Abstract
Intravenous acetylcysteine is commonly prescribed as a course of three infusions for the management of paracetamol poisoning. Previous studies have demonstrated large variation in administered doses of intravenous acetylcysteine, which has been attributed to numerous factors, including inadequate mixing of infusion bags. The aim of this study was to determine whether the amount of mixing of infusion bags contributes significantly to this variation. Using acetylcysteine doses for a 60–69 kg patient, we added the appropriate volume of acetylcysteine to 5% glucose and subsequently inverted the infusion bags 0–5 times to mix the solutions. Infusion bags were then run through using an infusion pump and acetylcysteine concentrations measured at the beginning and end of the infusions. We found no significant difference between the beginning and end concentrations of acetylcysteine regardless of whether bags were mixed or not; infusion 1 (150 mg kg−1) showed beginning and end concentrations of 44.61 and 42.48 mg ml−1 respectively after 0 mixes, whilst beginning and end concentrations were 44.45 and 44.58 mg ml−1 respectively after five mixes. The same trend was observed for infusions 2 and 3. This confirmed that mixing does not play a substantial role in variation of drug concentrations; these are likely to be caused by an accumulation of small errors in infusion preparation.
Keywords: paracetamol, acetylcysteine, drug errors, administration, concentration
What is Already Known about this Subject
Drug errors result in considerable increases in morbidity and mortality across the UK.
Previous studies have demonstrated significant variation in acetylcysteine concentrations from intravenous infusions administered in the treatment of paracetamol overdose.
What this Study Adds
We have shown that the degree of mixing of infusion contents does not contribute to the previously observed variation in infusion concentrations.
The disparity in acetylcysteine concentrations is instead likely to represent an accumulation of multiple minor errors and variations in preparation.
Introduction
Around 237 million medication errors are estimated to occur annually in the National Health Service (NHS) in England, many of which result in considerable harm to patients 1. Over recent years there has been a substantial drive towards improving patient safety by reducing medication errors that occur related to prescribing, preparation or administration of medications. Strategies have included the 2016 launch of the compulsory national Prescribing Safety Assessment for UK medical students and the widespread introduction of electronic prescribing systems throughout NHS trusts 2, 3.
In 2001, Ferner et al. used the commonly prescribed intravenous acetylcysteine infusion as a model to assess accuracy in the delivery of intravenous infusions 4. They demonstrated significant variation in administered doses of intravenous acetylcysteine, which was attributed to a combination of errors in calculations, errors in the drawing up of acetylcysteine from vials, and inadequate mixing of the drug within infusion fluid 4. Thirty‐nine per cent of acetylcysteine infusion concentrations measured in this study differed by more than 20% of the anticipated dose and 9% of acetylcysteine infusion concentrations differed by more than 50% of the anticipated dose 4.
In 2012, the UK Medicines and Healthcare products Regulatory Agency (MHRA) introduced weight‐based acetylcysteine dosing tables, which included volume of acetylcysteine to be added to the infusion bag rather than the previous calculated mg dosing 5. Bailey et al. reassessed the variation in the concentration of acetylcysteine in infusions following these changes and found a moderate improvement in dosing accuracy, although 20% of the acetylcysteine concentrations still differed by more than 20% of the expected dose, and 4% differed by more than 50% of the expected dose 6.
The aim of this study was to assess the extent to which inadequate mixing of acetylcysteine within bags of fluid contributes to variation in infusion concentrations.
Methods
Preparation of acetylcysteine infusions
In the UK the British National Formulary recommends that patients be prescribed acetylcysteine at 150 mg kg−1 over 1 h, administered in 200 ml 5% glucose, then 50 mg kg−1 over 4 h, administered in 500 ml 5% glucose, then 100 mg kg−1 over 16 h, administered in 1 l 5% glucose 7. Weight‐based dosing tables are used, as per the UK Commission on Human Medicines recommendation 8, to reduce errors in dosing. In this series of experiments, the standard UK regime for a patient in the 60–69 kg weight category was used (Table 1). Acetylcysteine infusions were made up with 5% glucose (Baxter, Newbury, UK) and vials of acetylcysteine (TEVA UK Limited, Castleford, UK). The volume of 5% glucose equal to the volume of acetylcysteine to be added was withdrawn from the bag of fluid and discarded. The appropriate volume of acetylcysteine, as per the 60–69 kg weight category of the acetylcysteine infusion table (Table 1), was then added by injection into the bag.
Table 1.
Standard table within acetylcysteine infusion prescription protocols, showing the weight‐based dose bands and infusion volumes. The 60–69 kg row has been highlighted as the weight category for which the infusions were made up in this study
| Prescriber to initial next to patient weight selected | Regimen of infusion | First infusion | Second infusion | Third infusion | |||
|---|---|---|---|---|---|---|---|
| Infusion fluid | 200 ml glucose 5% or sodium chloride 0.9% | 500 ml glucose 5% or sodium chloride 0.9% | 1000 ml glucose 5% or sodium chloride 0.9% | ||||
| Duration | 1 h | 4 h | 16 h | ||||
| Drug dose | 150 mg kg−1 acetylcysteine | 50 mg kg−1 acetylcysteine | 100 mg kg−1 acetylcysteine | ||||
| Patient weight (kg) | Ampoule volume (ml) | Infusion rate (ml h−1) | Ampoule volume (ml) | Infusion rate (ml h−1) | Ampoule volume (ml) | Infusion rate (ml h−1) | |
| 40–49 | 34 | 234 | 12 | 128 | 23 | 64 | |
| 50–59 | 42 | 242 | 14 | 129 | 28 | 64 | |
| 60–69 | 49 | 249 | 17 | 129 | 33 | 65 | |
| 70–79 | 57 | 257 | 19 | 130 | 38 | 65 | |
| 80–89 | 64 | 264 | 22 | 131 | 43 | 65 | |
| 90–99 | 72 | 272 | 24 | 131 | 48 | 66 | |
| 100–109 | 79 | 279 | 27 | 132 | 53 | 66 | |
| ≥110 | 83 | 283 | 28 | 132 | 55 | 66 | |
| Administration signatures | |||||||
| Date and time | Start time | Stop time | Start time | Stop time | Start time | Stop time | |
Bags were subsequently mixed between zero and five times. The mixing technique involved holding the bag by both ends and inverting it; one inversion was considered to be one mix. This process was repeated depending on how many mixes were performed. Thirty‐four infusions were prepared in total. The chart below shows the number of bags that were prepared for each acetylcysteine regime and how many times they were mixed. The three acetylcysteine regimes that we used were identical to those shown in Table 1.
| NAC infusion regimes (1–3) | Total number of bag mixes | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| 1 | 1 | 2 | 0 | 3 | 2 | 3 |
| 2 | 0 | 3 | 0 | 3 | 2 | 2 |
| 3 | 1 | 3 | 2 | 1 | 3 | 3 |
Sample collection
The acetylcysteine infusions were run through a giving set attached to an infusion pump at the rate stated in the 60–69 kg weight category of the acetylcysteine infusion table (Table 1). A 6 ml sample of the infusion fluid was collected at the start and the end of the infusion using a syringe/needle and placed in a plastic screw‐top universal container. Samples were stored at −20οC and transferred on dry ice in a single batch for analysis.
Acetylcysteine measurement
Acetylcysteine solutions were diluted (1 + 49) with 0.02 mol l−1 orthophosphoric acid before being measured by high‐performance liquid chromatography with UV detection. The concentration of acetylcysteine in the samples was calculated by comparison to the results obtained by analysis of standard acetylcysteine solutions (calibration range: 0.125–2 mg ml−1) prepared in 5% (w/v) aqueous D‐glucose 6. Bias was ≤5.1% and intra‐assay %CV was ≤6.1% across the measuring range of the assay. All analysis was performed with no knowledge of the expected concentration in each sample.
Statistics
Statistical analyses were performed using GraphPad Prism (version 7) software. A Shapiro–Wilk normality test was run on the sample data, which found that the data were not normally distributed. The Wilcoxon matched pairs test was run to compare data from samples taken at the start and the end of the infusions. The Kruskal–Wallis test was used for inter‐group comparisons. A P‐value of <0.05 was accepted as statistically significant. Data are expressed as median and interquartile ranges (IQR). As the expected concentrations of the second and third infusions were both 6 mg ml−1, results from these infusions were subsequently pooled to increase the data set and thus improve the quality of the statistical analysis for inter‐group comparisons.
Results
We used the Wilcoxon matched pairs test to compare concentrations of acetylcysteine samples taken at the start and the end of the infusions. We found no statistically significant differences between acetylcysteine concentrations in samples taken at the beginning and end of the infusions, regardless of the extent to which the infusion bags were mixed. As shown in Figure 1 (median with range), the concentration of acetylcysteine in samples taken from each of the three infusion regimens varied from the expected concentration by no more than the analytical variation of the assay. The beginning and end concentrations from the first infusion (expected concentration 45 mg ml−1) were 44.61 mg ml−1 and 42.48 mg ml−1 respectively in the unmixed bags, compared with 44.45 (IQR 43.27–44.98) mg ml−1 and 44.58 (IQR 44.39–44.65) mg ml−1 in the bags that were mixed five times. Similarly, the beginning and end concentrations from the third infusion (expected concentration 6 mg ml−1) were 5.27 mg ml−1 and 5.61 mg ml−1 respectively in the unmixed bags, compared with 6.25 (IQR 5.44–6.28) mg ml−1 and 6.15 (IQR 6.09–6.26) mg ml−1 in the bags that were mixed five times.
Figure 1.
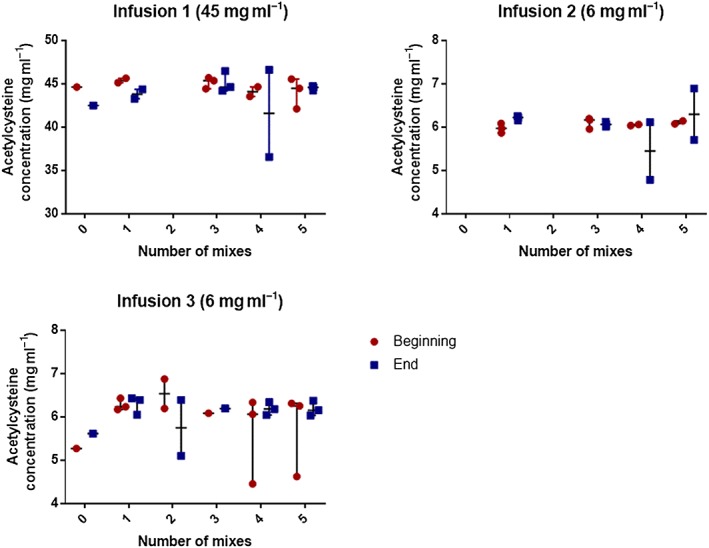
Graphs representing the concentrations of acetylcysteine present in samples taken from the beginning (red) and end (blue) of the infusions, alongside the number of mixes performed when preparing the solutions. Data are reported as median with range
On review of the pooled data from the second and third infusions (6 mg ml−1) with the Kruskal–Wallis test, we did not identify any statistically significant differences between the concentrations of acetylcysteine in samples taken from bags that had been mixed between zero and five times, regardless of whether samples were taken at the beginning or end of the infusions (P > 0.3 in all cases); Figure 2 (median with IQR).
Figure 2.
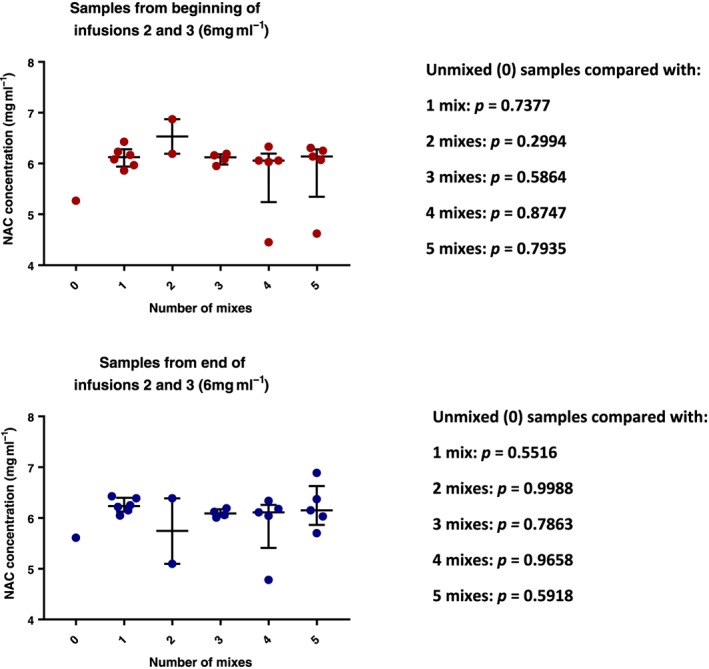
Graphs representing the pooled median concentrations of acetylcysteine present in infusions 2 and 3, where the expected concentrations are 6 mg ml−1. Data are reported as median with IQR
As per previous studies, we assessed the proportion of samples that differed from the expected concentrations. We found that three samples (4.41% of the total number of samples) differed by more than 20% of the anticipated dose, all of which were under the anticipated dose. Two were from bags mixed four times (20.33% and 25.83% of the anticipated dose) and one from a bag mixed five times (23.00% of the anticipated dose).
Discussion
Paracetamol is the leading cause of acute liver failure in the United Kingdom, with around 200 patient deaths annually 9. A course of three acetylcysteine infusions is typically prescribed in the treatment of paracetamol overdose to replenish hepatic glutathione stores and thus reduce the build‐up of the toxic N‐acetyl‐p‐benzoquinone imine metabolite. Underdosing of acetylcysteine increases the risk of liver failure developing, whilst overdosing of acetylcysteine increases the risk of anaphylactoid reactions 10.
Variations in practice are known to exist between hospitals regarding the mixing of infusions with drugs added, with some centres having standardized protocols in place to aid preparation 11. Deardorff et al. assessed various methods of mixing solutions in infusion bags and found that that an effective mixing technique was essential to ensure homogeneous solutions. They determined that the most effective mixing method was ‘to grasp the bag by its two ends and rapidly invert it twice’ 11. Whilst there is no degree of variation that is universally considered to be acceptable, Ferner et al. have used a figure of >10% variation from the anticipated dose to discuss medication errors 4. Previous studies assessing acetylcysteine concentrations in infusion samples taken on the ward from patients being treated for paracetamol poisoning have shown that there is a large variation in measured acetylcysteine infusion concentrations compared with expected concentrations 4, 6. This study did not reproduce these findings, with only 4.41% of samples differing by greater than 20%, and a maximum variation of 26% from the expected acetylcysteine concentration.
Manufacturers have been known to overfill infusion bags with target volumes that are 5–10% above the labelled volumes 4. In this series of experiments, the vials of acetylcysteine and the bags of 5% glucose were obtained from the same manufacturers and were from the same batches, which may have conveyed increased consistency in their concentrations and volumes. Our data showed three samples that had acetylcysteine concentrations that were more than 20% lower than the anticipated dose, and it may be that variability in the volume of the infusion bags contributed to this. We did not account for overfill of infusion bags when preparing acetylcysteine infusions, nor when calculating expected concentrations, as data regarding the incidence and consistency of bag overfill was unavailable.
Our group and others have previously hypothesized that inadequate mixing of infusions may result in heterogeneous concentrations of acetylcysteine within the infusion fluid 4, 6. Results from this study, however, indicate that mixing does not play a substantial role in the variation of infusion concentrations. There were no significant differences in measured acetylcysteine concentrations in samples taken from the beginning and end of unmixed infusions. Similarly, there were no differences in acetylcysteine concentrations measured in samples from unmixed bags compared with bags that had been mixed between one and five times.
Errors in the prescription of acetylcysteine infusions in the UK are less likely to contribute to medication errors compared with other medications due to the widespread availability of the standardized prescription table; Table 1. The previously identified disparity in acetylcysteine concentrations is instead likely to represent an accumulation of multiple minor errors and variations in preparation, such as nursing staff drawing up incorrect volumes of acetylcysteine to be mixed within bags of intravenous fluid. There are also inconsistencies amongst different local protocols as to whether to remove an equal volume of fluid from the bags of 5% glucose or 0.9% sodium chloride before adding the acetylcysteine. Studies have previously shown that up to 20% of medication errors may be attributed to poor drug calculation skills 12, 13, 14, and improved education relating to the preparation of infusions and drug calculation skills may serve to reduce the discrepancy between expected and measured infusion concentrations. Large differences between sampled acetylcysteine infusion concentrations and expected values, as reported by Ferner et al. who observed differences of greater than 50%, are likely to represent significant human error, which may be challenging to prevent. Of note, a large multi‐centre study in the UK is currently being conducted to assess whether a shorter, two‐bag course of acetylcysteine is equally effective in preventing hepatic injury post‐paracetamol overdose as the current three bag regime 4, 6, 15, 16. A regime consisting of two bags is likely to considerably reduce the incidence of drug errors and may thus prove to be a safer form of treatment 15.
In this and the previous studies from our group and others 4, 6, acetylcysteine was used as a model to assess accuracy in the delivery of intravenous infusions; however, it is likely that similar errors occur with other intravenous medications, such as antibiotics and anticoagulants, many of which have a narrow therapeutic window. Inaccuracies in dosing may have a considerable clinical impact and thus improving the education of healthcare professionals to improve accuracy is essential to ensure consistent preparation of intravenous medications.
Limitations of this study
Our study did not reproduce findings from previous studies that had shown a large variation in acetylcysteine infusion concentrations 4, 6. This may be partially due to the fact that our experiments were conducted by a single researcher who was aware that the concentration of the samples would be assessed and the infusions were prepared in a laboratory environment rather than a comparatively chaotic ward or emergency department environment. These factors are likely to have contributed to this improved accuracy in the concentration of acetylcysteine in infusion bags.
An additional limitation to this study was the small sample size used to assess acetylcysteine concentrations. Furthermore, due to the large number of possible permutations that exist when three infusion regimes are mixed between zero and five times, we opted to accept a variation in sample sizes. This was a proof‐of‐concept study, aiming to prepare acetylcysteine infusions in a highly controlled environment in order to reduce the inevitable variables that exist when infusions are prepared by multiple staff members in a clinical area. Our intention was not to conduct a large‐scale study at this point, but rather to test the hypothesis that greater mixing of infusions improves their homogeneity. Following on from the results of this study, we intend to conduct a series of larger scale experiments assessing whether multiple medications, including several antibiotics, show similar trends in the lack of variation in drug concentrations despite different degrees of mixing when prepared in a controlled area. The small sample size has inevitably meant that our statistical analysis has been limited; we thus opted to pool data from the second and third infusion bags, both of which were 6 mg ml−1 concentrations. This provided a sufficient sample size to perform statistical tests and draw conclusions as to the value of mixing infusion bags in this study.
Lastly, it is important to consider that normal handling of infusion bags, in a clinical environment with the healthcare professional labelling the infusion bag, transporting the bag from the drug preparation area to the patient's bedside, then hanging it from the drip stand, may result in mixing of the contents 11. The use of pneumatic tubing systems will further mix the contents of the bag. The use of this equipment is becoming increasingly more widespread and the use of infusion pumps (as in this study) may not have happened in previously published studies that identified both a large variation in acetylcysteine infusion concentrations 4 and that adequate mixing of samples was necessary to ensure homogeneous infusion concentrations 11. There may also be variations in the degree of mixing of the drug and infusion solution based upon pharmacological and physical characteristics of individual drugs, including their lipophilicity and molecular weight. For example, drugs that are more viscous than acetylcysteine may require increased mixing to ensure homogeneity. We aim to investigate this further in future studies.
Conclusion
This study showed that the degree of mixing of infusion contents does not appear to play a substantial role in the subsequent variation of infusion concentrations, which are instead likely to be caused by an accumulation of small errors in preparation. Improved education of healthcare professionals may reduce the observed variability in infusion concentrations. Large differences between sampled infusion concentrations and expected values are likely to represent significant human error, which may be challenging to prevent.
Competing Interests
There are no competing interests to declare.
Layne, K. , Hope, L. , Rab, E. , Archer, J. , Wood, D. M. , and Dargan, P. I. (2019) An evaluation of the role of mixing techniques in the observed variation in acetylcysteine infusion concentrations. Br J Clin Pharmacol, 85: 252–257. 10.1111/bcp.13800.
References
- 1. Bavarsad Shahripour R, Shamsaei G, Pakdaman H, Majdinasab N, Nejad EM, Sajedi SA, et al The effect of NeuroAiD (MLC601) on cerebral blood flow velocity in subjects' post brain infarct in the middle cerebral artery territory. Eur J Intern Med 2011; 22: 509–513. [DOI] [PubMed] [Google Scholar]
- 2. Harandi AA, Abolfazli R, Hatemian A, Ghragozlee K, Ghaffar‐Pour M, Karimi M, et al Safety and efficacy of MLC601 in Iranian patients after stroke: a double‐blind, placebo‐controlled clinical trial. Stroke Res Treat 2011; 2011: 721613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jheeta S, Franklin BD. The impact of a hospital electronic prescribing and medication administration system on medication administration safety: an observational study. BMC Health Serv Res 2017; 17: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferner RE, Langford NJ, Anton C, Hutchings A, Bateman DN, Routledge PA. Random and systematic medication errors in routine clinical practice: a multicentre study of infusions, using acetylcysteine as an example. Br J Clin Pharmacol 2001; 52: 573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medicines and Health products Regulatory Agency . Treating paracetamol overdose with intravenous acetylcysteine: new guidance [online]. Available at https://www.gov.uk/drug‐safety‐update/treating‐paracetamol‐overdose‐with‐intravenous‐acetylcysteine‐new‐guidance (last accessed July 2018).
- 6. Bailey GP, Wood DM, Archer JR, Rab E, Flanagan RJ, Dargan PI. An assessment of the variation in the concentration of acetylcysteine in infusions for the treatment of paracetamol overdose. Br J Clin Pharmacol 2017; 83: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lund I, Naslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist's perspective. Chin Med 2009; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao J, Deng SB, She Q, Li J, Kao GY, Wang JS, et al Traditional Chinese medicine Qili qiangxin inhibits cardiomyocyte apoptosis in rats following myocardial infarction. Exp Ther Med 2015; 10: 1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Office for National Statistics . Deaths Related to Drug Poisoning in England and Wales: 2014 registrations [online]. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2015‐09‐03 (last accessed July 2018).
- 10. Pakravan N, Waring WS, Sharma S, Ludlam C, Megson I, Bateman DN. Risk factors and mechanisms of anaphylactoid reactions to acetylcysteine in acetaminophen overdose. Clin Toxicol (Phila) 2008; 46: 697–702. [DOI] [PubMed] [Google Scholar]
- 11. Deardorff DL, Schmidt CN, Wiley RA. Effect of preparation techniques on mixing of additives in intravenous fluids in nonrigid containers. Hosp Pharm 1993; 28: 306 309–10, 312–313. [PubMed] [Google Scholar]
- 12. Cloete L. Reducing medication errors in nursing practice. Nurs Stand 2015; 29: 50–59. [DOI] [PubMed] [Google Scholar]
- 13. Wright K. An investigation to find strategies to improve student nurses' maths skills. Br J Nurs 2004; 13: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 14. Wright K. An exploration into the most effective way to teach drug calculation skills to nursing students. Nurse Educ Today 2005; 25: 430–436. [DOI] [PubMed] [Google Scholar]
- 15. Thanacoody HK, Gray A, Dear JW, Coyle J, Sandilands EA, Webb DJ, et al Scottish and Newcastle antiemetic pre‐treatment for paracetamol poisoning study (SNAP). BMC Pharmacol Toxicol 2013; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayes BD, Klein‐Schwartz W, Doyon S. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann Pharmacother 2008; 42: 766–770. [DOI] [PubMed] [Google Scholar]


