Abstract
Vitamin D deficiency and rickets are more common in non-western immigrants and refugees than in the native population. Severe vitamin D deficiency (serum 25-hydroxyvitamin D <25 nmol/l) may occur in up to 50% of children and adults of non-western origin. They are not used to sunshine exposure due to the often excessive sunshine in the country of origin. They usually have a more pigmented skin. Non-western immigrants and refugees often wear skin-covering clothes due to religious or cultural tradition. The food contains little vitamin D with the exception of fatty fish. In addition, many immigrants have a low calcium intake. Complaints may include fatigue, pain in shoulders, ribs, lower back and thighs. Neonates and young children may have spasms and convulsions due to hypocalcemia. Older children and adolescents may have bone pain, muscle weakness and skeletal deformities. Widening of the wrist, chest deformities and bowing of the legs may occur, and longitudinal growth is delayed. In adults, muscle weakness and bone pain are predominant. Laboratory examination may show hypocalcemia and hypophosphatemia and elevated alkaline phosphatase. The serum 25(OH)D is below 25 nmol/l in case of severe vitamin D deficiency with symptoms. Impaired 25-hydroxylation or 1α-hydroxylation may occur in case of severe liver or renal disease or by genetic causes. Radiographs of wrists or knees may show widening of the growth plates and cupping of radius and ulna may confirm the diagnosis. In adolescents and adults, radiographs of painful bones may show pseudofractures or Looser zones. Rickets and osteomalacia are treated by vitamin D3 2000 IU/d in infants, 3000–6000 IU/d in older children in combination with calcium 500 mg /d. In osteomalacia, the adult vitamin D3 dose is 2000–3000 IU/d, combined with calcium 1000–2000 mg/d. Prevention of vitamin D deficiency can be done with vitamin D3 400–800 IU/d, depending on age. Nutritional measures include fortification of milk or other foods.
Keywords: Vitamin D deficiency, Rickets, Osteomalacia, Immigrants, Prevention
Highlights
-
•
Severe vitamin D deficiency is common in non-western immigrants.
-
•
This is often due to low sunshine exposure, pigmented skin, low calcium intake.
-
•
Vitamin D deficiency in small children may cause spasms, muscle weakness, bone pain and deformities.
-
•
The clinical picture of osteomalacia is characterized by pain and muscle weakness.
-
•
Prevention of vitamin D deficiency is feasible with vitamin D3 400–800 IU/d depending on age.
1. Introduction
Vitamin D deficiency is common in non-western immigrants and refugees. In this review, the specific features of vitamin D deficiency in this risk group will be discussed, including causes, consequences, prevention and treatment.
2. Case history
A Dutch-Moroccan woman of 29 years old comes to the clinic with complaints of pain with walking for 3 months, which started in December. The pain around her pelvic girdle and upper legs is increasing, and stair climbing becomes very difficult. In addition, she has pain around her shoulders. She has one child, now 6 months old. She wears a veil, when outside, and avoids sunshine in the middle of the day. She does not consume dairy products except for some yoghurt once or twice a week. Her child receives vitamin D drops after visiting a children's clinic.
Clinical examination reveals a healthy obese woman with weak upper leg muscles. She cannot rise from a chair without using her hands.
On laboratory examination, the serum calcium in 1.96 mmol/l, phosphate 0.68 mmol/l, albumin 39 g/l, creatinine 85 μmol/l, alkaline phosphatase 175 U/l (ref limit <120 IU/l). The serum 25-hydroxyvitamin D (25(OH)D) is below 5 nmol/l, the detection limit.
The patient is treated with vitamin D3 800 IU/d, and when the serum 25(OH)D value becomes available, this is increased to 2000 IU/d and calcium 500 mg 3 tablets daily are added. The pain resolves within a week and muscle strength increases within weeks. After a month, stair climbing is no problem anymore. After 6 weeks, serum 25(OH)D is 54 nmol/l and the vitamin D dose is reduced to 800 IU/d.
3. Epidemiology of vitamin D deficiency in immigrants and refugees
The first reports on vitamin D deficiency in immigrants came from the United Kingdom. Vitamin D deficiency was very common in immigrants coming from Pakistan to the UK (Preece et al., 1973). Serum 25(OH)D was lower than 25 nmol/l in all 29 patients studied. In 19 of them alkaline phosphatase concentrations were elevated, and 5 of these had symptomatic rickets or osteomalacia. In the Netherlands, vitamin D deficiency was frequent in 8 year old Turkish and Moroccan children, with 42% of Turkish children in The Hague having a serum 25(OH)D < 20 nmol/l (Meulmeester et al., 1990). Decreased seasonal variation of serum 25(OH)D and high risk of osteomalacia was reported in Asians in London, with histological osteomalacia mainly in vegetarian Hindus (Finch et al., 1992). A similar observation was made a few years later (Solanki et al., 1995). Low serum 25(OH)D concentrations were also found in 2 year old Asian children living in England (Lawson and Thomas, 1999). The Oslo Immigrant Health Study showed a high prevalence of vitamin D deficiency in 5 groups of immigrants in Oslo (Holvik et al., 2005). Low serum 25(OH)D was observed in Turkish immigrants in Germany with a mean value of 38.1 nmol/l, women being more deficient than men, while German people had a mean serum 25(OH)D of 68.4 nmol/l (Erkal et al., 2006). A similar picture was found in immigrant mothers from Pakistan, Turkey and Somalia and their infants attending health clinics in Norway (Madar et al., 2009). A review of all rickets cases in Southern Denmark between 1985 and 2005 revealed 29 ethnic Danish cases and 83 cases among immigrants (Beck-Nielsen et al., 2009). Severe vitamin D deficiency (25(OH)D < 25 nmol/l) was also observed in >50% of pregnant non-western immigrant women in the Hague, the Netherlands (van der Meer et al., 2006). In a study from family practices in four large cities (The Hague, Haarlem, Amsterdam and Amersfoort), vitamin D deficiency was very common in all ethnic subgroups (van der Meer et al., 2008). Severe vitamin D deficiency, defined as a serum 25(OH)D lower than 25 nmol/l, was observed in 41% of Turkish adults, 36% of Moroccan, 51% of Surinam South Asian, 45% of Surinam Creole and 19% of Sub-Saharan African adults, compared to 6% of indigenous Dutch adults. Vitamin D status was very low in Pakistani immigrant children and adults in Denmark with a mean serum 25(OH)D of 10.9 nmol/l in girls, 12.0 in women and 20.7 nmol/l in men respectively (Andersen et al., 2008)
Similar observations were made in refugees asking asylum in Switzerland, all with serum 25(OH)D < 20 nmol/l (de Torrente dela et al., 2004). Vitamin D deficiency was common in children of refugees in the Netherlands with 55% of them having a serum 25(OH)D lower than 50 nmol/l (Stellinga-Boelen et al., 2007). Sub-Sahara immigrants and refugees older than 20 years living in Melbourne had a mean serum 25(OH)D level of 27.3 nmol/l, with vitamin D deficiency (serum 25(OH)D < 50 nmol/l) in 88% (Renzaho et al., 2011). In Calgary, Canada, 1217 refugee women and children were screened on vitamin D status. While mean serum 25(OH)D was 52 nmol/l, levels lower than 50 nmol/l were found in 61% of women and 42% of children (Aucoin et al., 2013). Immigrants, including refugees/asylum seekers, from Africa, the Middle East and Asia arriving in Norway, often were vitamin D deficient (serum 25(OH)D < 50 nmol/l) with a prevalence of vitamin D deficiency of 81% in those from the Middle East, 73% from Sub-Sahara Africa and 75% in those from South Asia, while the prevalence only was 24% in East Asians (Eggemoen et al., 2013). The prevalence of vitamin D deficiency (serum 25(OH)D < 50 nmol/l) in 386 North Korean refugees in South Korea was 87% (Kim et al., 2015).
4. Specific factors contributing to vitamin D deficiency in immigrants
Non-western immigrants and refugees often come from countries closer to the equator than the host country is situated. The higher northern or southern latitude means less effective sunshine (lower ultraviolet exposure) during the day for vitamin D production and absent vitamin D synthesis during the winter months. In addition, their skin is usually more pigmented, decreasing the effectivity of the ultraviolet light of the sun (Table 1). Premigration latitude is an important factor for vitamin D deficiency in immigrants (Ruwanpathirana et al., 2014). The longer the residence of the immigrant in the host country, the higher the risk of having a vitamin D deficiency (Martin et al., 2016). Cultural or religious habits often lead to skin-covering clothing style, further decreasing the potential for cutaneous vitamin D production and a 5 times higher risk of vitamin D deficiency (Erkal et al., 2006) (Table 1). Dietary vitamin D intake might compensate for this, but only fatty fish contains a substantial amount of vitamin D. The effects of vitamin D deficiency may be modified by calcium intake. However, dietary calcium intake often is low in non-western immigrants due to a low dairy intake and/or low absorption of calcium due to other dietary constituents such as chapatti (Preece et al., 1973). The consumption of vitamin D supplements is low in immigrants. Vitamin D status was lower in Turkish, Moroccan, Indian and sub-Sahara African populations in Europe than in their country of origin (van der Meer et al., 2011).
Table 1.
Risk factors for vitamin D deficiency in immigrants.
| Less UBV radiation exposure than in home country |
| Dark skin pigmentation |
| Premigration latitude |
| Duration of residence in host country |
| Cultural habits such as wearing a veil |
| Different dietary habits |
| Low intake of vitamin D supplements |
| Low calcium intake |
5. Consequences of severe vitamin D deficiency in immigrants
Musculo-skeletal pain, localized in shoulders, ribs, lower back and thighs, and fatigue were reported in 11 female asylum seekers in Lausanne, Switzerland (de Torrente dela et al., 2004). Non-specific musculo-skeletal pain was reported in immigrants by general practitioners in the Netherlands(Schreuder et al., 2012). A survey in Gujarati people (coming from the state of Gujarat in western India) in the UK showed a lower serum 25(OH)D concentration and a lower BMD compared to white people, 60% of the Gujarati men and 50% of the women having a serum 25(OH)D lower than the detection limit of the assay (12.5 nmol/l). BMD in the lumbar spine and in the hip was about 15% lower in Gujarati females than in white females. The difference between both groups of men was less and not significant (Hamson et al., 2003). Serum 25(OH)D and forearm bone mineral density were lower in Bangladeshi and Somali immigrant women than in Finnish women and both correlated well with each other (Islam et al., 2012).
6. Clinical picture
The clinical picture varies according to the age of the patient. In neonates and small children, symptoms of hypocalcemia may prevail, such as spasms of hands and feet and convulsions. These can be provoked by a blood pressure measurement, i.e. Trousseau's sign. In children above 6 months, bone and muscle symptoms occur, such as muscle weakness, bone pain and deformities. Painful widening of the wrist, chest deformities (rachitic rosary) and bowing of the legs occur, the classical picture of rickets. Longitudinal growth is delayed in older children. Clinical signs and symptoms may be less in older children (Beck-Nielsen et al., 2009). In adolescents and adults, muscle weakness and bone pain are the presenting symptoms of clinical vitamin D deficiency and osteomalacia. The myopathy is localized around shoulder and pelvic girdle. Standing up from a chair, walking and stair climbing can be very problematic.
7. Laboratory examination
Symptomatic vitamin D deficiency is associated with hypocalcemia and hypophosphatemia or serum calcium and phosphate in the lower normal range. The alkaline phosphatase concentration is elevated. Usually measurement of the serum 25(OH)D concentration to confirm vitamin D deficiency takes a few days to weeks, but with clinical suspicion of vitamin D deficiency treatment with vitamin D and calcium should not be delayed. The serum 25(OH)D concentration usually is very low (< 15–20 nmol/l), unless a very low dietary calcium intake is the cause of the complaints. Serum PTH is elevated, but less so with a higher dietary calcium intake, than with a lower intake (Patel et al., 2016). For the diagnosis, other causes of increased alkaline phosphatase should be excluded, such as Paget's disease or bone metastasis. Liver disease can be excluded by other liver function tests within reference limits. Increased bone turnover can be established by measurement of other markers of bone turnover such as CTx and P1NP.
For the differential diagnosis, impaired 25-hydroxylation and 1α-hydroxylation by severe liver or renal disease, or by genetic causes, or a mutated non-functioning vitamin D receptor are rare causes of rickets and osteomalacia. Hypophosphatemic rickets or osteomalacia are characterized by a very low serum phosphate and a low tubular reabsorption of phosphate (TRP), leading to renal phosphate loss. The TRP and the renal phosphate threshold (TmP/GFR) can be calculated from a fasting 2-hour urine sample and a blood sample at the same time (Walton and Bijvoet, 1975). Other causes are severe calcium deficiency and intoxications by aluminum or cadmium.
8. Radiology
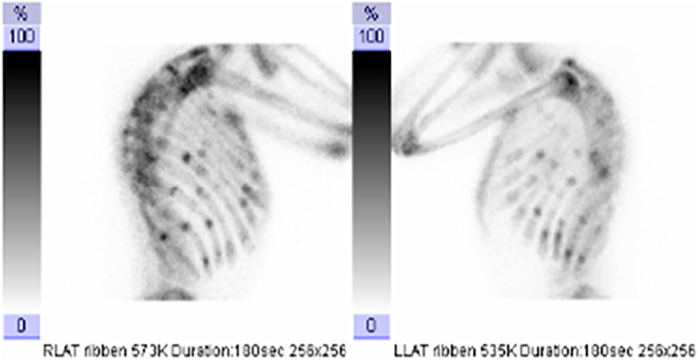
The diagnosis of rickets can be confirmed by radiographs of the wrists and/or knees, showing widening of the growth plate, and delayed mineralization shown as cupping of the radius and a low number of mineralization nuclei (Lips et al., 2013). In adolescents and adults, radiographs can be made of painful areas to show pseudofractures or fractures, e.g. of the ribs, pelvis and long bones. Pseudofractures or Looser zones are characterized by a radiolucent line through one cortex with sclerosis at the margins. Deformities of the legs may be either varus or valgus. Radiographs of the spine (Fig. 1) often show a milk-glass appearance, and are unsharp, due to the presence of non-mineralized bone (osteoid tissue). Bone scintigraphy may be done in adults to identify hot spots which may point to fractures and pseudofractures, as shown in Fig. 2 (Lips et al., 2013). However, imaging is not absolutely necessary to establish the diagnosis of osteomalacia due to vitamin D deficiency, which can be made on the base of the clinical picture and laboratory measurements. In case of severe osteomalacia, bone mineral density (BMD) can be very low, suggestive of severe osteoporosis. On appropriate treatment, the increase of BMD can be very fast and large, unlike changes in osteoporosis on treatment.
Fig. 1.

Spinal radiograph of a patient with osteomalacia. The vertebrae show a milk-glass appearance and unsharp edges, characteristics of osteomalacia.
Fig. 2.
Bone scintigraphy in a patient with osteomalacia showing multiple hot spots in the ribs, indicating fractures or pseudofractures.
9. Bone biopsy
The gold standard for the diagnosis of osteomalacia is the demonstration of an increased quantity of non-mineralized bone (osteoid tissue) in a bone biopsy (Lips et al., 2013). Transiliac bone biopsies are usually obtained after tetracycline double labeling. The biopsy should be included in methylmethacrylate without decalcification. The biopsy sections should be stained with Goldner's trichrome or von Kossa, and partly left unstained. The quantity of non-mineralized bone should be >5%, for a certain diagnosis >10% of bone tissue. Osteoid seams are thicker than 15 μm in typical cases. Unstained sections can be inspected in ultraviolet light for double tetracycline labels, which are absent in osteomalacia.
In a typical patient with nutritional osteomalacia, a bone biopsy often is not necessary. The patient of the case history had a typical clinical picture with pain and muscle weakness, low serum calcium and phosphate, elevated alkaline phosphatase, and very low serum 25-hydroxyitamin D, so that a bone biopsy was not needed. When the clinical picture and laboratory results are less clear and a different cause is suspected (e.g. hypophosphatemic rickets), a bone biopsy may be necessary to establish the diagnosis.
10. Prevention and treatment
In symptomatic vitamin D deficiency, especially when associated with rickets or osteomalacia, vitamin D deficiency is usually accompanied by a low calcium intake. Thus, treatment with calcium should almost always be combined with the vitamin D treatment. Rickets and osteomalacia can be healed with remarkably low doses of vitamin D. The Global Consensus on Nutritional Rickets recommends in infants in the first year of life vitamin D 2000 IU/d for 3 months, and 3000–6000 IU/d for older children (Munns et al., 2016). The oral calcium dose should be 500 mg/d at least, but higher doses may lead to faster improvement. Symptomatic hypocalcemia should be treated with intravenous calcium infusion until symptoms disappear.
Nutritional osteomalacia can even be healed with a low dose of vitamin D 800–1200 IU/d. Higher doses e.g. 2000–3000 IU/d are usually given to accelerate healing. Calcium should be given as a dose of 1000–2000 mg/d (calcium carbonate 2500–5000 mg/d). Higher doses may be necessary when intestinal calcium absorption is decreased e.g. in celiac disease.
Prevention of rickets and osteomalacia can be done with low doses of vitamin D 400–800 IU/d combined with a sufficient dietary calcium intake, usually by dairy products e.g. 500–1000 mg/d. Vitamin D can also be given in higher doses at larger intervals e.g. 25,000–50,000 IU/month. Even longer intervals have been tried. Vitamin D 150,000 IU in one dose or placebo were given to 84 non-western immigrants with non-specific musculo-skeletal pain (Schreuder et al., 2012). In a study in general practices in the Netherlands, 232 non-western immigrants were randomized to vitamin D3 800 IU/d, 100,000 IU/3 months or advice to increase sunlight exposure. The baseline serum 25(OH)D was 22.5 nmol/l, increasing to 53 nmol/l with 800 IU/d, to 50.5 nmol/l with 100,000 IU/3 months and to 29.1 nmol/l with sunlight advice (Wicherts et al., 2011).
11. Public health measures
Non-western immigrants are a special risk group for severe vitamin D deficiency. This applies even more to refugees, a vulnerable group, who can be malnourished and severely vitamin D deficient upon arrival (de Torrente dela et al., 2004).
In general, prevention of vitamin D deficiency in children <1 year is feasible with 400 IU/d. Older children and adults require 600 IU/d, while in older persons (>70 yr) 800 IU/d is recommended. To address risk groups such as non-western immigrants, other measures to prevent vitamin D deficiency may include a moderate increase of sun exposure, the consumption of vitamin D-rich food (fatty fish) and vitamin D-fortified foods. Vitamin D has been added to milk and other dairy products, to bread and flour, and to juices in some countries, but this is not yet common practice.
12. Conclusion
Non-western immigrants and refugees require special attention regarding the risk of severe vitamin D deficiency and symptomatic disease. General practitioners and family doctors should be aware of this. They should start vitamin D supplementation when suspicion of vitamin D deficiency is arising and blood samples have been obtained to confirm the diagnosis.
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Andersen R. Pakistani immigrant children and adults in Denmark have severely low vitamin D status. Eur. J. Clin. Nutr. 2008;62(5):625–634. doi: 10.1038/sj.ejcn.1602753. [DOI] [PubMed] [Google Scholar]
- Aucoin M. Vitamin D status of refugees arriving in Canada: findings from the Calgary refugee health program. Can. Fam. Physician. 2013;59(4):e188–e194. [PMC free article] [PubMed] [Google Scholar]
- Beck-Nielsen S.S. Nutritional rickets in Denmark: a retrospective review of children's medical records from 1985 to 2005. Eur. J. Pediatr. 2009;168(8):941–949. doi: 10.1007/s00431-008-0864-1. [DOI] [PubMed] [Google Scholar]
- van der Meer I.M. High prevalence of vitamin D deficiency in pregnant non-western women in The Hague, Netherlands. Am. J. Clin. Nutr. 2006;84(2):350–353. doi: 10.1093/ajcn/84.1.350. [DOI] [PubMed] [Google Scholar]
- van der Meer I.M. Fatty fish and supplements are the greatest modifiable contributors to the serum 25-hydroxyvitamin D concentration in a multiethnic population. Clin. Endocrinol. 2008;68(3):466–472. doi: 10.1111/j.1365-2265.2007.03066.x. [DOI] [PubMed] [Google Scholar]
- van der Meer I.M. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: an overview. Osteoporos. Int. 2011;22(4):1009–1021. doi: 10.1007/s00198-010-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggemoen A.R. Vitamin D status in recently arrived immigrants from Africa and Asia: a cross-sectional study from Norway of children, adolescents and adults. BMJ Open. 2013;3(10) doi: 10.1136/bmjopen-2013-003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkal M.Z. High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk factors. Osteoporos. Int. 2006;17(8):1133–1140. doi: 10.1007/s00198-006-0069-2. [DOI] [PubMed] [Google Scholar]
- Finch P.J. Blunted seasonal variation in serum 25-hydroxy vitamin D and increased risk of osteomalacia in vegetarian London Asians. Eur. J. Clin. Nutr. 1992;46(7):509–515. [PubMed] [Google Scholar]
- Hamson C. Comparative study of bone mineral density, calcium, and vitamin D status in the Gujarati and white populations of Leicester. Postgrad. Med. J. 2003;79(931):279–283. doi: 10.1136/pmj.79.931.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvik K. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo immigrant health study. Eur. J. Clin. Nutr. 2005;59(1):57–63. doi: 10.1038/sj.ejcn.1602033. [DOI] [PubMed] [Google Scholar]
- Islam M.Z. Prevalence of vitamin D deficiency and secondary hyperparathyroidism during winter in pre-menopausal Bangladeshi and Somali immigrant and ethnic Finnish women: associations with forearm bone mineral density. Br. J. Nutr. 2012;107(2):277–283. doi: 10.1017/S0007114511002893. [DOI] [PubMed] [Google Scholar]
- Kim K.J. Vitamin D status and associated metabolic risk factors among North Korean refugees in South Korea: a cross-sectional study. BMJ Open. 2015;5(11) doi: 10.1136/bmjopen-2015-009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M., Thomas M. Vitamin D concentrations in Asian children aged 2 years living in England: population survey. BMJ. 1999;318(7175):28. doi: 10.1136/bmj.318.7175.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P., van Schoor N.M., Bravenboer N. Vitamin D-related disorders, in Primer on the metabolic bone diseases and disorders of mineral metabolism. In: Rosen C.J., editor. The American Society for Bone and Mineral Research. Wiley Blackwell; 2013. pp. 613–623. [Google Scholar]
- Madar A.A., Stene L.C., Meyer H.E. Vitamin D status among immigrant mothers from Pakistan, Turkey and Somalia and their infants attending child health clinics in Norway. Br. J. Nutr. 2009;101(7):1052–1058. doi: 10.1017/S0007114508055712. [DOI] [PubMed] [Google Scholar]
- Martin C.A., Gowda U., Renzaho A.M. The prevalence of vitamin D deficiency among dark-skinned populations according to their stage of migration and region of birth: a meta-analysis. Nutrition. 2016;32(1):21–32. doi: 10.1016/j.nut.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Meulmeester J.F. Vitamin D status, parathyroid hormone and sunlight in Turkish, Moroccan and Caucasian children in the Netherlands. Eur. J. Clin. Nutr. 1990;44(6):461–470. [PubMed] [Google Scholar]
- Munns C.F. Global consensus recommendations on prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016;101(2):394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D3 (25OHD) concentration and parathyroid hormone (PTH) concentration. Arch. Dis. Child. 2016;101(4):316–319. doi: 10.1136/archdischild-2015-308985. [DOI] [PubMed] [Google Scholar]
- Preece M.A. Vitamin-D deficiency among Asian immigrants to Britain. Lancet. 1973;1(7809):907–910. doi: 10.1016/s0140-6736(73)91361-5. [DOI] [PubMed] [Google Scholar]
- Renzaho A.M. Prevalence of vitamin D insufficiency and risk factors for type 2 diabetes and cardiovascular disease among African migrant and refugee adults in Melbourne: a pilot study. Asia Pac. J. Clin. Nutr. 2011;20(3):397–403. [PubMed] [Google Scholar]
- Ruwanpathirana T. Assessment of vitamin D and its association with cardiovascular disease risk factors in an adult migrant population: an audit of patient records at a community health Centre in Kensington, Melbourne, Australia. BMC Cardiovasc. Disord. 2014;14:157. doi: 10.1186/1471-2261-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder F., Bernsen R.M., van der Wouden J.C. Vitamin D supplementation for nonspecific musculoskeletal pain in non-western immigrants: a randomized controlled trial. Ann. Fam. Med. 2012;10(6):547–555. doi: 10.1370/afm.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki T. Are elderly Asians in Britain at a high risk of vitamin D deficiency and osteomalacia? Age Ageing. 1995;24(2):103–107. doi: 10.1093/ageing/24.2.103. [DOI] [PubMed] [Google Scholar]
- Stellinga-Boelen A.A. Vitamin D levels in children of asylum seekers in The Netherlands in relation to season and dietary intake. Eur. J. Pediatr. 2007;166(3):201–206. doi: 10.1007/s00431-006-0221-1. [DOI] [PubMed] [Google Scholar]
- de Torrente de la Jara G., Pecoud A., Favrat B. Musculoskeletal pain in female asylum seekers and hypovitaminosis D3. BMJ. 2004;329(7458):156–157. doi: 10.1136/bmj.329.7458.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton R.J., Bijvoet O.L. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2(7929):309–310. doi: 10.1016/s0140-6736(75)92736-1. [DOI] [PubMed] [Google Scholar]
- Wicherts I.S. Sunlight exposure or vitamin D supplementation for vitamin D-deficient non-western immigrants: a randomized clinical trial. Osteoporos. Int. 2011;22(3):873–882. doi: 10.1007/s00198-010-1343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.