Abstract
Aims
To determine the risk of mycotic infections associated with the use of sodium–glucose co‐transporter 2 inhibitors (SGLT2i) in a real‐world setting.
Methods
We conducted a prescription sequence symmetry analysis using data from Truven Health MarketScan (2009–2015). We selected continuously enrolled patients newly initiating both an SGLT2i and an antifungal between 1 April 2013 and 31 December 2015 within time periods of 30, 60, 90, 180 or 365 days of each other. Adjusted sequence ratios (ASR) were calculated for each time period as the ratio of patients initiating SGLT2i first over those initiating an antifungal first adjusted for time trends in prescribing. Analyses were stratified by sex and type of SGLT2i.
Results
There were 23 276 patients who newly initiated both SGLT2i and an antifungal in our study period. These patients were further classified into those initiating the two drugs within 365 (n = 17 504), 180 (n = 11 873), 90 (n = 7697), 60 (n = 5856) or 30 (n = 3650) days of each other. Increased risks of mycotic infections were present across all time periods, with the strongest effect observed in the 90‐day interval [ASR 1.53 (confidence interval, CI 1.43–1.60)]. Findings differed by sex [90‐day ASR females: 1.65 (CI 1.56–1.74); males 1.25 (CI 1.14–1.36)] and by SGLT2i [90‐day ASR canagliflozin 1.57 (CI 1.49–1.66); non‐canagliflozin 1.42 (CI 1.31–1.55)].
Conclusion
Initiation of SGLT2i was associated with an increased risk for mycotic infections. Findings from this commercially insured population in the real world are consistent with evidence available from clinical trials.
Keywords: diabetes, pharmacoepidemiology, drug safety, evidence‐based medicine
What is Already Known about this Subject
Clinical trials of sodium–glucose co‐transporter 2 inhibitors report a 2–6‐fold increase in the risk for genital mycotic infections.
This increase in risk has not been confirmed in a real‐world setting.
Prescription sequence symmetry analysis is a validated approach for postmarketing safety surveillance.
What this Study Adds
Sodium–glucose co‐transporter 2 inhibitor use in the first 90 days is associated with 53% increased risk for mycotic infections requiring use of a prescription antifungal in a real‐world setting.
Introduction
Type 2 diabetes is a chronic metabolic disease that is characterized by elevated levels of glucose in the blood 1, 2, 3. Diabetes affects over 30 million people living in the USA and 422 million people worldwide 4, 5. Chronic hyperglycaemia has serious effects on the whole body, making diabetes one of the leading causes of blindness, renal failure, nontraumatic amputations and death worldwide. Sodium–glucose co‐transporter 2 inhibitors (SGLT2i) are a new class of oral antihyperglycemic agents that prevent the resorption of glucose in the proximal renal tubule and promote glucosuria 6. In addition to their antihyperglycaemic effect, clinical trials of SGLT2i have demonstrated significant cardio‐ and reno‐protective effects 7, 8.
Genital mycotic infections are one of the most common adverse effects of treatment with SGLT2i reported in clinical trials 9, 10, 11. Meta‐analyses of data from clinical trials have found a 2–6‐fold increase in the incidence of vulvovaginitis in women and balanitis in men following treatment with canagliflozin, dapagliflozin or empagliflozin relative to an active comparator (another oral antidiabetic) 6, 12, 13, 14. Most cases reported occur as singular episodes that are resolved by treatment with an anti‐fungal medication. The mechanism of action by which treatment with SGLT2i results in genital mycotic infections remains unclear 15. It has been postulated that increased urinary glucose excretion favours fungal colonization but a similar increase in risk has not been observed in patients with familial renal glucosuria 15. Evidence related to the relationship between SGLT2i and genital mycotic infections comes almost exclusively from clinical trials. Patient characteristics and behaviours may vary widely outside of a controlled setting such as a clinical trial. For example, adherence to antidiabetic medications is suboptimal in actual practice whereas strictly enforced in clinical trials 16. We therefore considered it important to study this relationship in a real‐world setting. Evidence from observational research and clinical trials can be used together to allow patients and healthcare providers to make informed decisions about treatments. To the best of our knowledge, no large population‐based studies have been conducted in the USA to determine the extent of the risk of SGLT2i related mycotic infections in a real‐world setting.
Methods
Our objective was to determine the risk of mycotic infections associated with the initiation of SGLT2i in a real‐world setting. To accomplish this, we performed prescription sequence symmetry analysis (PSSA), a case‐based observational study design used in pharmacovigilance research for rapid detection of specific medication safety concerns or for efficiently mining vast amounts of data in active surveillance 17.
PSSA
The design of PSSA studies has been described previously 18, 19, 20. Briefly, this case‐based study design examines the distribution of sequences in which a pair of drugs are initiated within a patient. In studies of drug safety, the pair of drugs includes (i) an index drug and (ii) a remedy drug that is prescribed for an adverse effect caused by the index drug. In the absence of an adverse effect, the sequence of drug initiation within a patient is random and patients are equally likely to initiate either drug first. That is, there is symmetry in the distribution of sequences in which drugs are initiated. However, in the presence of an adverse effect the distribution of sequences favors the index drug first followed by the remedy. That is, there is an asymmetry in the distribution of sequences with a greater number of patients initiating the index drug first. The effect measure is a sequence ratio defined as the number of patients initiating the index drug first over those initiating the index drug last. In theory, these sequence ratios approximate incidence rate ratios. Given that this approach only studies patients that initiated both index and remedy drugs, its main advantage is that the analysis remains unaffected by confounders that are time invariant within a patient. Moreover, this approach is efficient since it only relies on prescription data. However, its validity in studies of drug safety relies on the specificity of the remedy drug that serves as a surrogate for the actual outcome and other assumptions, such as no bias, being true.
Data source
Our PSSA was conducted using data from the 2009–2015 Truven Health MarketScan Commercial and Medicare Supplemental databases. The MarketScan databases aggregate adjudicated patient‐level healthcare resource utilization data from commercial health insurance plans run by approximately 350 private‐sector employers and payers across the USA. Healthcare resource utilization data include diagnoses, procedures recorded during inpatient admissions or outpatient encounters. In addition, the data also capture prescription medications dispensed in an outpatient setting. Drugs dispensed are identified by National Drug Codes. Additional prescribing information available includes date of dispensing, days' supply, metric quantity, and costs borne by the patient and the payer. The MarketScan databases have been widely used in observational research examining treatment utilization and health outcomes resulting in over 300 peer‐reviewed publications as of 2016 21. Evidence generated using such data is generalizable to commercially insured individuals living in the USA.
Study population
Our study included patients initiating both index (SGLT2i) and remedy (antifungal) drugs within a certain time period during the study period. Our study period was 1 April 2013 (SGLT2i first approved in the USA) to 31 December 2015 (end of our data). Time periods studied included 30, 60, 90, 180 and 365 days between the initiation of index and remedy drugs. Index drugs included canagliflozin, dapagliflozin and empagliflozin. Remedy drugs included azole antifungals that are commonly indicated for treatment of genital mycotic infections, including butoconazole, clotrimazole, ketoconazole, fluconazole, miconazole, nystatin, sulfanilamide, itraconazole, terconazole and tioconazole. Treatment initiation was defined as no prior use of SGLT2i or antifungals during the baseline period. The baseline period was defined as 12 months prior to the first prescription for index or remedy drug. Patients were excluded from the analysis if they lacked continuous enrolment during the baseline period or if they initiated both index and remedy drugs on the same date.
Statistical analysis
A crude sequence ratio (CSR) was calculated for each time period as the ratio of number of patients initiating a SGLT2i first over number of those initiating an antifungal first. Given that temporal trends in drug utilization can extraneously influence sequences of drug initiation, a corrective measure called a null effect ratio (NER) was calculated. The NER incorporates probabilities of daily incident therapies and was calculated as follows:
Variables included in the calculation of a are:
m denotes days of the survey period, 1 April 2013 to 31 December 2015.
n denotes day m + 1 of the survey period.
u is the last day of the survey period, 31 December 2015.
SGLT2i m is number of incident SGLT2i therapies on day m.
AFn is number of incident antifungal therapies on consecutive day n.
An adjusted sequence ratio (ASR) was calculated for each time period as the CSR divided by the NER. Confidence intervals (95% CI) were constructed around CSRs and ASRs using the binomial distribution as follows 22, 23:
Where standard error was calculated as:
Findings were considered statistically significant at an α level of 0.05 if the 95% CI did not include the null (1.0). No adjustments were made for multiple comparisons.
Subgroup analyses
The risk of mycotic infections was assessed in dichotomous subgroups of sex and type of SGLT2i initiated (canagliflozin vs. non‐canagliflozin).
Study and analytic datasets were developed using SAS software Version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical analyses were performed using Microsoft Excel 2010 for Windows 7 (Microsoft Corporation, Redmond, WA, USA). The University of Illinois at Chicago Office for the Protection of Research Subjects determined that this study does not meet the definition of human subject research.
Results
There were 23 276 patients who initiated both a SGLT2i and an antifungal medication during the period 1 April 2013 to 31 December 2015 and met the selection criteria. Of these, 17 504 initiated index and remedy drugs within 365 days of each other. Patients were further classified into those who initiated both drugs within 30 (n = 3650), 60 (n = 5856), 90 (n = 7697) or 180 (n = 11 873) days. Of the 17 504 patients who initiated both drugs a maximum of 365 days apart, 69.4% were female. The average age was 49.5 (standard deviation, ± 0.6) years. Canagliflozin was the most frequently initiated index drug (68.9%), followed by dapagliflozin (22.4%). Fluconazole was the most frequently initiated remedy drug (58.3%), followed by nystatin (15.6%) and clotrimazole (15.3%).
In the analysis where index and remedy drugs were initiated within 365 days, the majority (56.6%) initiated SGLT2i first in the sequence resulting in a CSR of 1.31 (CI 1.27–1.35). The NER for this period was 1.05, indicating that trends in prescribing SGLT2i and antifungals were relatively stable within the period. Adjusting for prescribing trends resulted in an ASR of 1.24 (CI 1.20–1.28), indicating that initiating an SGLT2i is associated with a 24% increase in the rate of mycotic infections requiring use of antifungal medication use in a 12‐month period post‐treatment initiation. Table 1 describes the sequences in the other time periods. Figure 1 shows ASRs for all time periods. In general, there is an increased risk for mycotic infections following treatment with SGLT2i, but the risk is highest during the first 90 days [ASR 1.53 (CI 1.43–1.60)].
Table 1.
Crude and adjusted sequence ratios, overall and by subgroups
| Analysis | Number initiating SGLT2i and antifungal | SGLT2i first | Antifun gal first | CSR (95% CI) | NER | ASR (95% CI) |
|---|---|---|---|---|---|---|
| Overall | ||||||
| ±30 days | 3650 | 2113 | 1537 | 1.37 (1.28–1.46) | 1.01 | 1.35 (1.26–1.44) |
| ±60 days | 5856 | 3537 | 2319 | 1.53 (1.45–1.61) | 1.03 | 1.48 (1.40–1.56) |
| ±90 days | 7697 | 4756 | 2941 | 1.62 (1.55–1.70) | 1.06 | 1.53 (1.46–1.60) |
| ±180 days | 11 873 | 7212 | 4661 | 1.55 (1.49–1.61) | 1.09 | 1.42 (1.37–1.47) |
| ±365 days | 17 504 | 9911 | 7593 | 1.31 (1.27–1.35) | 1.05 | 1.24 (1.20–1.28) |
| Female | ||||||
| ±30 days | 2759 | 1658 | 1101 | 1.51 (1.40–1.63) | 1.02 | 1.48 (1.37–1.60) |
| ±60 days | 4380 | 2734 | 1646 | 1.66 (1.56–1.77) | 1.04 | 1.60 (1.50–1.70) |
| ±90 days | 5684 | 3617 | 2067 | 1.75 (1.66–1.85) | 1.06 | 1.65 (1.56–1.74) |
| ±180 days | 8493 | 5268 | 3225 | 1.63 (1.56–1.71) | 1.09 | 1.50 (1.44–1.57) |
| ±365 days | 12 147 | 6976 | 5171 | 1.35 (1.30–1.40) | 1.04 | 1.29 (1.24–1.34) |
| Male | ||||||
| ±30 days | 891 | 455 | 436 | 1.04 (0.92–1.19) | 1.01 | 1.04 (0.91–1.18) |
| ±60 days | 1476 | 803 | 673 | 1.19 (1.08–1.32) | 1.02 | 1.17 (1.05–1.29) |
| ±90 days | 2013 | 1139 | 874 | 1.30 (1.19–1.42) | 1.05 | 1.25 (1.14–1.36) |
| ±180 days | 3380 | 1944 | 1436 | 1.35 (1.26–1.45) | 1.09 | 1.24 (1.16–1.33) |
| ±365 days | 5357 | 2935 | 2422 | 1.21 (1.15–1.28) | 1.07 | 1.13 (1.07–1.19) |
| Canagliflozin | ||||||
| ±30 days | 2533 | 1494 | 1039 | 1.44 (1.33–1.45) | 1.02 | 1.41 (1.31–1.53) |
| ±60 days | 4068 | 2488 | 1580 | 1.57 (1.48–1.68) | 1.03 | 1.53 (1.43–1.63) |
| ±90 days | 5371 | 3348 | 2023 | 1.65 (1.57–1.75) | 1.05 | 1.57 (1.49–1.66) |
| ±180 days | 8265 | 5091 | 3174 | 1.60 (1.53–1.68) | 1.08 | 1.48 (1.42–1.55) |
| ±365 days | 12 053 | 7003 | 5050 | 1.39 (1.34–1.44) | 1.06 | 1.30 (1.25–1.35) |
| Non‐canagliflozin | ||||||
| ±30 days | 1117 | 619 | 498 | 1.24 (1.10–1.40) | 1.02 | 1.22 (1.09–1.37) |
| ±60 days | 1788 | 1049 | 739 | 1.42 (1.29–1.56) | 1.04 | 1.36 (1.24–1.49) |
| ±90 days | 2326 | 1408 | 918 | 1.53 (1.41–1.67) | 1.08 | 1.42 (1.31–1.55) |
| ±180 days | 3608 | 2121 | 1487 | 1.43 (1.33–1.52) | 1.11 | 1.29 (1.21–1.38) |
| ±365 days | 5451 | 2908 | 2543 | 1.14 (1.08–1.20) | 1.05 | 1.09 (1.03–1.15) |
ASR, adjusted sequence ratio; CI, confidence interval; CSR, crude sequence ratio; NER, null effect ratio; SGLT2i, sodium–glucose co‐transporter 2 inhibitor
Figure 1.
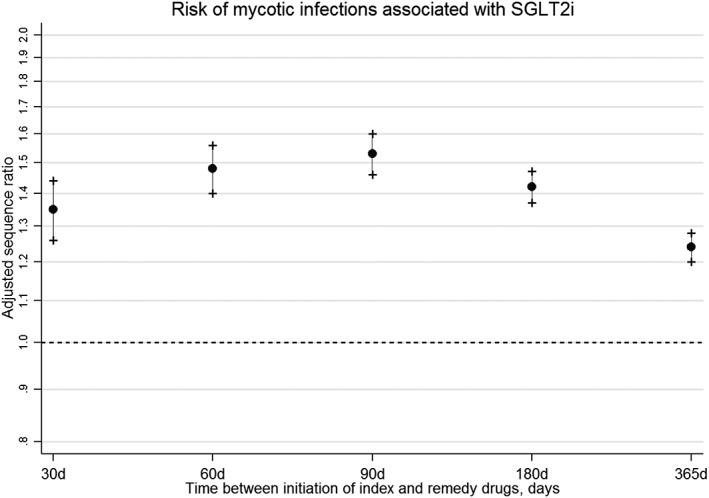
Risk of mycotic infections associated with sodium–glucose co‐transporter 2 inhibitors
Figure 2 shows the asymmetry in the incidence of antifungal medication use in relation to time since initiation of SGLT2i. There were more incident antifungal therapies in the months following SGLT2i initiation than before initiation of SGLT2i. In the 12 months prior to SGLT2i use, antifungal prescribing was relatively stable over time. There was a sharp increase in incident antifungal use immediately following initiation of SGLT2i. Antifungal prescribing remained high in the first 3 months post SGLT2i‐initiation, after which antifungal prescribing returned to rates observed prior to SGLT2i use.
Figure 2.
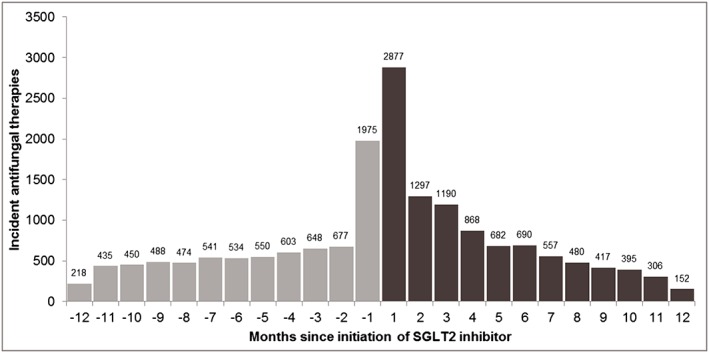
Incident antifungal use in relation to initiation of sodium–glucose co‐transporter 2 (SGLT2) inhibitor
In subgroup analyses by sex (Figure 3), ASRs for risk of developing mycotic infections requiring prescription antifungal use were consistently elevated among women; whereas ASRs indicating increased risk in men were lower than that in women and had CIs including 1.0 in early time periods. Risk in women was generally higher across all analysis periods, but the highest risk observed in these stratified analyses were within the first 90 days [90‐day ASR females: 1.65 (CI 1.56–1.74); males: 1.25 (CI 1.14–1.36)].
Figure 3.
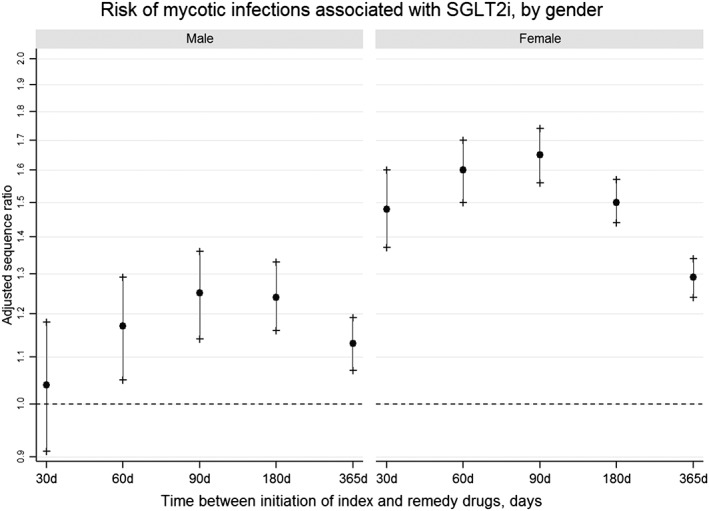
Risk of mycotic infections associated with sodium–glucose co‐transporter 2 inhibitors, by sex
Risk estimates for mycotic infections also differed slightly by the type of SGLT2i initiated (Figure 4). Antifungal use was higher following treatment with canagliflozin compared to treatment with dapagliflozin or empagliflozin [90‐day ASR canagliflozin: 1.57 (CI 1.49–1.66); non‐canagliflozin: 1.42 (CI 1.31–1.55)].
Figure 4.
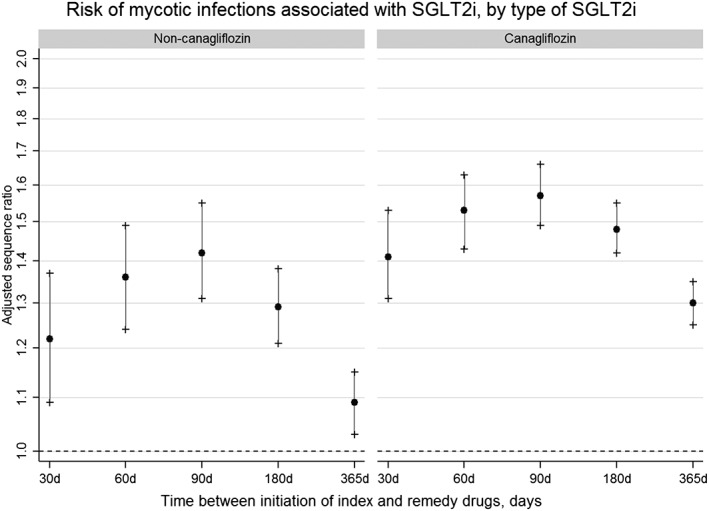
Risk of mycotic infections associated with sodium–glucose co‐transporter 2 inhibitors, by type of inhibitor
Discussion
We conducted a large, population‐based observational study of commercially insured individuals residing in the USA to determine the risk of genital mycotic infections associated with the initiation of SGLT2i in a real‐world setting. Our analysis of patients newly initiating both SGLT2i and azole antifungal medications within predefined time periods between April 2013 and December 2015 examined the sequence in which these two drugs were initiated. Across all time periods analyzed, we demonstrated an increased risk for mycotic infections following initiation of SGLT2i. The observed risk was highest among women, patients newly initiating canagliflozin, and within the first 90 days post‐SGLT2i initiation.
Study findings in the context of current evidence
The observed increase in risk in this study is consistent with results from several meta‐analyses of clinical trials 24, 25, 26, 27. In one recent meta‐analysis, the increase in risk for genital mycotic infections relative to placebo was 4.9‐ and 5.2‐fold for canagliflozin 100 mg and 300 mg, respectively; 4.3‐ and 5.0‐fold for dapagliflozin 5 mg and 10 mg, respectively; and 3.8‐ and 3.7‐fold for empaglifozin 10 mg and 25 mg, respectively 15. Relative to an active comparator, another meta‐analysis reported the following risk ratios for genital tract infections: canagliflozin 4.96 (3.35–7.34); dapagliflozin 4.21 (2.85–6.23); empagliflozin 2.69 (1.43–5.06) 12. In comparison with clinical trials, we observed a more modest elevation in risk.
The magnitude of risk for mycotic infections was higher among patients initiating canagliflozin. While the mechanism of antihyperglycemic action is similar across all SGLT2i, canagliflozin alone has been associated with an increased risk for other adverse events 28, 29. It is unclear if differences observed in the metabolism and elimination of these agents contributes to different safety profiles 9, 10, 11. A recent meta‐analysis also noted a larger effect for canagliflozin 12. However, there are no direct head‐to‐head comparisons across SGLT2i. One network meta‐analysis indirectly compared across SGLT2i and found no significant differences among canagliflozin vs. dapagliflozin or canagliflozin vs. empagliflozin 24. Direct comparisons may be investigated further in future research.
In a study of patients with diabetes in an Australian general practice setting, increased risk for genital infections was higher with SGLT2i use relative to dipeptidyl peptidase‐4 inhibitors (DPP4i), another second‐line antihyperglycaemic medication 30. The study compared new users of SGLT2i (n = 1977) and DPP4i (n = 1964) followed from date of first prescription until the earliest occurrence of an infection, death or end of follow up. The rate of infections among SGLT2i users was 2.9% vs. 0.9% in DPP4i users, resulting in an adjusted hazard ratio of 3.5 (95% CI 1.95–5.89). The authors reported that the majority of infections in the SGLT2i cohort occurred within the first 12 weeks. This latency period between SGLT2i initiation and development of mycotic infection is consistent with our study and clinical trials 26, which demonstrate the greatest risk within the first 90 days of treatment.
The magnitude of our findings (1.2–1.5‐fold increased risk) was modest compared to findings from clinical trials (2–6‐fold increased risk) or from the Australian general practice setting (3.5‐fold increased risk). While our data only captured prescription antifungals dispensed in an outpatient setting, the majority of uncomplicated mycotic infections in the USA are treated with antifungal medications available over‐the‐counter, possibly explaining the differences in our estimates. Instead, our findings are likely to be representative of patients receiving prescription antifungals for a more severe or persistent mycotic infection 31. Therefore, our findings probably underestimate the total burden of this clinically significant adverse effect and may be interpreted as the risk of mycotic infections requiring treatment with a prescription antifungal.
Study limitations
The asymmetry in drug sequence initiation in our study where there was increased incidence of antifungal use in the months after SGLT2i initiation is consistent with findings from clinical trials of these medications. However, inferences regarding symmetry and asymmetry in this type of study design hold true under the assumptions that there are no biases due to confounding. Common sources of bias in this design include confounding by indication, confounding by disease severity, reverse causality and time‐dependent confounding. Figure 2 helps us evaluate the presence of confounding by disease severity. In the months before SGLT2i initiation, incident antifungal use is relatively stable. We observed some increases in the use of antifungals in the 1 month before SGLT2i initiation, possibly as a result of prophylactic antifungal prescribing where patients have the remedy medication on hand in anticipation of this common adverse event. Alternately, this is a demonstration of confounding by disease severity where poorly controlled diabetes may trigger both events – mycotic infections and prescription of SGLT2 inhibitors. Research on prescribing practices at the time of initiation of SGLT2i may help clarify these findings. Future research incorporating negative control exposures may also help evaluate confounding by indication.
Our design is also vulnerable to two sources of bias that may lead to an over estimation of effects. The first is due to differential misclassification. We cannot measure over‐the‐counter use of antifungals. We may be seeing a higher number of patients initiating the remedy drug second as their recent change in antidiabetic treatment may lead to their use of a prescription antifungal vs. over‐the‐counter antifungal. The second is due to reverse causation. Prescription sequence symmetry analysis, when used in investigations of documented adverse effects, may be susceptible to bias due to reverse causation 32. Effect estimates may be overestimated due to spuriously high number of patients initiating the remedy drug second, given that prescribers with the knowledge of the adverse effect may avoid prescribing the index drug second. The degree to which this affects our findings is unknown, given that we do not know how prescribers weigh benefits of SGLT2 inhibitors (antihyperglycaemic effect, cardio‐ and renoprotective effects) against the risk of mycotic infections at the point of care. Moreover, our effect estimates were modest in comparison to findings from clinical trials (1.2–1.5‐fold increase in risk in our study as opposed to 2–6‐fold increase in risk in clinical trials).
Finally, we have limitations related to the use of claims data. The use of prescription antifungals serves as a surrogate for our outcome of interest. We included all possible agents used to treat a mycotic infection, however, since our outpatient dispensing data lack an indication, we cannot be certain that it is a genital mycotic infection (as opposed to other fungal infections) that provoked the use of the antifungal. While the use of administrative pharmacy records is a reliable and reproducible method to describe health care utilization, it is unknown whether the dispensed medication was taken as directed.
Study strengths
It has been noted that most genital mycotic infections in the primary care setting do not result in an office visit. Many patients resolve uncomplicated, symptomatic mycotic infection by receiving consultation from care providers by phone and practicing self‐care with over‐the‐counter antifungals. Therefore, the association between SGLT2i and persistent or severe mycotic infections in a real‐world setting may be better elucidated using prescribing data. PSSA has demonstrated moderate sensitivity (61%) and high specificity (93%) for safety signal detection using administrative prescribing data 33. Through the case‐based approach utilized in PSSA, individuals serve as their own controls. Therefore, our findings are robust to confounding by race, family history, genetic factors and time‐invariant factors within each individual 34.
Conclusions
To the best of our knowledge, ours is the first study conducted in a real‐world setting in the USA. We found up to 1.5‐fold increase in risk for mycotic infections requiring the use of prescription antifungals following treatment with SGLT2i with the greatest risk occurring within the first 90 days of treatment. Our findings may help guide patient education at the initiation of treatment with SGLT2i for diabetes.
Competing Interests
There are no competing interests to declare.
The authors wish to express their sincere gratitude to Dr Tianxiu Wang, at the Center for Clinical and Translational Science at the University of Illinois at Chicago (funded by grant UL1TR002003), for providing assistance with the calculation of CIs.
Adimadhyam, S. , Schumock, G. T. , Calip, G. S. , Smith Marsh, D. E. , Layden, B. T. , and Lee, T. A. (2019) Increased risk of mycotic infections associated with sodium–glucose co‐transporter 2 inhibitors: a prescription sequence symmetry analysis. Br J Clin Pharmacol, 85: 160–168. 10.1111/bcp.13782.
References
- 1. Triplitt CL, Repas T, Alvarez C. Chapter 57. Diabetes mellitus In: Pharmacotherapy: A Pathophysiologic Approach, 9e, eds DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. New York, NY: The McGraw‐Hill Companies, 2014. Available at accesspharmacy.mhmedical.com/content.aspx?aid=57496051 (last accessed 19 June 2017). [Google Scholar]
- 2. Association AD . 2. Classification and diagnosis of diabetes. Diabetes Care 2017; 40 (Suppl. 1): S11–S24. 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 3. Fowler MJ. Diagnosis, classification, and lifestyle treatment of diabetes. Clin Diabetes 2010; 28: 79–86. [Google Scholar]
- 4. American Diabetes Association . Fast Facts ‐ Data and Statistics About Diabetes|American Diabetes Association. Available at http://professional.diabetes.org/content/fast‐facts‐data‐and‐statistics‐about‐diabetes (last accessed 3 May 2017).
- 5. World Health Organization . Global Report on Diabetes. Geneva: World Health Organization, 2016. [Google Scholar]
- 6. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 9. INVOKANA® [prescribing information] . Titusville, NJ: Janssen Pharmaceuticals, Inc., 2015. Available at https://www.invokanahcp.com/dosing‐prescribing (last accessed 17 July 2017).
- 10. FARXIGA [package insert] . Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2016. Available at https://www.farxiga-hcp.com/ (last accessed 17 July 2017).
- 11. JARDIANCE [prescribing information] . Ridgefort, CT: Boehringer Ingelheim Pharmaceuticals, Inc., 2015. Available at https://jardiancehcp.com/ (last accessed 17 July 2017).
- 12. Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT‐2 inhibitors and the risk of infections: a systematic review and meta‐analysis of randomized controlled trials. Acta Diabetol 2018; 55: 503–514. [DOI] [PubMed] [Google Scholar]
- 13. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co‐transport‐2 inhibitors in type 2 diabetes: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16: 457–466. [DOI] [PubMed] [Google Scholar]
- 14. Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, et al Benefits and harms of sodium‐glucose co‐transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta‐analysis. PLoS One 2016; 11: e0166125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium‐glucose co‐transporter 2 inhibitors: a meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2017; 19: 348–355. [DOI] [PubMed] [Google Scholar]
- 16. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care 2017; 40: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 17. Lai ECC, Pratt N, Hsieh CY, Lin SJ, Pottegård A, Roughead EE, et al Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol 2017; 32: 567–582. [DOI] [PubMed] [Google Scholar]
- 18. Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology 1996; 7: 478–484. [PubMed] [Google Scholar]
- 19. Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf 2009; 18: 483–491. [DOI] [PubMed] [Google Scholar]
- 20. Pratt NL, Ilomäki J, Raymond C, Roughead EE. The performance of sequence symmetry analysis as a tool for post‐market surveillance of newly marketed medicines: a simulation study. BMC Med Res Methodol 2014; 14: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang S. Health Research Data for the Real World: the MarketScan Databases. 2011. Available at http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf (last accessed 19 September 2017).
- 22. van Boven JFM, de Jong‐van den Berg LT, Vegter S. Inhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysis. Drug Saf 2013; 36: 231–236. [DOI] [PubMed] [Google Scholar]
- 23. Morris JA, Gardner MJ. Statistics in medicine: calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J Clin Res Ed 1988; 296: 1313–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium‐glucose co‐transporter‐2 inhibitors in type 2 diabetes mellitus: systematic review and network meta‐analysis. Diabetes Obes Metab 2016; 18: 783–794. [DOI] [PubMed] [Google Scholar]
- 25. Wu JHY, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2016; 4: 411–419. [DOI] [PubMed] [Google Scholar]
- 26. Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, et al Genital mycotic infections with canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin 2014; 30: 1109–1119. [DOI] [PubMed] [Google Scholar]
- 27. Huilin Tang M, Jingjing Zhang MD, Yiqing Song MD. Adverse effects and safety of SGLT2 inhibitor use among patients with type 2 diabetes: findings from RCT evidence. North Am J Med Sci 2017; 10: 78–82. [Google Scholar]
- 28. US Food and Drug Administration . Drug Safety and Availability ‐ FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. Available at https://www.fda.gov/Drugs/DrugSafety/ucm500965.htm (last accessed 16 March 2018).
- 29. US Food and Drug Administration . Safety Alerts for Human Medical Products ‐ Invokana and Invokamet (canagliflozin): Drug Safety Communication ‐ New Information on Bone Fracture Risk and Decreased Bone Mineral Density. Available at http://wayback.archive-it.org/7993/20170112164004/http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm461876.htm (last accessed 15 August 2017).
- 30. Gadzhanova S, Pratt N, Roughead E. Use of SGLT2 inhibitors for diabetes and risk of infection: analysis using general practice records from the NPS MedicineWise MedicineInsight program. Diabetes Res Clin Pract 2017; 130: 180–185. [DOI] [PubMed] [Google Scholar]
- 31. Gurwitz JH, McLaughlin TJ, Fish LS. The effect of an Rx‐to‐OTC switch on medication prescribing patterns and utilization of physician services: the case of vaginal antifungal products. Health Serv Res 1995; 30: 672–685. [PMC free article] [PubMed] [Google Scholar]
- 32. Pouwels KB, Visser ST, Bos HJ, Hak E. Angiotensin‐converting enzyme inhibitor treatment and the development of urinary tract infections: a prescription sequence symmetry analysis. Drug Saf 2013; 36: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 33. Wahab IA, Pratt NL, Wiese MD, Kalisch LM, Roughead EE. The validity of sequence symmetry analysis (SSA) for adverse drug reaction signal detection. Pharmacoepidemiol Drug Saf 2013; 22: 496–502. [DOI] [PubMed] [Google Scholar]
- 34. Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med 2013; 125: 33–46. [DOI] [PubMed] [Google Scholar]


