formulation, permitting regulatory approval to be determined (‘known formulations’, Table 1); 13/132 FDCs were described as ‘combinations’, naming the antibiotic but not the other formulation components. ‘Combinations’ comprised <1% of antibiotic sales in 2011–12 (Table S1). The study analyses are based on the 118 ‘known formulations’. There were 86 SDF antibiotics listed in PharmaTrac.
Table 1.
Systemic antibiotic sales volumes in India, November 2007 to October 2012, described by total sales, sales of fixed‐dose combination (FDC) formulations and of single‐drug formulations (SDFs), sales of FDC and SDF formulations with Central Drugs Standard Control Organization (CDSCO) approval and with no record of approval, and by sales of dual antimicrobial FDC formulations. Sales are reported in millions of units, where a unit is a strip of 10 tablets or capsules, one bottle (oral liquid formulations) or one injection (parenteral drugs)
| Systemic antibiotic sales volumes in India November 2007 to October 2012 (millions of units) | Nov 2007 to Oct 2008 | Nov 2008 to Oct 2009 | Nov 2009 to Oct 2010 | Nov 2010 to Oct 2011 | Nov 2011 to Oct 2012 |
|---|---|---|---|---|---|
| Total antibiotic sales (All FDCs + all SDFs) | 2055.86 m units | 2161.58 m units | 2348.08 m units | 2411.27 m units | 2583.07 m units |
| All FDC formulations a (% of total antibiotic sales) | 632.78 (30.78%) | 699.84 (32.38%) | 750.66 (31.97%) | 786.33 (32.61%) | 872.02 (33.76%) |
| All SDF formulations (% of total antibiotic sales) | 1423.08 (69.22%) | 1461.74 (67.62%) | 1597.43 (68.03%) | 1624.93 (67.39%) | 1711.04 (66.24%) |
| Sales arising from known b FDC formulations (% of all FDC sales) | 631.07 m units (99.73%) | 697.67 m units (99.69%) | 748.66 m units (99.73%) | 784.52 m units (99.77%) | 869.81 m units (99.75%) |
| Sales of CDSCO‐approved FDC formulations (% of FDC known formulation sales) | 334.83 (53.1%) | 390.07 (56%) | 445.69 (59.5%) | 492.41 (62.8%) | 569.55 (65.5%) |
| Sales of FDC formulations unapproved by CDSCO (% of FDC known formulation sales) | 296.24 (46.9%) | 307.6 (44%) | 302.97 (40.5%) | 292.11 (37.2%) | 300.26 (34.5%) |
| Sales of FDC formulations approved by MHRA/EMA and/or FDA (% of FDC known formulation sales) | 161.9 (25.7%) | 192.6 (27.6%) | 216.9 (29%) | 237.8 (30.3%) | 268.6 (30.9%) |
| Sales of dual antimicrobial FDC formulations (% of FDC known formulation sales, % of total antibiotic sales) | 408.72 (64.77%, 19.88%) | 446.63 (64.02%, 20.66%) | 442.53 (59.11%, 18.85% | 458.24 (58.41%, 19.00% | 488.55 (56.17%, 18.91%) |
| Sales arising from SDFs | 1423.08 m units | 1461.74 m units | 1597.43 m units | 1624.93 m units | 1711.04 m units |
| Sales of CDSCO‐approved c SDFs (% of all SDF sales) | 1384.81 (97.31%) | 1422.60 (97.32%) | 1556.63 (97.45%) | 1586.60 (97.64%) | 1670.17 (97.61%) |
| Sales of unapproved formulations (% of all SDF sales) | 38.28 (2.69%) | 39.15 (2.68%) | 40.79 (2.55%) | 38.34 (2.36%) | 40.87 (2.39%) |
EMA, European Medicines Agency; FDA, Food and Drug Administration; MHRA, Medicines and Healthcare Products Regulatory Agency
All systemic FDC formulations, including those with full formulation information and combinations where only the antibiotic name was specified
Known formulations; includes 118 FDCs for which complete formulation information was available in the PharmaTrac sales database and for which CDSCO approval could be determined; excludes 13 FDCs listed as ‘combinations’ with incomplete formulation details and for which CDSCO approval could not be determined
CDSCO‐approved/likely to be approved SDFs (see text).
Regulatory approval status in India, the UK and the US
FDCs
Of the 118 FDC formulations, 43 (36%) were CDSCO approved and 75 (64%) had no record of approval; 4(3.4%) were approved in the UK and US; two (2%) were NLEM listed (Figure 1).
Figure 1.
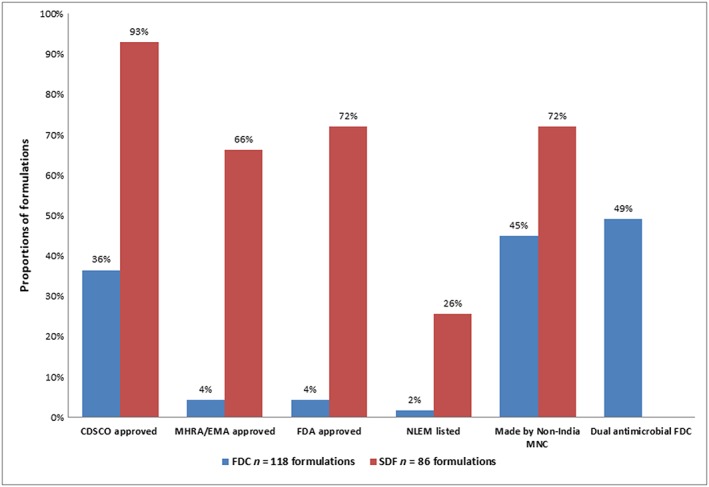
Fixed‐dose combination (FDC) and single‐drug formulations (SDFs) of systemic antibiotics listed on the market in India: regulatory approval of FDCs and SDFs in India [Central Drugs Standard Control Organization (CDSCO)], the UK [Medicines and Healthcare Products Regulatory Agency (MHRA)/ European Medicines Agency (EMA)] and the US [Food and Drug Administration (FDA)], the National List of Essential Medicines (NLEM‐India 2011) listing, proportions of FDC formulations comprising dual antimicrobials, and proportions of FDC and SDFs made by multinational company (MNC) manufacturers. N = 118 FDCs and 86 SDFs
Sixty FDC formulations (51%) comprised one antibiotic plus agents including beta‐lactamase inhibitors, lactobacillus, mucolytic agents and secretolytic agents. Of these, 28/60 (47%) were CDSCO‐approved, 32/60 (53%) had no record of approval and 3/60 (5%) were approved by the MHRA/EMA and/or FDA (amoxycillin plus clavulanic acid, piperacillin plus tazobactam, imipenem plus cilastatin) (Table S1_FDC118).
Fifty‐eight FDC formulations (49%) comprised dual antimicrobials: 36 were two antibiotics; 17 were an antibiotic plus an antiprotozoal drug; and five were an antibiotic with another antimicrobial (e.g. antifungal, antiviral) (Figure 1). Fifteen dual antimicrobial formulations (26%) were CDSCO approved and 43 (74%) were unapproved; one (trimethoprim plus sulfamethoxazole) was approved by the MHRA/EMA and FDA (Table S1_FDC118).
SDFs
Of the 86 SDF antibiotics, 80 (93%) were or were likely to be, CDSCO approved (Figure 1): 62 had approval listed; six had CDSCO‐granted European import licences; two were NLEM 2011 listed; and 10 had been discovered in the 1950s–1960s, with approval possibly granted prior to 1971, the earliest CDSCO SDF approval record. Six SDFs (7%) had no record of CDSCO approval. Twenty‐two SDFs (26%) were NLEM listed, 57 (66%) were MHRA/EMA approved, and 62 (72%) were FDA approved (Figure 1).
Total systemic antibiotic sales
Table 1 shows the systemic antibiotic 12‐monthly sales volumes during the 5‐year period examined (November 2007–October 2012). Total systemic sales (FDCs plus SDFs) increased by 26%, from 2055.86 million units in 2007–08 to 2583.07 million units in 2011–12, with FDCs rising by 38%, and SDFs by 20%. The FDC proportion of annual sales increased from 31% to 34%, while the SDF proportion fell from 69% to 66% (Table 1).
FDC sales
In 2007–08, 53% of FDC sales comprised CDSCO‐approved formulations (Table 1, Figure 2). By 2011–12, this had increased to 66%. Dual antimicrobials comprised 56% of FDC sales in 2011–12, most being unapproved formulations (Figure 2). In total, less than half of FDC sales were of MHRA/EMA‐approved and/or FDA‐approved formulations, and fewer than one‐third were NLEM listed (Figure 2).
Figure 2.
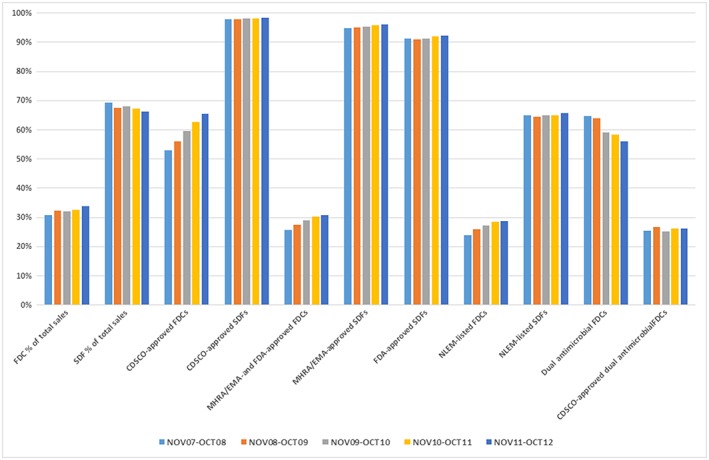
Proportions of 12‐monthly total antibiotic sales volumes (units) annually, 2007–2012, comprising fixed‐dose combination (FDC) formulations and single‐drug formulations (SDFs), and proportions of FDC and SDF sales volumes approved by the Central Drugs Standard Control Organization (CDSCO), Medicines and Healthcare Products Regulatory Agency (MHRA)/European Medicines Agency (EMA) and Food and Drug Administration (FDA), and national lists of essential medicines (NLEM) listed, and including dual antimicrobials. Volume is expressed in millions of units, where a unit is a strip of 10 tablets or capsules, one bottle (oral liquid formulations) or one injection (parenteral drugs)
SDF sales
Almost all SDF sales were of CDSCO‐approved formulations – 97% in 2007–08 and 98% in 2011–12. Over 90% were MHRA/EMA approved and/or FDA approved, and two‐thirds were NLEM listed (Figure 2).
Top‐selling formulations, 2011–12
FDCs
Amoxicillin plus clavulanic acid was the top‐selling formulation, followed by ampicillin plus cloxacillin, trimethoprim plus sulfamethoxazole, and ofloxacin plus ornidazole. The 20 top‐selling FDC formulations, listed in Table 2, comprised 61% of total FDC sales in 2011–12; 10/20 were dual antimicrobial formulations; 7/20 formulations had no record of CDSCO approval; 17/20 were unapproved by the MHRA/EMA and FDA. The two NLEM‐listed FDCs (amoxicillin plus clavulanic acid and trimethoprim plus sulfamethoxazole) together accounted for 29% of sales in 2011–12.
Table 2.
Top‐selling 20 fixed‐dose combination (FDC) antibiotic formulations by volume, 2011–12, according to dual antimicrobial formulation, regulatory approval status in India [Central Drugs Standard Control Organization (CDSCO)], the UK [Medicines and Healthcare Products Regulatory Agency (MHRA)/European Medicines Agency (EMA)] and the US [Food and Drug Administration (FDA)], the National List of Essential Medicines (NLEM; India 2011) listing, multinational company (MNC) manufacture, and volume sold. Volume is expressed in millions of units, where a unit is a strip of 10 tablets or capsules, one bottle (oral liquid formulations) or one injection (parenteral drugs)
| Antibiotic FDCs: top 20 formulations by volume of sales 2011–12 | Dual antimicrobial | Record of CDSCO approval | Approved MHRA/EMA and FDA | Listed NLEM 2011 | Made by MNC | Volume sold (millions of units) |
|---|---|---|---|---|---|---|
| AMOXYCILLIN + CLAVULANIC ACID | N | Y | Y/Y | Y | Y | 161.8 |
| AMPICILLIN + CLOXACILLIN | Y | N | N/N | N | Y | 110 |
| TRIMETHOPRIM + SULFAMETHOXAZOLE | Y | Y | Y/Y | Y | Y | 88.6 |
| OFLOXACIN + ORNIDAZOLE | Y | Y | N/N | N | Y | 63 |
| CEFTRIAXONE + SULBACTUM | N | Y | N/N | N | Y | 34.1 |
| CEFIXIME + OFLOXACIN | Y | Y | N/N | N | Y | 31.2 |
| CEFTRIAXONE + TAZOBACTUM | N | Y | N/N | N | Y | 31 |
| FURAZOLIDONE + METRONIDAZOLE | Y | N | N/N | N | Y | 30.4 |
| NORFLOXACIN + METRONIDAZOLE | Y | N | N/N | N | Y | 23.7 |
| CEFPODOXIME + CLAVULANIC ACID | N | Y | N/N | N | Y | 23.4 |
| LOMOFEN (furazolidone/atropine/diphenoxylate) | N | N | N/N | N | N | 20.9 |
| OFLOXACIN + METRONIDAZOLE | Y | N | N/N | N | N | 19.6 |
| CEFIXIME + CLAVULANIC ACID | N | Y | N/N | N | Y | 17.3 |
| AMOXYCILLIN + CLOXACILLIN | Y | N | N/N | N | Y | 16.8 |
| CEFOTAXIME + SULBACTUM | N | Y | N/N | N | Y | 16.2 |
| PIPERACILLIN + TAZOBACTAM | N | Y | Y/Y | N | Y | 16 |
| CIPROFLOXACIN + TINIDAZOLE | Y | N | N/N | N | Y | 15.8 |
| CEFOPERAZONE + SULBACTUM | N | Y | N/N | N | Y | 15.7 |
| AMOXYCILLIN + CLOXACILLIN + LACTOBACILLUS | Y | Y | N/N | N | Y | 10.7 |
| CEFIXIME + LACTOBACILLUS | N | Y | N/N | N | Y | 10.4 |
| Proportion affirming | 50% | 65% | 15% | 10% | 90% | |
| top 20 FDC volume (millions of units) | 530.3 m | |||||
| Proportion of total FDC volume (872.02 m units) | 60.80% |
N, no; Y, yes
SDFs
Ceftriaxone was the top‐selling SDF, followed by cefixime, metronidazole and cefotaxime. The 20 top‐selling SDFs, listed in Table 3, comprised 87% of total SDF sales in 2011–12. All had or were likely to have, CDSCO approval; all had MHRA/EMA approval; 19/20 had FDA approval. NLEM‐listed SDFs made up 65% of SDF sales.
Table 3.
Top‐selling 20 SDF antibiotics by volume, 2011–12, according to regulatory approval status in India [Central Drugs Standard Control Organization (CDSCO)], the UK [Medicines and Healthcare Products Regulatory Agency (MHRA)/European Medicines Agency (EMA)] and the US [Food and Drug Administration (FDA)], the National List of Essential Medicines (NLEM; India 2011) listing, multinational company (MNC) manufacture, and volume sold in 2011–12. Volume is expressed in millions of units, where a unit is a strip of 10 tablets or capsules, one bottle (oral liquid formulations) or one injection (parenteral drugs)
| Antibiotic SDFs: top 20 formulations by volume of sales 2011–12 | Record of CDSCO approval | Approved MHRA/EMA and FDA | Listed NLEM 2011 | Made by MNC | Volume sold (millions of units) |
|---|---|---|---|---|---|
| CEFTRIAXONE | Y | Y/Y | Y | Y | 146.1 |
| CEFIXIME | Y | Y/Y | Y | Y | 136.1 |
| METRONIDAZOLE | Y | Y/Y | Y | Y | 128.2 |
| CEFOTAXIME | Y | Y/Y | Y | Y | 125.7 |
| AZITHROMYCIN | Y | Y/Y | Y | Y | 119.2 |
| AMIKACIN | Y | Y/Y | Y | Y | 103.2 |
| CEFPODOXIME | Y | Y/Y | N | Y | 83.3 |
| OFLOXACIN | Y | Y/N | Y | Y | 72.9 |
| GENTAMICIN | Likely | Y/Y | Y | Y | 68.9 |
| PENICILLIN G | Likely | Y/Y | N | Y | 67.6 |
| AMOXYCILLIN | Y | Y/Y | Y | Y | 62.7 |
| CEFADROXIL | Y | Y/Y | N | Y | 59.1 |
| OXYTETRACYCLINE | Y | Y/Y | N | Y | 52.5 |
| CIPROFLOXACIN | Y | Y/Y | Y | Y | 51.7 |
| LEVOFLOXACIN | Y | Y/Y | N | Y | 45.9 |
| TETRACYCLINE | Y | Y/Y | N | Y | 36.4 |
| ERYTHROMYCIN | Likely | Y/Y | Y | Y | 35.6 |
| CEFUROXIME | Y | Y/Y | N | Y | 35.3 |
| LINCOMYCIN | Y | Y/Y | N | Y | 32.8 |
| CEFALEXIN | Likely | Y/Y | Y | Y | 31.3 |
| Proportion affirming | 100% | 100% / 95% | 60% | 100% | |
| top 20 SDF volume (millions of units) | 1494.3 | ||||
| Proportion of total SDF volume (1711.04 m units) | 87.3% |
N, no; Y, yes.
‘Likely’ = likely to have CDSCO approval on the basis of having an export licence, being listed on the NLEM, and/or marketed prior to CDSCO records commencing (1971).
Highest priority critically important antibiotic sales, 2011–12
FDCs
In 2011–12, 42% of FDC sales in India included WHO‐designated highest priority critically important antibiotics (Table S1). Eight formulations were dual antimicrobial FDCs: azithromycin plus ofloxacin, cefixime plus azithromycin, cefixime plus ofloxacin, cefpodoxime plus azithromycin, cefpodoxime plus levofloxacin, cefpodoxime plus ofloxacin, ceftriaxone plus vancomycin, levofloxacin plus azithromycin. They made up 5% of FDC sales volumes in 2011–12. Two formulations were CDSCO approved (cefixime plus ofloxacin, cefpodoxime plus ofloxacin). None was FDA approved, MHRA/EMA approved or NLEM listed.
SDFs
Among SDFs, highest priority critically important antibiotics comprised 54% of sales in 2011–12, and carbapenems and polymixins comprised 0.4% of sales.
Numbers of brand‐named products and manufacture by MNCs
FDCs
The 118 FDC formulations gave rise to 3307 brand‐named products, made by 476 manufacturers, of which 464 were based in India and 11 were MNCs. The FDCs with the greatest numbers of products were: ofloxacin plus ornidazole, 382 branded products made by 279 manufacturers; amoxicillin plus clavulanic acid, 293 products, 189 manufacturers; and ciprofloxacin plus tinidazole, 208 products, 147 manufacturers (Table S1_FDCproducts).
MNCs manufactured 53/118 (45%) FDC formulations (Figure 1; Table S1_FDCproducts). These gave rise to 148 brand‐named products, manufactured by 11 MNCs including Abbott, Astra Zeneca, Bayer, Eli Lilly, GlaxoSmithKline (GSK), Merck/MSD, Novartis, Pfizer, Sanofi‐Aventis and Wyeth. Twenty‐eight MNC‐manufactured formulations were dual antimicrobials: 19 with two antibiotics (e.g. ampicillin plus dicloxacillin, ceftriaxone plus vancomycin); and nine with an antibiotic plus antiprotozoal (e.g. ciprofloxacin plus tinidazole, ofloxacin plus ornidazole) (Table S1_FDCproducts).
Thirty‐three MNC‐manufactured formulations (33/53; 62%) were CDSCO approved; 4/53(8%) were MHRA/EMA and FDA approved (amoxicillin plus clavulanic acid, imipenem plus cilastatin, piperacillin plus tazobactam, trimethoprim plus sulfamethoxazole). Of the 20 formulations without CDSCO approval, 18 were manufactured by Abbott. Some other MNCs each manufactured 1–3 unapproved formulations (Table S1_FDCproducts).
Of the eight formulations comprising two highest priority critically important antibiotics, all were manufactured by Indian pharmaceutical companies and five were manufactured by Abbott but none were manufactured by other MNCs.
SDFs
The 86 SDF antibiotics gave rise to 4934 branded products, made by 532 manufacturers; 515 were based in India and 13 were MNCs. The antibiotics with the greatest numbers of products were: ofloxacin, 443 branded products made by 303 manufacturers; azithromycin, 370 products, 263 manufacturers; cefixime, 341 products; 228 manufacturers (Table S1_SDFproducts).
MNCs manufactured 62/86 (72%) SDF formulations (Figure 1; Table S1_SDFproducts). These gave rise to 250 brand‐named products, manufactured by 13 MNCs. Fifty‐eight MNC‐manufactured SDFs (58/62; 94%) were CDSCO approved/likely to be approved, 47/62 (76%) were FDA approved and 44/62 (71%) were MHRA/EMA approved (Figure 1).
MNC‐manufactured antibiotic sales
MNC‐manufactured FDCs and SDFs comprised approximately 19% of both FDC and SDF sales annually. Among the 20 top‐selling FDC and SDF formulations, 90% and 100%, respectively, were MNC manufactured (Tables 3 and 4).
Discussion
India was recently shown to be the largest consumer of antibiotics per capita among 71 countries, and to have increasing levels of consumption 8. Our study demonstrated, for the first time, that its high consumption is led by rising sales of FDCs of antibiotics, the majority unapproved by the national drugs regulator, the CDSCO. The study confirmed parliamentary committee observations that most FDCs available in India have not been approved by regulators elsewhere, and also found that MNCs are among those manufacturing unapproved FDC formulations.
Of 118 antibiotic FDC formulations on the market during 2007–2012, almost two‐thirds had no record of CDSCO approval and only four were approved by UK and/or US regulators. In contrast, most of the 86 antibiotic SDFs on the Indian market were approved by the CDSCO and by UK and US regulators. Almost half of the FDC formulations included dual antimicrobials, some combining two highest priority critically important antibiotics. India's National List of Essential Medicines was found to include only two of the antibiotic FDCs and 22 of the SDFs marketed.
In the analysis of sales volumes, FDC sales increased by 38% over the 5‐year period 2007–2012, compared with 20% for antibiotic SDFs. By 2011–12, antibiotic FDCs accounted for over one‐third of total antibiotic sales. This is strikingly high. By comparison, in the UK, 5% of community‐dispensed systemic antibiotics in 2012 were FDCs, and 95% were SDFs 34. Although subject to no regulatory scrutiny, unapproved FDC formulations comprised more than one‐third of the FDC sales annually in India.
SDFs accounted for two‐thirds of total antibiotic sales volumes, almost all of which were CDSCO‐approved formulations. Of these, two‐thirds of sales were for NELM‐listed antibiotics and over half were for highest priority critically important antibiotics 7. There were found to be thousands of brand‐named FDC and SDF products on the market, manufactured by several hundreds of Indian companies and fewer than 20 MNCs.
MNC manufacture in India of FDCs unapproved in the UK and US
MNCs manufactured almost 20% of the systemic antibiotic FDC and SDF volumes sold in India annually. Twenty MNC‐manufactured FDC formulations had no record of CDSCO approval. Only four of the 53 FDC formulations made in India by MNCs had UK or US regulatory approval. The leading manufacturer was Abbott, whose role in the manufacture of unapproved dual‐antimicrobial FDC formulations has been criticized 35, 36, 37. In contrast to FDCs, most MNC‐manufactured SDF formulations were CDSCO approved and over 70% had UK/US regulatory approval. Pharmaceutical companies, including some manufacturers of unapproved FDCs, made a declaration in 2016 on their commitment to combating AMR 38.
Pharmacological problems of FDCs
Many dual antimicrobial formulations had been poorly considered pharmacologically. Combinations of broad‐spectrum antibiotics with antiprotozoal drugs were among the most highly prescribed FDCs – e.g. ofloxacin plus ornidazole and norfloxacin plus metronidazole. Used to treat diarrhoea, their intention is to cover possible bacterial and amoebic causes but as antimicrobials are not first‐line diarrhoea treatment, the combinations are inappropriate and may exacerbate diarrhoea owing to their effects on normal gut flora.
FDC component drugs were found to be commonly pharmacologically incompatible, having different half‐life durations requiring different dosing frequencies that cannot be accommodated in FDC formulations. For example, ofloxacin, dosed once daily, was combined with ornidazole or tinidazole, both needing twice‐daily dosing or, with metronidazole, needing 8‐hourly dosing. Similarly, azithromycin (once daily) was combined with cefpodoxime (twice daily). Some of the combinations have potentially serious interactions. In the case of azithromycin plus ofloxacin or levofloxacin, all of these, antibiotics are associated with prolongation of the cardiac QT‐interval, and the combinations are potentially harmful for vulnerable individuals. Gatifloxacin was withdrawn by regulators, including the FDA, in 2006 owing to associations with glycaemic disorders, but the present study found it to be available in India, in combination with both ornidazole and metronidazole.
Drug regulatory weaknesses
Our study exposed the consequences of acknowledged weaknesses in India's drug regulatory system. Although the government has convened reviews of regulation, actions to improve matters have been ineffective 10, 11, 12, 13, 39, 40. In February 2016, the Kokate Committee, constituted ‘for examining the safety and efficacy of unapproved FDCs which were licensed by State Drug Licensing Authorities without due approval of [the Drugs Controller General (India)] DCG(I)’ published its report to government 13. Over 6000 brand‐named products were examined, (including 163 systemic antibiotic FDC products) and categorized by the committee as ‘irrational’, ‘requiring further deliberation’, ‘rational’ or ‘requiring further generation of data’ 40. In March 2016, the government banned 344 FDC formulations from sale because none had ‘therapeutic justification’ and each was ‘likely to involve risk to human beings, whereas safer alternatives to the said drug are available’ 41. Following appeals, the ban was overturned by the Delhi High Court in November 2016 42. The matter continues, with the Supreme Court recently upholding the government's right to ban drugs in the public interest 43. Included in the ban were 16 unapproved systemic antibiotic FDC formulations listed in PharmaTrac. Of these, 11 were dual antimicrobials and six were manufactured by MNCs. In 2011–12, these 16 formulations accounted for 14% of antibiotic FDC sales. The government did not explain the selection criteria for banning. Many alternative approved formulations similar to those classed by the Kokate Committee as ‘irrational’ are available, so even if the ban had been enforced, the impact on the sales of antibiotic FDCs would have been negligible.
In a further delay to improving regulation, the government announced in June 2016 that it was withdrawing the Drugs and Cosmetics (Amendment) Bill, 2013, introduced in the Rajya Sabha on 29 August 2013, and instead would ‘comprehensively review the existing law with two‐fold objectives viz. to facilitate the ease of doing business and substantially enhancing the quality and efficacy of our products’ 44.
Actions needed
The sale of unapproved, unscrutinized FDC antibiotics undermines measures to control AMR. Definitive regulatory action to ensure that antibiotic formulations sold in India are rigorously evaluated and approved by the drugs regulator would permit India to participate effectively in AMR control measures. A starting point would be a government ban on the manufacture and sale of unapproved antibiotic formulations, commencing with dual antimicrobial FDCs (Table S1). The marketing of centrally unapproved formulations of new drugs is illegal 15. This approach would not deprive patients of clinically needed antibiotics because approved SDFs are available. In all cases, the evidence base supporting CDSCO systemic antibiotic approvals, both FDC and SDF, should be made publicly available. In relation to MNC‐manufactured antibiotic FDCs, the MNCs should be required to justify the sale of products in India that do not have the approval of their own national regulators – and, in multiple cases, not even the approval of the Indian regulator. Enactment of policies to reduce inappropriate antimicrobial use is poor in India 45. Work is needed to understand why prescribers are choosing antimicrobial FDCs, not adhering to the NLEM and frequently prescribing unapproved formulations. If these actions were to be implemented, India could participate effectively in the solutions proposed for national 46 and global 1, 23, 47, 48 action on resistance.
Limitations
We used publicly available CDSCO information to determine antibiotic approvals. We assumed that records were accurate, and cross‐checked sources extensively, but it is possible that we overlooked information. Pharmatrac® drug sales data are estimates determined using standard sampling methods and sales volume, formulation, brand‐name and manufacturer information cannot be double‐checked. In common with similar datasets, they do not distinguish prescription from nonprescription sales, but with high levels of nonprescription antibiotic use in India, sales data provide the most accurate estimate of national antibiotic use. When we categorized the Pharmatrac® sales volumes by antibiotic class, the rankings matched those reported using another commercial data source 8, 49.
Conclusions
The present systematic examination of Indian sales data and regulatory information confirmed government claims about antimicrobial use. FDCs comprise increasing proportions of antibiotic sales, but most of the formulations sold are unapproved by the CDSCO and only a handful are approved by regulators in the UK and US. Their manufacture by MNCs contradicts stated commitments on combating AMR. The use of unapproved, unscrutinized antibiotic FDC formulations is likely to contribute to India's rising AMR. Until definitive action is taken to ban most systemic antibiotic FDCs from manufacture and sale, AMR initiatives in India are likely to be undermined and the global action plan impeded.
Competing Interests
There are no competing interests to declare.
We thank Professor Nilima A. Kshirsagar, National Chair of Clinical Pharmacology, ICMR Government of India, and Dr Dhvani Mehta of the Public Health and Environmental Justice Initiative at the Vidhi Centre for Legal Policy, New Delhi, for their time, comments and discussion of the drug regulatory landscape in India. We thank Chinu Srinivasan, All India Drug Action Network, and Professor Roger Jeffery, School of Social and Political Science, University of Edinburgh, for facilitating data access. We thank Graham Kirkwood, University of Newcastle, UK, for assistance with Tables. We wish, especially, to acknowledge the valued contributions of the late Professor Ranjit Roy Chaudhury.
Contributors
Concept for the paper: P.M. conceptualized the study; P.M. and A.K. carried out drug sales data extraction and checking; P.M. and P.R. carried out regulatory approval information extraction; P.M., P.R. and A.P. planned the analyses; P.M. and A.K. performed the analyses; P.M., A.K., P.R. and A.P. interpreted the findings; P.M. wrote the first draft of the manuscript; P.M., P.R. and A.P revised the manuscript; P.M., P.R., A.K. and A.P agreed the final draft.
Editorial Note
Since this article was first published online on the 21st of February 2018, Pfizer has informed the British Journal of Clinical Pharmacology that it has never manufactured FURAZOLIDONE + METRONIDAZOLE and LEVOFLOXACIN + TINIDAZOLE. Pfizer has further stated that it has never marketed AMOXYCILLIN + CLOXACILLIN under the brand name ‘Megamycin’ for human use. AIOCD Pharmasofttech AWACS Pvt Ltd, the market research company maintaining the Pharmatrac database, has agreed to Pfizer's request to retrospectively amend the database. Pfizer does not challenge the CDSCO record. This note was added on the 19th of November 2018.
Supporting information
Table S1 Systemic Antibiotic Approvals and Sales India Data
McGettigan, P. , Roderick, P. , Kadam, A. , and Pollock, A. (2019) Threats to global antimicrobial resistance control: Centrally approved and unapproved antibiotic formulations sold in India. Br J Clin Pharmacol, 85: 59–70. 10.1111/bcp.13503.
References
- 1. O'Neill J. The review on antimicrobial resistance. Tackling drug‐resistant infections globally: final report and recommendations. 19 May 2016. Available at http://amr‐review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (last accessed 21 November 2017).
- 2. Laxminarayan R, Matsosp P, Pant S, Brower C, Rottigen J‐A, Klugman K, et al Antimicrobials: access and sustainable effectiveness 1. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–175. [DOI] [PubMed] [Google Scholar]
- 3. Holmes A, Moore LSP, Sundsfjord A, Stienbakk M, Regmi S, Karkey A, et al Understanding the mechanisms and drivers of antimicrobial resistance. Lancet: 2016. ; 387: 176–187. [DOI] [PubMed] [Google Scholar]
- 4. Mendelsom M, Rottingen JA, Gopinathan U, Hamer D, Wertheim H, Basnyat B, et al Maximising access to achieve appropriate human antimicrobial use in low‐income and middle‐income countries. Lancet 2016; 387: 188–198. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Global action plan on antimicrobial resistance. Available at http://www.who.int/drugresistance/global_action_plan/en/ (last accessed 31 January 2018).
- 6. World Health Organization . List of essential medicines, India, 2011. Available at http://apps.who.int/medicinedocs/documents/s18693en/s18693en.pdf.
- 7. World Health Organization . Critically important antimicrobials for human medicine. 3rd revision, 2011. Available at http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf (last accessed 21 November 2017).
- 8. Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell B, Levin S, et al Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14: 742–750. [DOI] [PubMed] [Google Scholar]
- 9. Patel V, Parikh R, Nandraj S, Balasubramaniam P, Narayan K, Paul VK, et al Asssuring health coverage for all in India. Lancet 2015; 386: 2422–2435. [DOI] [PubMed] [Google Scholar]
- 10. Department‐Related Standing Committee on Health and Family Welfare . 59th Report on the functioning of the Central Drugs Standard Control Organization (CDSCO). Parliament of India, New Delhi, 2012. Available at http://164.100.47.5/newcommittee/reports/englishcommittees/committee%20on%20health%20and%20family%20welfare/59.pdf (last accessed 21 November 2017).
- 11. Department‐Related Standing Committee on Health and Family Welfare . 66th Report on action taken by the government on the recommendations/observations contained in the 59th Report on the functioning of Central Drugs Standards Control Organization (CDSCO). Parliament of India, New Delhi, 2013. Available at http://www.searchingforsafety.net/uploads/2/6/2/1/26218021/rajya_sabha_april_2013.pdf (last accessed 21 November 2017).
- 12. Chaudhury R. Report of the Prof Ranjit Roy Chaudhury Expert Committee to formulate policy and guidelines for approval of new drugs, clinical trials and banning of drugs. Parliament of India, New Delhi, 2013. Available at http://www.indiaenvironmentportal.org.in/files/file/clinical%20trials1.pdf (last accessed 21 November 2017).
- 13. Kokate CK. Report by the Expert Committee constituted for examination of fixed dose combinations (FDCs) permitted for manufacture for sale in the country without due approval from office of DCG‐(I) regarding. Parliament of India, New Delhi, 2016.
- 14.Available at http://www.cdsco.nic.in/writereaddata/fdcreport10_2_2016.pdf (last accessed 31 January 2018).
- 15. Government of India . Ministry of Health and FamilyWelfare . Manufacturing and Marketing of Banned/ Unapproved Drugs. 2013. Available at http://pib.nic.in/newsite/PrintRelease.aspx?relid=101213 (last accessed 31 January 2018).
- 16. Roderick P, Mahajan R, McGettigan P, Pollock A. India should introduce a new Drugs Act. Lancet 2014; 383: 206–209. [DOI] [PubMed] [Google Scholar]
- 17. Medicines & Healthcare Products Regulatory Agency . Co‐amoxiclav 500/125 tablets. Available at http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1515734933668.pdf (last accessed 31 January 2018).
- 18. Medicines & Healthcare Products Regulatory Agency . Septrin® (trimethoprim + sulfamethoxazole). Available at http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1496991840650.pdf.
- 19. British National Formulary. Available at https://bnf.nice.org.uk/treatment-summary/antibacterials-principles-of-therapy.html (last accessed 31 January 2018).
- 20. National Institute for Health and Care Excellence (NICE) . Guideline NG15; August 2015. Available at https://www.nice.org.uk/guidance/NG15/chapter/1‐Recommendations#recommendations‐for‐prescribers (last accessed 31 January 2018).
- 21. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 22. National Centre for Disease Control, India . National antibiotic treatment guidelines for antimicrobial use in infectious diseases. Available at http://pbhealth.gov.in/AMR_guideline7001495889.pdf (last accessed 31 January 2018).
- 23. Ministry of Health and Family Welfare, Government of India . Antibiotic policy. Available at http://pib.nic.in/newsite/PrintRelease.aspx?relid=117474 (last accessed 31 January 2018).
- 24. Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta‐analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev 2013; 26: 289–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. All Indian Origin Chemists and Distributors (AIOCD) Pharmasofttech, AWACS Pvt. Ltd. 2014. Available at http://www.aiocdawacs.com/ProductDetail.aspx (last accessed 21 November 2017).
- 27. Central Drugs Standard Control Organization (CDSCO) . FDCs approved 1961–2014. Available at http://cdsco.nic.in/writereaddata/Aprroved%20FDC%20list%20till%20november%202014.pdf (last accessed 21 November 2017).
- 28. Central Drugs Standard Control Organization (CDSCO) . SDF list of approved drugs CDSCO. 1971–1998. Available at http://www.cdsco.nic.in/writereaddata/1971‐98.pdf (last accessed 31 January 2018).
- 29. Central Drugs Standard Control Organization (CDSCO) . List of approved new drug. Available at http://www.cdsco.nic.in/forms/list.aspx?lid=2034&Id=11 (last accessed 31 January 2017).
- 30. Medicines and Healthcare Products Regulatory Agency . Medicines information: SPC & PILs. Available at http://www.mhra.gov.uk/Safetyinformation/Medicinesinformation/SPCandPILs/index.htm (last accessed 21 November 2017).
- 31. European Medicines Agency . European public assessment reports. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 (last accessed 21 November 2017).
- 32. US Food and Drug Administration . Orange Book: approved drug products with therapeutic equivalence evaluations. Available at https://www.accessdata.fda.gov/scripts/cder/ob/default.cfm (last accessed 21 November 2017).
- 33. Tacconelli E, Cataldo MA, Paul M, Kluytmans J, Schroeder W, Foschi F, et al STROBE‐AMS: recommendations to optimise reporting of epidemiological studies on antimicrobial resistance and informing improvement in antimicrobial stewardship. BMJ Open 2016; 6: e010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NHS Digital. Prescription cost analysis England . 2012. Available at http://www.hscic.gov.uk/catalogue/PUB10610 (last accessed 21 November 2017).
- 35. Abbott Healthcare, India . Therapy areas. Available at http://www.abbott.in/products/therapy‐areas.html (last accessed 31 January 2018).
- 36. Mahr K, Siddiqui Z. As combination drugs engulf India, an American pharmaceutical giant profits. Reuters, 15 December 2015. Available at http://www.reuters.com/investigates/special‐report/india‐medicine‐abbott/ (last accessed 21 November 2017).
- 37. Lane EJ. India's penchant for fixed‐dose drugs a big problem. Fierce Pharma, 15 December 2015. Available at http://www.fiercepharma.com/regulatory/india‐s‐penchant‐for‐fixed‐dose‐drugs‐a‐big‐problem (last accessed 21 November 2017).
- 38. Declaration by the pharmaceutical, biotechnology and diagnostics industries on combating antimicrobial resistance, 2016. Available at http://amr‐review.org/sites/default/files/Industry_Declaration_on_Combating_Antimicrobial_Resistance_UPDATED%20SIGNATORIES_MAY_2016.pdf (last accessed 21 November 2017).
- 39. Kokate CK. Report of expert committee on fixed dose combinations (FDCs) licensed by SLAs for manufacture without approval of DCG(I), applications of which have been received by CDSCO. Available at http://www.cdsco.nic.in/writereaddata/fdcexpert%2019.1.2016.pdf (last accessed 21 November 2017).
- 40. Kokate CK. Report of expert committee on applications of fixed dose combinations (FDCs) received by CDSCO for proving safety and efficacy categorised under category ‘a’. Available at http://www.cdsco.nic.in/writereaddata/fdc16.04.2015.pdf (last accessed 21 November 2017).
- 41. Government of India , Ministry of Health and Family Welfare . The Gazette of India. No 608. New Delhi. 10th March 2016. Available at http://www.cdsco.nic.in/writereaddata/GSR705E.pdf (last accessed 31 January 2018).
- 42. Jain A. Delhi High Court reverses ban on combination drugs. The Hindu, 2 December 2016. Available at http://www.thehindu.com/news/national/article16735149.ece. (last accessed 21 November 2017)
- 43. Rautray S, Raghavan P. Government need not consult statutory board to ban combo drugs: Supreme Court. Economic Times Healthworld. Available at https://health.economictimes.indiatimes.com/news/policy/government‐need‐not‐consult‐statutory‐board‐to‐ban‐combo‐drugs‐supreme‐court/61664489 (last accessed 6 December 2017).
- 44. Press Information Bureau. Government of India, 22 June 2016 Available at http://pib.nic.in/newsite/erelease.aspx?relid=146413 (last accessed 21 November 2017).
- 45. Holloway KA, Rosella L, Henry D. The impact of WHO essential medicines policies on inappropriate use of antibiotics. PLoS ONE 2016; 11: e0152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Government of India, Ministry of Health and Family Welfare . National action plan on antimicrobial resistance 2017–2021. Available at http://cseindia.org/userfiles/inap_amr_20170420.pdf (last accessed 21 November 2017).
- 47. Dar OA, Hasan R, Schlundt J, Harbarth S, Caleo G, Dar FK, et al Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet 2016; 387: 285–295. [DOI] [PubMed] [Google Scholar]
- 48. Ardal C, Outterson K, Hoffman SJ, Ghafur A, Sharland M, Ranganathan N, et al International cooperation to improve access to and sustain effectiveness of antimicrobials. Lancet 2016; 387: 296–307. [DOI] [PubMed] [Google Scholar]
- 49. IMS Health Midas Pharmaceutical Sales Data. Available at http://www.imshealth.com/en/solution‐areas/technology‐and‐applications/offerings/midas (last accessed 21 November 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Systemic Antibiotic Approvals and Sales India Data


